Open Access Article
Open Access ArticleSustainable decommissioning of perovskite solar cells: from waste to resources
Valentina
Larini†
,
Matteo
Degani
 ,
Silvia
Cavalli†
,
Silvia
Cavalli†
 and
Giulia
Grancini
and
Giulia
Grancini
 *
*
Department of Chemistry & INSTM, University of Pavia, Via T. Taramelli 14, 27100 Pavia, Italy. E-mail: giulia.grancini@unipv.it
First published on 24th June 2025
Abstract
Perovskite solar cells (PSCs) have witnessed a rapid progression as emerging alternatives for innovative photovoltaics (PVs). However, this promising growth also comes with challenges related to the end-of-life (EoL) management of exhausted devices. In this review, we discuss different studies on the implications of the decommissioning of PSCs from a sustainable perspective by reviewing current PSC recycling strategies as general guidelines in the field of EoL PSCs. We hope that this review would encourage the necessary development of more virtuous energy-efficient and environmentally friendly recycling protocols for PSC recovery, from lab- to large-scale application in view of perovskite-based PV technology's imminent jump to the market.
1. Introduction
One of the most urgent global issues is accomplishing the challenge of carbon “net zero” in the context of the UN sustainable development goals. Therefore, intensifying eco-friendly energy-conversion technologies is mandatory. In this respect, the International Renewable Energy Agency (IRENA) predicted that in the coming years, photovoltaics (PVs) will lead the industry of renewable energy production. However, this inspiring near-futuristic vision comes with a downside related to the end-of-life (EoL) management of exhausted solar panels. In fact, IRENA also estimated that global solar PV waste will reach 60 million metric tons by 2050.1 Other studies also envision scenarios of cumulative PV module waste production between 54 million and 160 million metric tons.2 Even though this may appear as a relevant quantity of waste, it is important to compare this quantity with the much higher waste production from other types of technologies (i.e. coal ash and oily sludge waste generated from fossil fuel energy). This means that continuous research on long-lasting high-yield PV modules together with their reuse and recycling is still of great relevance in contributing to decarbonization, and EoL strategies need to be addressed in the early stages of any solar cell technology development.In photovoltaics, perovskite solar cells (PSCs) have attracted great attention from the scientific community and from stakeholders and government3 as new emerging solar energy production devices4 owing to their various advantages. Among these, their low energy payback time (EPBT) and levelized cost of electricity (LCOE) together with a high record power conversion efficiency (PCE) make them ideal candidates in the solar arena. In less than 20 years, PSCs have approached lab-scale record efficiency (27%),5 which monocrystalline silicon technologies attained only after more than 50 years of research. The reason behind the success of PSCs lies in their extensive tolerance towards defects, besides being processed from solutions of the perovskite active material, which results in reduced charge carrier recombination and long carrier diffusion length, leading to remarkable PCEs.6,7 Perovskite materials can be integrated with other photovoltaic technologies to fabricate tandem or triple-junction solar cells, thereby exceeding the efficiency limitations of conventional single-junction devices. In the most widely studied tandem architectures, a perovskite absorber is deposited on top of another photovoltaic layer, such as a secondary perovskite layer (perovskite/perovskite) or a crystalline silicon (perovskite/silicon). Notably, in the 2-terminal perovskite/silicon configuration, recent advancements have enabled a PCE of 34.6%, demonstrating the potential of these hybrid approaches to revolutionize next-generation photovoltaics.8
Despite their high efficiencies and broad applicability, PSCs still suffer from device longevity with respect to the 20 years of stable operation required from commercial PV technologies.9 Extensive research efforts have been made to enhance the stability of PSCs,10,11 spanning from strategies that tackle intrinsic properties of perovskites to encapsulation procedures to secure the device from the external environment.12–14 Consequently, several companies have just started working on perovskite-based PV installed pilot lines and produced module prototypes for commercialization.15 Therefore, it is estimated that perovskite-based PVs will soon contribute to the global solar PV market and consequently also to PV waste generation.
In this respect, PSC decommissioning becomes an urgent task that, in our opinion, should be readily addressed at the device design stage. According to the European Union, 80% of a product's environmental impact is determined during its design phase.16 Moreover, in line with the 12th sustainable development goal of the Agenda 2030, the recovery of PSCs would meet the requirements of responsible consumption and production patterns.17 It is also important to keep in mind that just converting renewable solar energy into electricity does not directly translate to green production. Therefore, to achieve real sustainability, both the recyclability and the environmental impact of such a novel energy-conversion technology must be considered. In this regard, the circular economy, whose pillars are, in a priority order, reduce, reuse, recycle, and recover, is the model of choice for minimizing material waste and creating further value from depleted PSCs. Life cycle assessment (LCA) comparing landfill and recycling EoL scenarios for exhausted PSCs demonstrates that the recovery of critical components of PSCs reduces the environmental impact of such technology,18,19 as schematically depicted in Fig. 1. Moreover, recycling PSCs results in the safe management of their toxic contents, mainly represented by lead (Pb). It is well known that Pb represents a hazard to human health because it can enter the bloodstream by several paths (ingestion, inhalation or dermal contact) and can accumulate in the skeleton (with a half-life of 40–50 years).20,21 Furthermore, Pb is known to be genotoxic, and it can cause neurological and non-neurological different types of disorders.22 By collecting PbI2 or the entire perovskite from spent PSCs, lead pollution can be reduced, while PSC sustainability is enhanced.
For these reasons, in recent years, many efforts have been made to ensure the circular deployment of perovskite-based PVs, developing sustainable protocols, in particular, employing green solvents to manage EoL and remanufacturing of PSCs (e.g. recycling of toxic lead iodide component and reuse of expensive charge transporting materials and metal contacts). Meanwhile, it is compelling to adequately disseminate to the scientific community the major outcomes of this important research aspect to boost perovskite-based PV technology's full development and its imminent launch to the market.
Thus, in this review, we first give a brief introduction to PSC technology, mainly focusing on the architecture of the different available devices, as basic knowledge to allow full comprehension of the subject to non-specialised readers. Then, we present various studies on the implications of the decommissioning of PSCs from a sustainable perspective through an overview of the proposed LCA and techno-economic analysis (TEA) in the literature, demonstrating the fact that such types of environmentally friendly “best practices” applied to PSCs can produce valuable resources from waste even at the lab-scale. We further proceed by commenting on different recovery protocols by discussing several published works from lab- to large-scale. Specifically, we summarise the existing recovery strategies for each (or multiple) material(s) that comprises PSCs, providing an overview of related articles following the waste hierarchy reported in the Directive 2008/98/EC of the European Parliament. Then, we discuss the impact of the solvent choice on recycling strategies and the rationale behind its selection, with special attention paid to the implementation of the use of green solvents. Finally, we conclude by summarising the main outcomes as general guidelines in the field and providing future perspectives.
2. Perovskite photovoltaic technologies
Perovskites are a class of materials characterized by an ABX3 crystal structure, where “A” represents an organic or inorganic cation, such as cesium (Cs+), methylammonium (MA+, CH3NH3+), and formamidinium (FA+, CH3CH2NH3+). The “B” site is occupied by a metal cation, commonly tin (Sn2+) or lead (Pb2+), while “X” is a halide anion, including iodide (I−), chloride (Cl−), or bromide (Br−). By carefully selecting different combinations of these elements, it is possible to tailor the properties of perovskite materials for specific applications in photovoltaics and optoelectronics.4,23,24One of the key advantages of lead-based perovskite materials is their excellent optoelectronic properties. They exhibit a high absorption coefficient (∼5.7 × 104 cm−1 at 600 nm), low Auger recombination, efficient charge carrier mobility (1–10 cm2 V−1 s−1), and long charge diffusion lengths. These characteristics make perovskites highly attractive for applications in solar cells, light-emitting diodes (LEDs), photodetectors, and other optoelectronic devices.
Furthermore, tunable bandgaps ranging from 1.3 to 2.2 eV are extremely important for tandem and multi-junction solar cells (Fig. 2c and d).25 In fact, in the context of perovskite solar cells (PSCs), different device architectures can be employed.26 As schematically illustrated in Fig. 2a and b, the single-junction configuration follows either an n–i–p (NIP) or p–i–n (PIN) structure depending on the arrangement of the charge transport layers relative to the light path.27 Common hole transport materials (HTMs) include spiro-OMeTAD, NiO, PTAA, and SAM layers, while electron transport materials (ETMs) frequently used are C60, PCBM, and SnO2.28,29
 | ||
| Fig. 2 Schematic illustration of different perovskite device architectures: (a) NIP, (b) PIN, (c) tandem and (d) triple junction solar cells. | ||
Perovskite materials offer versatile applicability across various photovoltaic (PV) domains,30 including indoor PV, building-integrated photovoltaics (BIPV), and agrivoltaics.31,32 This broad adaptability stems from their facile processability, as perovskite films can be deposited using solution-based techniques or vacuum evaporation methods, enabling scalable and cost-effective manufacturing.33
3. Sustainability and economic considerations of decommissioning PSCs
From an eco-friendly broad context, green energy production plays a strong role in fighting the climate crisis, with the ambition of substituting fossil fuels with other renewable sources of energy. The selection of one type over another technology can be driven by several aspects, such as geographical collocation and material availability. For a total evaluation of new green energy-conversion technology, besides efficiency, lifetime, and ease of manufacturing, environmental sustainability and economic advantages are timely fundamental aspects to consider when developing novel techniques and must also be considered as important indicators when designing proper recovery strategies. In fact, each adopted process accounts for material and electricity consumption as well as for environmental impact, which needs to be carefully evaluated to render both the process and the recovery protocols advantageous from a commercialisation perspective. LCA and TEA have been performed to evaluate the competitiveness of different PV technologies34 and can also be useful types of studies to compare different recoveries of PV device processes.In the contest of perovskite-based PVs, works that compare circular EoL approaches with landfill EoL scenarios for PSCs are of fundamental importance to foresee a virtuous direction to sustainable PSC commercialisation that may start already at the lab-scale and are discussed in detail in the subsequent sections.35
3.1. Environmental impact of recovery strategies by life cycle assessment
As anticipated above, LCA is a powerful tool for assessing the environmental impact of a product. Usually performed as a comparative analysis, it evaluates the sustainability of all manufacturing, operational, and disposal processes occurring during a product's lifetime. Although some studies performed a comparative LCA to estimate the profitability of PSCs with respect to other PV technologies,36–38 other types of studies evaluated the effects of PSC recovery as opposed to landfilling.18,39 In this respect, the effect of material recovery on primary energy consumption (PEC), global warming potential (GWP), energy payback time (EPBT), and greenhouse gas (GHG) emissions are several environmental-related indicators to consider in an LCA analysis.Tian et al.18 performed an LCA on six PSC architectures and identified the most advantageous device stacks by considering several environmental-related indicators. As shown in Table 1, PSCs can be fully competitive with their silicon counterparts. According to the study, the EPBT for silicon photovoltaics is approximately 2.4 years under a landfill end-of-life scenario, which can be reduced to 1.3 years when recycling processes are implemented. In comparison, PSCs exhibit significantly shorter EPBT values in all analysed cases, ranging from 0.19 to 0.60 years under landfill conditions, with further reductions anticipated under recycling scenarios. Although some uncertainty remains regarding the precise LCOE values for perovskite technology, a reliable estimate ranges from 4.52 to 6.11 €cent per kW h, demonstrating cost competitiveness with silicon-based photovoltaics.40
| PSCs | Si PVs | |
|---|---|---|
| EPBT (years) | 0.19–0.60 | 1.3–2.4 |
| LCOE (€cents per kW h) | Estimated 4.52–6.11 | 4.1–9.2 |
Results showed that in all cases, the recovery of critical components of PSCs can reduce the environmental footprint of the technology (Fig. 3).
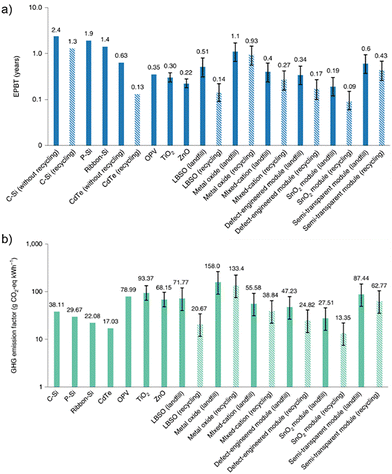 | ||
| Fig. 3 Comparison of (a) EPBT and (b) GHG emission factors among 13 PV modules based on different technologies. The solid bars correspond to landfill end-of-life scenarios for different PV modules, while the striped bars correspond to the recycling counterparts. The evaluations are based on rooftop-mounted installation in Southern Europe, with annual irradiation of 1700 kW h m−2 and a performance ratio of 75%. Error bars, 95% confidence intervals. Reproduced with permission.18 Copyright 2021, Nature. | ||
Similarly, in a recent work conducted by our group, we developed a circular recovery method to reuse and recycle key PSC materials and performed an LCA to assess its profitability with respect to a landfill end-of-life scenario. The analysis of full-spectrum midpoint impact categories revealed that the application of our circular recovery approach is definitively more beneficial with respect to the landfill EoL scenario.41 Finally, Rodriguez-Garcia et al.42 analysed 13 PSC recycling techniques reported in the literature that focused on transparent conductive oxide (TCO)-coated glass recovery. Surprisingly, their comparative LCA revealed that all considered processes, except for one, generated a higher environmental impact than the production of virgin substrates. In particular, the highest footprint was generated by chemicals used to dissolve the PV layers of the stack. Dimethylformamide (DMF), a toxic and environmentally harmful solvent,43,44 was employed in most of the impactful techniques analysed, while potassium hydroxide (KOH) aqueous solution was used in the process that truly resulted in a sustainable one. We can therefore conclude that the choice for using “greener” solvents, which is extensively discussed later in this review in a dedicated section, seems to be crucial in reducing the environmental impact of PSC recovery protocols.
3.2. Impact of recovery strategies on costs by techno-economic analysis
In a similar manner to LCA, TEA can be performed to estimate the economic profitability of a product in comparison to others, considering all lifetime stages, from production to disposal.The LCOE is a common indicator that allows for the calculation of the average cost of electricity in a currency per energy unit and can help assess the electricity generation costs of a technological process. Similarly, the minimum sustainable price (MSP) can be an important indicator to consider for providing the minimum rate of return (i.e. the efficiency of a technology in generating profits in relation to the resources used) necessary for a given industrial process to support a sustainable business over the long term.
Since most TEA studies on perovskite-based PVs neglect EoL impact, Martulli et al.39 proposed a model that considers both financial and environmental key indicators to obtain a complete environmental-techno-economic assessment (ETEA), evaluating the restoration of PSCs from an LCA perspective and estimating its economic advantages. Their study assessed the effects of different levels of recovery rates and retained PSC performance on device sustainability. The work also demonstrated that EPBT and GHG emission factors of PSCs can be decreased up to 23% and 13%, respectively, when high levels of recovery (>90%) and slightly reduced efficiency are achieved. Moreover, they calculated that the MSP and LCOE of PSCs can be reduced by 14% and 4%, respectively, if PSCs were recovered with high recovery levels (∼100%) and no performance loss.
Wu et al.45 compared the manufacturing costs of 1 m2 PSC fabricated with either fresh or recovered components. Their study reveals that the reuse of substrates and the recycling of the perovskite layer and HTL would reduce costs by 63.7% and 90.7% for devices fabricated at laboratory and industrial scales, respectively.
McGovern et al.46 presented a techno-economic study of perovskite PV technologies, comparing rigid and flexible single-junction perovskite modules to crystalline silicon PV (Si PV). They calculated the LCOE as a function of module efficiency and stability for a set of four modules. The LCOE equation demonstrated that low-weight flexible perovskite modules are promising. In fact, even though they are only slightly more interesting than rigid perovskite modules in competing against the Si PV utility sector, LCOE greatly benefited when considering the production of flexible low-weight modules by roll-to-roll manufacturing. Furthermore, LCA was performed for a representative flexible PSC device with 14% power conversion to evaluate the environmental impact of each layer in the flexible PSC architecture.
This type of comparison is important because flexible perovskite PVs can, in principle, broaden the range of PV applications, reaching novel PV market areas that are currently not achievable by exploiting both rigid perovskite and Si PVs.47
4. End-of-life management of perovskite solar cells
Considering the rapid growth in photovoltaic technology, circular recycling is trivial in PV deployment. Therefore, addressing the potential accumulation of large amounts of discarded solar panels is increasingly relevant for developing the sustainable decommissioning processes of photovoltaics. Presently, the recycling of commercial silicon PV modules is quite challenging owing to laborious component separation, leading to downcycling.48 In contrast, layer-by-layer PV devices, such as PSCs, could potentially allow for a relatively easy separation of material components through a selective dissolution strategy.49 Even though the perovskite PV device commercialisation process is still in its infancy,15 we believe that the intrinsic potential impact on recycling of such a type of device should already be addressed at the developing stage of this technology to pave the way towards fully sustainable PSCs.As extensively demonstrated in the previous section of this review, TEA and LCA confirmed the economic benefits of developing recycling procedures that can reduce environmental impacts at both the laboratory and industrial scales.
To program proper decommissioning strategies as a first step, it is important to identify the most critical components of PSCs. Once spotted, it is key to define directives for the design of dismissal processes. Fig. 4 presents a schematic representation of the waste hierarchy pyramid reported in the Directive 2008/98/EC of the European Parliament, where waste avoidance and waste treatment strategies are arranged from the most preferred (at the top) to the least preferred (at the bottom).50 When possible, reduction and reuse are to be pursued since they completely avoid waste generation. Recycling and recovery, instead, must be put in place before disposal when waste avoidance is impossible.
 | ||
| Fig. 4 (a) Waste hierarchy pyramid as reported in the Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain directives.50 Analysis of the (b) primary energy consumption, (c) cost analysis and (d) carbon footprint of perovskite solar cells. | ||
In this section, we summarise the existing recovery strategies for each (or multiple) material(s) that comprise PSCs, providing an overview of related articles following the waste hierarchy pyramid.
4.1. Evaluation of the economic and environmental impact of each perovskite solar cell component
The rational design of recovery strategies for PSCs involves determining the economic and environmental impact of each component of the technology. Through such analyses, materials can be ranked in terms of cost and sustainability, and their recovery urgency can be estimated. In Fig. 4b–d, the analysis of the primary energy consumption, costs and carbon footprint considering each perovskite solar cell component is graphically presented.Nazeeruddin and coworkers51 performed a cost analysis to produce perovskite solar panels. In their analysis, the material and equipment costs associated with perovskite PV production were estimated by comparing the impact of selecting different ETL and electrode materials in multiple locations. Significant variance was found in all metrics between the selected locations, which were considerably affected by local glass processing prices. Furthermore, in one of our studies,52 it emerged that transparent conductive oxide (TCO) glass coated with an electron transport layer (ETL) outweighs all other constituents, clearly indicating its high impact on manufacturing costs (23% of the cost analysis; pie chart depicted in Fig. 4c).
Furthermore, from an environmental footprint perspective, in the LCA proposed by Tian et al.18 mentioned in Section 3.1, the contribution that each component of the device has on its carbon footprint and PEC is evaluated, considering six different single-junction perovskite solar module (PSM) architectures. From an embedded material perspective, transparent conductive oxide (TCO)-coated glasses, such as indium tin oxide (ITO) and fluorine tin oxide (FTO), are widely used in solar cells owing to their excellent electrical conductivity and optical transparency, representing the most environmentally impacting component for all PSM architectures. Regarding manufacturing processes, although different architectures were characterized by different fabrication processes, those that required high temperatures or energy-intensive procedures were generally marked by higher PEC and carbon footprints.
Wu et al.45 demonstrated the high economic impact of the TCO glass, estimating the mass and cost composition of a 1 m2 PSM based on indium tin oxide (ITO)/tin oxide (SnO2)/methylammonium lead iodide (MAPbI3)/N2,N2′,N7,N7′-tetrakis(2,4-dimethoxyphenyl)-N2,N2′,N7,N7′-tetraphenyl-9,9′-spirobi[fluorene]-2,2′,7,7′-tetraamine (spiroOMeTAD)/Au. The ITO-coated substrate comprised almost the entire total mass (99.9%) and most of the total cost (58.3%) of the device. Furthermore, in 2020, NREL conducted a TEA, where manufacturing costs were estimated for a sheet-to-sheet single junction PSM produced in the United States.53 The analysis considered all steps of module production and installation, evaluating material cost, labour, utilities, maintenance, and depreciation. Thus, TCO was the most impacting component from both economic and environmental perspectives.
It is important to keep in mind different types of PSCs such as flexible PSC devices that employ alternative materials as substrates, such as plastic-based ITO using polyethylene terephthalate (PET) and polyethylene naphthalate (PEN), for which it is important to estimate costs and plan targeted recovery or recycling protocols. Even though the estimated costs of PET and PEN are lower than those of glass, they have petrochemical origins. Hence, their use may be associated with environmental pollution issues that need to be addressed differently from glass when considering their EoL management.54
Furthermore, the second highest GWP is the back glass used for encapsulating the device. Bogachuk et al.55 developed a novel thermally assisted mechanochemical approach to remove it along with most of the device constituents for remanufacturing PSCs. From a production perspective, it is therefore important to lower fabrication costs by developing highly effective and low-cost encapsulation materials as well as low-cost materials for charge transport layers and electrodes.56
Moreover, other impacting components are the metal contacts of PSCs. Industrial-scale PSC manufacturing is envisioned to also adopt different materials for metal contact (i.e. carbon) and different deposition methods that require additional recovery studies. For instance, Li. G et al.57 recently published a material cost analysis in which the costs of NIP and PIN PSCs were highly comparable ($86.49 and $81.31, respectively), while costs significantly reduced to $41.16 for carbon devices (49–52% reduction). This result is related to the fact that a carbon electrode is much cheaper than any noble metal electrode. Moreover, for carbon electrode deposition, a slot-die coating process associated with low energy consumption is needed. On the contrary, the deposition of noble metals requires an expensive physical vapor deposition process with high energy consumption. From a circular EoL management perspective, the reuse of collected metal contacts from spent devices is also an important aspect to consider, which is, however, still challenging. Many studies have described protocols for metal contact collection but do not reuse them in refabricated PSCs.58 This is related to the constraints that lab-scale fabrication imposes on metal contact deposition, which is usually performed by thermal evaporation and on metal contact choice, usually Au or Ag.
Cordell and coworkers59 presented a recent cost analysis of perovskite/silicon (Si) tandem modules with an efficiency of 25% and found that the choice of Si cell architecture, overall module efficiency, and factory throughput have the most significant impact on cost and competitiveness. In fact, they calculated an MSP of $0.428/WDC for their baseline two-terminal design and $0.423/WDC for their baseline four-terminal design, each at a module production of 3 GW per year in the United States.
Besides, considering all aspects previously mentioned in the introduction, the toxicity of Pb and the instability of perovskites prevent us from ignoring the threat that Pb-based PSCs might pose to the environment and human health. Several studies have demonstrated that when Pb enters the soil, it can be easily absorbed by plants60 and, if present in water, it can accumulate in aquatic animals,61 thus entering the food chain. In humans, Pb can enter the bloodstream by ingestion through intestinal absorption, inhalation or skin contact.62 It can then accumulate in organs and the skeleton and impair physiological functions and biochemical processes by mimicking biological ions such as Ca2+, Fe2+ and Zn2+.63 This issue results in neurotoxicity, detrimental effects on renal function and immunity, heart diseases and carcinogenicity.62,63 Zhang et al.62 calculated that even low fractions of Pb leaking from the PSCs into the food chain would exceed the threshold of the Pb weekly intake limit set by the Food and Agriculture Organization (FAO) of the United Nations.
In this respect, the issue related to Pb management has been addressed in several ways, such as by using lead-free perovskites (i.e. tin-based PSCs).64 However, so far, this alternative strategy has not yet proved sufficiently valid owing to the poor stability and low efficiency of these types of devices. Moreover, PbI2 sequestration using passivation or device encapsulation is a different attempted strategy for reducing the risks related to Pb use.30 Furthermore, Pb recycling is indeed an alternative strategy to mitigate its long-term risk. In fact, from a recovery perspective, the safe manipulation of EoL PSCs could contribute to the prevention of Pb pollution, complementing other practices and easing the way toward PSC commercialization.
Once the most impacting components are spotted, it is essential to define the directives for the disposing processes following the waste hierarchy.
4.2. Highest priority in the waste hierarchy: material reuse
As the most impacting components from both economic and environmental perspectives, extensive studies have been conducted to design the recovery of TCOs. Their reuse is made possible by the superior adhesion that exists between glass and TCO, which is derived from the commonly employed TCO deposition technique of magnetron sputtering. TCOs are usually exposed from spent PSCs by dissolution with the proper solvents of the upper layers of the stack and are then subjected to standard cleaning steps. They can be restored from both single- and multi-junction solar cells, and, in some cases, charge transport materials (CTMs) can be recovered together with the TCO substrate.For instance, fluorine-doped indium tin oxide (FTO) substrates were successfully restored by Chowdhury et al.65 and Huang et al.66 through the dissolution of the above layers with DMF. The as-obtained FTO glasses displayed similar crystallinity, optical and morphological properties with respect to pristine samples. Furthermore, Huang et al.66 demonstrated that the reuse of the FTO substrate does not affect the properties of the freshly deposited perovskite, thus enabling the fabrication of PSCs with performances similar to those produced with pristine substrates. Augustine et al.67 achieved similar results by employing a KOH solution in deionized water (DI H2O), instead of DMF, for the dissolution of the device components. Substrates refurbished with this strategy presented similar transmittance, conductivity, and roughness to pristine samples and a high degree of purity. Despite some K traces persisting on the surface of the recovered substrates, the performance of the refabricated PSCs was higher than those of fresh devices owing to the improved wettability that such K species conferred to the restored ITO surface. The recovery of the ITO glass substrate was also demonstrated for a perovskite–perovskite tandem solar cell and was hypothesized for a perovskite–silicon tandem by Tian et al.19 (Fig. 5a and b). First, device active layers were removed through subsequent cleaning steps in an aqueous cleaning solution, DI H2O, acetone and isopropyl alcohol (IPA). Then, the effectiveness of the recovery protocol was tested by comparing the PCE of perovskite–perovskite tandems fabricated with pristine and recovered ITO substrates. Solar cells fabricated with restored ITO glass demonstrated a higher efficiency of 22.2% (20.8% on average) with respect to the pristine substrate (21.7% for the champion device, 20.3% on average), which progressively increased after each recycling iteration, reaching a champion of 22.9% (21.4% on average) (Fig. 5c and d).
 | ||
| Fig. 5 Scheme of the recovery route for (a) ITO glass substrates constituting a perovskite–perovskite tandem solar cell and (b) silicon bottom cell constituting a perovskite–silicon tandem solar cell. (c) J–V curves and (d) PCE statistical analysis of the perovskite–perovskite tandem solar cell fabricated with ITO glass substrate recovered zero to four times. Reproduced with permission.19 Copyright 2023, the Royal Society of Chemistry. | ||
Feng et al.68 tested several dialkylamines to recover NiOx HTL-coated ITO substrates and ultimately selected a butylamine (BA):dipropylamine (DPA)-based 2-step approach. The improved PCE displayed by PSCs fabricated with restored substrates (18.65 ± 0.6% with respect to the pristine device, 16.46 ± 0.6%) was attributed to the ability of alkylamines to template the growth of high-quality perovskites and passivate the HTL/perovskite interface. Similarly, Zhu et al.69 fabricated PSCs that displayed higher PCE when refurbished substrates were employed. They noticed that the refurbished FTO/titanium oxide (TiO2) substrate contained high quantities of Ti3+ ions and some residual perovskite with a Pb-rich composition. The authors correlated the presence of these species to work function reduction and the conduction band minimum (CBM) narrowing. Although the former promoted electron–hole pair separation and suppressed recombination of charge carriers, the latter reduced interfacial recombination, ultimately leading to higher PCE. In a recent study, our group reported an enhancement of the average PCE upon the restoration of the SnO2 ETL-coated ITO by employing DMSO (and DMF for comparison) to dissolve the upper layers of the device (Fig. 6a–c).52 In this case, the efficiency improvement was strictly related to an average fill factor (FF) enhancement, which counterbalanced a slight open-circuit voltage (VOC) reduction (Fig. 6d and f). Although the average VOC decrease upon substrate restoration was correlated with the removal of K+ ions from the surface of ITO/SnO2, inducing higher charge-carrier recombination, the average FF increase was ascribed to the effect of PbI2 residuals on the surface of restored substrates. With a wider bandgap than the perovskite, PbI2 can trap holes generated in the ETL, preventing recombination at the ETL/perovskite interface and facilitating charge extraction, as suggested by transient photocurrent measurements (Fig. 6f). In contrast, although Kim et al.70 observed the presence of residual chemical species on the surface of restored FTO/mesoporous (mp)-TiO2 substrates, they did not witness an enhancement in the PV performance of their recovered PSCs. The morphological, structural and optical properties of refurbished substrates remained unchanged with respect to pristine samples, leading to almost the same efficiency of fresh devices, with minor PCE losses after iterating ten times the recovery process.
 | ||
| Fig. 6 (a) PCE, (b) FF and (c) VOC box charts of PSCs fabricated with fresh and recycled SnO2/ITO glass substrates. Time of flight-secondary ion mass spectroscopy (ToF-SIMS) (d) K+ and (e) Pb2+ surface maps of fresh and recycled SnO2/ITO glass substrates. (f) Transient photocurrent (TPC) decay curves of fresh and recycled SnO2/ITO glass substrates. Reproduced with permission.52 Copyright 2023, Wiley. | ||
Huang et al.71 demonstrated the reuse of both FTO/compact (c)-TiO2 and FTO/c-TiO2/mp-TiO2 substrates. After the dissolution of HTL and perovskite with DMF, both types of restored substrates displayed similar morphology and composition to the pristine samples. To further demonstrate the efficacy of the recovery process, a fresh perovskite was coated onto fresh and refurbished substrates, and its optical, structural and morphological properties were investigated. Ultimately, PSCs were fabricated by employing fresh and restored FTO/(c)-TiO2 and FTO/c-TiO2/mp-TiO2, which retained 90% and 85% of the original PCE, respectively, and 84% and 74% of the original PCE, respectively, after one additional recovery iteration. Types of mesoporous scaffolds other than mp-TiO2 have also been explored. Zhao et al.72 adopted super-aligned zinc oxide (ZnO) nanorods as mesostructured ETM; Ku et al.73 employed an mp-Ni counter electrode coated onto an FTO/TiO2/aluminum oxide (Al2O3) substrate, and Li et al.74 designed an FTO/mp-TiO2/mp-Al2O3/nanoporous (np)-Au:NiOx template. These three studies feature the dissolution of the perovskite with DMF and its reloading to fabricate refurbished PSCs. Bogachuk et al.75 went one step further, recovering not only the FTO-glass substrate coated with c-TiO2/mp-TiO2/mp-zirconium oxide (ZrO2) from a carbon-based PSM but also the back glass used for encapsulating the device, which is the component with the second highest GWP. The FTO substrate and the back sheet were manually separated after thermal treatment at 120–140 °C. The back glass was cleaned from polyisobutylene (PIB) and thermoplastic polyolefin (TPO) by peeling off after 1 hour in acetone. The FTO glass (10 × 10 cm2 plates) was restored by removing the perovskite in a bath of methylamine and ethanol (EtOH), followed by annealing at 400 °C. Finally, the encapsulated PSCs were refabricated, showing 88% of the original PCE. Interestingly, Kadro et al.76 envisioned the recovery of all components of an NIP by selectively dissolving all layers of the device. Although the fate of HTL, PbI2 and Au was discussed, only the FTO/mp-TiO2 substrate was eventually recovered. Refabricated PSCs retained the same PCE as fresh devices, even after the second recovery iteration of the TCO/ETL.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 4
DMSO = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, and the poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine (PTAA) HTL was removed with toluene (Fig. 7a). The recollected double-sided textured Si wafer was subjected to optical (Fig. 7b), morphological (Fig. 7c and d) and PV (Fig. 7e) investigations to ensure the preservation of its properties. When the refurbished Si bottom cell was employed to fabricate new perovskite–Si tandem devices, 98% of the original average efficiency was preserved, with a champion of 25.7% PCE (Fig. 7f and g).
1 mixture, and the poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine (PTAA) HTL was removed with toluene (Fig. 7a). The recollected double-sided textured Si wafer was subjected to optical (Fig. 7b), morphological (Fig. 7c and d) and PV (Fig. 7e) investigations to ensure the preservation of its properties. When the refurbished Si bottom cell was employed to fabricate new perovskite–Si tandem devices, 98% of the original average efficiency was preserved, with a champion of 25.7% PCE (Fig. 7f and g).
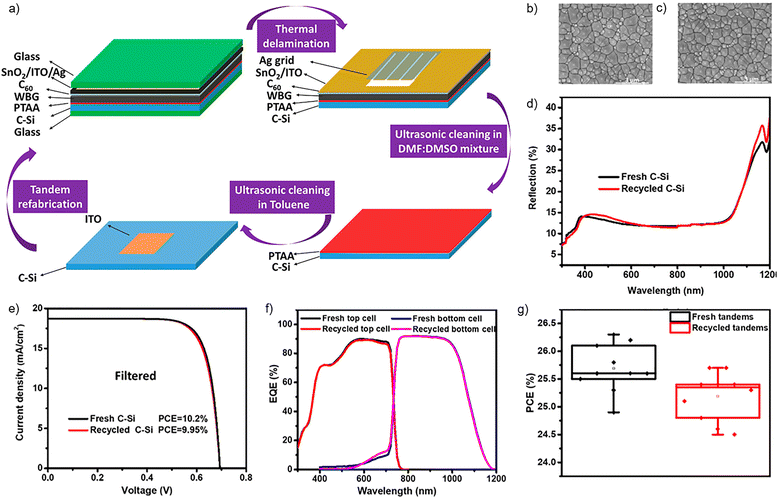 | ||
| Fig. 7 (a) Schematic recycling process of Si bottom cells from degraded, encapsulated perovskite–Si tandem solar cells. Top-view SEM images of (b) fresh and (c) recycled Si bottom cells. (d) Reflectance spectra of fresh and recycled Si bottom cells. (e) J–V curves of the recycled single-junction silicon bottom cells. (f) EQE spectra and (g) PCE box charts of fresh and refabricated tandem devices. Reproduced with permission.77 Copyright 2023, the American Chemical Society. | ||
4.3. Middle priority in the waste hierarchy: material recycling
According to the waste hierarchy, when the reuse of components is impossible, material recycling must be pursued. Regarding PSCs, critical materials other than the TCO, such as toxic PbI2 and some expensive CTMs, cannot be reused without any additional treatment and their recovery needs to go through some recycling steps. The recycling of costly CTMs, such as spiro-OMeTAD, is usually presented in the literature as part of more comprehensive recovery routes, where several device components are simultaneously recycled and reused. Thus, strategies addressing their recycling will be presented later in the section dedicated to the reuse and recycling of full device components. The first part of the section focuses on PbI2 recycling, for which various methods have been proposed, all aimed at reducing the risks associated with EoL management.Yang et al.78 employed a supramolecular complexation method based on chemical coordination and multidentate chelation between 2-hydroxypropyl-β-cyclodextrin (HPβCD)-1,2,3,4-butane tetracarboxylic acid (BTCA) complex and Pb2+ ions (Fig. 8a). HPβCD-BTCA complex was integrated into PSCs as a built-in network embedded in the active layer, and its Pb2+ sequestration capability was tested by subjecting damaged encapsulated devices to continuous water scouring for 1 h. The pristine sample showed a Pb leaking rate of 973 mg m−2 h−2, while the complex-containing sample displayed a leaking rate of 54 mg m−2 h−2 (Fig. 8b), which was further reduced to 14 mg m−2 h−2 (corresponding to 98.6% of Pb sequestration efficiency) when the encapsulating cover glass was replaced with a flexible polymer@HPβCD-BTCA-based sheet. HPβCD-BTCA@PbI2 complexes were recovered either to be redissolved for active layer fabrication or to collect PbI2 by dissolution in DI H2O and centrifugation (Fig. 8c).
 | ||
| Fig. 8 (a) Schematic of lead capturing by cross-linking HPβCD-BTCA supramolecular complex. (b) Comparison of Pb sequestration for the damaged PSCs with or without HPβCD-BTCA. (c) Illustration of the process of Pb recycling and management in PSCs. Reproduced with permission.78 Copyright 2023, Nature Publishing Group. | ||
Another approach based on chemical coordination was reported by Ren et al.,79 who employed zeolite to absorb Pb2+ ions. First, the HTL of the PSC was removed with ethyl acetate. Second, the perovskite active layer was dissolved in H2O, where Pb2+ ions could be absorbed by the zeolite (with an adsorption efficiency of 100%), driving the ion exchange reaction (eqn (1)) towards the complete dissolution of PbI2.
| PbI2(s) ⇌ Pb2+(aq) + I−(aq) | (1) |
Finally, PbI2 could be restored by the reaction of the I−-rich solution with Pb2+ ions desorbed from the zeolite. PSCs fabricated employing recycled PbI2 displayed an even higher champion efficiency (21.58%) than pristine devices (21.50%). Hong et al.80 compared the Pb2+ adsorption capacity of hydroxyapatite (HAP, Ca10(PO4)6(OH)2) with their synthesized whitlockite (WH, Ca18Mg2(HPO4)2(PO4)12), the first and second most abundant biominerals in human hard tissues, respectively. The results showed that WH had 1.68 times the absorption capacity of HAP, enabling 100% extraction of 3000 ppm of Pb2+ from a Pb(NO3)2 solution after 30 minutes. PbI2 was then recovered by treating WH with absorbed Pb2+ with HNO3 aqueous solution and KI. As-obtained recycled PbI2 was tested for the fabrication of the active layer of PSCs, which attained an average PCE of 19.0 ± 1.4%, only slightly lower than the pristine PCE (19.3 ± 0.9%). Similarly, Park et al.81 synthesized an iron (Fe)-incorporated HAP (HAP/Fe) hollow composite to induce Pb2+ adsorption. After PSC dissolution in DMF, HAP/Fe composites were added to the solution, absorbing Pb2+ ions with a collection yield of 99.99%. The HAP/Fe@Pb2+ complexes were removed from the solution by exploiting the magnetic properties induced by Fe incorporation by applying a magnetic field. PbI2 was recrystallized by reaction with 1 M potassium iodide (KI) solution under acidic conditions, with a 99.97% recovery yield. Finally, PSCs were fabricated by employing recycled and commercial PbI2, which displayed average PCEs of 16.0 ± 0.9% and 16.6 ± 1.0%, respectively.
Ultimately, Pb2+ can also be absorbed by organisms, such as fungi Cladosporium sp. strain F1, A. niger VKMF-1119 and M. ramannianus R-56, as demonstrated by Lee et al.82 Fungal strains were added to PbI2 solutions in DI H2O (pH 7), and their biosorption capacity was evaluated over time. Cladosporium sp. strain F1 exhibited the highest Pb2+ biosorption capacity, with a 94.1% extraction yield. PbI2 was recovered with a 99.7% yield from the hyphae of Cladosporium sp. strain F1 by solvent treatment with DMSO in an acidic aqueous solution (pH 2) at 70 °C.
 | ||
| Fig. 9 (a) Scheme of the H2O-based PbI2 recycling process. The process includes mechanical fragmentation of PSCs (1), hot aqueous extraction (2), solid–liquid separation (filtration) (3), PbI2 precipitation (cooling) (4) and recovery (5). PbI2 can be reused in new perovskites. Solid waste (W) is deprived of Pb compounds. (b) Metal extraction from a PSC during two consecutive extraction cycles. Reproduced with permission.83 Copyright 2023, Elsevier. (c) Schematic of in situ perovskite recycling from PSCs and sequential fabrication of new solar cells. (I) Removal of Ag electrode with adhesive tape. (II) Removal of the HTM by immersing in CB solvent. (III) Thermal decomposition of the perovskite into remained solid PbI2 and emitted organic gases. (IV) Development of new perovskite films by spin coating a MAI solution. (V) Preparation of spiro-MeOTAD layer. (VI) Evaporation of Ag electrode. (d) J–V characteristics and e) IPCE curves of PSCs fabricated with pristine PbI2 (sample 1), recycled PbI2 with optimized (sample 2) and non-optimised (sample 3) recycling procedure and second-time recycled PbI2 (sample 4). Reproduced with permission.84 Copyright 2017, Wiley. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (EG) = 1
ethylene glycol (EG) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 deep eutectic solvent to dissolve the perovskite. In both cases, the yields of Pb2+ dissolution were very high, namely 99.9% and 99.7%, respectively. Recovery yields were only reported by Wang et al.,85 who achieved 91.5% and 98.0% metal Pb deposition yields after the first and second electrolysis cycles, respectively.
2 deep eutectic solvent to dissolve the perovskite. In both cases, the yields of Pb2+ dissolution were very high, namely 99.9% and 99.7%, respectively. Recovery yields were only reported by Wang et al.,85 who achieved 91.5% and 98.0% metal Pb deposition yields after the first and second electrolysis cycles, respectively.
4.4. Recovery of multiple device components
To simultaneously overcome environmental and cost-related issues concerning PSCs, recovery strategies that simultaneously recycle and reuse more than one device component have been proposed. These processes are usually performed by sequentially dissolving each layer of the PSC stack, followed by specific treatments to recover each target material.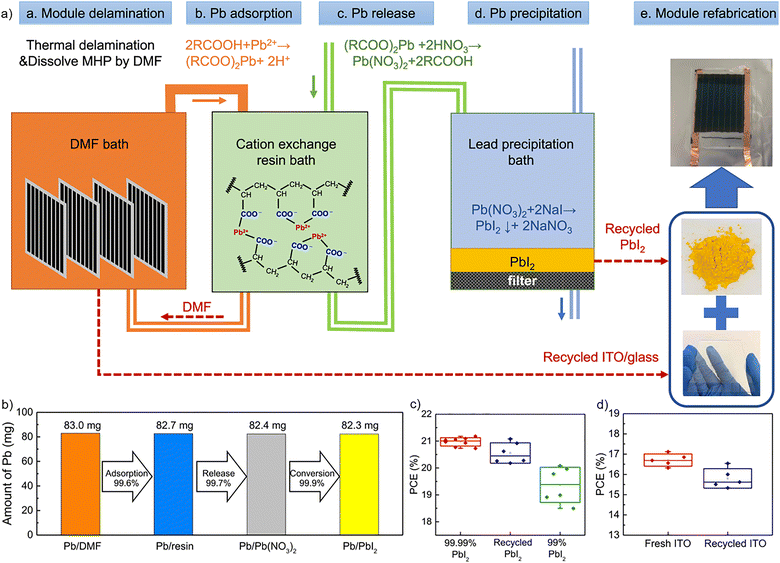 | ||
| Fig. 10 (a) Scheme of PSM recycling: a. Encapsulated perovskite solar modules were delaminated, and the perovskite active layer was dissolved using DMF; b. Pb2+ was removed from DMF by carboxylic acid cation-exchange resin; c. Absorbed Pb2+ ions on the resin were released to aqueous solution via resin regeneration process using HNO3; d. PbI2 was precipitated by pouring NaI into Pb(NO3)2-containing solution; e. PSMs were refabricated employing recycled materials. (b) Pb2+ adsorption, release and conversion yields from 10 mL of 40 mM PbI2 in DMF. (c) PCE box charts of PSCs (8 mm2 device size) fabricated with commercial 99.99% PbI2, recycled PbI2, and commercial 99% PbI2. (d) PCE box charts of PSMs (25 cm2 active area) fabricated on fresh and recycled ITO/glass. Reproduced with permission.88 Copyright 2021, Nature. | ||
First, the module encapsulation was removed by thermal delamination. Then, the ETL was washed with 1,2-dichlorobenzene, and PSMs were immersed in a DMF bath to dissolve the perovskite active layer. A carboxylic acid cation-exchange resin was employed to collect Pb2+ ions, exhibiting a 99.6% recovery yield (Fig. 10b). Pb2+ adsorption and release were governed by ion exchange between H+ and Pb2+ ions, as described in the following equation:
| 2R–COOH + Pb2+ ⇌ (R–COO)2Pb + 2H+ | (2) |
Since eqn (2) describes a reversible reaction, the addition of H+ ions could reverse the equilibrium towards Pb2+ release. Therefore, HNO3 aqueous solution was used to release Pb2+ ions from the resin (with a 99.7% recovery yield, Fig. 10b), which were further treated with sodium iodide to precipitate PbI2 (99.2% recovery yield). Recycled PbI2 was employed to fabricate the active layer of new PSMs, which displayed a median of 20.4% PCE for 8 mm2 devices, only 2.8% lower than the 21.0% PCE of pristine devices (Fig. 10c). Moreover, ITO/glass and back cover glass were collected from PSMs and reused to fabricate 25 cm2 active area modules. Devices produced with restored ITO/glass attained an average PCE of 15.9%, fairly similar to the average 16.7% PCE of pristine devices (Fig. 10d). Another approach was adopted by Deng et al.,89 who dissolved the perovskite in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 9
DMSO = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reused the resulting solution upon MAI addition to fabricate a recycled perovskite layer. Moreover, the same solvent composition was used to restore ITO/glass substrates. Champion PCEs of 16.6% and 15.3% were reported for devices produced with fresh and recycled materials, respectively. Zhang et al.90 employed DMF to dissolve the perovskite active layer, displaying a dissolution yield of 99.9%. Then, the solution was treated with ammonia (NH3) to precipitate lead hydroxide (Pb(OH)2), which was converted into PbI2 by reaction with hydroiodic acid (HI). The resulting PbI2, obtained with a 95.7% reaction yield, displayed 99.9% purity. Morphological, optical and structural properties of both recycled PbI2 and MAPbI3 fabricated with recycled PbI2 were assessed and compared with those displayed by fresh materials. Furthermore, PSCs produced using fresh and recycled PbI2 demonstrated similar PV performances, with 12.17% and 11.36% champion PCEs, respectively. Then, FTO/c-TiO2/m-TiO2 substrates were recollected and structurally and optically compared to pristine substrates. PSCs fabricated with reused FTO/c-TiO2/m-TiO2 displayed a 12.03% champion PCE, which is very close to the 12.21% champion PCE of fresh devices. Binek et al.91 achieved a 92–94% PbI2 recovery yield by employing a dissolution-crystallization method. After the organic component (MAI) of the perovskite layer was removed with DI H2O, PbI2 was dissolved in DMF and extracted from the solvent under reduced pressure. The FTO/TiO2 substrates were further treated with DMF to completely remove the ETL. The effects of PbI2 and FTO recycling on PSC performance were studied separately. Devices fabricated with fresh and recycled PbI2 displayed 14.6% and 12.1% champion efficiencies, respectively. However, the use of recovered FTO glass substrates produced average PCEs of 13.4 ± 1.1%, 12.8 ± 1.3% and 13.5 ± 1.5% for the 1st, 2nd and 3rd recovery iterations, respectively. Feng et al.92 treated the devices with a BA solution to dissolve the perovskite layer and the [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) ETL. After BA evaporation, the solid precipitate was washed with toluene to remove PCBM and with ethanol to dissolve butylammonium iodide (BAI) and bathocuproine (BCP). Recycled MAPbI3 crystals were then synthesized from recollected PbI2 by employing a temperature-lowering method. Although fresh and recycled perovskite displayed very similar morphology and crystallinity, a red-shifted PL emission peak for recycled MAPbI3 was attributed by the authors to the formation of shallow defects near the band edge. ITO/NiOx substrates were also refurbished and optically, morphologically and compositionally tested. Pristine and restored samples displayed similar properties, except for the presence of PbI2 traces on the surface of the recovered ITO/NiOx. Nonetheless, the PV performances of the recovered devices were not negatively affected by PbI2 residues. The recovered PSCs displayed a champion of 17.95% and an average of 17.27% PCE, which was even higher than the champion and average PCE of pristine devices, namely 17.84% and 17.18%, respectively.
1 and reused the resulting solution upon MAI addition to fabricate a recycled perovskite layer. Moreover, the same solvent composition was used to restore ITO/glass substrates. Champion PCEs of 16.6% and 15.3% were reported for devices produced with fresh and recycled materials, respectively. Zhang et al.90 employed DMF to dissolve the perovskite active layer, displaying a dissolution yield of 99.9%. Then, the solution was treated with ammonia (NH3) to precipitate lead hydroxide (Pb(OH)2), which was converted into PbI2 by reaction with hydroiodic acid (HI). The resulting PbI2, obtained with a 95.7% reaction yield, displayed 99.9% purity. Morphological, optical and structural properties of both recycled PbI2 and MAPbI3 fabricated with recycled PbI2 were assessed and compared with those displayed by fresh materials. Furthermore, PSCs produced using fresh and recycled PbI2 demonstrated similar PV performances, with 12.17% and 11.36% champion PCEs, respectively. Then, FTO/c-TiO2/m-TiO2 substrates were recollected and structurally and optically compared to pristine substrates. PSCs fabricated with reused FTO/c-TiO2/m-TiO2 displayed a 12.03% champion PCE, which is very close to the 12.21% champion PCE of fresh devices. Binek et al.91 achieved a 92–94% PbI2 recovery yield by employing a dissolution-crystallization method. After the organic component (MAI) of the perovskite layer was removed with DI H2O, PbI2 was dissolved in DMF and extracted from the solvent under reduced pressure. The FTO/TiO2 substrates were further treated with DMF to completely remove the ETL. The effects of PbI2 and FTO recycling on PSC performance were studied separately. Devices fabricated with fresh and recycled PbI2 displayed 14.6% and 12.1% champion efficiencies, respectively. However, the use of recovered FTO glass substrates produced average PCEs of 13.4 ± 1.1%, 12.8 ± 1.3% and 13.5 ± 1.5% for the 1st, 2nd and 3rd recovery iterations, respectively. Feng et al.92 treated the devices with a BA solution to dissolve the perovskite layer and the [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) ETL. After BA evaporation, the solid precipitate was washed with toluene to remove PCBM and with ethanol to dissolve butylammonium iodide (BAI) and bathocuproine (BCP). Recycled MAPbI3 crystals were then synthesized from recollected PbI2 by employing a temperature-lowering method. Although fresh and recycled perovskite displayed very similar morphology and crystallinity, a red-shifted PL emission peak for recycled MAPbI3 was attributed by the authors to the formation of shallow defects near the band edge. ITO/NiOx substrates were also refurbished and optically, morphologically and compositionally tested. Pristine and restored samples displayed similar properties, except for the presence of PbI2 traces on the surface of the recovered ITO/NiOx. Nonetheless, the PV performances of the recovered devices were not negatively affected by PbI2 residues. The recovered PSCs displayed a champion of 17.95% and an average of 17.27% PCE, which was even higher than the champion and average PCE of pristine devices, namely 17.84% and 17.18%, respectively.
4.5. Simultaneous TCO/ETL reuse and PbI2 and HTL recycling
Utilizing approaches that simultaneously combine TCO and ETL reuse together with PSC multi-component recycling helps to alleviate pollution risks, decrease waste generation during the device recycling process, and lower EoL recycling costs. The recycling techniques discussed in this section focus on developing protocols for multiple material recovery. In the context of multiple-material recovery, the recycling of the top CTM of PSCs has also been included in several recovery strategies as an additional step in substrate reuse and PbI2 recycling. Interestingly, all proposed processes are designed on the NIP and employ expensive spiro-OMeTAD as HTL.Among these contributions, Wang et al.93 demonstrated the recovery of ITO/NiOx substrates, the perovskite layer and the spiro-OMeTAD HTL using a “one-key bleacher” solution composed of methylamine and tetrahydrofuran (THF). Protocols based on layer-by-layer schemes for disassembly require several steps to obtain proper material to reuse in refabricated PSCs, resulting in economically expensive protocols. In this work, the bleacher solution simultaneously dissolved the entire stack of the device, exposing the ITO/NiOx substrate (Fig. 11). Spiro-OMeTAD was recovered from THF by rotary evaporation, achieving 98.9% purity, and the methylamine solution containing the liquefied perovskite was utilised upon acetonitrile (ACN) addition to reform a perovskite layer with morphological and crystalline properties similar to those of fresh samples. PSCs fabricated with fresh and recycled components displayed similar performances, namely 20.6 ± 0.6% and 20.3 ± 0.6% respectively, and minimal PCE loss was displayed after repeating the recycling protocol two times (20.1 ± 0.6% PCE). In a recent study, Wu et al.45 demonstrated the recovery of ITO/SnO2 substrates, MAPbI3 perovskite and spiro-OMeTAD HTL with almost 100%, 87% and 66% recovery yields, respectively. Spiro-OMeTAD was removed by dissolution in chlorobenzene (CB) and purified from its dopants through column chromatography. MAPbI3 was then dissolved in γ-butyrolactone (GBL) and recrystallised with EtOH. Finally, additional SnO2 deposition was performed onto recovered ITO/SnO2 substrates to improve the PV performances of recycled devices. After the comparison of fresh and recycled material properties, PSCs were fabricated by employing pristine and recovered components, demonstrating a champion 17.1% PCE, which is very similar to the champion 17.7% PCE of fresh devices. Additionally, the TCA and LCA of the proposed protocol were conducted, demonstrating the benefits of the adoption of such a recycling process with respect to a landfill EoL scenario. Similar results were demonstrated by our group41 for the recovery of ITO/SnO2 substrate, PbI2 and spiro-OMeTAD, employing green solvents. Recycling yields were close to 100%, 99.4% and 89.2% for ITO/SnO2, PbI2 and spiro-OMeTAD, respectively. Spiro-OMeTAD was dissolved in EtOAc and purified from its dopants by MilliQ H2O extraction. Formamidinium lead iodide (FAPbI3) perovskite was removed from the ITO/SnO2 substrate by ultrasonication in DI H2O, and PbI2 was recrystallized with EtOH. Finally, ITO/SnO2 substrates were cleaned by sequential ultrasonication in the washing solution, DI H2O, acetone and IPA. The properties of recovered materials were compared to those displayed by fresh components, and PSCs were fabricated to assess the impact of the recycling procedure on PV performance. Recycled PSCs displayed an average of 18.9% PCE, which is only 1.6% lower than the pristine average PCE (19.2%). The environmental advantages of the adoption of this recycling protocol with respect to a landfill EoL scenario were demonstrated by LCA. Moreover, the entire recovery protocol was repeated three times, and although PSCs displayed progressive PCE loss after each iteration, the energy return on investment (EROI) assessment revealed that the third iteration was still more convenient than landfilling. Finally, the solvents employed in the recycling protocol were recovered by distillation.
 | ||
| Fig. 11 (a) Cross section SEM image, showing the architecture adopted for PSCs, and schematic of how the bleacher solution simultaneously recycles multiple components: (i) Au and SnO2-coated ITO/glass, (ii) liquefied perovskite, and (iii) spiro-OMeTAD. (b) SEM images of fresh (top), liquified (middle) and recycled (bottom) perovskites. (c) XRD pattern of fresh and recycled perovskite films. (d) PCE box charts of PSCs fabricated with fresh and recycled components, with the recovery protocol iterated twice. Reproduced with permission.93 Copyright 2021, Cell Press. | ||
In summary, two major recycling methods for perovskite solar cells are generally applied. The first one, described in most of the procedures, involves the sequential dissolution of each single device layer (the layer-by-layer approach). Usually, the top metal contact is typically removed through a tape-assisted lift-off process, followed by a series of sequential chemical treatments that enable the selective dissolution of the constituent layers of the device. The second method, less common, uses a “special” solution that can simultaneously dissolve the entire stack of the device in a single step. On the one hand, the layer-by-layer approach has been consolidated in several studies as an effective strategy for recycling methods for PSCs. On the other hand, the possibility of simultaneously recycling all components of perovskite-based devices in one step may represent a promising route to simplify the recycling complexity and reduce the LCOE of PSCs.
Importantly, it appears that the most virtuous PSC EoL management procedures (efficient in reducing energy requirements and environmental footprints) consider the use of “green solvents” or water-based solutions and exploit recycling protocols that allow recovering the most critical components of the device stack to reuse purified materials in multi-component refabricated PSCs, lowering waste and costs in the EoL procedure. Additionally, in this case, the use of aqueous solutions or less toxic and environmentally friendly solvents is crucial for achieving efficient and sustainable decommissioning.
5. Importance of solvents (manipulation and or substitution towards green solvents)
As already mentioned, solvents significantly affect recovery techniques.42 The shift towards ecofriendly solvents represents a significant step towards real sustainability in PSC EoL. Therefore, this part of the review is dedicated to their selection. Specific criteria for their evaluation based on their impact on human health and the environment are first discussed. Finally, special attention is dedicated to their purification after use from a real circular economy perspective.5.1. Criteria for solvent selection
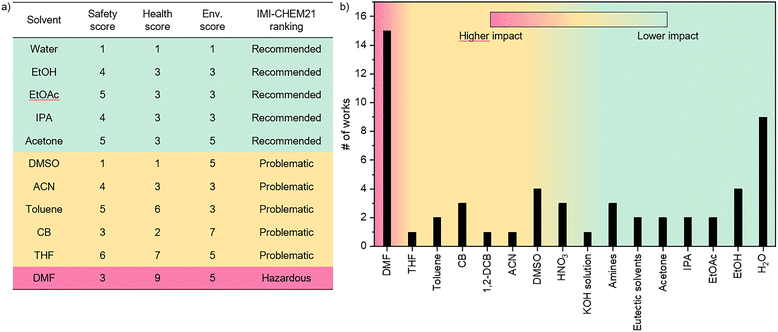
First, a strategy that univocally identifies solvent hazards and environmental impacts must be defined. Several studies evaluate solvents based on combined LCA and environmental, health and safety (EHS) properties. Therefore, by coupling hazard estimations with full life-cycle resource exploitation and pollutant emissions, one can determine how green is a solvent.94,95 According to these guidelines, the Innovative Medicines Initiative (IMI)-CHEM21 public–private partnership consortium developed a solvent guide that combines the EHS criteria with LCA for common and bio-derived solvents, expanding the results previously reported by Prat et al.96,97 In their ranking, solvents are classified as recommended (or preferred), problematic, hazardous and highly hazardous based on the constraints that one should consider when employing them at the lab-scale or in a pilot line. Fig. 12a presents the IMI-CHEM21 ranking of solvents commonly employed for PSC manufacturing and recovery. Although only DMF is ranked as hazardous, owing to its high toxicity and environmental impact, many other common solvents, such as DMSO, THF and CB, are identified as problematic and are, therefore, not ideal to be employed in large-scale production. Among recommended solvents, we reported IMI-CHEM21 ranking of H2O, EtOH, EtOAc, IPA and acetone, which were employed in some previously discussed recovery protocols. In this regard, Fig. 12b summarises the number of studies, among those analysed in the previous paragraphs, which report the use of a specific solvent as the main dissolution aid for the perovskite layer or CTMs and the impact of such solvent according to the IMI-CHEM21 ranking. Although several works adopted low-impact solvents, such as eutectic solvents, EtOH and H2O, problematic solvents have been widely employed, and DMF was used in 15 of the mentioned publications. These results suggest that, although some efforts towards the use of safer solvents have been made, more awareness is needed when designing recovery protocols, especially on solvent choice and solvent management. For example, in a very recent publication, Xiao et al.98 showed that the development of a water-based solution is efficient for perovskite recycling. In the best closed-loop system scenario, the choice of using “green solvents” should also be considered from a fabrication viewpoint.30,99,1005.2. Solvent purification
Rodriguez-Garcia et al.42 estimated that solvent purification can significantly reduce the environmental impact of recovery processes by 56–68%. Moreover, from an economic viewpoint, Wu et al.45 reported that by implementing solvent recycling into recovery strategies, the cost of PSMs fabricated with both refurbished materials and recycled solvents would be reduced by 18–20% with respect to the sole material reuse.In a recent work from our group,41 we presented the purification of solvents employed in the recovery protocol with the aim of creating a process that could be as circular as possible. EtOAc, EtOH and DI H2O were distilled, and their purification was evaluated with respect to specific contaminants, i.e. spiro-OMeTAD for EtOAc and PbI2 for EtOH and DI H2O, resulting in ∼100%, 99.8% and 97.4% removal, respectively. Similarly, Kim et al.70 reported that 99.99% of PbI2 could be removed from the solvent used to dissolve the perovskite layer (DMF) by adsorption with HAP. In the context of DMF recovery, the use of such refurbished solvent to manufacture new PSCs was demonstrated by Kim et al., who developed gelatine-conjugated hematite nanoparticles (HT NPs) that could effectively capture PbI2 from both wastewater (even in binary systems, with 99.9% extraction efficiency).101,102 In the latter case, purified DMF was used, together with recovered spiro-OMeTAD dissolved in CB, to fabricate new PSCs, which exhibited an average 24.02 ± 0.30% PCE, very much similar to the 24.12 ± 0.31% PCE of devices produced with fresh solvents.102
6. Conclusions and future perspectives
This review provided an important overview of the EoL management of depleted PSCs, which is a crucial issue that we encourage to take into consideration at the design stage, long before the commercialization of devices. In fact, since some PSC components are toxic, such as Pb and Pb-derived materials, inappropriate disposal of exhausted devices may lead to serious waste pollution, thus resulting in public environmental and health hazards.Herein, we first presented the economic and environmental types of analysis that should be performed to assess the implications behind the recycling of PSCs. Such type of study is of fundamental importance to obtain a complete ETEA for evaluating PSC restoration, estimating both sustainability and economic advantages at once. As extensively demonstrated in the dedicated section of this review, TEA and LCA confirmed the economic benefits of developing recycling procedures that can reduce environmental impacts, both at the laboratory and industrial scales.
As TCO is the most impacting component, from both economic and environmental perspectives, extensive studies have been conducted to design its recovery. Overall, the reuse and restoration of TCO is a crucial step toward achieving a closed-loop system for perovskite solar cells, ensuring that these devices are both high-performing and environmentally friendly. Challenges in designing efficient close-looped strategies also come from the need to address the proper reuse of other impacting components, such as the encapsulation back glass and metal contacts.
Thus, following the waste hierarchy pyramid, aside from TCO/ETL reuse, it is also important to provide useful recycling protocols to simultaneously recover all materials composing the device stack, such as PbI2 and HTL, to refabricate new high performance PSCs.
From a green chemistry perspective, we highlighted the major importance of solvent choice within recovery protocols, emphasizing the need for future studies to select solvents carefully based on their toxicity and environmental impact. Using “green solvents” should also be considered at the fabrication stage to achieve a total circular system.
In fact, designing recovery protocols that will be easily integrated into pilot lines and industrial manufacturing processes is fundamental to advancing towards PSC sustainable commercialization.
Furthermore, it is important to keep in mind different types of PSCs; for example, flexible devices that enlarge the field of application of perovskite-based PV technology. Since such devices employ alternative materials as substrates, it is urgent to plan new protocols for targeting proper recovery or recycling. In this respect, we envision new challenges in the recycling of flexible PSC substrates.
Finally, with the rapid advancement of the Internet of Things (IoTs) in our society, the application of PSC as indoor photovoltaics (IPVs) can offer a promising solution to fulfil new requests in terms of the type of energy font by providing lightweight power sources for IoT devices that can adapt to diverse indoor lighting conditions. In this respect, we foresee the rapid development of “ad hoc” and more and more virtuous sustainable and cost-efficient strategies to achieve proper EoL management integrated with a smart device design and environmentally friendly fabrication. This is essential to rapidly move from lab- to large-scale manufacturing in view of the forthcoming launch of perovskite-based PV technology to the market from a circular economy perspective.
Conflicts of interest
There are no conflicts to declare.Data availability
All the presented data were extracted from previously published papers with the authors' obtained copyrights and processed using commonly available imaging software. Additional information is available from the corresponding author upon reasonable request.Acknowledgements
This work was supported by FARE Ricerca in Italia Project (EXPRESS, no. R18ENKMTA3) and Fondazione Cariplo Economia Circolare 2021 Project (FLHYPER, no. 20201067). Authors acknowledge support from the GOPV project (CSEAA_00011), which received funds from Bando Ricerca di Sistema-CSEA-TIPO A Piano triennale 2019–2021 Decreto direttoriale 27 Ottobre 2021 del Ministero della Transizione Ecologica; MASE-(ex MITE); and Ministero dell’Università e della Ricerca and University of Pavia through the programme Dipartimenti di Eccellenza 2023–2027. Authors are grateful to Riccardo Pallotta for his precious support in the revision of the manuscript.Notes and references
- End of Life Management: Solar Photovoltaic Panels (IRENA, 2016).
- H. Mirletz, H. Hieslmair, S. Ovaitt, T. L. Curtis and T. M. Barnes, Nat. Phys., 2023, 19, 1376–1378 Search PubMed.
- V. Larini, L. Ardito, A. Messeni Petruzzelli, F. Matteucci and G. Grancini, Chem, 2023, 9, 2738–2756 CAS.
- M. A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS.
- National Renewable Energy Laboratory (NREL), Best Research-Cell Efficiency Chart.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- J. Y. Kim, J. W. Lee, H. S. Jung, H. Shin and N. G. Park, Chem. Rev., 2020, 120, 7867–7918 CrossRef CAS PubMed.
- C. Zuo, H. J. Bolink, H. Han, J. Huang, D. Cahen and L. Ding, Adv. Sci., 2016, 3, 1500324 CrossRef PubMed.
- H. Zhu, S. Teale, M. N. Lintangpradipto, S. Mahesh, B. Chen, M. D. McGehee, E. H. Sargent and O. M. Bakr, Nat. Rev. Mater., 2023, 8, 569–586 CrossRef.
- R. Pallotta, S. Cavalli, M. Degani and G. Grancini, Small Struct., 2024, 5, 2300448 CrossRef CAS.
- C. C. Boyd, R. Cheacharoen, T. Leijtens and M. D. McGehee, Chem. Rev., 2019, 119, 3418–3451 CrossRef CAS PubMed.
- B. Ding, Y. Ding, J. Peng, J. Romano-deGea, L. E. K. Frederiksen, H. Kanda, O. A. Syzgantseva, M. A. Syzgantseva, J. N. Audinot, J. Bour, S. Zhang, T. Wirtz, Z. Fei, P. Dörflinger, N. Shibayama, Y. Niu, S. Hu, S. Zhang, F. F. Tirani, Y. Liu, G. J. Yang, K. Brooks, L. Hu, S. Kinge, V. Dyakonov, X. Zhang, S. Dai, P. J. Dyson and M. K. Nazeeruddin, Nature, 2024, 628, 299–305 CrossRef CAS PubMed.
- D. P. McMeekin, P. Holzhey, S. O. Fürer, S. P. Harvey, L. T. Schelhas, J. M. Ball, S. Mahesh, S. Seo, N. Hawkins, J. Lu, M. B. Johnston, J. J. Berry, U. Bach and H. J. Snaith, Nat. Mater., 2023, 22, 73–83 CrossRef CAS PubMed.
- P. Mariani, M. Á. Molina-García, J. Barichello, M. I. Zappia, E. Magliano, L. A. Castriotta, L. Gabatel, S. B. Thorat, A. E. Del Rio Castillo, F. Drago, E. Leonardi, S. Pescetelli, L. Vesce, F. Di Giacomo, F. Matteocci, A. Agresti, N. De Giorgi, S. Bellani, A. Di Carlo and F. Bonaccorso, Nat. Commun., 2024, 15, 1–15 Search PubMed.
- S. P. Feng, Y. Cheng, H. L. Yip, Y. Zhong, P. W. K. Fong, G. Li, A. Ng, C. Chen, L. A. Castriotta, F. Matteocci, L. Vesce, D. Saranin, A. Di Carlo, P. Wang, J. Wei Ho, Y. Hou, F. Lin, A. G. Aberle, Z. Song, Y. Yan, X. Chen, Y. M. Yang, A. A. Syed, I. Ahmad, T. Leung, Y. Wang, J. Y. Lin, A. M. C. Ng, Y. Li, F. Ebadi, W. Tress, G. Richardson, C. Ge, H. Hu, M. Karimipour, F. Baumann, K. Tabah, C. Pereyra, S. R. Raga, H. Xie, M. Lira-Cantu, M. V. Khenkin, I. Visoly-Fisher, E. A. Katz, Y. Vaynzof, R. Vidal, G. Yu, H. Lin, S. Weng, S. Wang and A. B. Djurišic, J. Phys. Mater., 2023, 6, 032501 CrossRef CAS.
- European Commission, A new Circular Economy Action Plan For a cleaner and more competitive Europe, Brussels, 2020.
- United Nations, Transforming our World: the 2030 Agenda for Sustainable Development, 2015.
- X. Tian, S. D. Stranks and F. You, Nat. Sustainability, 2021, 4, 821–829 CrossRef.
- X. Tian, B. Roose, S. D. Stranks and F. You, Energy Environ. Sci., 2023, 16, 5551–5567 RSC.
- G. Schileo and G. Grancini, Lead or No Lead? Availability, Toxicity, Sustainability and Environmental Impact of Lead-Free Perovskites Solar Cells Search PubMed.
- V. K. Ravi, B. Mondal, V. V. Nawale and A. Nag, ACS Omega, 2020, 5, 29631–29641 CrossRef CAS PubMed.
- T. Sanders, Y. Liu, V. Buchner and P. B. Tchounwou, Neurotoxic effects and biomarkers of lead exposure: a review, Rev. Environ. Health, 2009, 24, 15–45 CAS.
- F. Faini, V. Larini, A. Scardina and G. Grancini, MRS Bull., 2024, 49, 1059–1069 CrossRef.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 CrossRef CAS PubMed.
- F. Wang, X. Zou, M. Xu, H. Wang, H. Wang, H. Guo, J. Guo, P. Wang, M. Peng, Z. Wang, Y. Wang, J. Miao, F. Chen, J. Wang, X. Chen, A. Pan, C. Shan, L. Liao and W. Hu, Adv. Sci., 2021, 8, 2100569 CrossRef CAS PubMed.
- X. Wu, B. Li, Z. Zhu, C. C. Chueh and A. K. Y. Jen, Chem. Soc. Rev., 2021, 50, 13090–13128 RSC.
- R. Pallotta, M. Degani, F. Toniolo, S. Cavalli, F. Turci, W. Hua Bi, A. Girella, C. Milanese, A. Schüler, A. Magrez and G. Grancini, Mater. Today Adv., 2024, 24, 100541 CrossRef CAS.
- M. Degani, R. Pallotta, G. Pica, M. Karimipour, A. Mirabelli, K. Frohna, M. Anaya, T. Xu, C. Q. Ma, S. D. Stranks, M. L. Cantù and G. Grancini, Adv. Energy Mater., 2025, 15, 2404469 CrossRef CAS.
- R. Pallotta, F. Faini, F. Toniolo, V. Larini, M. Schmidt, S. Marras, G. Pica, S. Cavalli, S. Mattioni, L. E. Hueso, B. Martin-Garcia, B. Ehrler and G. Grancini, Joule, 2025, 9(6), 101964 CrossRef.
- C. Yang, W. Hu, J. Liu, C. Han, Q. Gao, A. Mei, Y. Zhou, F. Guo and H. Han, Light: Sci. Appl., 2024, 13, 227 CrossRef CAS PubMed.
- A. S. R. Bati, Y. L. Zhong, P. L. Burn, M. K. Nazeeruddin, P. E. Shaw and M. Batmunkh, Commun. Mater., 2023, 4, 2 CrossRef CAS.
- E. L. Unger, L. Kegelmann, K. Suchan, D. Sörell, L. Korte and S. Albrecht, J. Mater. Chem. A, 2017, 5, 11401–11409 RSC.
- F. U. Kosasih, E. Erdenebileg, N. Mathews, S. G. Mhaisalkar and A. Bruno, Joule, 2022, 6, 2692–2734 CrossRef CAS.
- N. Bartie, L. Cobos-Becerra, F. Mathies, J. Dagar, E. Unger, M. Fröhling, M. A. Reuter and R. Schlatmann, J. Ind. Ecol., 2023, 27, 993–1007 CrossRef CAS.
- X. Wu, D. Zhang, X. Wang, X. Jiang, B. Liu, B. Li, Z. Li, D. Gao, C. Zhang, Y. Wang and Z. Zhu, EcoMat, 2023, 5, e12352 CrossRef CAS.
- A. Maalouf, T. Okoroafor, Z. Jehl, V. Babu and S. Resalati, Renewable Sustainable Energy Rev., 2023, 186, 113652 CrossRef CAS.
- M. L. Parisi, S. Maranghi, L. Vesce, A. Sinicropi, A. Di Carlo and R. Basosi, Renewable Sustainable Energy Rev., 2020, 121, 109703 CrossRef.
- M. De Bastiani, V. Larini, R. Montecucco and G. Grancini, Energy Environ. Sci., 2022, 16, 421–429 RSC.
- A. Martulli, N. Rajagopalan, F. Gota, T. Meyer, U. W. Paetzold, S. Claes, A. Salone, J. Verboven, R. Malina, B. Vermang and S. Lizin, Prog. Photovoltaics Res. Appl., 2023, 31, 180–194 CrossRef.
- Fraunhofer institute for solar energy systems ise. levelized cost of electricity renewable energy technologies June 2021.
- V. Larini, C. Ding, B. Wang, R. Pallotta, F. Faini, L. Pancini, Z. Zhao, S. Cavalli, M. Degani, M. De Bastiani, F. Doria, C.-Q. Ma, F. You and G. Grancini, EES Sol., 2025 10.1039/D4EL00004H.
- G. Rodriguez-Garcia, E. Aydin, S. De Wolf, B. Carlson, J. Kellar and I. Celik, ACS Sustainable Chem. Eng., 2021, 9, 15239–15248 CrossRef CAS.
- R. Vidal, J. A. Alberola-Borràs, S. N. Habisreutinger, J. L. Gimeno-Molina, D. T. Moore, T. H. Schloemer, I. Mora-Seró, J. J. Berry and J. M. Luther, Nat. Sustainability, 2021, 4, 277–285 CrossRef.
- European Chemical Agency (ECHA), Substance Infocard: N,N-dimethylformamide, https://echa.europa.eu/substance-information/-/substanceinfo/100.000.617, (accessed 24 July 2024).
- Z. Wu, M. Sytnyk, J. Zhang, G. Babayeva, C. Kupfer, J. Hu, S. Arnold, J. Hauch, C. Brabec and I. M. Peters, Energy Environ. Sci., 2024, 17, 4248–4262 RSC.
- L. McGovern, E. C. Garnett, S. Veenstra and B. van der Zwaan, Sustainable Energy Fuels, 2023, 7, 5259–5270 RSC.
- Y. Hu, T. Niu, Y. Liu, Y. Zhou, Y. Xia, C. Ran, Z. Wu, L. Song, P. Müller-Buschbaum, Y. Chen and W. Huang, ACS Energy Lett., 2021, 6, 2917–2943 CrossRef CAS.
- R. Deng, N. L. Chang, Z. Ouyang and C. M. Chong, Renewable Sustainable Energy Rev., 2019, 109, 532–550 CrossRef CAS.
- A. Binek, M. L. Petrus, N. Huber, H. Bristow, Y. Hu, T. Bein and P. Docampo, ACS Appl. Mater. Interfaces, 2016, 8, 12881–12886 CrossRef CAS PubMed.
- European Parliament, Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives (Text with EEA relevance), 2008.
- P. Čulík, K. Brooks, C. Momblona, M. Adams, S. Kinge, F. Maréchal, P. J. Dyson and M. Khaja Nazeeruddin, Design and Cost Analysis of 100 MW Perovskite Solar Panel Manufacturing Process in Different Locations, ACS Energy Lett., 2022, 7, 3039–3044 CrossRef.
- V. Larini, C. Ding, F. Faini, G. Pica, G. Bruni, L. Pancini, S. Cavalli, M. Manzi, M. Degani, R. Pallotta, M. De Bastiani, C. Q. Ma and G. Grancini, Adv. Funct. Mater., 2023, 2306040 Search PubMed.
- B. L. Smith, M. Woodhouse, K. A. W. Horowitz, T. J. Silverman, J. Zuboy and R. M. Margolis, Photovoltaic (PV) Module Technologies: 2020 Benchmark Costs and Technology Evolution Framework Results, 2021 Search PubMed.
- L. McGovern, E. C. Garnett, S. Veenstra and B. van der Zwaan, Sustainable Energy Fuels, 2023, 7, 5259–5270 RSC.
- D. Bogachuk, P. van der Windt, L. Wagner, D. Martineau, S. Narbey, A. Verma, J. Lim, S. Zouhair, M. Kohlstädt, A. Hinsch, S. D. Stranks, U. Würfel and S. W. Glunz, ACS Sustainable Resour. Manage., 2024, 1, 417–426 CrossRef CAS PubMed.
- C. Zhang and N. G. Park, Commun Mater., 2024, 5, 194 CrossRef CAS.
- G. Li and H. Chen, Sol. RRL, 2024, 8, 2400540 CrossRef CAS.
- E. S. Akulenko, M. Hadadian, A. Santasalo-Aarnio and K. Miettunen, Heliyon, 2023, 9, e13584 CrossRef CAS PubMed.
- J. J. Cordell, M. Woodhouse and E. L. Warren, Joule, 2025, 9, 101781 CrossRef CAS.
- C. Zhu, S. Li, Y. Li, K. Liu, J. Chen, B. Lu and X. Li, Environ. Sci. Pollut. Res., 2023, 30, 43472–43479 CrossRef CAS PubMed.
- Y. Zhu, Y. Kang, H. Huang, D. Zhuang, M. Li, Z. Ling, K. Peng, L. Zhai and C. Zou, J. Mater. Chem. A, 2023, 12, 2916–2923 RSC.
- H. Zhang, J. W. Lee, G. Nasti, R. Handy, A. Abate, M. Grätzel and N. G. Park, Nature, 2023, 617, 687–695 CrossRef CAS PubMed.
- C. H. Chen, S. N. Cheng, L. Cheng, Z. K. Wang and L. S. Liao, Adv. Energy Mater., 2023, 13, 2204144 CrossRef CAS.
- M. M. Byranvand, W. Zuo, R. Imani, M. Pazoki and M. Saliba, Chem. Sci., 2022, 13, 6766–6781 RSC.
- M. S. Chowdhury, K. S. Rahman, V. Selvanathan, A. K. M. Hasan, M. S. Jamal, N. A. Samsudin, M. Akhtaruzzaman, N. Amin and K. Techato, RSC Adv., 2021, 11, 14534–14541 RSC.
- L. Huang, Z. Hu, J. Xu, X. Sun, Y. Du, J. Ni, H. Cai, J. Li and J. Zhang, Sol. Energy Mater. Sol. Cells, 2016, 152, 118–124 CrossRef CAS.
- B. Augustine, K. Remes, G. S. Lorite, J. Varghese and T. Fabritius, Sol. Energy Mater. Sol. Cells, 2019, 194, 74–82 CrossRef CAS.
- X. Feng, S. Wang, Q. Guo, Y. Zhu, J. Xiu, L. Huang, Z. Tang and Z. He, J. Phys. Chem. Lett., 2021, 12, 4735–4741 CrossRef CAS PubMed.
- W. Zhu, W. Chai, D. Chen, H. Xi, D. Chen, J. Chang, J. Zhang, C. Zhang and Y. Hao, ACS Appl. Mater. Interfaces, 2020, 12, 4549–4557 CrossRef CAS PubMed.
- B. J. Kim, D. H. Kim, S. L. Kwon, S. Y. Park, Z. Li, K. Zhu and H. S. Jung, Nat. Commun., 2016, 7, 1–9 Search PubMed.
- L. Huang, J. Xu, X. Sun, R. Xu, Y. Du, J. Ni, H. Cai, J. Li, Z. Hu and J. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 3261–3269 CrossRef CAS.
- X. Zhao, H. Shen, R. Sun, Q. Luo, X. Li, Y. Zhou, M. Tai, J. Li, Y. Gao, X. Li and H. Lin, Sol. RRL, 2018, 2, 1700194 CrossRef.
- Z. Ku, X. Xia, H. Shen, N. H. Tiep and H. J. Fan, Nanoscale, 2015, 7, 13363–13368 RSC.
- M. H. Li, Y. S. Yang, K. C. Wang, Y. H. Chiang, P. S. Shen, W. C. Lai, T. F. Guo and P. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 41845–41854 CrossRef CAS PubMed.
- D. Bogachuk, P. van der Windt, L. Wagner, D. Martineau, S. Narbey, A. Verma, J. Lim, S. Zouhair, M. Kohlstädt, A. Hinsch, S. D. Stranks, U. Würfel and S. W. Glunz, ACS Sustainable Resour. Manage., 2024, 1, 417–426 CrossRef CAS PubMed.
- J. M. Kadro, N. Pellet, F. Giordano, A. Ulianov, O. Müntener, J. Maier, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2016, 9, 3172–3179 RSC.
- G. Yang, M. Wang, C. Fei, H. Gu, Z. J. Yu, A. Alasfour, Z. C. Holman and J. Huang, ACS Energy Lett., 2023, 8, 1639–1644 CrossRef CAS.
- M. Yang, T. Tian, Y. Fang, W. G. Li, G. Liu, W. Feng, M. Xu and W. Q. Wu, Nat. Sustainability, 2023, 6, 1455–1464 CrossRef.
- M. Ren, Y. Miao, T. Zhang, Z. Qin, Y. Chen, N. Wei, X. Qian, T. Wang and Y. Zhao, ACS Sustainable Chem. Eng., 2021, 9, 16519–16525 CrossRef CAS.
- J. S. Hong, H. J. Kim, C. H. Sohn, O. Y. Gong, J. H. Choi, K. H. Cho, G. S. Han, K. T. Nam and H. S. Jung, Energy Environ. Mater., 2023, 6, 1–7 Search PubMed.
- S. Y. Park, J. S. Park, B. J. Kim, H. Lee, A. Walsh, K. Zhu, D. H. Kim and H. S. Jung, Nat. Sustainability, 2020, 3, 1044–1051 CrossRef.
- J. Lee, Y. Ko, S. Kim and H. G. Hur, J. Hazard. Mater., 2023, 442, 130106 CrossRef CAS PubMed.
- F. Schmidt, M. Amrein, S. Hedwig, M. Kober-Czerny, A. Paracchino, V. Holappa, R. Suhonen, A. Schäffer, E. C. Constable, H. J. Snaith and M. Lenz, J. Hazard. Mater., 2023, 447, 130829 CrossRef CAS PubMed.
- J. Xu, Z. Hu, L. Huang, X. Huang, X. Jia, J. Zhang, J. Zhang and Y. Zhu, Prog. Photovoltaics Res. Appl., 2017, 25, 1022–1033 CrossRef CAS.
- H. Wang, X. Chen, X. Li, J. Qu, H. Xie, S. Gao, D. Wang and H. Yin, Chem. Eng. J., 2022, 447, 137498 CrossRef CAS.
- C. G. Poll, G. W. Nelson, D. M. Pickup, A. V. Chadwick, D. J. Riley and D. J. Payne, Green Chem., 2016, 18, 2946–2955 RSC.
- P. Chhillar, B. P. Dhamaniya, V. Dutta and S. K. Pathak, ACS Omega, 2019, 4, 11880–11887 CrossRef CAS PubMed.
- B. Chen, C. Fei, S. Chen, H. Gu, X. Xiao and J. Huang, Nat. Commun., 2021, 12, 1–10 CrossRef CAS PubMed.
- F. Deng, S. Li, X. Sun, H. Li and X. Tao, ACS Appl. Mater. Interfaces, 2022, 14, 52163–52172 CrossRef CAS PubMed.
- S. Zhang, L. Shen, M. Huang, Y. Yu, L. Lei, J. Shao, Q. Zhao, Z. Wu, J. Wang and S. Yang, ACS Sustainability Chem. Eng., 2018, 6, 7558–7564 CrossRef CAS.
- A. Binek, M. L. Petrus, N. Huber, H. Bristow, Y. Hu, T. Bein and P. Docampo, ACS Appl. Mater. Interfaces, 2016, 8, 12881–12886 CrossRef CAS PubMed.
- X. Feng, Q. Guo, J. Xiu, Z. Ying, K. W. Ng, L. Huang, S. Wang, H. Pan, Z. Tang and Z. He, Cell Rep. Phys. Sci., 2021, 2, 1–15 Search PubMed.
- K. Wang, T. Ye, X. Huang, Y. Hou, J. Yoon, D. Yang, X. Hu, X. Jiang, C. Wu, G. Zhou and S. Priya, Matter, 2021, 4, 2522–2541 CrossRef CAS.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–993 RSC.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2015, 18, 288–296 RSC.
- X. Xiao, N. Xu and X. Tian, et al., Aqueous-based recycling of perovskite photovoltaics, Nature, 2025, 638, 670–675, DOI:10.1038/s41586-024-08408-7.
- R. Vidal, J. A. Alberola-Borràs, S. N. Habisreutinger, J. L. Gimeno-Molina, D. T. Moore, T. H. Schloemer, I. Mora-Seró, J. J. Berry and J. M. Luther, Nat. Sustainability, 2021, 4, 277–285 CrossRef.
- H. J. Kim, Y. J. Kim, G. S. Han and H. S. Jung, Green Solvent Strategies toward Sustainable Perovskite Solar Cell Fabrication, Sol. RRL, 2024, 8, 2300910, DOI:10.1002/solr.202300910.
- H. J. Kim, J. M. Lee, J. H. Choi, D. H. Kim, G. S. Han and H. S. Jung, J. Hazard. Mater., 2021, 416, 125696 CrossRef CAS PubMed.
- H. J. Kim, O. Y. Gong, Y. J. Kim, G. W. Yoon, G. S. Han, H. Shin and H. S. Jung, ACS Energy Lett., 2023, 8, 4330–4337 CrossRef CAS.
Footnote |
| † These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2025 |