 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Overcoming (some) rules in synthesis design
Reinhard W.
Hoffmann

Fachbereich Chemie der Philipps Universität Marburg, Hans Meerwein Str.4, D-35032 Marburg, Germany. E-mail: rwho@chemie.uni-marburg.de
First published on 28th February 2025
Abstract
The experience gained in the development of science is codified in dogmas, rules, and directives. Such originally helpful rules are not necessarily cast in concrete, as they may become obsolete because of newer challenges and developments. This is manifested by breakthroughs violating such rules. Three examples concerning synthesis design in Organic Chemistry are discussed in this essay.
Introduction
Science, the accumulation of knowledge in a specific area, is worked out by the universe of scientists. The knowledge as well as experience, accumulated over generations, forms the canon of the particular branch of science. This canon also contains rules and dogmas, which influence the activities of the individual scientists, by pointing out e.g. no-go areas. Such (negative) directives have been more or less rationalized in most cases, giving credence to the rules. Thus, such rules act as walls, which confine the aims/areas of activity of the scientists (Fig. 1). Working orthodox within the confines of the rules/dogmas will surely enlarge knowledge in a predictable and confirmative manner. Yet, chances to explore new avenues leading to transformative changes in science remain obviously meagre.In new research areas, where confining dogmas do not yet exist because the boundaries have yet to be set, researchers will explore even weird ideas spontaneously. In the classic research areas, in turn, fundamental advances will most likely be achieved only by breaking through the confining walls, proving that some of the rules are not so solid or not as widely applicable as hitherto believed. To overcome an obstructing wall, the easiest option is to take a ladder; a less conspicuous approach is to dig a hole/tunnel under the wall. Yet, these approaches mark only one-time solutions to overcome an obstacle. A lasting solution is attained by breaking through the wall. Concerning rules in the canon of science, this happened when scientists explicitly challenged, – or at least simply ignored – a certain rule in their research projects.
Some breakthroughs in strategies of organic synthesis
The field of natural product/complex molecule synthesis has been in constant development.1 Aiming at increasing efficiency in synthesis, distinct strategies in synthesis planning have been developed and codified,2,3 with the yardstick being step-economy.4 The development of this field over time, hence, implied repeated breakthroughs overcoming existent rules. In this essay, three examples (subjectively chosen) will be given, in which hitherto discouraged strategies in complex molecule synthesis were shown to be workable and profitable.Avoid medium sized rings and macrocycles as intermediates in the synthesis of bridged polycyclic compounds
Corey (1975) examined strategies toward the synthesis of bridged polycyclic compounds.5 He stated that the highest structural simplification in retrosynthesis would result from cutting a bond in the most highly bridged ring. In the case of a simple target, such as ibogamine (1) (Scheme 1), this would be the ring highlighted in 1a. Nevertheless, not all bonds in the most highly bridged ring serve equally well cf.1b. Especially, cutting the bonds marked in red, would lead to a precursor molecule, which contains a medium-sized ring cf. e.g.1c. As medium sized rings were difficult to synthesize at that time, an approach to 1via a strategy involving 1c in the forward direction – i.e. a transannular ring closure in a medium sized ring – was red-flagged, Rule 4, of Corey's analysis.Regarding completed ibogamine syntheses,6 most use a building block approach and anellated the seven-membered ring to a prefabricated iso-quinuclidine unit, allowing short syntheses within 7–11 steps, cf.1d.
There is just a single synthesis,7 in which one of the green-flagged bonds marks the key skeleton-forming step, a synthesis completed by P. Grieco in just nine steps cf.1e. In contrast (Scheme 2), there are two early syntheses of ibogamine, in which the key skeleton-forming step involved the red-flagged bonds, i.e. involved medium-sized ring precursors, cf.1c8 and 1f.9 The attendant lengthier routes with 16 and 21 steps respectively may well have contributed to the formulation of the Corey rules.
Given these findings, the Corey rules make sense. Nevertheless, how stringent are they, as being dependent on the states of art in synthesis? It did thus not come as a surprise that a breakthrough synthesis of ibogamine was published in 2023,10 which generated the target in merely nine steps, despite forming a red-flagged bond as the key step by going through a nine-membered ring precursor, cf.1c in Scheme 3.
 | ||
| Scheme 3 Key step of Townsend's synthesis of ibogamine.10 | ||
Actually, the feasibility of generating bridged polycyclic compounds effectively via bridging medium-ring sized precursors was already demonstrated when Fukuyama (2004)11 completed a synthesis of strychnine, in which he chose just such an approach (Scheme 4).
 | ||
| Scheme 4 Key steps of Fukuyama's synthesis of strychnine.11 | ||
In preparing the key intermediate (2), the formation of the nine-membered ring was not an obstacle at all. Fukuyama's strychnine synthesis thus implied that Corey's reservation concerning medium sized rings as intermediates in complex molecule synthesis is not necessarily warranted. Right from its institution, Corey's rule 4 was contingent upon the state of art in synthesizing medium-sized rings. In due course, with the advent of improved methods to make medium sized rings, such as ring-closing metathesis,12 the rule was bound to become obsolete. An impressive example is given by E. Carreira's second synthesis of euphorikanin A13 in Scheme 5 with the following sequence of transformations.
 | ||
| Scheme 5 Key steps of Carreira's synthesis of euphorikanin A.13 | ||
The key step consists of an intraannular aldol-addition in the ten-membered ring followed in situ by a semi-pinacol rearrangement to furnish the bridged polycyclic lactone. Thus, Corey's rule 4 may now be relegated to history. Yet, Corey's rule does not crumble readily. Challenging it, remains what it is, “challenging”.14
Avoid functional group (and protective group) remodelling steps in multistage synthesis
With increasing complexity of the target structures, syntheses tend to become excessively long and longer. This caused Hendrickson (1975)15 to consider, which types of steps are essential to a synthesis and which are concession steps. Hendrickson concluded that only the skeleton-forming steps are essential, and that functional-group introduction and remodelling steps could be avoided, when one succeeds in generating the required functional groups simultaneously in the skeleton-forming steps. The avoidance of concession steps in an “ideal synthesis”16 would reduce the step count to that of the essential skeleton forming steps. The biggest sin would accordingly be, to separate skeleton formation and functional group introduction into two phases of the synthetic operation. Ironically, this is just what nature does in the biosynthesis of complex terpenes.17 Hence, when decade's later biomimetic synthesis got into focus, such an unorthodox, yet biomimetic approach was scrutinized.18 What rendered such an approach challenging was a lack of methods to functionalize a complex hydrocarbon skeleton in a position- and stereo-specific manner. In due course, with the growing field of C–H-activation chemistry,19 research dedicated to the synthesis of eudesmane terpenes20 revealed a surprising number of options: A single functional group – e.g. hydroxyl – in the initial intermediate suffices to guide subsequent oxygenation operations specifically20 (Scheme 6). | ||
| Scheme 6 Position selective oxyfunctionalization of model compounds in a study directed towards eudesmane terpenes.20 | ||
Based on these experiences Baran embarked on a two-phase synthesis of taxol.21 In the first phase, compound 3 was generated,22 a compound that attains the taxol skeleton (lacking C-20, cf. structure 4) and containing two carbonyl groups at C-2 and C-4 to initiate the oxygenation sequence in phase 2 (Scheme 7).
 | ||
| Scheme 7 Access to a naked taxane skeleton.22 | ||
Phase 2 was a major challenge, to decorate the intermediate 3 with six further oxygen functions in addition to attaching C-20. This goal has been eventually attained (Scheme 8) in a heroic effort by eighteen additional steps applying a variety of sometimes-delicate oxygenation methods.21
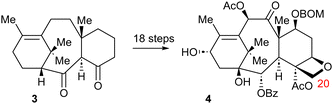 | ||
| Scheme 8 Elaboration of the taxane skeleton to a fully functionalized taxol precursor.21 | ||
The taxol (+2 steps) precursor 4 was thus reached in 24 operational steps, in which the hydrocarbon backbone was assembled first, followed by a phase of oxygenation operations to decorate the skeleton with the appropriate functional groups. This approach undercutted significantly the length of the hitherto 10 de novo syntheses of taxol, which ranged from about 40 to more than 60 steps. This achievement constitutes a breakthrough, in demonstrating that a complex molecule synthesis – separating skeleton formation and functional group introduction into two phases of the synthetic operation – can be efficient in contrast to Hendrickson's definition of an ideal synthesis.
Hendrickson's definition of the ideal synthesis15 provides a logic approach to minimize the step count of a synthesis endeavour. It gives kind of utopian directions to the Promised Land. As long as actual syntheses are substantially remote from this ideal,15 it is not imperative to follow Hendrickson's directions strictly. Rather it depends on the availability of suitable methods to minimize the step count of a synthesis. The latter is the dominant goal and may perhaps be easier reached by following other theorems than that of Hendrickson.
Try to reach an exponential increase of complexity along the synthesis route. Avoid passing through intermediates with excess complexity
Syntheses generally start from simple(r) starting materials and lead to a target compound of high(er) complexity. I.e., the molecular complexity – which can be numerically defined23 – of the intermediates, is increasing along the synthesis sequence. Bertz (1982)24 traced complexity of the intermediates over step number for several syntheses of the same target and found that overall yield decreased, the higher the sum of molecular complexities of the intermediates turned out. I.e. it affected synthetic complexity25 of the route to the target. The interrelation of molecular and synthetic complexity has recently reviewed comprehensively.26To translate this into practical advice, the following rule seems to apply: keep overall complexity of the intermediates along the synthesis path low, by (1) a late (= exponential) increase in molecular complexity, and (2) by avoiding intermediates, whose complexity exceeds that of following intermediates or the target.
This advice was never strictly abided by in the years that followed, as several syntheses passed through an intermediate(s) with excess complexities, “overbred intermediates” see e.g.3,26,27 This could be justified, when the choice of this particular intermediate allowed to short-cut much lengthier detours in the synthesis. Yet, the outright violation of this advice would be to generate the intermediate of highest complexity at the beginning of a synthesis sequence and to work from there “downwards” to reach eventually a target of lower complexity. This has been proposed and realized by T. Gaich (2021)28 by opening routes to the different cyclotaxane families in a divergent synthesis scheme. She had just completed the extraordinary synthesis of the probably most complex member – canataxpropellane 5 (Scheme 9) – of the taxane family.29 T. Gaich realized that – in essentially her own words28 – “selective and sequential fragmentation of distinct transannular bonds in the canataxpropellane framework will result in the synthesis of all four carboskeletons of the other cyclotaxanes ad libitum”
 | ||
| Scheme 9 Canataxpropellane.29 | ||
In realizing this concept, T. Gaich did not start from the completed canataxpropellane, but – for practical reasons – from an intermediate 6 (Scheme 10) in the canataxpropellane synthesis, that came close to the complexity of the envisioned other cyclotaxanes.
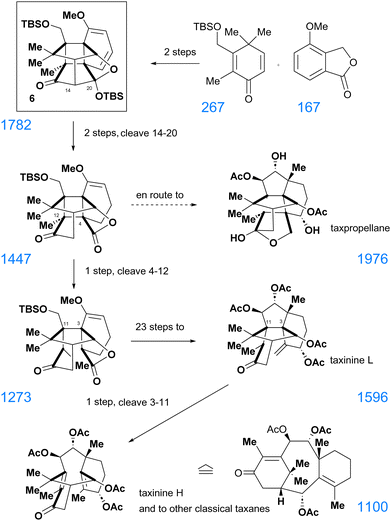 | ||
| Scheme 10 Divergent routes to members of the cyclotaxane family31 with SPS-scores32 of the structures added in blue. | ||
Such an undertaking is, however, meaningful only, when compound 6, the base camp for divergent synthesis,30 can be reached quickly and in high yield. This indeed holds for compound 6, which has been generated in a convergent manner in merely two steps from simple precursors with eruption-like increase in complexity (Scheme 10). From thereon, the completion of such chemical metamorphoses of taxane diterpenes,31 as judged by the simple step count, is by no means excessive for taxane syntheses. These findings thus fly in the face of the Bertz directives for managing complexity along a synthesis route. To illustrate this, the SPS-complexity scores32 have been added to the structures that appear in Scheme 10. Thus, the key intermediate 6 has a far higher complexity than most of the subsequent intermediates or targets.
The Bertz directives are a heuristic rule based on the examination of a small number of syntheses. It is accordingly expected, that with a substantially larger number of examples significant outliers, such as the cyclotaxane-metamorphoses will show up. Moreover, the Bertz rules refer originally to overall yield of synthesis sequences and only indirectly to step count. Nevertheless, the Gaich accomplishments are a breakthrough in that the handling of the high complexity intermediates did not entail an overall excessive number of steps.
Conclusions
These three examples illustrate how certain rules (dogmas, directives) controlling the planning of syntheses of complex molecules have been initially helpful, lasted for a while, and became eventually simple guidelines because of competing factors. These examples provide a glimpse into how complex molecule synthesis changes over time, driven not only by advances in synthetic methodology but foremost by flexibility in thinking. To reach breakthrough solutions in complex molecule synthesis, one should not be bashful. Rules are good to reach decent solutions for regular synthesis problems. Nevertheless, novel challenges in synthesis call for an out of the box approach. Hence, some rules – being the confining walls of the box – have to be overcome.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- R. W. Hoffmann, Angew. Chem., 2013, 125, 133–140 (
Angew. Chem.
, 2013
, 52
, 123–130
) CrossRef
.
-
(a)
E. J. Corey and X.-M. Cheng, The Logic of Chemical Synthesis, J. Wiley & Sons, New York, 1989 Search PubMed
; (b) E. J. Corey, Angew. Chem., 1991, 103, 469–479 ( Angew. Chem., Int. Ed. Engl. , 1991 , 30 , 455–465 ) CrossRef CAS
.
-
R. W. Hoffmann, Elements of Synthesis Planning, Springer, Heidelberg, 2009 Search PubMed
.
- P. A. Wender, V. A. Verma, T. J. Paxton and T. H. Pillow, Acc. Chem. Res., 2008, 41, 40–49 CrossRef CAS PubMed
.
- E. J. Corey, W. J. Howe, H. W. Orf, D. A. Pensak and G. Petersson, J. Am. Chem. Soc., 1975, 97, 6116–6124 CrossRef CAS
.
- G. K. Jana, S. Paul and S. Sinha, Org. Prep. Proced. Int., 2011, 43, 541–573 CrossRef CAS
.
- K. J. Henry, P. A. Grieco and W. J. DuBay, Tetrahedron Lett., 1996, 37, 8289–8292 CrossRef CAS
.
- G. Büchi, D. L. Coffen, K. Kocsis, P. E. Sonnet and F. E. Ziegler, J. Am. Chem. Soc., 1965, 87, 2073–2075 CrossRef
.
- J. P. Kutney, W. J. Cretney, P. Le Quesne, B. McKague and E. Piers, J. Am. Chem. Soc., 1970, 92, 1712–1720 CrossRef CAS PubMed
.
- A. J. Hughes and S. D. Townsend, Org. Lett., 2023, 25, 4567–4570 CrossRef CAS PubMed
.
- Y. Kaburagi, H. Tokuyama and T. Fukuyama, J. Am. Chem. Soc., 2004, 126, 10246–10247 CrossRef CAS PubMed
.
- H.-G. Schmalz, Angew. Chem., 1995, 107, 1981–1984 (
Angew. Chem., Int. Ed. Engl.
, 1995
, 34
, 1833–1836
) CrossRef
.
- M. J. Classen, B. Kicin, V. A. P. Ruf, A. Hamminger, L. Ribadeau-Dumas, W. M. Amberg and E. M. Carreira, J. Am. Chem. Soc., 2023, 145, 27225–27229 CrossRef CAS PubMed
.
- B. A. Wright, T. Okada, A. Regni, G. Luchini, S. Sowndarya., S. V. Nattawadee Chaisan, S. Kölbl, S. F. Kim, R. S. Paton and R. Sarpong, J. Am. Chem. Soc., 2024, 146, 33130–33148 Search PubMed
.
- J. B. Hendrickson, J. Am. Chem. Soc., 1975, 97, 5784–5800 Search PubMed
.
- T. Gaich and P. S. Baran, J. Org. Chem., 2010, 75, 4657–4673 CrossRef CAS PubMed
.
-
(a) M. Davis and R. Croteau, Top. Curr. Chem., 2000, 209, 53–95 Search PubMed
; (b) R. Kaspera and R. Croteau, Phytochem. Rev., 2006, 5, 433–444 CrossRef CAS PubMed
.
- Y. Ishihara and P. S. Baran, Synlett, 2010, 1733–1745 CAS
.
-
Handbook of C-H Transformations, ed. G. Dyker, Wiley VCH, Weinheim, 2005 Search PubMed
.
-
(a) K. Chen and P. S. Baran, Nature, 2009, 459, 824–828 CrossRef CAS PubMed
; (b) K. Chen, Y. Ishihara, M. M. Galan and P. S. Baran, Tetrahedron, 2010, 66, 4738–4744 CrossRef CAS
.
- Y. Kanda, H. Nakamura, S. Umemiya, R. K. Puthukanoori, V. R. M. Appala, G. K. Gaddamanugu, B. R. Paraselli and P. S. Baran, J. Am. Chem. Soc., 2020, 142, 10526–10533 CrossRef CAS PubMed
.
- A. Mendoza, Y. Ishihara and P. S. Baran, Nat. Chem., 2012, 4, 21–25 CrossRef CAS PubMed
.
-
(a) S. H. Bertz, New J. Chem., 2003, 27, 860–869 RSC
; (b) J. Li and M. D. Eastgate, Org. Biomol. Chem., 2015, 13, 7164–7176 RSC
.
-
(a) S. H. Bertz, J. Am. Chem. Soc., 1982, 104, 5801–5803 CrossRef CAS
; (b) S. H. Bertz and T. J. Sommer, Organic Synthesis, Theory and Applications, ed. T. Hudlicky, JAI Press, London, 1993, vol. 2, p. 67 Search PubMed
.
- C. W. Coley, L. Rogers, W. H. Green and K. F. Jensen, J. Chem. Inf. Model., 2018, 58, 252–261 Search PubMed
.
- B. A. Wright and R. Sarpong, Nat. Rev. Chem., 2024, 8, 776–792 CrossRef CAS PubMed
.
-
(a) E. C. Cherney, J. C. Green and P. S. Baran, Angew. Chem., 2013, 125, 9189–9192 (
Angew. Chem., Int. Ed.
, 2013
, 52
, 9019–9022
) CrossRef
; (b) B. A. Wright, A. Chaisan, N. Regni and R. Sarpong, J. Am. Chem. Soc., 2024, 146, 1813–1818 CrossRef CAS PubMed
.
- F. Schneider, L. Pan, M. Ottenbruch, T. List and T. Gaich, Acc. Chem. Res., 2021, 54, 2347–2360 CrossRef CAS PubMed
.
- F. Schneider, K. Samarin, S. Zanella and T. Gaich, Science, 2020, 367, 676–681 CrossRef CAS PubMed
.
- L. Li, Z. Chen, X. Zhang and Y. Jia, Chem. Rev., 2018, 118, 3752–3832 CrossRef CAS PubMed
.
- L. Pan, F. Schneider, M. Ottenbruch, R. Wiechert, T. List, P. Schoch, B. Mertes and T. Gaich, Nature, 2024, 632, 543–549 CrossRef PubMed
.
- A. Krzyzanowski, A. Pahl, M. Grigalunas and H. Waldmann, J. Med. Chem., 2023, 66, 12739–12750 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2025 |




