 Open Access Article
Open Access ArticleIntracellular aggregation of exogenous molecules for biomedical applications
Da-Yong
Hou†
 abc,
Haoran
Wang†
abc,
Haoran
Wang†
 *d,
Yue-Ze
Wang
*d,
Yue-Ze
Wang
 ac,
Dong-Bing
Cheng
ac,
Dong-Bing
Cheng
 *e,
Ben Zhong
Tang
*e,
Ben Zhong
Tang
 *cf and
Wanhai
Xu
*cf and
Wanhai
Xu
 *a
*a
aNHC Key Laboratory of Molecular Probe and Targeted Theranostics, Heilongjiang Key Laboratory of Scientific Research in Urology, Harbin Medical University, Harbin 150001, China. E-mail: xuwanhai@hrbmu.edu.cn
bDepartment of PET-CT/MRI, Harbin Medical University Cancer Hospital, Harbin 150001, China
cSchool of Science and Engineering, Shenzhen Institute of Aggregate Science and Technology, The Chinese University of Hong Kong, Shenzhen (CUHK-Shenzhen), Shenzhen, Guangdong 518172, China. E-mail: tangbenz@cuhk.edu.cn
dFaculty of Materials Science, Shenzhen MSU-BIT University, Shenzhen 518115, China. E-mail: wanghr@smbu.edu.cn
eSchool of Chemistry, Chemical Engineering & Life Science, Wuhan University of Technology, No. 122 Lushi Road, Wuhan 430070, China. E-mail: chengdb@whut.edu.cn
fDepartment of Chemistry, Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, The Hong Kong University of Science and Technology, Hong Kong, China
First published on 6th June 2025
Abstract
Most biomolecules play important roles in aggregated states, as exemplified by proteins and DNA. Inspired by biomacromolecule formation, the exploration of intracellular bioactive materials derived from exogenous molecules has drawn considerable interest. In cells, exogenous molecules may assemble into macromolecules and supermolecules and thus help monitor disease processes or regulate the cell fate, which provides a new approach to disease treatment. The diverse cellular microenvironments (reductive in the cytoplasm, oxidative in mitochondria, and acidic in lysosomes) can be exploited to achieve controllable and precise intracellular aggregation using intelligent molecular design. Moreover, the intracellular polymerization and organelle targeting–triggered aggregation of exogenous molecules can be used for cell fate manipulation. This review deals with the intracellular aggregation of exogenous molecules activated by intracellular stimuli, exogenous stimuli, and organelle targeting and discusses the related molecular mechanisms and biomedical applications, providing guidance for the design of bioactive materials and discovery of theranostic agents.
1. Introduction
Cells, the basic structural and functional units of life, are heavily reliant on the intricate aggregation of biomolecules within their boundaries to sustain diverse life functions1 ranging from growth and reproduction to metabolism and communication. The modulation of cell behavior and fate by endogenous biomolecules, such as polysaccharides,2–5 proteins,6,7 and nucleic acids,8–11 has been extensively researched. Given the importance of these biomolecules for regulating cellular processes and determining overall cell health and function,12 considerable attention has been drawn to their roles in cancer treatment, antiviral therapy, and degenerative neurological disorder management.13The precise control of cellular behavior through the intracellular clustering of exogenous molecules has emerged as a focal point of biomedical research.14 By introducing foreign molecules into cells and manipulating their aggregation patterns, one can exert fine-tuned control over cellular processes15 and revolutionize the treatment of diseases and disorders by enabling more targeted and effective interventions.16 The realization of controllable in situ aggregation of biocompatible exogenous molecules inside cells holds promise for creating novel biomaterials and pharmaceuticals specifically designed to interact with and modify cellular processes.17 Thus, this approach has the potential to advance biomaterial and pharmaceutical sciences and promote the development of new treatments for diverse medical conditions.18–20
However, the implementation of the above strategy is hindered by the diversified characteristics of organisms and complexity of the intracellular environment,21 as well as other constraints.22,23 The exogenous molecules must be biocompatible, i.e., harmoniously coexist with the cellular machinery without eliciting adverse reactions.24 Biological barriers, such as cell membranes and intracellular organelles, present physical and chemical obstacles that must be navigated. Furthermore, these molecules should maintain their bioactivity within the cellular environment, as function loss can compromise the intended effects. The conditions causing aggregation are highly specific and must be carefully controlled.25 Intracellular conditions, including pH, temperature, and the presence of various biomolecules, can profoundly influence the aggregation process.26 Another critical factor is the interference due to intracellular reactive species,27 including reactive oxygen species (ROS) and reactive nitrogen species, which can disrupt the aggregation process and lead to side reactions or inactive aggregate formation.
The folding of exogenous macromolecules within cells adds another layer of complexity.28,29 The intricate three-dimensional structure of proteins and other macromolecules is essential for their functions, and achieving the correct fold in vivo is a formidable challenge.30 Despite these challenges, intracellular aggregation has a broad range of applications in cancer therapy, bioimaging, tissue engineering, and other biomedical fields. By artificially regulating cells and tissues through the controlled aggregation of exogenous molecules within the cell, one can unlock new therapeutic strategies, imaging techniques, and tissue regeneration methods.31–33 This cutting-edge field holds great promise, motivating researchers to fully tap the potential of intracellular aggregation.34
This review deals with the intracellular aggregation of exogenous molecules due to external stimuli or alterations in the cellular microenvironment,35 discussing the progress in this field and providing a multidisciplinary biomedical perspective (Fig. 1). We examine the related aggregation mechanisms, highlighting key findings, advancements, and limitations. These limitations encompass technical barriers, such as the difficulty in accurately measuring and visualizing aggregation processes at the nanoscale, as well as biological ones, including the dynamic nature of the cellular microenvironment and potential for aggregation to disrupt normal cellular functions.
To facilitate a clearer understanding of the content of this review, Table 1 provides a structured summary of currently reported intracellular aggregation strategies for exogenous molecules. The strategies are systematically categorized based on three key dimensions: mechanism of aggregation (the underlying principles governing molecular aggregation within cells), molecular design (the structural or chemical features engineered to induce aggregation), and biomedical application (the therapeutic, diagnostic, or imaging-related purposes these strategies serve in biological contexts).
| Mechanism of aggregation | Molecular design | Biomedical application | |
|---|---|---|---|
| Intracellular microenvironment | pH | Schiff base | Intracellular pH imaging36 |
| Base-pairing DNA | Cell behavior regulation37 | ||
| pH-responsive peptide | Inhibit autophagy38 | ||
| Selective cell death39 | |||
| Increase the anti-prion activity40 | |||
| Phenylboronate | Monitoring intracellular structural change41 | ||
| Abundant periphery NH2 groups | Enhancing antigen presentation42 | ||
| Liposome-encapsulated spiky nanoparticles | Phototherapy applications43 | ||
| Protonation of CuPc–SO4 | Enhance sonodynamic therapy44 | ||
| Mixed-charge nanoparticles | Selective death of cancer cells45 | ||
| Oligonucleotides | Chemo-photothermal synergetic therapy46 | ||
| ROS | Thioketal bond47 | Selectively inducing apoptosis of cancer cells | |
| Te–O oxidative polymers48 | |||
| Organotellurides49 | |||
| Aniline dimer derivative | Phototheranostics of tumor50 | ||
| Y-shaped diacetylene | Inhibited tumor metastasis51 | ||
| Thiolated tripeptide | Intracellular artificial enzyme52 | ||
| Selenoxide peptide | Anti-inflammatory treatment53 | ||
| Thiol-modified oligo (p-phenylene vinylene) | Enhancing drug efficacy54 | ||
| Tz-conjugated assembly precursor | Self-reporting prodrug activation55 | ||
| GSH | GSH-responsive peptide | Abolish liver tumor growth and metastasis56 | |
| MR imaging of caspase 3/757 | |||
| High-efficacy cancer therapy58–60 | |||
| Proteolysis targeting chimera61 | |||
| Enhanced dual-modal imaging and photothermal therapy62 | |||
| Selective tumor ferroptosis and pyroptosis63 | |||
| MR contrast of tumor64 | |||
| N-Hydroxyethyl acrylamide | Self-inflicted apoptosis of cancer cells65 | ||
| Thio-disulfide exchange reaction | Enhanced chemotherapy66 | ||
| Enzyme | Alkaline phosphatase (ALP)-responsive peptide derivative | Selectively enhancing radiosensitivity67 | |
| Overcome drug-resistant cancer cells68 | |||
| Enhanced photoacoustic imaging69 | |||
| Quantitative imaging70 | |||
| Protein kinase A (PKA)-responsive phosphorylated polymer | Chemosensitization71 | ||
| Matrix metalloproteinase-7 (MMP-7)-responsive peptide derivative | Cancer cell death72 | ||
| Tyrosinase-catalyzed peptide | Cancer immunotherapy73 | ||
| Caspase-3-responsive peptide derivative | Drug delivery and cancer imaging74 | ||
| Imaging of protease activity75 | |||
| Gelatinase-responsive peptide derivative | Bacterial infection detection76 | ||
| Furin-responsive peptide derivative | MicroPET tumor imaging77 | ||
| Overcoming multidrug resistance (MDR)78 | |||
| Caspase-1-responsive AIEgen–peptide conjugate | Sensitive detection and eradication of intracellular bacterial infections79 | ||
| Cathepsin B-responsive AIEgen–peptide conjugate | Promote the tumor photodynamic therapy80 | ||
| SIRT5-responsive peptide derivative | Mitochondrial activity modulation81 | ||
| β-Galactosidase (β-Gla)-responsive peptide derivative | Selective identification and removal of senescent cells82 | ||
| Exogenous element | Light | Photochromic arylazopyrazole | Intertubular aggregation of microtubules83 |
| Acrylic and methacrylic monomers84 | Modulate cellular function and behaviour | ||
| Tyr-containing proteins85 | |||
| Photoactivatable prodrug | Inhibits tumor growth and metastasis86 | ||
| Poly(ethylene glycol) diacrylate | Immunoengineering87 | ||
| Temperature | Poly(N-isopropylacrylamide) (PNIPAM)88–91 | Intracellular temperature sensing | |
| Carbazole-based fluorescent organic nanomaterials92 | |||
| Dispersed fluorescent nanodiamonds93 | |||
| Poly(N-vinylcaprolactam) (PNVCL)94 | |||
| Polymer–peptide conjugates (PPCs) | Deep tumor therapy95 | ||
| Monitoring tumor therapy96 | |||
| Hydroxychalcone-based polymers (HCPs) | Mitochondrial temperature sensing97 | ||
| Other | Molecules based on click reaction | Photothermal therapy98 | |
| Cell imaging and cell killing99 | |||
| Immuno-chemotherapy100 | |||
| DNA aptamer | Enhanced intracellular retention101 | ||
| Tumor therapy102 | |||
| Peptide foldamer | Cancer cell death103 | ||
| Silica nanoparticles | Ultrasound-guided cell implantation104,105 | ||
| Lycobetaine (LBT) | Modulate mitochondrial connectivity106 | ||
| Zoledronate | Stress macrophage mitochondria107 | ||
| Organelle targeting | Mitochondria | Cyclometalated platinum(II) compounds108 | Mitochondria imaging |
| Host–guest conjugates109 | |||
| Triphenylphosphonium (TPP) conjugation | Overcome proteolytic degradation110 | ||
| Controls cancer cell fate111 | |||
| Mitochondria-targeting nucleopeptide | Treatment of age-related diseases112 | ||
| Host–guest conjugates | Ferroptosis of cancer cells113 | ||
| TPP-modified DNA114,115 | Cancer cell apoptosis | ||
| TPP-modified peptide116,117 | |||
| Mitochondria-targeting nucleopeptide118 | |||
| Lysosome | DNA nanoparticles | Including cell movement and cell autophagy37 | |
| DNA-ceria nanocomplex | Reducing cell mortality119 | ||
| Peptide derivative | Inhibit autophagy38 | ||
| Triggering apoptosis of cells120 | |||
| Addressing MDR121 | |||
| Enhanced antitumor immunity42,122 | |||
| DNA nanoribbons | Cancer cell imaging123 | ||
| Nanocrystals | Photothermal therapy124 | ||
| Drug–silane conjugates | Addressing MDR125 | ||
| Augmentation of chemotherapy126 | |||
| Nucleus | Drug–peptide conjugates | Abolish tumor growth and metastasis56 | |
| DNA-aptamer | Antitumor immunity127 | ||
| Phosphopeptide assemblies | Selective killing of tumor cells128,129 | ||
| Anthracene-guanidine derivative | Cell sensing and imaging130 | ||
In addressing these limitations, we propose innovative solutions and potential research directions, including the development of advanced imaging techniques to better visualize aggregation processes, utilization of biocompatible materials to minimize cellular function disruption, and exploration of novel strategies to control and manipulate aggregation pathways for therapeutic purposes. By presenting these potential solutions, we aim to stimulate new ways of thinking and foster further advancements in the field of intracellular aggregation research.
2. Strategies for the intracellular delivery of exogenous molecules
The intracellular aggregation of molecules can trigger specific biological processes and functions, e.g., the aggregation of amyloid protein fibrils at the lesion site leads to Alzheimer's disease (AD),131 whereas the aggregation of the tumor suppressor protein p53 in tumor cells is involved in cancer progression.132 Similarly, certain exogenous molecules, such as small-molecule drugs and macromolecules,133 can be engineered to aggregate within cells and thus alter their physiological state or achieve a particular function.134 Usually, the realization of the intracellular aggregation of exogenous molecules is dependent on the pathway of their entry into cells, which is related to the molecular state, i.e., the physicochemical properties of these molecules play a crucial role in determining how and where they enter the cell. The molecular state can influence factors such as molecule size, shape, and charge, which, in turn, affect the ability of exogenous molecules to traverse the cell membrane.135 For instance, small uncharged molecules may freely diffuse through the membrane, while larger or charged molecules may require specific transport proteins or other mechanisms to facilitate their entry.The entry pathway can be influenced by the internal environment of the cell and its regulatory mechanisms.136,137 Cells have a complex network of signaling pathways and regulatory proteins that control the in- and outward movement of molecules to ensure that only certain molecules enter and are directed to the correct location within the cell.138 Thus, the realization of intracellular aggregation is a multifaceted process that is heavily influenced by the entry pathway, which is related to the molecular state of the substances involved. Understanding these relationships is crucial for developing effective strategies to manipulate or control intracellular aggregation in biological and medical applications.
2.1. Free diffusion
Free diffusion is a process by which exogenous molecules spontaneously and passively permeate through the cell membrane, driven solely by concentration gradients and random molecular motion.139 The molecules simply diffuse across the phospholipid bilayer of the membrane, moving from higher- to lower-concentration areas until an equilibrium is reached. This intracellular delivery method does not involve active transport proteins or energy-consuming processes and is therefore straightforward but often inefficient.140 Li et al. reported a small molecule that diffused into cells, underwent polymerization catalyzed by intracellular transglutaminase, and subsequently in situ aggregated into elastin-like polypeptides (Fig. 2A).141 Various topological nanostructures were constructed (Fig. 2B and C) and shown to be substantially cytotoxic despite exhibiting different biological functions (Fig. 2D–F). | ||
| Fig. 2 (A) Intracellular polymerization catalyzed by TGase and controllable in situ nanostructure construction. (B) Intracellular polymerization of peptide monomers by TG2 monitored using fluorescence resonance energy transfer techniques. (C) Confocal images showing the nanostructures (green) formed from P4 at 4 °C and from P7 and P9 at 37 °C. (D) Thermoprecipitation of FITC-P4 from cells. (E) Time-dependent growth of P4 within cells, with the FITC-labeled P4 collected at various time points via thermoprecipitation. (F) Thermodynamic self-assembly of DBD-P4 in HeLa cells during cooling. Adapted with permission from ref. 141. Copyright 2017 Springer Nature. | ||
2.2. Endocytosis
Endocytosis is a fundamental cellular process by which cells internalize extracellular material, including nutrients, signaling molecules, and pathogens,142 and is characterized by the formation of vesicles that bud off from the plasma membrane and encapsulate extracellular material for delivery into the cell. Exogenous molecules capable of intracellular aggregation typically enter cells through receptor-mediated endocytosis and macropinocytosis. Receptor-mediated endocytosis involves specific interactions between a receptor on the cell surface and its cognate ligand, leading to the internalization of the ligand–receptor complex. This highly specific and regulated form of endocytosis plays a crucial role in the internalization of various exogenous molecules, including nutrients, hormones, growth factors, and antibodies.143 Macropinocytosis is a form of endocytosis that allows cells to internalize large volumes of extracellular fluid and solutes and is characterized by the formation of macropinosomes, which are large vesicles derived from the plasma membrane. Macropinocytosis can be used to deliver large molecules, particles, or even cells into the target cell interior. By engineering molecules or particles to trigger macropinocytosis, one can efficiently internalize them while bypassing the traditional barriers of cellular uptake. Xu's group demonstrated that receptor-mediated endocytosis and macropinocytosis are the main endocytic pathways of exogenous molecules (Fig. 3A). Owing to the aggregation of molecules occurring within cells, the intracellular fluorescence intensity of these molecules was significantly higher than that in the control group. This indicates their efficient enrichment inside the cells (Fig. 3B). The intracellular aggregation of molecules was accompanied by a progressive enhancement in fluorescence intensity over the course of laser irradiation, and this phenomenon may be attributed to the laser-mediated escape of these molecules from lysosomes (Fig. 3C). Moreover, the selective aggregation of nanomaterials through endocytosis in cancer cells, which can be realized under the action of tumor-specific enzymes (Fig. 3D and E).58,144,145 | ||
| Fig. 3 (A) Mechanism of endocytic molecular uptake. (B) Images of HeLa cells incubated for 24 h at 37 °C with 500 mM NBDff-es-tau-(O), washed three times, and treated with a compound-free live-cell imaging solution. Scale bar: 50 mm. (C) Electron microscopy images confirming the endocytosis of NBDff-es-tau-(O) assemblies. Adapted with permission from ref. 145. Copyright 2017 Elsevier BV. (D) Endoplasmic reticulum stress induction through the tandem molecular self-assembly of exogenous molecules. (E) LC spectrum and optical images illustrating the conversion from Comp. 1 to Comp. 3 under the influence of a tumor-specific enzyme. Optical and transmission electron microscopy (TEM) images of the solution formed by adding alkaline phosphatase (ALP) to Comp. 1 (200 μM) for 24 h and the precipitate created by adding rat liver microsomes and NADPH to Comp. 2 for 24 h (scale bar: 100 nm). Adapted with permission from ref. 58. Copyright 2019 American Association for the Advancement of Science. | ||
3. Intracellular aggregation mechanisms of exogenous molecules
The design and synthesis of exogenous molecules can affect their ability to aggregate within the cell. This aggregation can be achieved by relying on the cellular microenvironment alone without external interventions, as exemplified by the tumor cell microenvironment (ROS, pH, hypoxia, glutathione (GSH), proteases, etc.).146–149 and organelles (lysosomes, mitochondria, endoplasmic reticulum, etc.) (Fig. 4). The aggregation of exogenous molecules in cells can also be achieved by exposure to light, ultrasound, heat, and other conditions under artificial control with external interventions.150,151 Overall, the process and outcome of exogenous molecule polymerization in cells are determined by a combination of factors, including the physical and chemical conditions of the intracellular environment, nature and design of the exogenous molecules, and cell–molecule interactions. Diverse aggregation schemes based on these complex intra- and extracellular conditions have been developed, from the intracellular assembly of peptides, small-molecule aggregation-induced fluorescence emission, and supramolecular host–guest interactions to the chain-growth and stepwise polymerization of small molecules, to explore the related biomedical prospects, find therapies for major diseases such as cancer, and promote the development of nanobiomedicine.3.1. Intracellular microenvironment-induced aggregation of exogenous molecules
Yamamoto et al. reported a peptide amphiphile aggregating into entangled nanofibers (hydrogel) in the acidic intracellular environment and used it to induce selective tumor cell death and achieve a high anti-tumor activity (Fig. 5A and B).39 Lin et al. developed a strategy for the pH-responsive intracellular aggregation of inorganic molecules based on the protonation of CuPc–SO4 and BSA under the acidic intracellular conditions and the resulting interaction weakening, which led to Fe3+ release and self-aggregation into nanorods (Fig. 5C and D). This acidity-sensitive aggregation was vital for achieving specific accumulation within the tumor microenvironment and thereby enhancing retention and sonodynamic therapy (SDT) efficacy.44
 | ||
| Fig. 5 (A) Selective cell death caused by the self-assembly of an intracellular pH-responsive peptide amphiphile. (B) Gelation tests of PBS after 24 h at various pH performed using 0.15 wt% C16-VVAEEE. TEM images of freeze-dried hydrogel and PBS solution at pH 6.8 and 7.4. (C) Acid-responsive aggregation behavior of CuPc–Fe@BSA. (D) TEM images of CuPc–Fe@BSA after variable-duration exposure to media with various pH. Adapted with permission from ref. 44. Copyright 2024 Elsevier BV. | ||
pH-responsive intracellular aggregation can not only facilitate the escape of chemotherapeutics from lysosomes but also synergistically augment photothermal therapy (PTT), markedly improving tumor treatment efficacy. Moreover, aggregates formed in the acidic environment of lysosomes can impact cell metabolism, directly regulating cell fate or causing cell death.37,38,42,46 Borkowska et al. developed nanoparticles that featured a blend of positively and negatively charged ligands in precise ratios and targeted lysosomes in cancer cells while demonstrating a negligible cytotoxicity toward normal cells. This specificity was attributed to a sequence of pH-responsive aggregation processes. Initially, small clusters prone to endocytosis formed on the cell surface and then transformed into large structured assemblies and crystals inside cancer lysosomes under acidic conditions. These assemblies could not be removed by exocytosis, causing lysosome swelling that progressively compromised lysosomal membrane integrity and ultimately disrupted lysosomal functions and initiated cell death.45
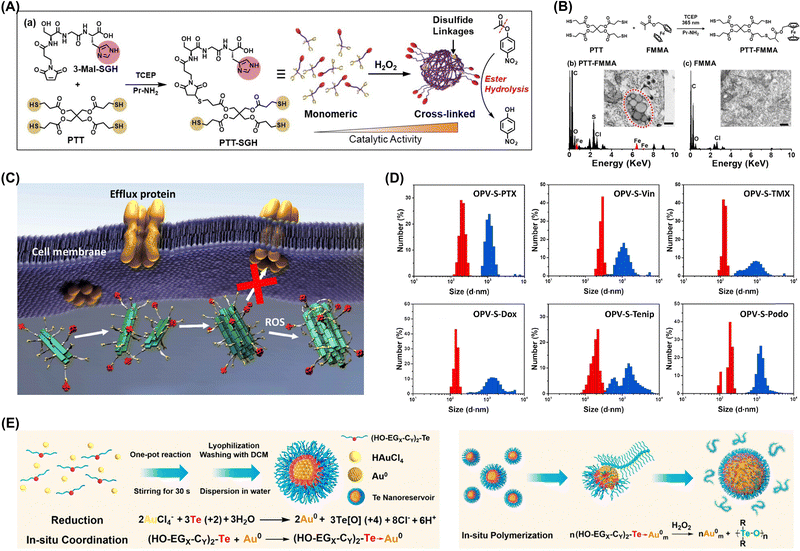 | ||
| Fig. 6 (A) Overview of artificial enzyme synthesis and thiol group crosslinking under oxidative conditions enhancing ester hydrolysis activity. (B) Synthesis of PTT-FMMA, bio-TEM and energy-dispersive spectra of nanostructures in A549 cells after 24 h of incubation with 100 μM PTT-FMMA or FMMA. Adapted with permission from ref. 52. Copyright 2022 Wiley-VCH. (C) Assembly and aggregation of OPV-S-Drugs in cancer cells. (D) Hydrodynamic diameters of OPV-S-Drugs measured by DLS (red: without H2O2; blue: with H2O2). Adapted with permission from ref. 54. Copyright 2019 Chinese Chemical Society. (E) In situ polymerization and aggregation processes in cells. Adapted with permission from ref. 48. Copyright 2021 American Chemical Society. | ||
ROS-induced aggregation is involved in the development and progression of various diseases, including neurodegenerative disorders, cardiovascular diseases, and cancer. Understanding the mechanisms underlying ROS-induced aggregation and developing strategies to mitigate its effects are crucial for advancing therapeutic interventions and improving human health.50,51 Xu's group developed an oxidative polymerization reaction exploiting the chemical properties of organotellurides and intracellular redox environment. This polymerization occurred within cells without external stimuli and was initiated by intracellular ROS, enabling selective cancer cell targeting (Fig. 6E).48 The process triggered apoptosis through a self-amplification mechanism, and the polymer products interacted with selenoproteins, disrupting intracellular antioxidant systems and increasing oxidative stress to create a positive feedback loop that further promoted oxidative polymerization and activated ROS-related apoptosis pathways. The same group developed a hyperbranched polymerization process within living cells utilizing the oxidative polymerization of organotellurides in redox environments. The resultant hyperbranched polymers assembled into branched nanostructures capable of efficiently bypassing drug pumps and minimizing drug efflux and thereby enabled sustained treatment through polymerization.49
 | ||
| Fig. 7 (A) GSH-responsive intracellular reassembly of NSBSO selectively inducing ferroptosis or pyroptosis in tumor cells based on intracellular GSH levels. (B) TEM images of NSBSO obtained after 6 h exposure to 0 and 1 mM GSH at 37 °C. (C) Molecular structures of NSBSO and NCBSO and optical and TEM images acquired after 24 h exposure to 10 mM GSH at 37 °C. Adapted with permission from ref. 63. Copyright 2022 Springer Nature. (D) GSH-controlled self-assembly turning 19F NMR signals off and Casp3/7-controlled disassembly turning them on. (E) Chemical structures of the peptide probe. (F) TEM images of 1-NPs (top) and 1-Scr-NPs (bottom) following 30 min exposure to 2 mM GSH and subsequent incubation with Casp3. (G) 19F MRI of 1 (top) and 1-Scr (bottom) in RIPA lysis buffer and cell lysate incubated with HepG2 cells and apoptotic HepG2 cells. Adapted with permission from ref. 57. Copyright 2015 American Chemical Society. | ||
Magnetic resonance imaging (MRI) is a common diagnostic tool known for its noninvasiveness, soft tissue contrast, high imaging resolution, and lack of ionizing radiation. To improve the sensitivity and specificity of MRI, Liang's group developed a contrast agent that enhanced the detection of early-stage cancer through the aggregation of peptide probes induced by intracellular GSH (Fig. 7D). This smart and controllable intracellular aggregation approach enables the enrichment of low-dose 19F probes through locally self-assembling and disassembling nanoparticles, thereby avoiding rapid transverse relaxation of the probes. The designed contrast agent (Fig. 7E) is capable of self-assembling in response to glutathione (GSH) and subsequently disassembling the formed nanoparticles under the action of caspase 3/7 (Fig. 7F). By employing this intelligent strategy, we successfully utilized probe to consecutively detect the activities of GSH and Casp3/7 in vitro and within cells. Moreover, under low-dose conditions and with the aid of a 14.1 T magnetic field, we achieved imaging of Casp3/7 activity in cells and zebrafish (Fig. 7G).57,64
In certain cases, enzyme-induced intracellular aggregation can generate toxic aggregates inducing the death of tumor cells. Li et al. used enzyme-instructed self-assembly to develop intracellular supramolecular structures markedly increasing the effectiveness of cisplatin against drug-resistant ovarian cancer cells (Fig. 8A).68 The synthesized small peptide precursors acted as substrates for carboxylesterase, which cleaved the preinstalled ester bond to afford peptides spontaneously forming nanofibers in aqueous environments. At optimal concentrations, these peptide precursors were nontoxic to cells and effectively enhanced cisplatin activity against drug-resistant ovarian cancer cells. Wang's group developed a caspase-3/7-triggered intracellular aggregation system, integrating imaging probes and therapeutic agents. When this system was applied to mouse models, intracellular aggregation enhanced drug accumulation within solid tumors because of the recognition-induced self-assembly effect (Fig. 8B and C).74
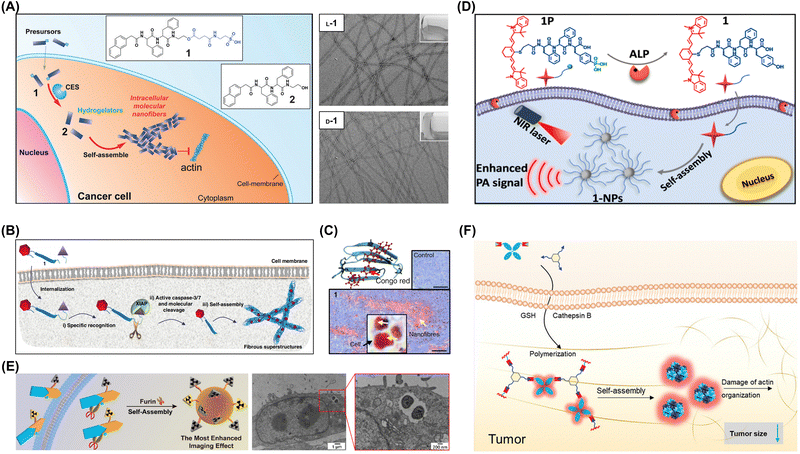 | ||
| Fig. 8 (A) Enzymatic transformation of precursor 1 by carboxylesterase to hydrogelator 2 for intracellular self-assembly. Adapted with permission from ref. 68. Copyright 2015 Wiley-VCH. (B) Mechanism of specific recognition, molecular cleavage, and in situ self-assembly leading to β-sheet nanostructures enhancing accumulation in tumor tissue. (C) Light microscopy images of Congo red-stained tumor sections with yellow arrows indicating nanofibers. Adapted with permission from ref. 74. Copyright 2019 Springer Nature. (D) ALP-triggered self-assembly of near-infrared (NIR) nanoprobes for the enhanced photoacoustic imaging of tumors. Adapted with permission from ref. 69. Copyright 2018 American Chemical Society. (E) Intracellular synthesis of hybrid gallium-68 nanoparticles in cancer cells for enhanced microPET imaging. Adapted with permission from ref. 77. Copyright 2021 American Chemical Society. (F) Enzyme-mediated intracellular reduction and condensation of peptides affording aggregation-induced emission luminogen (AIEgen)-based nanostructures enhancing fluorescence and tumor treatment efficacy. Adapted with permission from ref. 80. Copyright 2021 Wiley-VCH. | ||
Understanding the mechanisms and outcomes of the enzyme-induced intracellular aggregation of exogenous molecules is crucial for various fields, including toxicology, pharmacology, and biotechnology. By studying these interactions, one can gain insights into how cells process and respond to foreign substances and develop strategies to manipulate or effectively utilize aggregation events. Wu et al. designed a near-infrared (NIR) probe for the photoacoustic imaging of alkaline phosphatase (ALP) activity. Under the catalytic action of ALP, the probe can undergo efficient hydrolysis, followed by self-assembly to form nanoparticles. The formed nanoparticles exhibit a 6.4-fold enhancement in the photoacoustic (PA) signal at 795 nm. In vivo tumor photoacoustic imaging results demonstrate that, compared to the ALP inhibitor-treated control group, the tumor photoacoustic contrast in the experimental group increases by 2.3-fold at 4 hours post-probe injection (Fig. 8D).69 Chen et al. reported two precursor molecules capable of synthesizing hybrid gallium-68 nanoparticles in furin-overexpressing cancer cells and enhancing microPET tumor imaging in mice. In vivo experimental results revealed that when these two molecules were co-injected, the radiotracer exhibited the longest retention time in the bloodstream, the highest radioactivity in tumor regions, and the most pronounced enhancement in microPET tumor imaging within mice (Fig. 8E).77 Liu et al. developed an enzyme-mediated aggregation-induced emission luminogen (AIEgen), promoting its accumulation and retention within tumors and thus enabling extended imaging and enhanced tumor growth inhibition (Fig. 8F).80 Upon the action of tumor-specific cathepsin proteases, the cleavage of the peptide triggers a condensation polymerization reaction between the exposed cysteine residue and the 2-cyanobenzothiazole group. This reaction facilitates AIEgen accumulation at the tumor site, amplifying the fluorescence signal. Enzyme-catalyzed polymerization disrupts actin architecture, altering cell motility and suppressing proliferation. Furthermore, the AIEgen's inherent photosensitization combined with tumoral accumulation markedly boosts in vivo PDT antitumor efficacy upon light exposure.
3.2. Exogenous stimuli-induced aggregation of exogenous molecules
Exogenous molecules may undergo aggregation in response to physical, chemical, or biological interactions prompted by exogenous stimuli. Such aggregation phenomena can have important implications in various fields, including biology, chemistry, and materials science, where understanding and controlling the behavior of exogenous molecules is crucial for the development of novel technologies and applications. | ||
| Fig. 9 (A) Strategy for 365 nm illumination-induced polymerization within living cells using HPMA as a monomer and irgacure 2959 as an initiator. (B) Comparison of untreated cells and polymerized cells with actin filament staining (F-actin) after 48 and 72 h; scale bar: 10 μm. (C) Polymerization of FMMA in HeLa cells. Adapted with permission from ref. 84. Copyright 2019 Springer Nature. (D) Molecular structures of a paclitaxel-modified cyclodextrin ternary supramolecular assembly. Adapted with permission from ref. 83. Copyright 2018 Wiley-VCH. (E) Illustration of G-DC preparation through the direct permeation of hydrogel monomers and photoinitiators into cells. (F) Cryo-SEM images revealing the topographical features of GC-MPs, which maintained stability and structural integrity over 30 days. Adapted with permission from ref. 87. Copyright 2021 Wiley-VCH. | ||
Several factors influence the extent and nature of light-induced aggregation.167 The intensity, wavelength, and duration of light exposure strongly affect the aggregation degree. The chemical structure of exogenous molecules, including their functional groups and charge distributions, plays a pivotal role in determining their aggregation behavior. Moreover, the solvent environment, such as polarity, viscosity, and the presence of ions or other solutes, can markedly affect the aggregation process. Intracellular hydrogelation was swiftly achieved with minimal disruption to cellular morphology and surface antigen presentation by directly permeating dendritic cells with poly(ethylene glycol) diacrylate hydrogel monomers and using ultraviolet-activated radical polymerization (Fig. 9E). This process resulted in highly durable and adaptable cell–gel hybrid structures enabling the exchange of peptide antigens. These structures could be preserved through freezing and lyophilization and functionalized with cytokine-releasing carriers to modulate T-cell activity (Fig. 9F).87
 | ||
| Fig. 10 (A) Schematic of triggered microdomain assembly in living cells showing the solubility of GFP-ELPs in the cytosol prior to heating. (B) Illustration of polymer phase separation in a valine library of elastin-like polypeptides. Adapted with permission from ref. 168. Copyright 2012 American Chemical Society. (C) Stimuli-instructed construction of controllable nanoaggregates for monitoring tumor therapy responses. Adapted with permission from ref. 96. Copyright 2017 American Chemical Society. | ||
The temperature-sensitive properties of molecules are often used to achieve the aggregation of materials within cells. Poly(N-isopropylacrylamide) is a prominent temperature-sensitive polymer because of its unique lower critical solution temperature.90,91 This characteristic enables rapid phase transitions at physiological temperatures, which subsequently trigger aggregation within cellular environments. Qiao et al. used poly(N-isopropylacrylamide) to create functional nanoaggregates for the in vivo sensing and monitoring of cellular physiological processes (Fig. 10C).96,169 This strategy involved designing thermosensitive polymer–peptide conjugates (PPCs) forming nanoaggregates within cells because of their isothermal phase transition behavior. The phase transition characteristics of PPCs were studied by analyzing various critical parameters, including chain length, hydrophilicity, grafted peptide ratio, and concentration. In response to specific intracellular stimuli, the PPCs underwent customized changes and formed nanoaggregates with long-term retention properties.
Yin et al. developed a nanohybrid capable of capturing, converting, and utilizing the excess copper found in tumor cells.98 This approach provided dual therapeutic effects, inducing photothermal damage to primary tumors and inhibiting metastasis via copper deficiency. The nanohybrid consisted of gold nanoparticles (AuNPs) comodified with 3-azidopropylamine, 4-ethynylaniline, and N-aminoethyl-N′-benzoylthiourea (BTU). During therapy, BTU selectively chelated copper after endocytosis, diminishing intracellular copper levels and thus suppressing vascularization and tumor migration. Concurrently, BTU converted copper to cuprous ions, facilitating a click reaction between the azido and alkynyl groups on the AuNP surface and resulting in on-demand AuNP aggregation (Fig. 11A and B). Being unnatural to living cells, click reactions hold promise for advancing biological research.
 | ||
| Fig. 11 (A) Synthesis and therapeutic mechanism of ABAE nanoparticles, which target overexpressed copper in tumor cells for primary tumor elimination and metastasis inhibition through copper capture, state conversion, and cuprous ion-induced photothermal therapy. Adapted with permission from ref. 98. Copyright 2023 Wiley-VCH. (B) TEM images of ABAE nanoparticles before and after Cu2+ chelation. (C) Intracellular spontaneous amino-yne click polymerization and synthesis of poly(β-aminoacrylate). Adapted with permission from ref. 99. Copyright 2023 Science China Press. (D) Self-assembly of DNA hyperbranched aggregates in cancer cells mediated by microRNA-21. Adapted with permission from ref. 101. Copyright 2024 Wiley-VCH. | ||
Tang et al. introduced a “lab-in-cell” concept using a novel spontaneous amino-yne click polymerization.99 This method involved the spontaneous polymerization of a carbonyl-activated terminal diyne with a tetraphenylethene (TPE)-containing primary diamine within cells, resulting in a polymer with a weight-average molecular weight of 7300 Da. By leveraging the in vivo amino-yne click polymerization and aggregation-induced emission properties of TPE, the authors achieved a “turn-on” capability for cell imaging (Fig. 11C).
Specific interactions with intracellular molecules can induce their aggregation, which helps regulate intracellular molecule concentrations and enhance biomolecule activity.103,105,107,172 Li et al. used a DNA framework along with the hybridization chain reaction (HCR) to achieve the targeted assembly of hyperbranched aggregates within cancer cells.101 HCR is known for its signal amplification and linear extension features, enabling the precise triggering of precursor morphological transformations by minimal amounts of endogenous microRNA-21. The confined spatial environment of the framework and varied orientations of hairpins sped up the hyperbranched network assembly, and the produced micrometer-sized aggregates demonstrated improved intracellular retention capabilities (Fig. 11D).
3.3. Organelle targeting-induced aggregation of exogenous molecules
The rapid progress in biotechnology and nanoscience has drawn attention to methods of precisely manipulating the localization and function of exogenous molecules within cells. Organelles serve as highly specialized structural and functional units within cells, undertaking a variety of biochemical reactions and material transport tasks. Achieving the precise regulation of their functions through the induction of exogenous molecule aggregation on specific organelles is a promising strategy. Molecular recognition mechanisms can be used to direct exogenous molecules (such as drugs, nanoparticles, and proteins) to specific organelles and induce their aggregation thereon to regulate organelle functions. Organelle (mitochondria, lysosomes, nuclei, etc.) targeting-induced aggregation has enabled remarkable progress in targeted therapies (Fig. 12).173 | ||
| Fig. 13 (A) Trapping of adenosine triphosphate (ATP) within the mitochondria of cancer cells facilitated by MNP interacting with adenosine diphosphate (ADP) to trigger cell apoptosis. (B) TEM images of control cells and those exposed to the MNP/ADP complex for 4 h at a concentration of 50 mM. Adapted with permission from ref. 118. Copyright 2022 Royal Society of Chemistry. (C) Intramitochondrial formation of Mito-FF. (D) Mitochondrial colocalization of Mito-FF assessed using MitoTracker Red FM. Notable accumulation within the mitochondria is observed (scale bar: 5 μm). Adapted with permission from ref. 117. Copyright 2017 Springer Nature. (E) (i) Assemblies generated via host–guest interactions between Fc-TPP1 and WP6 molecules and (ii) nanostructures resulting from the enzyme-mediated self-assembly of these host–guest complexes. Adapted with permission from ref. 113. Copyright 2022 American Chemical Society. | ||
The high levels of hydrogen peroxide near mitochondria enable mitochondria-targeted aggregates to effectively catalyze the Fenton reaction and thus generate considerable amounts of ROS, which enhance the ability of these aggregates to kill tumor cells.176 Yang et al. showed that host–guest complexation is an effective strategy for modulating the enzymatic self-assembly of peptides. This complexation inhibited the enzymatic kinetics of peptide assemblies on the cell surface, promoting their internalization. Once internalized, the complex dissociated in the acidic environment of lysosomes to release the peptides, which then self-assembled within mitochondria. The buildup of these assemblies induced ferroptosis in cancer cells, leading to cell death both in vitro and in tumor-bearing mouse models (Fig. 13E).113
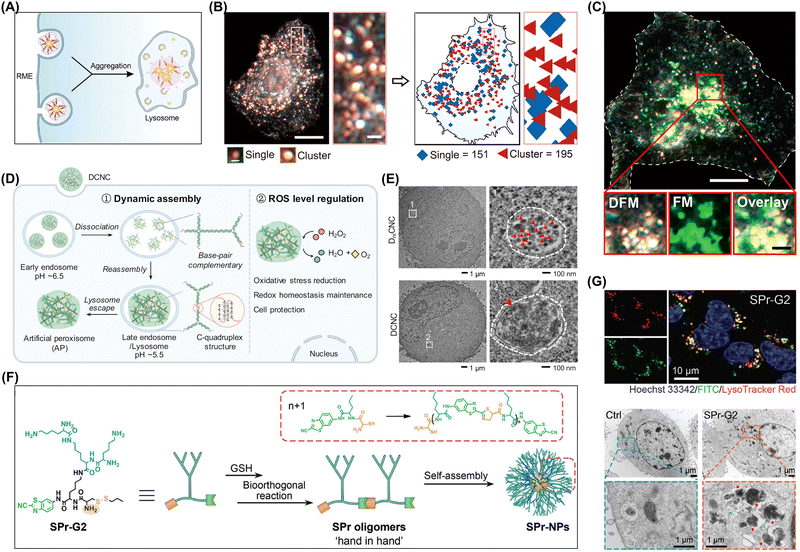 | ||
| Fig. 14 (A) Aggregation of lipo-SNPs induced by intracellular lysosome targeting during cellular uptake. (B) Left: Representative DFM images; right: corresponding pseudoimages obtained using the CBA method. Scale bars: 10 μm, 1 μm (enlarged), and 500 nm (bottom). (C) DFM-FM images revealing the colocalization of lipo-SNPs (bright yellow) alongside lysosomes (green), with scale bars measuring 10 and 2 μm (insets). Adapted with permission from ref. 43. Copyright 2024 American Chemical Society. (D) Assembly of DCNC into artificial peroxisomes under acidic conditions within lysosomes. (E) Representative TEM images and a partial enlargement of MCF-7 cells treated with DniCNC and DCNC for 6 h. Adapted with permission from ref. 119. Copyright 2022 Springer Nature. (F) Plausible mechanism of aggregation induced by intracellular lysosome targeting and representative bio-TEM images of 4T1 cells treated with PBS or SPr-G2 (200 × 10−6 M) for 3 h. Adapted with permission from ref. 42. Copyright 2022 Springer Nature. | ||
The aggregation of exogenous molecules within lysosomes is not only closely related to the metabolic and regulatory mechanisms within cells but may also impact their normal physiological functions. The aggregation of certain exogenous molecules may interfere with the normal functioning of lysosomes, leading to abnormal degradation or the accumulation of intracellular biomacromolecules and thus triggering cell damage or disease. The aggregation of exogenous molecules within lysosomes can also be used to develop new principles and methods of treating certain diseases.123,124,178 Yang's group developed a dynamic assembly system using a DNA–ceria nanocomplex for the intracellular in situ construction of artificial peroxisomes (APs) (Fig. 14D).119 These nanocomplexes were created with branched DNA featuring an i-motif structure that responded to acidic lysosomal conditions and enabled the transformation from a nanocomplex to bulk-scale APs (Fig. 14E). The initial nanoscale size promoted cellular uptake, while the bulk scale of the APs enhanced retention within the cell. The APs demonstrated enzyme-like catalytic activities, scavenging ROS through the decomposition of H2O2 into O2 and H2O. Luo et al. reported a low-generation lysine dendrimer (SPr-G2) that underwent bioorthogonal in situ polymerization in response to intracellular GSH within mouse breast cancer cells to afford large assemblies (Fig. 14F).42 These assemblies interacted with lysosomes, causing their expansion and enhancing lysosomal membrane permeabilization (Fig. 14G). This, in turn, upregulated major histocompatibility complex class I on the tumor cell surfaces and resulted in tumor cell death. Furthermore, the therapeutic efficacy of camptothecin, a chemotherapeutic, was enhanced by conjugating it to SPr-G2.
 | ||
| Fig. 15 (A) Enzyme-instructed self-assembly involving 1Lp or 1Dp and leading to the formation of intranuclear assemblies. Following cellular entry, the micelle transforms into nanoribbons through ALP-catalyzed dephosphorylation and subsequently coassembles with histone proteins within the cell nuclei. (B) TEM images of 1Lp and 1Dp at a concentration of 200 μM in PBS, along with the resulting 1L and 1D, obtained 24 h after ALP addition. (C) CLSM images of SJSA-1 cells treated with 1Lp or 1Dp at 200 μM for 4 h. Adapted with permission from ref. 128. Copyright 2022 Wiley-VCH. (D) Proposed self-adaptive mechanism of membrane-to-nucleus delivery via an organelle-mimicking cascade process. Adapted with permission from ref. 56. Copyright 2022 KeAi Communications Co. | ||
Zhan et al. developed a peptide conjugate (HpYss) incorporating 10-hydroxycamptothecin (HCPT) to mimic the transformation of artificial extracellular vesicles for targeted liver cancer therapy (Fig. 15D). This conjugate underwent sequential self-assembly triggered by extracellular ALP and intracellular GSH, leading to a morphological change from nanoparticles to nanofibers. An experimental phase diagram was created to illustrate the effects of varying ALP and GSH levels on self-assembly. Within HepG2 cells, HpYss formed dynamically transforming organelle-mimetic structures facilitating the efficient delivery of HCPT to the nucleus. The transition from extracellular nanoparticles (50–100 nm) to intracellular nanofibers (4–9 nm wide) was found to be essential for effective nuclear delivery.56 The nucleus targeting-induced aggregation of exogenous molecule features broad application prospects and challenges. Continuous in-depth research and technological innovation are expected to expand the scope of applications in disease treatment, gene editing, and other fields.129,179
4. Biomedical applications of intracellular aggregation
Intracellular molecular aggregation has broad biomedical application prospects, revealing the microscopic mechanisms of life activities while providing new insights for disease diagnosis and treatment. Endogenous biomolecular aggregation represents certain normal cellular processes, such as the formation of biomolecular condensates, which is an efficient means of organization and regulation developed by cells during their evolution. These condensates function as small intracellular communities concentrating proteins or other biomolecules to perform specific tasks. Molecular aggregation facilitates gene expression processes by increasing the opportunities for intermolecular binding and thereby promotes gene expression.By designing exogenous molecules to achieve precise and controllable aggregation within organisms, one can exploit the properties of molecular aggregates to further advance disease diagnosis and treatment. For example, exogenous molecules capable of aggregation-induced emission (AIE) and having specific targeting capabilities can be designed to aggregate within specific cells or tissues and thus enable precise disease localization and imaging. Additionally, by regulating the aggregation state of exogenous molecules within cells, one can achieve therapeutic effects. The aggregated molecules can also serve as tools for screening drugs or treatment methods. By observing aggregation state changes, one can assess the impact of drugs or treatment methods on cells or tissues and thereby screen out potentially effective drugs or therapies. Furthermore, molecular aggregation can be used to construct biosensors or bioreactors for monitoring environmental changes within organisms or producing specific biological products.
4.1. Exploration of life processes
Xu et al. used driving primer-triggered polymerization-mediated metastable assembly to construct a well-structured metastable DNA nanostructure solely comprising two hairpin probes (HAPs), which is an important development in assembly techniques (Fig. 16A).194 The characteristics and functions of this nanostructure were examined using atomic force microscopy and gel electrophoresis. Despite existing in a metastable state, the structure exhibited a considerable stability at physiological temperatures. This assembly technique could be modified for the in situ imaging of microRNA-21 within cancer cells by tagging one of the hairpin probes with a fluorophore and quencher. In contrast to the conventional fluorescence probe-based in situ hybridization method, this DNA nanoassembly markedly improved imaging effectiveness within cancer cells and provided a sensitive indication of microRNA-21 expression levels, as revealed by confocal imaging (Fig. 16B).
 | ||
| Fig. 16 (A) DNA nanoassembly triggered by microRNA-21 designed for the in situ visualization of miRNAs within cells. (B) In situ images of microRNA-21 within MCF-7 cells acquired using a mixture of SHAP and LHAP in the absence (upper panel) and presence (lower panel) of polymerase. Adapted with permission from ref. 194. Copyright 2017 Elsevier BV. (C) General strategy for probe design focusing on the cRGD-targeted imaging of intracellular thiols via αvβ3 integrin-mediated cellular uptake, which leads to disulfide bond cleavage and subsequent fluorescence activation. Adapted with permission from ref. 195. Copyright 2014 Royal Society of Chemistry. | ||
Selectivity and specificity, as well as the stability and controllability of aggregation mechanisms and the efficiency and accuracy of signal amplification, are crucial factors.196–200 Consequently, future research should further optimize and refine these strategies to enhance detection sensitivity and accuracy.201 To achieve this goal, researchers need to delve deeper into the underlying principles of these strategies, exploring innovative approaches to improve their performance. This may involve refining the design of selective and specific recognition elements, enhancing the stability and predictability of aggregation processes, and optimizing signal amplification techniques to minimize noise and errors.202–209 By concentrating on these aspects, one can improve detection technologies and realize more precise and dependable outcomes across diverse domains, including biomedicine, environmental monitoring, and food safety.210–212 Liu's group developed a targeted light-up probe for intracellular thiol imaging in cells expressing integrin avβ3 (Fig. 16C).195 This probe was carefully engineered using a highly soluble targeted cyclic RGD (cRGD) peptide containing five aspartic acid residues (referred to as D5), a TPE fluorogen for fluorescence, and a thiol-reactive disulfide linker cleavable under certain conditions. The related emission spectra revealed a positive correlation between GSH concentration and fluorescence intensity, which was ascribed to the increase in the quantity of TPE aggregates forming in aqueous environments with elevated GSH concentrations. The probe was suitable for live cell imaging.
 | ||
| Fig. 17 (A) Chemical structures of 1 and 1C and cartoon illustrating the intracellular reduction-controlled condensation of 1 resulting in the self-assembly of 1-NPs for tumor imaging with persistent phosphorescence. (B) Time-course phosphorescence images of nude mice xenografted with HepG2 tumor cells acquired at 0.25, 1, 2, 4, 12, and 24 h after probe injection. Adapted with permission from ref. 226. Copyright 2018 Royal Society of Chemistry. (C) CTSB-guided intracellular formation of Gd-CBT-NPs aimed at enhancing T2-weighted magnetic resonance imaging at 9.4 T. (D) TEM image of MDA-MB-231 cells treated with 300 μM VC-Gd-CBT for 8 h. Adapted with permission from ref. 227. Copyright 2023 Wiley-VCH. (E) Reduction-induced self-assembly of a γ-glutamyltransferase (GGT)-targeted probe into nanoparticles for in vivo PET imaging. Adapted with permission from ref. 228. Copyright 2020 American Chemical Society. | ||
Moreover, Liang group further leveraged the biocompatible condensation reaction to craft a “smart” contrast agent, VC-Gd-CBT, which is responsive to CTSB and based on gadolinium (Gd) (Fig. 17C).226 This agent can autonomously assemble into larger Gd-laden nanoparticles within cells, facilitated by glutathione reduction and CTSB cleavage (Fig. 17D). This transformation amplifies the T2-weighted magnetic resonance (MR) contrast for imaging of tumors. Ye et al. designed a small-molecule probe labeled with fluorine-18, and this probe was crafted using a biocompatible CBT-Cys condensation reaction and cleverly adorned with a γ-glutamate (γ-Glu) substrate, which is recognizable by gamma-glutamyltransferase (GGT) (Fig. 17E).220 This innovation aims to enhance PET imaging for detecting GGT levels in tumors of living nude mice.
Liang's group developed a NIR AIEgen (Ac-Trp-Glu-His-Asp-Cys(StBu)-Pra(QMT)-CBT (QMT-CBT)) reacting through a CBT-Cys click reaction upon caspase-1 activation (Fig. 18A).229 This reaction formed cyclic dimers, which then aggregated into nanoparticles. This dual aggregation process activated the AIE signal, improving the AD imaging capability. Molecular dynamics simulations indicated that QMT within the QMT-NPs stacked more tightly than in the single aggregates of the control compound. The dual aggregation enhanced fluorescence intensity 1.9-, 1.7-, and 1.4-fold compared with single aggregation states in vitro, within cells, and in a living AD mouse model, respectively (Fig. 18B and C).
 | ||
| Fig. 18 (A) Caspase-1-instructed dual aggregations of QMT-CBT activating NIR fluorescence and enhancing Alzheimer's disease (AD) imaging. (B) Bio-TEM images of Aβ25–35-treated PC12 cells incubated with 10 μM QMT-CBT for 1 h. (C) Ex vivo fluorescence images displaying the brain and major organs of WT and AD mice. Adapted with permission from ref. 229. Copyright 2023 American Chemical Society. (D) Step-by-step strategy used to overcome the limitations of commercial ThT and leading to the development of ultrasensitive off–on NIR probes. (E) In vivo mapping illustrating Aβ deposition in an AD model (APP/PS1 transgenic) mouse. Comparison of fluorescence images acquired 20 min after the intravenous injection of QM-FN-SO3 into wild-type and APP/PS1 mice. Adapted with permission from ref. 230. Copyright 2019 American Chemical Society. | ||
Changes in amyloid-beta (Aβ) and tau protein levels in the cerebrospinal fluid can occur years before clinical symptoms of AD appear. Fu et al. developed a strategy for the rational design of NIR AIE-active probes specifically targeting Aβ plaques (Fig. 18D).230 The authors incorporated a lipophilic π-conjugated thiophene bridge to shift the emission wavelength into the NIR range and improve blood–brain barrier permeability. The QM-FN-SO3 probe successfully addressed the AIE challenge of balancing lipophilicity with aggregation in aqueous environments, enabling the high-fidelity detection of Aβ plaques in vivo with an exceptional binding affinity. This probe is a superior alternative to commercial probes, such as ThT or ThS (Fig. 18E). The dosage and safety of exogenous molecules should be controlled to prevent unnecessary nervous system damage, and monitoring results should be interpreted alongside other biomarkers and clinical manifestations for a comprehensive assessment.
 | ||
| Fig. 19 (A) Peptidoglycan metabolic labeling AIEgen (TPAPy-D-Ala) for rapid and precise Mycobacterium tuberculosis diagnosis and in situ elimination in macrophage cells. (B) Labeling of intracellular BCG with TPAPy-D-Ala. Adapted with permission from ref. 235. Copyright 2023 Cell Press. (C) Macrophage chemotaxis-instructed detection of Staphylococcus aureus infection in vivo and the molecular component of the probe (MPC). (D) MPC is actively targeted to the mannose receptor of the macrophage cell. Under the pathogen-associated molecular patterns, the activated caspase-1 inside the macrophage cells specifically tailors MPC to induce self-assembly and retention. Adapted with permission from ref. 236. Copyright 2018 American Chemical Society. | ||
The intracellular invasion and persistence of Staphylococcus aureus within phagocytic cells contribute to the challenges in treating S. aureus infections and potential for developing antibiotic resistance.76,237 Identifying phagocytic cells infected by S. aureus is crucial for guiding antibiotic therapy and mitigating drug resistance. Wang's group reported a peptide-chlorophyll photoacoustic probe that utilized the activation of caspase-1 enzymes in immune cells in response to infected sites and was subsequently cleaved and assembled to enable the imaging of bacterial infection sites (Fig. 19C).236 Once the probe entered macrophages through receptor-mediated endocytosis, caspase-1 enzymes were activated and cleaved the probe. Owing to the change in the hydrophilic and hydrophobic properties, the cleaved substrate molecules aggregated and assembled through hydrophobic interactions, achieving a good photoacoustic imaging effect and enabling the detection of intracellularly hidden bacteria (Fig. 19D). This method is effective for detecting early-stage infections and assessing treatment efficacy in later stages. Additionally, photosensitizers can be used to effectively kill intracellular host-associated bacteria and thus reduce the emergence of resistant strains and recurrent infections.
4.2. Regulation of cell fate
Intracellular biomolecular aggregates can be used as drug reservoirs to extend the residence time of drugs in drug-resistant cancer cells. Du et al. developed a tripeptide, phenylalanine-phenylalanine-tyrosine (Phe-Phe-Tyr or FFY), that underwent oxidation and spontaneously assembled into nanoparticles strongly interfering with microtubules and effectively reversing drug resistance in melanoma (Fig. 20A).241 In the presence of tyrosinase, FFY was converted into a melanin-like dimer (mFFY), which self-assembled into mFFY assemblies. These assemblies inhibited tubulin self-polymerization, resulting in a notable G2/M cell cycle arrest (13.9% higher compared with the control) (Fig. 20B).
 | ||
| Fig. 20 (A) Tyrosinase-induced tripeptide assemblies and their apoptotic effects on cisplatin-resistant melanoma cells. (B) Intracellular localization of green (mFFY) and red (microtubule) fluorescence signals after 24 h of treatment. Adapted with permission from ref. 241. Copyright 2022 American Chemical Society. (C) Proposed mechanism of ATP sequestration by NP1 assemblies in multidrug-resistant cells leading to the deceleration of drug efflux and drug efficacy enhancement. (D) NP1 assemblies with ATP formed in the presence of glucose. Adapted with permission from ref. 242. Copyright 2018 Wiley-VCH. | ||
In vivo experiments showed that the peritumoral injections of FFY reduced resistant melanoma tumor volumes by 87.4% compared with controls. Wang et al. demonstrated that nucleopeptide assemblies can selectively capture ATP in complex environments, such as serum and cytosol (Fig. 20C).242 These assemblies preferentially sequester ATP over adenosine diphosphate (ADP), and enzymes that convert ATP and ADP influence the nanostructures formed by nucleopeptides and nucleotides. The nucleopeptides efficiently sequester ATP within cells to decelerate efflux pumps in multidrug-resistant cancer cells and thus enhance the effectiveness of DOX (Fig. 20D).
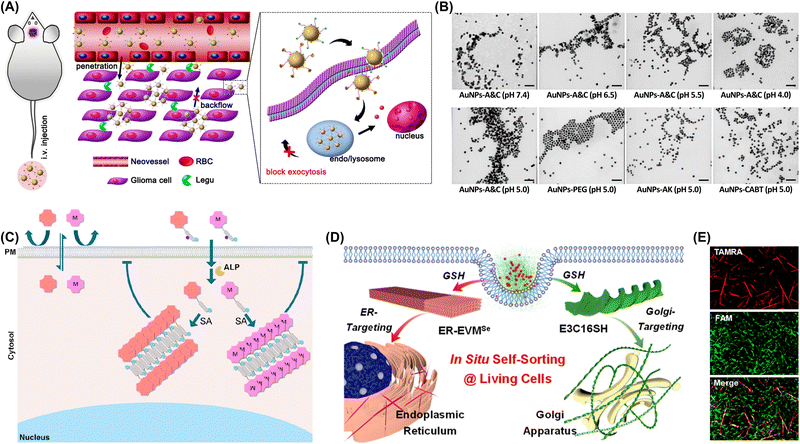 | ||
| Fig. 21 (A) In vivo behavior of AuNPs-doxorubicin-A&C following intravenous injection (increased accumulation is highlighted). (B) TEM images of AuNPs-A&C incubated with legumain at various pH for 12 h and those of control nanoparticles incubated at pH 5.0 for the same duration. Scale bar: 100 nm. Adapted with permission from ref. 253. Copyright 2016 American Chemical Society. (C) Immobilization of macromolecules or macromolecule–metal complexes within the cytosol. Adapted with permission from ref. 254. Copyright 2022 American Chemical Society. (D) In situ self-sorting peptide assemblies within living cells. (E) SIM images of mixed E3C16 (0.5 mM) and EVMSe (0.5 mM), with FAM-E3C16 (1 μM) and TAMRA-EVMSe (1 μM) as fluorescence probes, viewed under channel I (FAM), channel II (TAMRA), or merged channels. Adapted with permission from ref. 259. Copyright 2022 American Chemical Society. | ||
Despite the potential of the intracellular aggregation of exogenous molecules for enhancing drug targeting, this strategy faces certain challenges, as exemplified by the need to (i) precisely control the size, shape, and stability of molecular aggregates; (ii) avoid toxicity or damage to cells caused by aggregated drugs; and (iii) further optimize the mechanisms and conditions for drug release.255–258 Yu's group introduced a GSH-responsive in situ self-sorting peptide assembly system targeting multiple organelles within cancer cells to induce their combinatorial dysfunction and death (Fig. 21D).259 This system utilized two peptides derived from lipid-inspired peptide interdigitating amphiphiles (E3C16E6) and peptide bola-amphiphiles (EVMSeO). The unique organization patterns of these peptides enabled GSH-induced self-sorting into isolated nanofibrils within cells facilitated by the cleavage of disulfide-linked hydrophilic domains or reduction of selenoxide groups. The self-sorting behavior of these peptide assemblies within HeLa cells was characterized using super-resolution structured illumination microscopy (Fig. 21E). With the continuous development of biotechnology, nanotechnology, and materials science, these challenges will be overcome, and the application of exogenous intracellular molecular aggregation in drug targeting will become more extensive and thorough. The use of the intracellular aggregation of exogenous molecules to enhance drug targeting is an innovative and promising strategy. By precisely designing and optimizing the structure and properties of molecular aggregates, one can realize specific recognition and binding to target cells or tissues and thereby improve drug efficacy and safety.
Guo et al. used heterogeneous peptide–protein assembly to selectively phosphorylate proteins and thereby activate the necroptotic signaling pathway and facilitate cell necroptosis (Fig. 22A).263 Inspired by natural necrosome structures formed by receptor-interacting protein kinases (RIPKs) 1 and 3, the authors designed kinase-biomimetic peptides using natural or D-amino acids or by connecting D-amino acids in a retro-inverso (DRI) configuration. These peptides self-assembled into nanofibrils and accelerated the assembly of PR3 when mixed with the same. The DRI–PR1 peptide exhibited a strong binding affinity for the RIPK3 protein, showing a specific cytotoxicity toward colon cancer cells overexpressing RIPK3 (Fig. 22B). Mechanistic studies indicated that RIPK1-biomimetic peptides enhanced the phosphorylation of RIPK3, activating the necroptotic signaling pathway responsible for cell death while not substantially increasing inflammatory cytokine secretion (Fig. 22C).
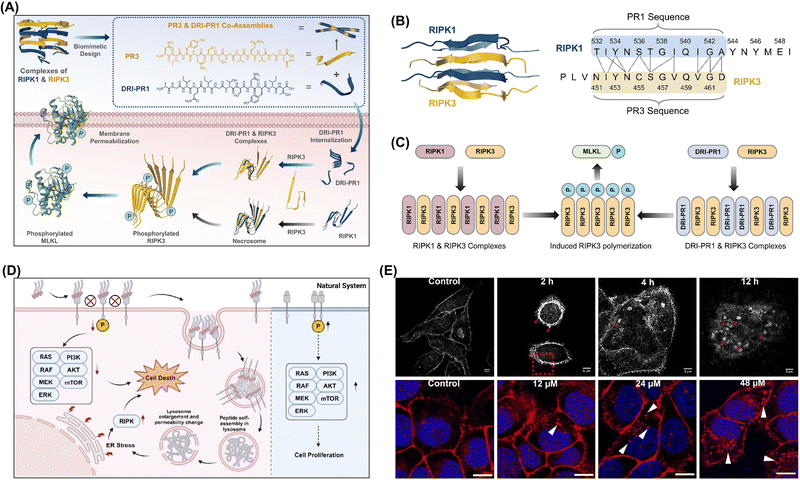 | ||
| Fig. 22 (A) Design of kinase-mimetic peptides PR3 and DRI–PR1 and role of DRI–PR1 in promoting RIPK3 aggregation to activate the necroptotic signaling pathway and induce cell necroptosis. (B) Design principle of RIPK1-mimetic peptide PR1 and RIPK3-mimetic peptide PR3 showcasing the truncation of the RIHM motifs from RIPK1 and RIPK3, respectively. (C) Natural necroptosis induced by RIPK1–RIPK3 complexes compared with necroptosis induced by RIPK1-mimetic peptides; DRI–PR1–RIPK3 complexes act as artificial necrosomes, inducing RIPK3 aggregation and phosphorylation and thus activating the necroptotic signaling pathway and promoting cell death. Adapted with permission from ref. 263. Copyright 2023 Wiley-VCH. (D) Peptide assemblies aimed at modulating the clustering of the membrane protein EGFR to inhibit cancer cells. (E) Structured illumination microscopy images showing EGFR clusters on the cell membrane and aggregates in the cytoplasm of HeLa cells treated with varying concentrations of PAD-1 (12, 24, and 48 μM) for 24 h and revealing the concentration-dependent formation of EGFR aggregates. Adapted with permission from ref. 264. Copyright 2024 American Chemical Society. | ||
The aggregation of exogenous molecules within cells may trigger signaling pathways closely related to apoptosis regulation.218,265,266 For instance, certain exogenous molecules can activate the caspase pathway and thus initiate apoptosis. The aggregation of exogenous molecules may also impact the function of organelles such as mitochondria and the endoplasmic reticulum, which play crucial roles in apoptosis. Damage to mitochondria can trigger the onset of apoptosis.267,268 The abovementioned aggregation may also affect cell cycle progression. By interfering with the regulatory mechanisms of the cell cycle, exogenous molecules can induce apoptosis at specific stages. In cancer treatment, the effective inhibition of tumor growth and spreading can be achieved by inducing apoptosis in tumor cells.
Wang's group developed a self-assembled scaffold using a cyclic peptide recognizing the membrane protein EGFR and disrupting its signaling pathway through multivalent interactions driven by assembly-induced aggregation (Fig. 22D).264 Upon introduction to cells, PAD-1 oligomers targeted and bound to overexpressed EGFR on cancer cell membranes, effectively inhibiting its function. This binding triggered endocytosis, leading to the accumulation of PAD-1 and EGFR within lysosomes. Inside the lysosomes, PAD-1 and EGFR formed nanofibers to induce lysosomal membrane permeabilization (LMP) (Fig. 22E). This permeabilization disrupted EGFR homeostasis and halted the downstream signaling vital for cancer cell survival while leading to the release of protein aggregates that induce endoplasmic reticulum stress and ultimately resulting in the selective apoptosis or necrosis of cancer cells.
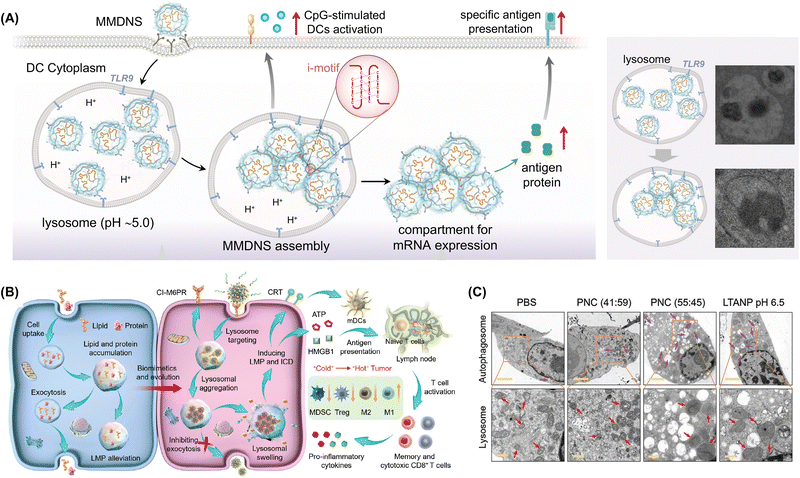 | ||
| Fig. 23 (A) Intracellular acidic environments induce the assembly of MMDNS in lysosomes, allowing the assembled aggregates to escape into the cytoplasm and create compartments for mRNA expression. Adapted with permission from ref. 273. Copyright 2024 American Association for the Advancement of Science. (B) LTANP-targeted aggregation in lysosomes inducing lysosomal membrane permeabilization and immunogenic cell death for cancer immunotherapy via a biomimetic strategy. (C) Bio-TEM images of B16F10 cells treated with various formations (scale bar: 2 μm). Regions of interest are highlighted with orange rectangles (scale bar: 0.5 μm), with purple and red arrows indicating autophagosomes and lysosomes, respectively. Adapted with permission from ref. 274. Copyright 2024 Wiley-VCH. | ||
The intracellular aggregation of exogenous molecules provides a novel approach to tumor immunotherapy. However, further research and exploration are required to ensure the effectiveness and safety of this strategy. In particular, efficient, specific, and low-toxicity exogenous molecules should be identified to guarantee treatment safety and efficacy. Precise immune response regulation is also needed to avoid autoimmune reactions or immune escape phenomena caused by overactivation. Xing et al. developed lysosomal-targeting aggregated nanoparticles (LTANPs) for cancer treatment inspired by lysosomal swelling caused by the excessive accumulation of nondegraded substances. These nanoparticles were engineered with a specific surface composition, properties, and interparticle interactions to facilitate accumulation in tumors and selective aggregation within cancer cell lysosomes (Fig. 23B).274 This aggregation resulted in irreversible lysosomal swelling and induced LMP in cancer cells (Fig. 23C). LMP triggered by nanoparticle aggregation effectively initiated immunogenic cell death by disrupting the autophagy-lysosome pathway, leading to robust antitumor immune responses and converting tumor immunogenicity from cold to hot in a melanoma model. Additionally, the LTANPs could be combined with clinically approved programmed death ligand-1 antibodies to enhance T cell-mediated antitumor immunity, which notably improved antitumor efficacy and reduced tumor recurrence and metastasis.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (Fig. 24A).282 This photosensitizer was integrated with a ferrocene-containing amphiphilic block copolymer (PEG-b-PMAEFc). In the tumor microenvironment, this combination catalyzed H2O2 decomposition via the Fenton reaction, producing hydroxyl radicals (˙OH). These radicals promoted an addition reaction between the abundant water-soluble GSH in tumor cells and C
C bonds (Fig. 24A).282 This photosensitizer was integrated with a ferrocene-containing amphiphilic block copolymer (PEG-b-PMAEFc). In the tumor microenvironment, this combination catalyzed H2O2 decomposition via the Fenton reaction, producing hydroxyl radicals (˙OH). These radicals promoted an addition reaction between the abundant water-soluble GSH in tumor cells and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of TMPP, improving the hydrophilicity of the photosensitizer and reducing aggregation. In vitro and in vivo studies showed that this approach considerably enhances the therapeutic efficacy of PDT.
C bonds of TMPP, improving the hydrophilicity of the photosensitizer and reducing aggregation. In vitro and in vivo studies showed that this approach considerably enhances the therapeutic efficacy of PDT.
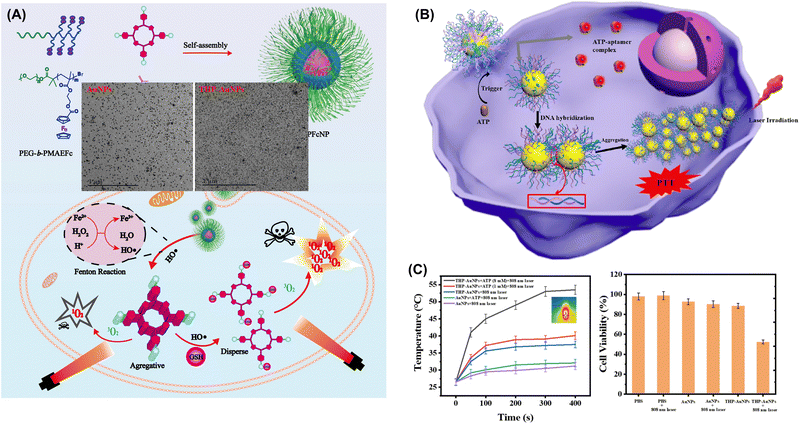 | ||
| Fig. 24 (A) In the acidic microenvironment of tumors, TPFcNP catalyzes H2O2 decomposition to generate hydroxyl radicals (˙OH), which break down TPFcNP and release aggregated TMPP. The hydroxyl radicals can further react with overexpressed hydrophilic glutathione and aggregative TMPP to enhance the hydrophilicity of the latter and reduce its aggregation. Under light exposure, the porphyrin dispersion generates large amounts of highly toxic singlet oxygen to kill tumor cells. Adapted with permission from ref. 282. Copyright 2020 American Chemical Society. (B) Design and construction of THP–AuNPs and the ATP-triggered aggregation of THP–AuNPs for the imaging and photothermal therapy of tumors. (C) Time-dependent heat generation by AuNPs and THP–AuNPs and illustration of the photothermal effect of THP–AuNPs exposed to an 808 nm laser in the presence of ATP, along with the viability of MCF-7 cells after treatment and irradiation. Adapted with permission from ref. 289. Copyright 2022 American Chemical Society. | ||
Photothermal conversion efficiency is a key factor determining PTT efficacy.283,284 When exogenous molecules (such as photothermal agents) aggregate within cells, they may form more effective photothermal conversion structures, which helps increase the photothermal conversion efficiency and generate higher temperatures to enhance the therapeutic effect of PTT.285,286 Aggregated exogenous molecules within cells can form hotspot regions with temperatures markedly exceeding those in the surrounding tissues. This localized hyperthermia can effectively kill tumor cells while reducing damage to normal tissues.287,288 By designing exogenous molecules as nanoparticles or utilizing other delivery systems, one can achieve their effective accumulation within and penetration into tumor tissues. These nanoparticles or delivery systems can release exogenous molecules within cells and promote their aggregation to further enhance the therapeutic effect of PTT. Zhang et al. developed a molecular switch comprising a triple-helix probe (THP) and MUC1 aptamer-functionalized AuNPs for fluorescence imaging and PTT (Fig. 24B).289 The MUC1 aptamer directed the THP–AuNP conjugates to tumor cells. At high levels of ATP, the triple-helix probes underwent structural changes, causing the intracellular aggregation of AuNPs. These aggregates remained trapped at the tumor site, enabling concurrent tumor imaging and PTT (Fig. 24C).
 | ||
| Fig. 25 (A) Aggregation-enhanced photodynamic therapy mechanism mediated by TBmA-Glu. GGT activation of TBmA-Glu leads to the formation of TBmA aggregates, which induce ferroptosis in cancer cells. Adapted with permission from ref. 296. Copyright 2024 Springer Nature. (B) Mechanisms of apoptosis and ferroptosis induced by 1-NBS@CeO2 in tumor cells under light irradiation. (C) CLSM images depicting ROS levels in A375 cells following various treatments under normoxic and hypoxic conditions (scale bar: 20 μm). Adapted with permission from ref. 300. Copyright 2024 Wiley-VCH. | ||
The aggregation of exogenous molecules may inhibit the antioxidant system, reducing the cellular antioxidant capacity and ROS accumulation and ultimately triggering ferroptosis. Additionally, exogenous molecules may possess iron-chelating abilities or affect the functions of iron metabolism-related proteins, resulting in intracellular an iron overload.297–299 This overload can initiate the Fenton reaction, generating a large amount of ROS, further exacerbating lipid peroxidation and cellular damage, and promoting ferroptosis. Luo et al. developed a nanoreactor (1-NBS@CeO2) integrating type I PDT with CeO2 nanozymes. This system comprised spherical nanoparticles containing a peptide amphiphile (1-NBS) and CeO2 and exhibited enhanced SOD- and POD-like activities (Fig. 25B).300 Under light irradiation, 1-NBS@CeO2 generated superoxide radicals (O2˙−) that were converted into toxic hydroxyl radicals. This led to increased ROS and decreased GSH levels in A375 tumor cells, causing mitochondrial dysfunction and promoting apoptosis and ferroptosis (Fig. 25C).
Conclusions
Although the intracellular aggregation of exogenous molecules has promising biomedicinal applications, it faces several challenges and limitations.301,302 One critical concern lies in ensuring that polymer molecules are accurately synthesized and folded within the cellular environment.303 This process requires meticulous control over the chemical and biological conditions to prevent misfolding or aggregation in unintended ways, which could lead to cellular dysfunction or toxicity. Moreover, the need to avoid adverse effects on the cell is another hurdle. The introduction of exogenous molecules into cells must be carefully managed to prevent the disruption of normal cellular processes and induction of unwanted immune responses. Thus, a deep understanding of cell biology and the mechanisms by which these molecules interact with cellular components is required.Effective delivery systems and precise regulatory mechanisms are also areas of considerable concern.304 Developing robust and efficient methods for delivering exogenous molecules into cells while ensuring precise control over their aggregation and activity is crucial for the success of numerous biomedical applications. This includes the design of novel carriers, such as liposomes, nanoparticles, or viral vectors, capable of protecting the molecules during delivery and releasing them at the desired location within the cell. Recent advances in the intracellular aggregation of exogenous molecules have considerably contributed to gene therapy, drug delivery, and metabolic engineering.305 The self-assembly properties of certain molecules within cells have been used to develop novel therapeutic agents that demonstrate improved efficacy and reduced toxicity compared with traditional treatments. This progress opens up new avenues for more effective therapeutic strategies.
In recent years, coacervates-dynamic liquid–liquid phase-separated droplets-have emerged as cornerstone models for unraveling the formation and functions of membraneless organelles, which lack lipid membranes yet orchestrate critical cellular processes. These droplets hold transformative potential across three fronts: macromolecular drug delivery, biomimetic reaction chambers, and minimal cellular mimics. While exogenous molecule-induced coacervate formation within living cells remains underexplored due to technical hurdles, their advantages over rigid aggregates are striking. Unlike static aggregation, coacervates act as membraneless compartments that enhance enzyme activity by concentrating substrates up to 100-fold while preserving protein flexibility-a dual benefit that rigid aggregates lack. Moreover, these droplets can reprogram cellular workflows by sequestering or releasing regulatory factors, effectively altering metabolic pathways without genetic modification. Thus, engineering biologically active coacervates via exogenous molecules represents one of most promising direction in intracellular molecular aggregation, bridging the gap between synthetic chemistry and cell biology.
As interdisciplinary research and technological convergence accelerate, the intracellular aggregation of exogenous molecules is emerging as a cornerstone of next-generation biomedical innovation. In our view, this field is transitioning from a tool-driven paradigm to a programmable therapeutic platform, where controlled molecular self-assembly enables real-time modulation of cellular behavior with subcellular precision. For instance, integrating stimuli-responsive polymers (e.g., light- or pH-sensitive materials) could allow clinicians to dynamically trigger aggregation dynamics within tumor microenvironments or diseased compartments, minimizing off-target toxicity and maximizing therapeutic efficacy. A parallel frontier lies in co-opting endogenous cellular machinery-such as chaperone proteins or autophagy pathways-to regulate synthetic aggregate formation and clearance, transforming these constructs into transient, self-correcting therapeutic entities. This synergy between synthetic biology and cellular physiology may also revolutionize regenerative medicine, enabling in situ tissue engineering by directing stem cell differentiation or extracellular matrix remodeling through programmable aggregation of bioactive molecules.
Yet realizing this vision demands rigorous scrutiny of long-term safety and scalability. While preclinical studies underscore the promise of engineered aggregates, critical questions persist regarding their biodegradability, immunogenicity, and inter-patient variability. The intracellular aggregation of exogenous molecules represents a paradigm shift: a move from static drugs to adaptive, biology-integrated tools capable of tackling intractable diseases (e.g., neurodegeneration, genetic disorders) where traditional approaches have stalled. Ascribing to the unique properties of AIE molecules, which may be poised to exert a significant translational impact within the next few years, which holds substantial promise for precise disease diagnosis and therapy. Ultimately, with the advances in technology and research, the intracellular aggregation of exogenous molecules will play an increasingly important role in biomedicine, holding promise for revolutionizing our approach to treating diseases and improving human health.
Author contributions
Da-Yong Hou and Haoran Wang contribute equally to this work. D.-Y. Hou: conceptualization; writing – original draft; data curation; project administration. Y.-Z. Wang: conceptualization; data curation. H.-R. Wang, D.-B. Cheng, B.-Z. Tang and W.-H. Xu: supervision; validation; funding acquisition; writing – review & editing. All authors have read and approved the final version of the manuscript.Data availability
All data are obtained from peer-reviewed articles, books, and patents as reported in the references list. No other datasets have been used.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFB3801000), National Natural Science Foundation of China (82427806, 82302266, 52173138, and 22375155), Hong Kong Scholar Project (XJ2024052), Key Research and Development Program of Heilongjiang Province (2022ZX06004), Heilongjiang Provincial Natural Science Foundation of China (PL2024H159), Harbin Medical University Cancer Hospital Haiyan Foundation (JJYQ2024-03 and JJZD2024-17), the Open Fund of Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, Guangzhou 510640, China (South China University of Technology) (2023B1212060003) and Nn10 program of Harbin Medical University Cancer Hospital (Nn102024-01).Notes and references
- Z. Liu, J. Guo, Y. Qiao and B. Xu, Acc. Chem. Res., 2023, 56, 3076–3088 CrossRef CAS PubMed.
- L. Shi, Int. J. Biol. Macromol., 2016, 92, 37–48 CrossRef CAS PubMed.
- Y. M. Zhang, Y. H. Liu and Y. Liu, Adv. Mater., 2020, 32, e1806158 CrossRef PubMed.
- K. Liu, D. Zheng, H. Lei, J. Liu, J. Lei, L. Wang and X. Ma, ACS Biomater. Sci. Eng., 2018, 5, 1730–1737 Search PubMed.
- M. Rinaudo, Polym. Int., 2008, 57, 397–430 CrossRef CAS.
- R. Kalluri and V. S. LeBleu, Science, 2020, 367, eaau6977 CrossRef CAS PubMed.
- H. V. Goodson and E. M. Jonasson, Cold Spring Harbor Perspect. Biol., 2018, 10, a022608 CrossRef PubMed.
- M. Grasso, P. Piscopo, A. Confaloni and M. Denti, Molecules, 2014, 19, 6891–6910 CrossRef PubMed.
- F. Ding, S. Zhang, Q. Chen, H. Feng, Z. Ge, X. Zuo, C. Fan, Q. Li and Q. Xia, Small, 2023, 19, e2206228 CrossRef PubMed.
- Z. Zhang, J. Conniot, J. Amorim, Y. Jin, R. Prasad, X. Yan, K. Fan and J. Conde, J. Controlled Release, 2022, 350, 80–92 CrossRef CAS PubMed.
- Y. Zhao, X. Zuo, Q. Li, F. Chen, Y. Chen, J. Deng, D. Han, C. Hao, F. Huang, Y. Huang, G. Ke, H. Kuang, F. Li, J. Li, M. Li, N. Li, Z. Lin, D. Liu, J. Liu, L. Liu, X. Liu, C. Lu, F. Luo, X. Mao, J. Sun, B. Tang, F. Wang, J. Wang, L. Wang, S. Wang, L. Wu, Z. Wu, F. Xia, C. Xu, Y. Yang, B. Yuan, Q. Yuan, C. Zhang, Z. Zhu, C. Yang, X. Zhang, H. Yang, W. Tan and C. Fan, Sci. China Chem., 2021, 64, 171–203 CrossRef CAS PubMed.
- W. Yi, P. Xiao, X. Liu, Z. Zhao, X. Sun, J. Wang, L. Zhou, G. Wang, H. Cao, D. Wang and Y. Li, Signal Transduction Targeted Ther., 2022, 7, 386 CrossRef CAS PubMed.
- Y. Luo, X. He, Q. Du, L. Xu, J. Xu, J. Wang, W. Zhang, Y. Zhong, D. Guo, Y. Liu and X. Chen, Exploration, 2024, 4, 20230134 CrossRef CAS PubMed.
- F. Li, X. Lan and X. Liang, Aggregate, 2021, 2, e27 CrossRef CAS.
- W. Du, X. Hu, W. Wei and G. Liang, Bioconjugate Chem., 2018, 29, 826–837 CrossRef CAS PubMed.
- M. Dergham, S. Lin and J. Geng, Angew. Chem., Int. Ed., 2022, 61, e202114267 CrossRef CAS PubMed.
- W. Wang, Z. Xin, D. Liu, Q. Liu, Y. Liu, Z. Qiu, J. Zhang, P. Alam, X. Cai, Z. Zhao and B. Z. Tang, Biosens. Bioelectron., 2025, 267, 116800 CrossRef CAS PubMed.
- H. Wang, W. Ouyang and H. Liu, Nano TransMed, 2024, 3, 100032 CrossRef.
- W. Zhu, X. Zhang and W. Hu, Sci. Bull., 2021, 66, 512–520 CrossRef CAS PubMed.
- H. Jiang, S. Zhu, Z. Cui, Z. Li, Y. Liang, J. Zhu, P. Hu, H. Zhang and W. Hu, Chem. Soc. Rev., 2022, 51, 3071–3122 RSC.
- X. Feng, X. Wang, C. Redshaw and B. Z. Tang, Chem. Soc. Rev., 2023, 52, 6715–6753 RSC.
- J. A. Finbloom, F. Sousa, M. M. Stevens and T. A. Desai, Adv. Drug Delivery Rev., 2020, 167, 89–108 CrossRef CAS PubMed.
- U. E. Ewii, A. L. Onugwu, V. C. Nwokpor, I. Akpaso, T. E. Ogbulie, B. Aharanwa, C. Chijioke, N. Verla, C. Iheme, C. Ujowundu, C. Anyiam and A. A. Attama, Nano TransMed, 2024, 3, 100042 CrossRef.
- Z. Zhou, K. Maxeiner, D. Y. W. Ng and T. Weil, Acc. Chem. Res., 2022, 55, 2998–3009 CrossRef CAS PubMed.
- D. Wu, J. Lei, Z. Zhang, F. Huang, M. Buljan and G. Yu, Chem. Soc. Rev., 2023, 52, 2911–2945 RSC.
- J. An, H. Hong, M. Won, H. Rha, Q. Ding, N. Kang, H. Kang and J. S. Kim, Chem. Soc. Rev., 2023, 52, 30–46 RSC.
- S. Chagri, D. Y. W. Ng and T. Weil, Nat. Rev. Chem., 2022, 6, 320–338 CrossRef PubMed.
- X. Zhang, J. Wang, Y. Zhang, Z. Yang, J. Gao and Z. Gu, Chem. Soc. Rev., 2023, 52, 8126–8164 RSC.
- J. Liu and B. Liu, Prog. Polym. Sci., 2022, 129, 101545 CrossRef CAS.
- N. Song, F. Tian, Y. Zou and Z. Yu, ACS Appl. Mater. Interfaces, 2024, 16, 45821–45829 CrossRef CAS PubMed.
- F. Tian, R. Guo, C. Wu, X. Liu, Z. Zhang, Y. Wang, H. Wang, G. Li and Z. Yu, Angew. Chem., Int. Ed., 2024, 63, e202404703 CrossRef CAS PubMed.
- W. Wang and S. Wang, Lab Chip, 2022, 22, 1042–1067 RSC.
- P. Cen, J. Huang, C. Jin, J. Wang, Y. Wei, H. Zhang and M. Tian, Aggregate, 2023, 4, e352 CrossRef CAS.
- X. Mo, Z. Zhang, J. Song, Y. Wang and Z. Yu, J. Mater. Chem. B, 2024, 12, 4289–4306 RSC.
- X. Liang, Y. Zhang, J. Zhou, Z. Bu, J. Liu and K. Zhang, Coord. Chem. Rev., 2022, 473, 214824 CrossRef CAS.
- Y. Z. Yang, N. Xiao, S. G. Liu, L. Han, N. B. Li and H. Q. Luo, Mater. Sci. Eng., C, 2020, 108, 110401 CrossRef CAS PubMed.
- S. Yang, Y. Cheng, M. Liu, J. Tang, S. Li, Y. Huang, X. Kou, C. Yao and D. Yang, Nano Today, 2024, 56, 102224 CrossRef CAS.
- J. Wang, L. Zhou and H. Wang, Nano Today, 2024, 57, 102317 CrossRef CAS.
- S. Yamamoto, K. Nishimura, K. Morita, S. Kanemitsu, Y. Nishida, T. Morimoto, T. Aoi, A. Tamura and T. Maruyama, Biomacromolecules, 2021, 22, 2524–2531 CrossRef CAS PubMed.
- M. Waqas, W. Jeong, Y. Lee, D. Kim, C. Ryou and Y. Lim, Biomacromolecules, 2017, 18, 943–950 CrossRef CAS PubMed.
- D. Cheng, G. Qi, J. Wang, Y. Cong, F. Liu, H. Yu, Z. Qiao and H. Wang, Biomacromolecules, 2017, 18, 1249–1258 CrossRef CAS PubMed.
- D. Zhong, X. Hou, D. Pan, Z. Li, Q. Gong and K. Luo, Adv. Mater., 2024, 36, e2403588 CrossRef PubMed.
- L. Huang, X. Mao, B. Liu, Z. Fan, J. Li, C. Fan, Y. Tian, S. Luo and M. Liu, ACS Nano, 2024, 18, 8051–8061 CrossRef CAS PubMed.
- Q. Bai, M. Wang, K. Wang, J. Liu, F. Qu and H. Lin, J. Colloid Interface Sci., 2024, 671, 577–588 CrossRef CAS PubMed.
- M. Borkowska, M. Siek, D. V. Kolygina, Y. I. Sobolev, S. Lach, S. Kumar, Y. Cho, K. Kandere-Grzybowska and B. A. Grzybowski, Nat. Nanotechnol., 2020, 15, 331–341 CrossRef CAS PubMed.
- J. Zhang, Y. Cui, X. Feng, M. Cheng, A. Tang and D. Kong, ACS Appl. Mater. Interfaces, 2019, 11, 39624–39632 CrossRef CAS PubMed.
- D. Cheng, X. Zhang, Y. Gao, L. Ji, D. Hou, Z. Wang, W. Xu, Z. Qiao and H. Wang, J. Am. Chem. Soc., 2019, 141, 7235–7239 CrossRef CAS PubMed.
- Y. Dai, T. Li, Z. Zhang, Y. Tan, S. Pan, L. Zhang and H. Xu, J. Am. Chem. Soc., 2021, 143, 10709–10717 CrossRef CAS PubMed.
- C. Liu, B. Xianyu, Y. Dai, S. Pan, T. Li and H. Xu, ACS Nano, 2023, 17, 11905–11913 CrossRef CAS PubMed.
- A. Wang, H. Li, H. Feng, H. Qiu, R. Huang, Y. Wang, S. Ji, H. Liang, X. Shen and B. Jiang, ACS Appl. Mater. Interfaces, 2023, 15, 5870–5882 CrossRef CAS PubMed.
- N. Lv, T. Ma, H. Qin, Z. Yang, Y. Wu, D. Li, J. Tao, H. Jiang and J. Zhu, Sci. China Mater., 2022, 65, 2861–2870 CrossRef CAS.
- Y. Di, E. Zhang, Z. Yang, Q. Shen, X. Fu, G. Song, C. Zhu, H. Bai, Y. Huang, F. Lv, L. Liu and S. Wang, Angew. Chem., Int. Ed., 2022, 61, e202116457 CrossRef CAS PubMed.
- H. Wang, Y. Song, W. Wang, N. Chen, B. Hu, X. Liu, Z. Zhang and Z. Yu, J. Am. Chem. Soc., 2024, 146, 330–341 CrossRef CAS PubMed.
- L. Zhou, F. Lv, L. Liu and S. Wang, CCS Chem., 2019, 1, 97–105 CrossRef CAS.
- Y. Zhao, Q. Yao, J. Chen, R. Zhang, J. Song and Y. Gao, Biomater. Sci., 2022, 10, 5662–5668 RSC.
- J. Zhan, Y. Wang, S. Ma, Q. Qin, L. Wang, Y. Cai and Z. Yang, Bioact. Mater., 2022, 9, 120–133 CAS.
- Y. Yuan, H. Sun, S. Ge, M. Wang, H. Zhao, L. Wang, L. An, J. Zhang, H. Zhang, B. Hu, J. Wang and G. Liang, ACS Nano, 2015, 9, 761–768 CrossRef CAS PubMed.
- D. Zheng, Y. Chen, S. Ai, R. Zhang, Z. Gao, C. Liang, L. Cao, Y. Chen, Z. Hong, Y. Shi, L. Wang, X. Li and Z. Yang, Research, 2019, 2019, 4803624 CAS.
- D. S. Yang, Y. H. Yang, Y. Zhou, L. L. Yu, R. H. Wang, B. Di and M. M. Niu, Adv. Funct. Mater., 2020, 30, 1904969 CrossRef CAS.
- J. Zhan, J. Huang, Q. Xiao, Z. Yu, Y. Wang, X. Wang, F. Liu, Y. Cai, Z. Yang and L. Zheng, Anal. Chem., 2024, 96, 12630–12639 CrossRef CAS PubMed.
- N. Y. Zhang, D. Y. Hou, X. J. Hu, J. X. Liang, M. D. Wang, Z. Z. Song, L. Yi, Z. J. Wang, H. W. An, W. Xu and H. Wang, Angew. Chem., Int. Ed., 2023, 62, e202308049 CrossRef CAS PubMed.
- A. Wang, Q. Mao, M. Zhao, S. Ye, J. Fang, C. Cui, Y. Zhao, Y. Zhang, Y. Zhang, F. Zhou and H. Shi, Anal. Chem., 2020, 92, 16113–16121 CrossRef CAS PubMed.
- Y. Gao, Y. Li, H. Cao, H. Jia, D. Wang, C. Ren, Z. Wang, C. Yang and J. Liu, J. Nanobiotechnol., 2022, 20, 390 CrossRef CAS PubMed.
- Z. Hai, Y. Ni, D. Saimi, H. Yang, H. Tong, K. Zhong and G. Liang, Nano Lett., 2019, 19, 2428–2433 CrossRef CAS PubMed.
- Q. Shen, Y. Huang, Y. Zeng, E. Zhang, F. Lv, L. Liu and S. Wang, ACS Mater. Lett., 2021, 3, 1307–1314 CrossRef CAS.
- L. Feng, Z. Chen, W. Dong, A. Xie, X. Zang and J. Li, New J. Chem., 2023, 47, 21960–21968 RSC.
- Y. Gao, J. Gao, G. Mu, Y. Zhang, F. Huang, W. Zhang, C. Ren, C. Yang and J. Liu, Acta Pharm. Sin. B, 2020, 10, 2374–2383 Search PubMed.
- J. Li, Y. Kuang, J. Shi, J. Zhou, J. E. Medina, R. Zhou, D. Yuan, C. Yang, H. Wang, Z. Yang, J. Liu, D. M. Dinulescu and B. Xu, Angew. Chem., Int. Ed., 2015, 54, 13307–13311 CrossRef CAS PubMed.
- C. Wu, R. Zhang, W. Du, L. Cheng and G. Liang, Nano Lett., 2018, 18, 7749–7754 CrossRef CAS PubMed.
- Y. Zhao, X. Zhang, Z. Li, S. Huo, K. Zhang, J. Gao, H. Wang and X. J. Liang, Adv. Mater., 2017, 29, 1601128 CrossRef PubMed.
- D. Liu, Z. Miao, C. Wu, F. He, P. Ren, S. Bai, X. Jiang and Y. Gao, Chem. Sci., 2020, 11, 1132–1139 RSC.
- A. Tanaka, Y. Fukuoka, Y. Morimoto, T. Honjo, D. Koda, M. Goto and T. Maruyama, J. Am. Chem. Soc., 2015, 137, 770–775 CrossRef CAS PubMed.
- Q. Zhang, D. Zheng, X. Dong, P. Pan, S. Zeng, F. Gao, S. Cheng and X. Zhang, J. Am. Chem. Soc., 2021, 143, 5127–5140 Search PubMed.
- H. An, L. Li, Y. Wang, Z. Wang, D. Hou, Y. Lin, S. Qiao, M. Wang, C. Yang, Y. Cong, Y. Ma, X. Zhao, Q. Cai, W. Chen, C. Lu, W. Xu, H. Wang and Y. Zhao, Nat. Commun., 2019, 10, 4861 CrossRef PubMed.
- Z. Chen, M. Chen, K. Zhou and J. Rao, Angew. Chem., Int. Ed., 2020, 59, 7864–7870 Search PubMed.
- L. L. Li, H. L. Ma, G. B. Qi, D. Zhang, F. Yu, Z. Hu and H. Wang, Adv. Mater., 2016, 28, 254–262 CrossRef CAS PubMed.
- P. Chen, H. Wang, H. Wu, P. Zou, C. Wang, X. Liu, Y. Pan, Y. Liu and G. Liang, Anal. Chem., 2021, 93, 6329–6334 CrossRef CAS PubMed.
- Y. Yuan, L. Wang, W. Du, Z. Ding, J. Zhang, T. Han, L. An, H. Zhang and G. Liang, Angew. Chem., Int. Ed., 2015, 54, 9700–9704 Search PubMed.
- G. B. Qi, F. Hu, Kenry, L. L. Shi, M. Wu and B. Liu, Angew. Chem., Int. Ed., 2019, 58, 16229–16235 Search PubMed.
- G. Qi, X. Liu, L. Shi, M. Wu, J. Liu and B. Liu, Adv. Mater., 2022, 34, e2106885 Search PubMed.
- L. Yang, R. Peltier, M. Zhang, D. Song, H. Huang, G. Chen, Y. Chen, F. Zhou, Q. Hao, L. Bian, M. He, Z. Wang, Y. Hu and H. Sun, J. Am. Chem. Soc., 2020, 142, 18150–18159 Search PubMed.
- T. Xu, Y. Cai, X. Zhong, L. Zhang, D. Zheng, Z. Gao, X. Pan, F. Wang, M. Chen and Z. Yang, Chem. Commun., 2019, 55, 7175–7178 RSC.
- Y. M. Zhang, N. Y. Zhang, K. Xiao, Q. Yu and Y. Liu, Angew. Chem., Int. Ed., 2018, 57, 8649–8653 CrossRef CAS PubMed.
- J. Geng, W. Li, Y. Zhang, N. Thottappillil, J. Clavadetscher, A. Lilienkampf and M. Bradley, Nat. Chem., 2019, 11, 578–586 CrossRef CAS PubMed.
- M. Zhu, S. Wang, Z. Li, J. Li, Z. Xu, X. Liu and X. Huang, Nat. Commun., 2023, 14, 3598 CrossRef CAS PubMed.
- Y. Zhang, Q. Gao, W. Li, R. He, L. Zhu, Q. Lian, L. Wang, Y. Li, M. Bradley and J. Geng, JACS Au, 2022, 2, 579–589 CrossRef CAS PubMed.
- J. C. Lin, C. Y. Hsu, J. Y. Chen, Z. S. Fang, H. W. Chen, B. Y. Yao, G. H. M. Shiau, J. S. Tsai, M. Gu, M. Jung, T. Y. Lee and C. M. J. Hu, Adv. Mater., 2021, 33, e2101190 CrossRef PubMed.
- N. Yin, X. Wang, Y. Shu and J. Wang, J. Colloid Interface Sci., 2025, 679, 519–528 Search PubMed.
- J. Qiao, L. Qi, Y. Shen, L. Zhao, C. Qi, D. Shangguan, L. Mao and Y. Chen, J. Mater. Chem., 2012, 22, 11543 RSC.
- J. Wang, Y. Wang, S. Qiao, M. Mamuti, H. An and H. Wang, Natl. Sci. Rev., 2022, 9, nwab159 Search PubMed.
- R. Sharma, N. Vijay, B. J. Kim and H. Lee, Eur. Polym. J., 2023, 201, 112545 CrossRef CAS.
- R. S. Fernandes and N. Dey, ACS Appl. Nano Mater., 2023, 6, 5168–5176 CrossRef CAS.
- T. Wu, X. Chen, Z. Gong, J. Yan, J. Guo, Y. Zhang, Y. Li and B. Li, Adv. Sci., 2022, 9, e2103354 CrossRef PubMed.
- B. Saha, B. Ruidas, S. Mete, C. D. Mukhopadhyay, K. Bauri and P. De, Chem. Sci., 2020, 11, 141–147 RSC.
- F. Liu, Y. Cong, G. Qi, L. Ji, Z. Qiao and H. Wang, Nano Lett., 2018, 18, 6577–6584 Search PubMed.
- S. Qiao, Y. Ma, Y. Wang, Y. Lin, H. An, L. Li and H. Wang, ACS Nano, 2017, 11, 7301–7311 CrossRef CAS PubMed.
- Q. Zhang, Q. Zhang, Y. Sun, X. Tao, Y. Zhao, F. Guo, Z. Li, Z. Wang, Z. Liang and C. Yi, ACS Biomater. Sci. Eng., 2024, 10, 7167–7175 CrossRef CAS PubMed.
- T. Yin, T. Yang, L. Chen, R. Tian, C. Cheng, L. Weng, Y. Zhang and X. Chen, Acta Biomater., 2023, 166, 485–495 CrossRef CAS PubMed.
- R. Hu, X. Chen, T. Zhou, H. Si, B. He, R. T. K. Kwok, A. Qin and B. Z. Tang, Sci. China Chem., 2019, 62, 1198–1203 CrossRef CAS.
- M. Deng, R. Guo, S. Zang, J. Rao, M. Li, X. Tang, C. Xia, M. Li, Z. Zhang and Q. He, ACS Appl. Mater. Interfaces, 2021, 13, 18033–18046 Search PubMed.
- X. Q. Li, Y. L. Jia, Z. X. Wang, Y. W. Zhang, H. Y. Chen and J. J. Xu, Adv. Funct. Mater., 2024, 34, 2401711 CrossRef CAS.
- J. Shi, W. Nie, X. Zhao, X. Yang, H. Cheng, T. Zhou, Y. Zhang, K. Zhang and J. Liu, Adv. Mater., 2022, 34, e2201049 Search PubMed.
- M. Wang, L. Yi, Y. Li, R. Xu, J. Hu, D. Hou, C. Liu and H. Wang, J. Am. Chem. Soc., 2024, 146, 28669–28676 Search PubMed.
- S. Qi, P. Zhang, M. Ma, M. Yao, J. Wu, E. Mäkilä, J. Salonen, H. Ruskoaho, Y. Xu, H. A. Santos and H. Zhang, Small, 2019, 15, e1804332 Search PubMed.
- J. V. Jokerst, C. Khademi and S. S. Gambhir, Sci. Transl. Med., 2013, 5, 177ra35 Search PubMed.
- X. Zhao, F. Wang, C. Kam, M. Wu, J. Zhang, C. Xu, K. Bao, Q. He, R. Ye, B. Z. Tang and S. Chen, ACS Nano, 2024, 18, 21447–21458 Search PubMed.
- Y. Liu, Y. Wang, C. Wang, T. Dong, H. Xu, Y. Guo, X. Zhao, Y. Hu and J. Wu, Adv. Sci., 2022, 9, e2203027 Search PubMed.
- X. Q. Zhou, M. Mytiliniou, J. Hilgendorf, Y. Zeng, P. Papadopoulou, Y. Shao, M. P. Dominguez, L. Zhang, M. B. S. Hesselberth, E. Bos, M. A. Siegler, F. Buda, A. M. Brouwer, A. Kros, R. I. Koning, D. Heinrich and S. Bonnet, Adv. Mater., 2021, 33, e2008613 CrossRef PubMed.
- N. Chu, L. Cong, J. Yue, W. Xu and S. Xu, ACS Omega, 2022, 7, 34268–34277 CrossRef CAS PubMed.
- M. T. Jeena, S. Lee, A. K. Barui, S. Jin, Y. Cho, S. Hwang, S. Kim and J. Ryu, Chem. Commun., 2020, 56, 6265–6268 RSC.
- S. Kim, B. Jana, E. M. Go, J. E. Lee, S. Jin, E. An, J. Hwang, Y. Sim, S. Son, D. Kim, C. Kim, J. Jin, S. K. Kwak and J. Ryu, ACS Nano, 2021, 15, 14492–14508 Search PubMed.
- S. Kim, J. Chae, D. Kim, C. Park, Y. Sim, H. Lee, G. Park, J. Lee, S. Hong, B. Jana, C. Kim, H. Chung and J. Ryu, J. Am. Chem. Soc., 2023, 145, 21991–22008 Search PubMed.
- X. Yang, B. Wu, J. Zhou, H. Lu, H. Zhang, F. Huang and H. Wang, Nano Lett., 2022, 22, 7588–7596 Search PubMed.
- Y. Guo, S. Li, Z. Tong, J. Tang, R. Zhang, Z. Lv, N. Song, D. Yang and C. Yao, J. Am. Chem. Soc., 2023, 145, 23859–23873 Search PubMed.
- F. Li, Y. Liu, Y. Dong, Y. Chu, N. Song and D. Yang, J. Am. Chem. Soc., 2022, 144, 4667–4677 Search PubMed.
- S. Jin, M. T. Jeena, B. Jana, M. Moon, H. Choi, E. Lee and J. Ryu, Biomacromolecules, 2020, 21, 4806–4813 CrossRef CAS PubMed.
- M. T. Jeena, L. Palanikumar, E. M. Go, I. Kim, M. G. Kang, S. Lee, S. Park, H. Choi, C. Kim, S. Jin, S. C. Bae, H. W. Rhee, E. Lee, S. K. Kwak and J. Ryu, Nat. Commun., 2017, 8, 26 CrossRef CAS PubMed.
- H. Choi, G. Park, E. Shin, S. W. Shin, B. Jana, S. Jin, S. Kim, H. Wang, S. K. Kwak, B. Xu and J. Ryu, Chem. Sci., 2022, 13, 6197–6204 Search PubMed.
- C. Yao, Y. Xu, J. Tang, P. Hu, H. Qi and D. Yang, Nat. Commun., 2022, 13, 7739 CrossRef CAS PubMed.
- X. Ge, Y. Cao, X. Zhu, B. Yuan, L. He, A. Wu and J. Li, Adv. Healthcare Mater., 2023, 12, e2300265 Search PubMed.
- J. Wang, L. Hu, H. Zhang, Y. Fang, T. Wang and H. Wang, Adv. Mater., 2022, 34, e2104704 Search PubMed.
- S. Ji, J. Li, X. Duan, J. Zhang, Y. Zhang, M. Song, S. Li, H. Chen and D. Ding, Angew. Chem., Int. Ed., 2021, 60, 26994–27004 CrossRef CAS PubMed.
- X. Ouyang, N. Jia, J. Luo, L. Li, J. Xue, H. Bu, G. Xie and Y. Wan, JACS Au, 2023, 3, 2566–2577 CrossRef CAS PubMed.
- M. Pileni, J. Phys. Chem. C, 2021, 125, 20143–20156 CrossRef CAS.
- D. Hou, M. Wang, N. Zhang, S. Xu, Z. Wang, X. Hu, G. Lv, J. Wang, M. Lv, L. Yi, L. Wang, D. Cheng, T. Sun, H. Wang and W. Xu, Nano Lett., 2022, 22, 3983–3992 Search PubMed.
- D. Y. Hou, N. Y. Zhang, M. D. Wang, S. X. Xu, Z. J. Wang, X. J. Hu, G. T. Lv, J. Q. Wang, X. H. Wu, L. Wang, D. B. Cheng, H. Wang and W. Xu, Angew. Chem., Int. Ed., 2022, 61, e202116893 Search PubMed.
- X. Zhao, K. Zhang, Y. Wang, W. Jiang, H. Cheng, Q. Wang, T. Xiang, Z. Zhang, J. Liu and J. Shi, Adv. Funct. Mater., 2022, 32, 2108883 CrossRef CAS.
- S. Liu, Q. Zhang, H. He, M. Yi, W. Tan, J. Guo and B. Xu, Angew. Chem., Int. Ed., 2022, 61, e202210568 CrossRef CAS PubMed.
- S. Liu, Q. Zhang, A. N. Shy, M. Yi, H. He, S. Lu and B. Xu, J. Am. Chem. Soc., 2021, 143, 15852–15862 Search PubMed.
- P. J. Pacheco-Liñán, C. Alonso-Moreno, A. Ocaña, C. Ripoll, E. García-Gil, A. Garzón-Ruíz, D. Herrera-Ochoa, S. Blas-Gómez, B. Cohen and I. Bravo, ACS Appl. Mater. Interfaces, 2023, 15, 44786–44795 CrossRef PubMed.
- D. Eisenberg and M. Jucker, Cell, 2012, 148, 1188–1203 CrossRef CAS PubMed.
- J. L. Silva, E. A. Cino, I. N. Soares, V. F. Ferreira and G. A. P. De Oliveira, Acc. Chem. Res., 2018, 51, 181–190 CrossRef CAS PubMed.
- K. Bae, S. Ng, L. Li and M. Kurisawa, Biofunct. Mater., 2023, 1, 2 Search PubMed.
- Y. Deng, W. Zhan and G. Liang, Adv. Healthcare Mater., 2021, 10, e2001211 Search PubMed.
- Y. Chen, B. S. N. Tan, Y. Cheng and Y. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202410579 Search PubMed.
- W. Poon, B. R. Kingston, B. Ouyang, W. Ngo and W. C. W. Chan, Nat. Nanotechnol., 2020, 15, 819–829 Search PubMed.
- W. C. W. Chan, BME Front., 2023, 4, 16 Search PubMed.
- Y. Guo, P. Li, X. Guo, C. Yao and D. Yang, ACS Nano, 2024, 18, 30224–30246 Search PubMed.
- H. Cui, A. Glidle and J. M. Cooper, ACS Appl. Mater. Interfaces, 2022, 14, 31586–31593 Search PubMed.
- Y. Gao, J. Shi, D. Yuan and B. Xu, Nat. Commun., 2012, 3, 1033 CrossRef PubMed.
- L. Li, S. Qiao, W. Liu, Y. Ma, D. Wan, J. Pan and H. Wang, Nat. Commun., 2017, 8, 1276 Search PubMed.
- J. J. Rennick, A. P. R. Johnston and R. G. Parton, Nat. Nanotechnol., 2021, 16, 266–276 Search PubMed.
- V. L. Shepherd, Trends Pharmacol. Sci., 1989, 10, 458–462 CrossRef CAS PubMed.
- H. He, J. Wang, H. Wang, N. Zhou, D. Yang, D. R. Green and B. Xu, J. Am. Chem. Soc., 2018, 140, 1215–1218 Search PubMed.
- J. Zhou, X. Du, C. Berciu, S. J. Del Signore, X. Chen, N. Yamagata, A. A. Rodal, D. Nicastro and B. Xu, Mol. Ther., 2018, 26, 648–658 Search PubMed.
- Y. Huang, L. Ning, X. Zhang, Q. Zhou, Q. Gong and Q. Zhang, Chem. Soc. Rev., 2024, 53, 1090–1166 RSC.
- P. She, Y. Qin, X. Wang and Q. Zhang, Adv. Mater., 2022, 34, e2101175 Search PubMed.
- J. Ochi, K. Tanaka and Y. Chujo, Angew. Chem., Int. Ed., 2020, 59, 9841–9855 CrossRef CAS PubMed.
- D. P. Karothu, J. Mahmoud Halabi, E. Ahmed, R. Ferreira, P. R. Spackman, M. A. Spackman and P. Naumov, Angew. Chem., Int. Ed., 2022, 61, e202113988 CrossRef CAS PubMed.
- Y. Liu, L. Wang, L. Zhao, Y. Zhang, Z. Li and F. Huang, Chem. Soc. Rev., 2024, 53, 1592–1623 Search PubMed.
- J. Zhang, K. Liu, K. Mullen and M. Yin, Chem. Commun., 2015, 51, 11541–11555 RSC.
- L. Zhang, J. Huang, D. Buratto, P. Han, Z. Yang and R. Zhou, Aggregate, 2024, 5, e434 CrossRef CAS.
- N. Lin, X. Chen, S. Yan, H. Wang, Z. Lu, X. Xia, M. Liang, Y. Wu, L. Zheng, Q. Cao and Z. Ding, RSC Adv., 2016, 6, 25416–25419 RSC.
- Z. Cao, D. Li, L. Zhao, M. Liu, P. Ma, Y. Luo and X. Yang, Nat. Commun., 2022, 13, 2038 Search PubMed.
- Y. Cote, I. W. Fu, E. T. Dobson, J. E. Goldberger, H. D. Nguyen and J. K. Shen, J. Phys. Chem. C, 2014, 118, 16272–16278 CrossRef CAS PubMed.
- Z. Cheng, Y. Cheng, Q. Chen, M. Li, J. Wang, H. Liu, M. Li, Y. Ning, Z. Yu, Y. Wang and H. Wang, Nano Today, 2020, 33, 100878 CrossRef CAS.
- A. Nicolas Boluda, Z. Yang, I. Dobryden, F. Carn, N. Winckelmans, C. Péchoux, P. Bonville, S. Bals, P. M. Claesson, F. Gazeau and M. P. Pileni, Adv. Funct. Mater., 2020, 30, 2004274 CrossRef CAS.
- H. Wu, Z. Zhang, Y. Cao, Y. Hu, Y. Li, L. Zhang, X. Cao, H. Wen, Y. Zhang, H. Lv and X. Jin, Adv. Sci., 2024, 11, e2401047 CrossRef PubMed.
- M. Pieszka, S. Han, C. Volkmann, R. Graf, I. Lieberwirth, K. Landfester, D. Y. W. Ng and T. Weil, J. Am. Chem. Soc., 2020, 142, 15780–15789 CrossRef CAS PubMed.
- D. Wei, Y. Sun, H. Zhu and Q. Fu, ACS Nano, 2023, 17, 23223–23261 Search PubMed.
- Z. Zheng, P. Chen, M. Xie, C. Wu, Y. Luo, W. Wang, J. Jiang and G. Liang, J. Am. Chem. Soc., 2016, 138, 11128–11131 Search PubMed.
- H. Fan, Y. Li, J. Yang and X. Ye, J. Phys. Chem. B, 2017, 121, 9708–9717 CrossRef CAS PubMed.
- J. Zhan, Y. Cai, S. He, L. Wang and Z. Yang, Angew. Chem., Int. Ed., 2018, 57, 1813–1816 CrossRef CAS PubMed.
- M. Shahriari, M. Zahiri, K. Abnous, S. M. Taghdisi, M. Ramezani and M. Alibolandi, J. Controlled Release, 2019, 308, 172–189 CrossRef CAS PubMed.
- C. Wang, W. Du, C. Wu, S. Dan, M. Sun, T. Zhang, B. Wang, Y. Yuan and G. Liang, Angew. Chem., Int. Ed., 2022, 61, e202114766 CrossRef CAS PubMed.
- Y. Zhang, C. Xu, X. Yang and K. Pu, Adv. Mater., 2020, 32, e2002661 CrossRef PubMed.
- X. Zhang, D. Cheng, L. Ji, H. An, D. Wang, Z. Yang, H. Chen, Z. Qiao and H. Wang, Nano Lett., 2020, 20, 1286–1295 Search PubMed.
- M. K. Pastuszka, S. M. Janib, I. Weitzhandler, C. T. Okamoto, S. Hamm-Alvarez and J. A. MacKay, Biomacromolecules, 2012, 13, 3439–3444 CrossRef CAS PubMed.
- W. Li, Y. Zhou, S. He, T. Tong, C. Wang, P. Shi, S. Li, X. Wang, L. Yang, X. Cao and Z. Tian, Aggregate, 2024, 5, e496 Search PubMed.
- F. Liu, Y. Guo, Y. Hu, X. Zhang and X. Zheng, Anal. Bioanal. Chem., 2019, 411, 5845–5854 CrossRef CAS PubMed.
- M. Giel and Y. Hong, Aggregate, 2023, 4, e336 CrossRef CAS.
- T. Miki, K. Kajiwara, S. Nakayama, M. Hashimoto and H. Mihara, ACS Synth. Biol., 2022, 11, 2144–2153 CrossRef CAS PubMed.
- H. Liu and H. Wang, Adv. Drug Delivery Rev., 2024, 209, 115327 CrossRef CAS PubMed.
- K. Qian, S. Gao, Z. Jiang, Q. Ding and Z. Cheng, Exploration, 2024, 4, 20230063 CrossRef CAS PubMed.
- F. Wang, Q. Zhang, M. Yang, B. Yin and S. H. D. Wong, Biomed. Eng. Commun., 2024, 3, 12–16 CrossRef.
- Y. Li, X. He, P. Wang, B. Yuan, Y. Pan, X. Hu, L. Lu, A. Wu and J. Li, Small, 2024, 20, e2308621 CrossRef PubMed.
- X. Yang, H. Lu, Y. Tao, L. Zhou and H. Wang, Angew. Chem., Int. Ed., 2021, 60, 23797–23804 CrossRef CAS PubMed.
- B. Jana, S. Jin, E. M. Go, Y. Cho, D. Kim, S. Kim, S. K. Kwak and J. Ryu, J. Am. Chem. Soc., 2023, 145, 18414–18431 CrossRef CAS PubMed.
- C. P. Brangwynne, Soft Matter, 2011, 7, 3052 RSC.
- L. He, D. Lu, H. Liang, S. Xie, C. Luo, M. Hu, L. Xu, X. Zhang and W. Tan, ACS Nano, 2017, 11, 4060–4066 CrossRef CAS PubMed.
- Q. Huang, P. Ma, H. Li, B. Yin and B. Ye, ACS Appl. Bio Mater., 2020, 3, 2861–2866 CrossRef CAS PubMed.
- J. Li, D. Li, R. Yuan and Y. Xiang, ACS Appl. Mater. Interfaces, 2017, 9, 5717–5724 CrossRef CAS PubMed.
- Z. Yang, S. Zhang, H. Zhao, H. Niu, Z. Wu and H. Chang, Anal. Chem., 2018, 90, 13891–13899 CrossRef CAS PubMed.
- X. Li, F. Yang, C. Gan, R. Yuan and Y. Xiang, Biosens. Bioelectron., 2022, 197, 113809 CrossRef CAS PubMed.
- Z. Zhang, R. T. K. Kwok, Y. Yu, B. Z. Tang and K. M. Ng, J. Mater. Chem. B, 2018, 6, 4575–4578 RSC.
- H. Xu, X. Cheng, X. Sun, P. Chen, W. Zhan, X. Liu, X. Wang, B. Hu and G. Liang, Nano Lett., 2023, 23, 6178–6183 CrossRef CAS PubMed.
- B. Dong, S. Du, C. Wang, H. Fu, Q. Li, N. Xiao, J. Yang, X. Xue, W. Cai and D. Liu, ACS Nano, 2019, 13, 1421–1432 CAS.
- H. A. Ki, P. K. Naoghare, B. Oh, J. Choi and J. M. Song, Anal. Biochem., 2009, 388, 23–27 Search PubMed.
- L. Li, X. Fang, J. Le, Y. Zheng, X. Tan, Z. Jiang, H. Li, J. Xu and H. Xu, Talanta, 2022, 250, 123753 CrossRef CAS PubMed.
- L. Wen, Y. Lin, Z. Zhang, W. Lu, C. Lv, Z. Chen, H. Wang and D. Pang, Biomaterials, 2016, 99, 24–33 CrossRef CAS PubMed.
- L. Dai, W. Mao, L. Hu, J. Song, Y. Zhang, T. Huang and M. Wang, Dyes Pigm., 2022, 204, 110436 Search PubMed.
- D. Ye, G. Liang, M. L. Ma and J. Rao, Angew. Chem., Int. Ed., 2011, 50, 2275–2279 CrossRef CAS PubMed.
- R. Liu, S. Zhang, T. Zheng, Y. Chen, J. Wu and Z. Wu, ACS Nano, 2020, 14, 9572–9584 Search PubMed.
- J. Xu, Z. Wu, Z. Wang, J. Le, T. Zheng and L. Jia, Biomaterials, 2017, 120, 57–65 CrossRef CAS PubMed.
- Y. Yuan, R. T. K. Kwok, G. Feng, J. Liang, J. Geng, B. Z. Tang and B. Liu, Chem. Commun., 2014, 50, 295–297 Search PubMed.
- N. Singha, P. Gupta, B. Pramanik, S. Ahmed, A. Dasgupta, A. Ukil and D. Das, Biomacromolecules, 2017, 18, 3630–3641 Search PubMed.
- F. Sun, G. Zhang, D. Zhang, L. Xue and H. Jiang, Org. Lett., 2011, 13, 6378–6381 Search PubMed.
- J. Wang, S. Ma, K. Ge, R. Xu, F. Shen, X. Gao, Y. Yao, Y. Chen, Y. Chen, F. Gao and G. Wu, Anal. Chem., 2024, 96, 8922–8931 Search PubMed.
- L. Yang, Q. Wu, Y. Chen, X. Liu, F. Wang and X. Zhou, ACS Sens., 2019, 4, 110–117 Search PubMed.
- S. Zhou, Y. Xia, Y. Liu, Q. He and B. Song, Langmuir, 2017, 33, 5947–5956 Search PubMed.
- J. Tavakoli, Q. Hu, J. L. Tipper and Y. Tang, Aggregate, 2024, 5, e645 Search PubMed.
- X. Wei, Y. Wang, J. Shen, X. Chi, J. Xu, L. Jia, H. Xu and W. Liu, Sens. Actuators, B, 2025, 424, 136919 CrossRef CAS.
- W. Lv, M. Lin, R. Li, Q. Zhang, H. Liu, J. Wang and C. Huang, Chin. Chem. Lett., 2019, 30, 1410–1414 Search PubMed.
- X. Gong, R. Li, J. Zhang, P. Zhang, Z. Jiang, L. Hu, X. Liu, Y. Wang and F. Wang, Adv. Sci., 2024, 11, e2400517 Search PubMed.
- J. Liu, P. Du, J. Zhang, H. Shen and J. Lei, Chem. Commun., 2018, 54, 2550–2553 RSC.
- D. Li, Y. Wu, C. Gan, R. Yuan and Y. Xiang, Nanoscale, 2018, 10, 17623–17628 RSC.
- Y. Wu, Y. Gao, J. Su, Z. Chen and S. Liu, Chem. Commun., 2020, 56, 9008–9011 RSC.
- L. Dong, M. Zhang, H. Han, Y. Zang, G. Chen, J. Li, X. He and S. Vidal, Chem. Sci., 2021, 13, 247–256 RSC.
- S. Yang, H. Yu, X. Xu, T. Yang, Y. Wei, R. Zan, X. Zhang, Q. Ma, H. C. Shum and Y. Song, ACS Nano, 2023, 17, 8195–8203 CrossRef CAS PubMed.
- X. Sun, L. Xu, H. D. Xu, L. Xie, R. Wang, Z. Yang, W. Zhan, S. Shen and G. Liang, Adv. Healthcare Mater., 2024, 13, e2303472 CrossRef PubMed.
- Y. Yuan, S. Ge, H. Sun, X. Dong, H. Zhao, L. An, J. Zhang, J. Wang, B. Hu and G. Liang, ACS Nano, 2015, 9, 5117–5124 CrossRef CAS PubMed.
- Y. Yuan, F. Wang, W. Tang, Z. Ding, L. Wang, L. Liang, Z. Zheng, H. Zhang and G. Liang, ACS Nano, 2016, 10, 7147–7153 CrossRef CAS PubMed.
- M. Su, J. Ye, Q. Li, W. Ge, Y. Zhang, H. Jiang, C. Amatore and X. Wang, RSC Adv., 2015, 5, 74844–74849 RSC.
- M. Madhu, W. Tseng, Y. Chou, A. S. Krishna Kumar, C. Lu, P. Chang and W. Tseng, Anal. Chem., 2024, 96, 9007–9015 CrossRef CAS PubMed.
- A. Dutta, U. Goswami and A. Chattopadhyay, ACS Appl. Mater. Interfaces, 2018, 10, 19459–19472 CrossRef CAS PubMed.
- S. Kim, J. Kim, B. Jana and J. Ryu, RSC Adv., 2020, 10, 43383–43388 RSC.
- K. Gong, Q. Wu, H. Wang, S. He, J. Shang and F. Wang, Chem. Commun., 2020, 56, 1141–11413 RSC.
- Y. Yuan, Z. Ding, J. Qian, J. Zhang, J. Xu, X. Dong, T. Han, S. Ge, Y. Luo, Y. Wang, K. Zhong and G. Liang, Nano Lett., 2016, 16, 2686–2691 CrossRef CAS PubMed.
- Y. Wang, X. Li, P. Chen, Y. Dong, G. Liang and Y. Yu, Nanoscale, 2020, 12, 1886–1893 RSC.
- R. Zhang, R. Zhang, C. Zhao and X. Xu, Anal. Chim. Acta, 2022, 1193, 339395 CrossRef CAS PubMed.
- L. Qiu, K. Li, W. Dong, Y. Seimbille, Q. Liu, F. Gao and J. Lin, ACS Nano, 2021, 15, 18250–18259 CrossRef CAS PubMed.
- S. Liu, S. Fang, W. J. Jang, J. Yoon and L. Zhang, Anal. Chem., 2024, 96, 12794–12800 CrossRef CAS PubMed.
- J. Wang, M. Liu, X. Zhang, X. Wang, M. Xiong and D. Luo, Exploration, 2024, 4, 20230027 CrossRef CAS PubMed.
- S. Sun, H. Xu, Y. Yang, L. Wang, L. Ye, H. Jiang, C. Xue, Z. Shen and Z. Wu, Chem. Eng. J., 2022, 428, 131150 CrossRef CAS.
- Y. Jiang and K. Pu, Chem. Rev., 2021, 121, 13086–13131 CrossRef CAS PubMed.
- J. Li, Z. Hai, H. Xiao, X. Yi and G. Liang, Chem. Commun., 2018, 54, 3460–3463 RSC.
- M. Wei, L. Wang, Y. Wang, T. Zhang, C. Wang, C. Wu, C. Tian, G. Liang and Y. Yuan, Small, 2023, 19, e2300015 CrossRef PubMed.
- S. Ye, S. Wang, D. Gao, K. Li, Q. Liu, B. Feng, L. Qiu and J. Lin, Bioconjugate Chem., 2020, 31, 174–181 CrossRef CAS PubMed.
- L. Xu, H. Gao, W. Zhan, Y. Deng, X. Liu, Q. Jiang, X. Sun, J. Xu and G. Liang, J. Am. Chem. Soc., 2023, 145, 27748–27756 Search PubMed.
- W. Fu, C. Yan, Z. Guo, J. Zhang, H. Zhang, H. Tian and W. Zhu, J. Am. Chem. Soc., 2019, 141, 3171–3177 Search PubMed.
- J. Zhang, H. Guo, M. Liu, K. Tang, S. Li, Q. Fang, H. Du, X. Zhou, X. Lin, Y. Yang, B. Huang and D. Yang, Exploration, 2024, 4, 20230087 CrossRef CAS PubMed.
- M. Jiang, J. Kang and A. Dong, Biosens. Bioelectron., 2025, 267, 116873 CrossRef CAS PubMed.
- F. Hu, G. Qi, Kenry, D. Mao, S. Zhou, M. Wu, W. Wu and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 9288–9292 CrossRef CAS PubMed.
- C. Yang, F. Hu, X. Zhang, C. Ren, F. Huang, J. Liu, Y. Zhang, L. Yang, Y. Gao, B. Liu and J. Liu, Biomaterials, 2020, 244, 119972 CrossRef CAS PubMed.
- G. Dai, Y. Luo, M. Liao, P. Zhang, H. Pan, T. Yin, Q. Yang, S. Zheng, J. Liao, D. Liu, Z. He, W. Zhao, L. Song, P. Zhao, L. Cai, Z. Zhang and M. Zheng, Cell Rep. Phys. Sci., 2023, 4, 101238 CrossRef CAS.
- Q. Cai, Y. Fei, L. Hu, Z. Huang, L. Li and H. Wang, Nano Lett., 2018, 18, 6229–6236 CrossRef CAS PubMed.
- Q. Cai, Y. Fei, H. W. An, X. X. Zhao, Y. Ma, Y. Cong, L. Hu, L. L. Li and H. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 9197–9202 CrossRef CAS PubMed.
- J. Zhou, X. Du, J. Li, N. Yamagata and B. Xu, J. Am. Chem. Soc., 2015, 137, 10040–10043 CrossRef CAS PubMed.
- D. Hou, W. Xiao, J. Wang, M. Yaseen, Z. Wang, Y. Fei, M. Wang, L. Wang, H. Wang, X. Shi, M. Cai, H. Feng, W. Xu and L. Li, Biomaterials, 2022, 284, 121523 CrossRef CAS PubMed.
- L. Wang, B. Fu, D. Hou, Y. Lv, G. Yang, C. Li, J. Shen, B. Kong, L. Zheng, Y. Qiu, H. Wang, C. Liu, J. Zhang, S. Bai, L. Li, H. Wang and W. Xu, Biomaterials, 2023, 296, 122060 CrossRef CAS PubMed.
- M. Sun, C. Wang, M. Lv, Z. Fan and J. Du, J. Am. Chem. Soc., 2022, 144, 7337–7345 CrossRef CAS PubMed.
- H. Wang, Z. Feng, Y. Qin, J. Wang and B. Xu, Angew. Chem., Int. Ed., 2018, 57, 4931–4935 CrossRef CAS PubMed.
- J. Deng, W. Wan, M. Wang, R. Sun, W. Jin, D. Shen, Q. Xia, Z. Zhang, X. Dong, X. Sun and Y. Liu, Sens. Actuators, B, 2024, 413, 135891 CrossRef CAS.
- C. Wu, C. Wang, Y. Zheng, Y. Zheng, Z. Liu, K. Xu and W. Zhong, Adv. Funct. Mater., 2021, 31, 2104418 Search PubMed.
- Y. L. Fan, N. Y. Zhang, D. Y. Hou, Y. Hao, R. Zheng, J. Yang, Z. Fan, H. W. An and H. Wang, Adv. Healthcare Mater., 2023, 12, e2301162 CrossRef PubMed.
- Y. Wang, H. Li, Q. Jin and J. Ji, Chem. Commun., 2016, 52, 582–585 RSC.
- J. Song, C. Wu, Y. Zhao, M. Yang, Q. Yao and Y. Gao, Small, 2022, 18, e2104772 CrossRef PubMed.
- N. Chen, Z. Zhang, X. Liu, H. Wang, R. Guo, H. Wang, B. Hu, Y. Shi, P. Zhang, Z. Liu and Z. Yu, J. Am. Chem. Soc., 2024, 146, 10753–10766 CrossRef CAS PubMed.
- W. Ji, G. Liu, F. Wang, Z. Zhu and C. Feng, Chem. Commun., 2016, 52, 12574–12577 RSC.
- W. Tan, Q. Zhang, M. C. Quiñones-Frías, A. Y. Hsu, Y. Zhang, A. Rodal, P. Hong, H. R. Luo and B. Xu, J. Am. Chem. Soc., 2022, 144, 6709–6713 Search PubMed.
- J. Fan, Y. Li, Z. Wei, Y. Fan, X. Li, Z. Chen, D. Hou, W. Xiao, M. Ding, H. Wang and L. Wang, Nano Lett., 2021, 21, 6202–6210 Search PubMed.
- S. Zhang and Y. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 41105–41112 CrossRef CAS PubMed.
- S. Ruan, C. Hu, X. Tang, X. Cun, W. Xiao, K. Shi, Q. He and H. Gao, ACS Nano, 2016, 10, 10086–10098 CrossRef CAS PubMed.
- E. Du, Y. Tang, Q. Zhang, Z. Song, Y. Tao and Y. Zhang, Langmuir, 2022, 38, 4364–4370 CrossRef CAS PubMed.
- X. Liu, F. Tian, Z. Zhang, J. Liu, S. Wang, R. Guo, B. Hu, H. Wang, H. Zhu, A. Liu, L. Shi and Z. Yu, J. Am. Chem. Soc., 2024, 146, 24177–24187 CrossRef CAS PubMed.
- J. Li, D. Bullara, X. Du, H. He, S. Sofou, I. G. Kevrekidis, I. R. Epstein and B. Xu, ACS Nano, 2018, 12, 3804–3815 Search PubMed.
- E. Muro, A. Fragola, T. Pons, N. Lequeux, A. Ioannou, P. Skourides and B. Dubertret, Small, 2012, 8, 1029–1037 CrossRef CAS PubMed.
- W. Sun, J. Yin, L. Liu, Z. Wu, Y. Wang, T. Liu, H. Xiong, X. Liu, X. Wang and H. Jiang, Anal. Chem., 2023, 95, 14101–14110 CrossRef CAS PubMed.
- X. Liu, M. Li, J. Liu, Y. Song, B. Hu, C. Wu, A. Liu, H. Zhou, J. Long, L. Shi and Z. Yu, J. Am. Chem. Soc., 2022, 144, 9312–9323 Search PubMed.
- X. Lin, W. Li, Y. Wen, L. Su and X. Zhang, Biomaterials, 2022, 287, 121603 CrossRef CAS PubMed.
- J. Zhou, X. Du, X. Chen, J. Wang, N. Zhou, D. Wu and B. Xu, J. Am. Chem. Soc., 2018, 140, 2301–2308 CrossRef CAS PubMed.
- C. Gao, Q. Wang, J. Li, C. H. T. Kwong, J. Wei, B. Xie, S. Lu, S. M. Y. Lee and R. Wang, Sci. Adv., 2022, 8, eabn1805 CrossRef CAS PubMed.
- R. C. Guo, N. Wang, W. Wang, Z. Zhang, W. Luo, Y. Wang, H. Du, Y. Xu, G. Li and Z. Yu, Angew. Chem., Int. Ed., 2023, 62, e202314578 Search PubMed.
- Y. Li, L. Hu, J. Wang and H. Wang, Nano Lett., 2024, 24, 10681–10690 CrossRef CAS PubMed.
- X. Peng, J. Hao, W. Tao, D. Guo, T. Liang, X. Hu, H. Xu, X. Fan and C. Chen, J. Colloid Interface Sci., 2023, 640, 498–509 CrossRef CAS PubMed.
- Y. M. Zhang, J. H. Liu, Q. Yu, X. Wen and Y. Liu, Angew. Chem., Int. Ed., 2019, 58, 10553–10557 CrossRef CAS PubMed.
- B. Xie, H. Zhao, Y. Ding, Z. Wang, Y. Wang, C. Gao and R. Wang, J. Controlled Release, 2023, 357, 572–579 Search PubMed.
- P. Chandra Saha, R. S. Das, T. Chatterjee, M. Bhattacharyya and S. Guha, Bioconjugate Chem., 2020, 31, 1301–1306 CrossRef CAS PubMed.
- A. Hu, Y. Pu, N. Xu, Z. Cai, R. Sun, S. Fu, R. Jin, Y. Guo, H. Ai, Y. Nie and X. Shuai, Theranostics, 2023, 13, 1454–1469 CrossRef CAS PubMed.
- J. Lin, C. Chien, C. Lin, B. Yao, Y. Chen, Y. Liu, Z. Fang, J. Chen, W. Chen, N. Lee, H. Chen and C. J. Hu, Nat. Commun., 2019, 10, 1057 CrossRef PubMed.
- K. Morihiro, H. Osumi, S. Morita, T. Hattori, M. Baba, N. Harada, R. Ohashi and A. Okamoto, J. Am. Chem. Soc., 2023, 145, 135–142 CrossRef CAS PubMed.
- J. Peng, Y. Xiao, Q. Yang, Q. Liu, Y. Chen, K. Shi, Y. Hao, R. Han and Z. Qian, Acta Pharm. Sin. B, 2021, 11, 1069–1082 CrossRef CAS PubMed.
- X. Guo, M. Guo, R. Cai, M. Hu, L. Rao, W. Su, H. Liu, F. Gao, X. Zhang, J. Liu and C. Chen, Sci. Adv., 2024, 10, eadp3680 CrossRef CAS PubMed.
- Y. Xing, J. Yang, A. Peng, Y. Qian, Y. Liu, P. Pan and Q. Liu, Adv. Mater., 2024, 36, e2412730 CrossRef PubMed.
- R. Wang, X. Li and J. Yoon, ACS Appl. Mater. Interfaces, 2021, 13, 19543–19571 CrossRef CAS PubMed.
- X. Li, N. Kwon, T. Guo, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 11522–11531 Search PubMed.
- Y. Zhao, X. Zhang, Z. Chen, Y. Xu, H. Kim, H. Jeong, Y. R. Lee, J. Lee, X. Li and J. Yoon, Aggregate, 2024, 5, e514 CrossRef CAS.
- Y. Wang, W. Wu, D. Mao, C. Teh, B. Wang and B. Liu, Adv. Funct. Mater., 2020, 30, 2002431 Search PubMed.
- Y. C. Liu, G. J. Liu, W. Zhou, G. L. Feng, Q. Y. Ma, Y. Zhang and G. W. Xing, Angew. Chem., Int. Ed., 2023, 62, e202309786 CrossRef CAS PubMed.
- Y. Yang, Y. Hu, H. Du and H. Wang, Chem. Commun., 2014, 50, 7287–7290 Search PubMed.
- J. Shum, L. C. C. Lee, M. W. L. Chiang, Y. W. Lam and K. K. W. Lo, Angew. Chem., Int. Ed., 2023, 62, e202303931 CrossRef CAS PubMed.
- C. Wang, P. Zhao, D. Jiang, G. Yang, Y. Xue, Z. Tang, M. Zhang, H. Wang, X. Jiang, Y. Wu, Y. Liu, W. Zhang and W. Bu, ACS Appl. Mater. Interfaces, 2020, 12, 5624–5632 CrossRef CAS PubMed.
- J. Yang, L. Wu, L. Wang, R. Ren, P. Chen, C. Qi, H. Feng and B. Z. Tang, Aggregate, 2024, 5, e535 CrossRef CAS.
- M. Wang, H. Yi, Z. Zhan, Z. Feng, G. Yang, Y. Zheng and D. Zhang, Aggregate, 2024, 5, e622 CrossRef CAS.
- T. Du, Z. Shi, X. Mou and Y. Zhu, Colloids Surf., B, 2024, 234, 113706 CrossRef CAS PubMed.
- J. Chen, Y. Ma, W. Du, T. Dai, Y. Wang, W. Jiang, Y. Wan, Y. Wang, G. Liang and G. Wang, Adv. Funct. Mater., 2020, 30, 2001566 CrossRef CAS.
- N. Li, F. Shen, Z. Cai, W. Pan, Y. Yin, X. Deng, X. Zhang, J. O. Machuki, Y. Yu, D. Yang, Y. Yang, M. Guan and F. Gao, Small, 2020, 16, e2005511 CrossRef PubMed.
- W. Ye, H. Li, X. Li, X. Fan, Q. Jin and J. Ji, Bioconjugate Chem., 2019, 30, 1763–1772 CrossRef CAS PubMed.
- Y. Jiang, W. Zhao, H. Zhou, Q. Zhang and S. Zhang, Langmuir, 2022, 38, 3755–3764 CrossRef CAS PubMed.
- N. Kang, S. Son, S. Min, H. Hong, C. Kim, J. An, J. S. Kim and H. Kang, Chem. Soc. Rev., 2023, 52, 3955–3972 RSC.
- W. Kong, Q. Meng, R. M. Kong, Y. Zhao and F. Qu, Adv. Healthcare Mater., 2024, 13, e2402899 CrossRef PubMed.
- M. T. Jeena, J. Link, J. Zhang, I. Harley, P. Turunen, R. Graf, M. Wagner, L. A. Baptista, H. R. A. Jonker, L. Cui, I. Lieberwirth, K. Landfester, J. Rao, D. Y. W. Ng and T. Weil, Angew. Chem., Int. Ed., 2024, 63, e202412477 CrossRef CAS PubMed.
- X. Zhang, B. Zhang, Y. Zhang, Y. Ding, Z. Zhang, Q. Liu, Z. Yang, L. Wang and J. Gao, Angew. Chem., Int. Ed., 2024, 63, e202406602 Search PubMed.
- B. Wang, C. Li, D. He, K. Ding, Q. Tian, G. Feng, A. Qin and B. Z. Tang, Small, 2024, 20, e2307309 Search PubMed.
- H. Wang, D. Jiao, D. Feng, Q. Liu, Y. Huang, J. Hou, D. Ding and W. Zhang, Adv. Mater., 2024, 36, e2311733 CrossRef PubMed.
- W. Wang, R. Zhang, L. Zhang, L. Hao, X. Cai, Q. Wu, Z. Qiu, R. Han, J. Feng, S. Wang, P. Alam, G. Zhang, Z. Zhao and B. Z. Tang, Nat. Commun., 2024, 15, 9999 CrossRef CAS PubMed.
- X. Wu, T. Zhuang, M. Liu, X. Sun, L. Zhu, Q. Zhang, J. Han, L. Wang and R. Guo, Org. Chem. Front., 2024, 11, 6055–6063 RSC.
- Z. Zhang, B. Xie, X. Lu, L. Xiong, X. Li, Y. Zhang, C. Li and C. Wang, Nanoscale, 2024, 16, 903–912 RSC.
- M. Rong, J. Liu and L. Lu, Adv. Healthcare Mater., 2024, 13, e2400325 CrossRef PubMed.
- X. Luo, Q. Jiao, S. Pei, S. Zhou, Y. Zheng, W. Shao, K. Xu and W. Zhong, Adv. Healthcare Mater., 2024, 13, e2401787 CrossRef PubMed.
- Y. Li, R. Tian, H. Shi, J. Xu, T. Wang and J. Liu, Aggregate, 2023, 4, e317 CrossRef CAS.
- Q. Xu, Y. Ma, Y. Sun, D. Li, X. Zhang and C. Liu, Aggregate, 2023, 4, e333 CrossRef CAS.
- W. Li, Y. Zhou, S. He, T. Tong, C. Wang, P. Shi, S. Li, X. Wang, L. Yang, X. Cao and Z. Tian, Aggregate, 2024, 5, e496 CrossRef CAS.
- Z. Haidar, Biofunct. Mater., 2023, 1, 3 Search PubMed.
- B. Lin, A. Hung, W. Singleton, K. K. Darmawan, R. Moses, B. Yao, H. Wu, A. Barlow, M. Sani, A. J. Sloan, M. A. Hossain, J. D. Wade, Y. Hong, N. M. O'Brien-Simpson and W. Li, Aggregate, 2023, 4, e329 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and an h-index of 207.
000, and an h-index of 207.


