Open Access Article
Open Access ArticleProbing the electronic structure and dipole-bound state of the 7-azaindolide anion
Jisoo
Kang
 ,
Edward I.
Brewer
and
Lai-Sheng
Wang
,
Edward I.
Brewer
and
Lai-Sheng
Wang
 *
*
Department of Chemistry, Brown University, Providence, RI 02912, USA. E-mail: Lai-Sheng_Wang@brown.edu
First published on 29th October 2025
Abstract
We report an investigation of the electronic structure and dipole-bound state (DBS) of the cryogenically-cooled 7-azaindolide anion (7-AI−) using high-resolution photoelectron imaging, photodetachment spectroscopy, and resonant photoelectron spectroscopy. The electron affinity of the 7-AI radical is measured to be 2.6967(8) eV (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 ± 6 cm−1). Two excited electronic states of the neutral radical are observed at 0.8 eV and 1.4 eV above the ground state. Two minor isomers of 7-AI− due to deprotonation from the α- and β-carbon on the pyrrole ring are also detected with lower adiabatic detachment energies. A DBS is observed for the 7-AI− anion at 156 cm−1 below the detachment threshold, along with 16 vibrational Feshbach resonances. Resonant two-photon photoelectron imaging reveals that the DBS is relatively long-lived. Resonant photoelectron spectroscopy via the vibrational Feshbach resonances of the DBS gives rise to rich vibrational features for the 7-AI radical not accessible in conventional photoelectron spectroscopy. Fundamental vibrational frequencies for 16 vibrational modes of the 7-AI neutral radical are measured experimentally, including 7 bending modes. The current work provides extensive experimental electronic and vibrational information for the 7-AI− anion and the 7-AI radical.
751 ± 6 cm−1). Two excited electronic states of the neutral radical are observed at 0.8 eV and 1.4 eV above the ground state. Two minor isomers of 7-AI− due to deprotonation from the α- and β-carbon on the pyrrole ring are also detected with lower adiabatic detachment energies. A DBS is observed for the 7-AI− anion at 156 cm−1 below the detachment threshold, along with 16 vibrational Feshbach resonances. Resonant two-photon photoelectron imaging reveals that the DBS is relatively long-lived. Resonant photoelectron spectroscopy via the vibrational Feshbach resonances of the DBS gives rise to rich vibrational features for the 7-AI radical not accessible in conventional photoelectron spectroscopy. Fundamental vibrational frequencies for 16 vibrational modes of the 7-AI neutral radical are measured experimentally, including 7 bending modes. The current work provides extensive experimental electronic and vibrational information for the 7-AI− anion and the 7-AI radical.
1. Introduction
The 7-azaindole molecule, its photophysics, and electronic structure have attracted significant research interest, because the dimer of 7-azaindole involves double H-bonds and is considered to be an ideal model system for the base pairs in the double-stranded DNA.1–14 In particular, the excited-state double proton transfer in the 7-azaindole dimer has been investigated to gain insight into the stability and dynamics of the DNA base pairs.15–25 In the two-step model for the double proton transfer, the intermediate consists of an ion pair, in which a proton is transferred from one 7-azaindole molecule to the other, leaving behind a deprotonated 7-azaindole anion (i.e. 7-azaindolide or 7-AI−) and creating a protonated 7-azaindole cation. Thus, characterization of the 7-AI− anion is important for the understanding of the mechanisms of the double proton transfer in the 7-azaindole dimer. However, despite extensive investigations of the parent 7-azaindole molecule, there has been very little study on the 7-AI− anion or the 7-AI radical. Photoelectron spectroscopy (PES) and photodetachment spectroscopy (PDS) are the best techniques to characterize anions in the gas phase.26 Indeed, the group of Lineberger first tried to study the 7-AI− anion using PES.27 They measured a low resolution photoelectron (PE) spectrum at a photon energy of 3.494 eV, but their attempt to obtain high resolution PE spectra near threshold was not successful, because of the presence of vibrational hot bands and complicated autodetachment features attributed to the existence of a dipole-bound state (DBS). They were able to deduce an electron affinity (EA) of 2.699(1) eV for the 7-AI neutral radical.27 More recently, Noble et al. reported a PDS study of the 7-AI− anion cooled in a cryogenic ion trap by detecting the neutral products.28 They indeed observed a DBS at 160(10) cm−1 below the detachment threshold, as well as seven above-threshold vibrational levels of the DBS (a.k.a. vibrational Feshbach resonances). From the onset of the detachment threshold, Noble et al. obtained an EA of 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750(10) cm−1 (2.697 eV) for the 7-AI radical, consistent with the preliminary value obtained by the Lineberger group.
750(10) cm−1 (2.697 eV) for the 7-AI radical, consistent with the preliminary value obtained by the Lineberger group.
High-resolution photoelectron imaging of cryogenically-cooled anions has been demonstrated to be a powerful technique to probe the electronic and vibrational structures of the corresponding neutrals.29–32 We have shown that it is critical to conduct PDS on cryogenically-cooled anions to search for DBS.32–35 In particular, we have developed resonant PES (rPES) via the vibrational Feshbach resonances, which can not only yield highly non-Franck Condon PE spectra with rich vibrational information inaccessible in conventional PES, but is also important to assist the assignment of the DBS vibrational features.33,35–39 Combining PDS and rPES, we have investigated a number of complicated systems from simple aromatic anions to O-containing polycyclic aromatic hydrocarbon anions.40–51 Using resonant two-photon detachment (R2PD) PES, we have also been able to deduce dynamical information about the bound DBS levels.52–56
In this study, we use this integrated spectroscopic strategy to probe the electronic structure and DBS of the 7-AI− anion and its corresponding 7-AI radical. High-resolution PE spectra yield an EA of 2.6967(8) eV (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 ± 6 cm−1) for the 7-AI radical, while PES at high photon energies allows the observation of two low-lying excited electronic states for neutral 7-AI at 0.8 eV and 1.4 eV above its ground state. Two minor isomers due to deprotonation from the α- and β-carbon on the pyrrole ring are also detected at binding energies of 2.200(3) eV and 1.918(5) eV, respectively. A DBS is observed for the 7-AI− anion at 156(6) cm−1 below the detachment threshold, accompanied by 16 vibrational Feshbach resonances. R2PD PES via the zero-point level reveals that the DBS is relatively long-lived. The rPES via the 16 Feshbach resonances provides insights into the vibrational autodetachment processes and rich vibrational spectral features for the 7-AI neutral. The current study provides experimental values for 16 fundamental vibrational frequencies for the 7-AI radical, offering new electronic and spectroscopic benchmarks for further theoretical investigation of 7-AI− and its neutral counterpart.
751 ± 6 cm−1) for the 7-AI radical, while PES at high photon energies allows the observation of two low-lying excited electronic states for neutral 7-AI at 0.8 eV and 1.4 eV above its ground state. Two minor isomers due to deprotonation from the α- and β-carbon on the pyrrole ring are also detected at binding energies of 2.200(3) eV and 1.918(5) eV, respectively. A DBS is observed for the 7-AI− anion at 156(6) cm−1 below the detachment threshold, accompanied by 16 vibrational Feshbach resonances. R2PD PES via the zero-point level reveals that the DBS is relatively long-lived. The rPES via the 16 Feshbach resonances provides insights into the vibrational autodetachment processes and rich vibrational spectral features for the 7-AI neutral. The current study provides experimental values for 16 fundamental vibrational frequencies for the 7-AI radical, offering new electronic and spectroscopic benchmarks for further theoretical investigation of 7-AI− and its neutral counterpart.
2. Methods
2.1. Photoelectron imaging
The experiments were carried out using our home-built third-generation electrospray-ionization PES (ESI-PES) apparatus,57 incorporating a cryogenic Paul trap and a high-resolution velocity-map imaging (VMI) system.58–60 For the current study, the 7-AI− anions were produced via electrospray of a 1 mM solution of 7-azaindole (98%, Sigma-Aldrich) dissolved in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/water mixed solvent containing trace amount of NaOH to promote deprotonation. Anions from the ESI source were transported through a series of RF ion guides and subsequently accumulated and cooled in a Paul trap, which was maintained at 4.6 K using a closed-cycle helium refrigerator. Trapped ions are cooled by collisions with a 1 mTorr buffer gas consisting of a mixture of He/H2 in a 4/1 volume ratio. After an accumulation and cooling period of ∼100 ms, the ions were pulsed out at a 10 Hz repetition rate into the extraction region of a time-of-flight mass spectrometer. The 7-AI− anion was mass-selected and photodetached in the VMI interaction zone using either a tunable dye laser or the third (3.496 eV) and fourth (4.661 eV) harmonics of an Nd:YAG laser. The resulting photoelectrons were mapped onto a pair of 75 mm microchannel plates coupled to a phosphor screen, and the spatially-resolved images were recorded using a CCD camera. Image reconstruction and angular integration were performed using the MELEXIR algorithm.61 The energy scale was calibrated using the known PE spectrum of Au− at multiple photon energies. The system could achieve an energy resolution of ΔKE = 3.8 cm−1 at 55 cm−1, with a relative resolution of ∼1.5% for electrons above 1 eV.60 The VMI extraction voltages were adjusted to capture higher energy electrons and optimize image quality: −1200 V for the R2PD spectrum, −1000 V for the two PE spectra at 3.496 eV and 4.661 eV, −300 V for the rPES and the near-threshold PES.
1 methanol/water mixed solvent containing trace amount of NaOH to promote deprotonation. Anions from the ESI source were transported through a series of RF ion guides and subsequently accumulated and cooled in a Paul trap, which was maintained at 4.6 K using a closed-cycle helium refrigerator. Trapped ions are cooled by collisions with a 1 mTorr buffer gas consisting of a mixture of He/H2 in a 4/1 volume ratio. After an accumulation and cooling period of ∼100 ms, the ions were pulsed out at a 10 Hz repetition rate into the extraction region of a time-of-flight mass spectrometer. The 7-AI− anion was mass-selected and photodetached in the VMI interaction zone using either a tunable dye laser or the third (3.496 eV) and fourth (4.661 eV) harmonics of an Nd:YAG laser. The resulting photoelectrons were mapped onto a pair of 75 mm microchannel plates coupled to a phosphor screen, and the spatially-resolved images were recorded using a CCD camera. Image reconstruction and angular integration were performed using the MELEXIR algorithm.61 The energy scale was calibrated using the known PE spectrum of Au− at multiple photon energies. The system could achieve an energy resolution of ΔKE = 3.8 cm−1 at 55 cm−1, with a relative resolution of ∼1.5% for electrons above 1 eV.60 The VMI extraction voltages were adjusted to capture higher energy electrons and optimize image quality: −1200 V for the R2PD spectrum, −1000 V for the two PE spectra at 3.496 eV and 4.661 eV, −300 V for the rPES and the near-threshold PES.
2.2. Photoelectron angular distributions
VMI also provides photoelectron angular distributions (PAD), offering additional insight into the electronic characters of the detachment transitions. For randomly oriented molecules undergoing single-photon detachment by linearly polarized light, the differential cross section is given by: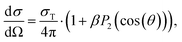 | (1) |
| I(θ) ∼ (1 + βP2(cos(θ))), | (2) |
The β parameter ranges from −1 (perpendicular) to +2 (parallel).62 In atomic systems, detachment from an s orbital (![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) = 0) leads to p-wave (
= 0) leads to p-wave (![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) = 1) photoelectrons with β = 2, while detachment from a p-orbital (
= 1) photoelectrons with β = 2, while detachment from a p-orbital (![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) = 1) produces both s and d partial waves (
= 1) produces both s and d partial waves (![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) = 0, 2) with β = −1. For molecular orbitals (MOs), the interpretation of the β parameter is more nuanced, but trends in β can still yield qualitative information about the orbital character of the electron being detached.
= 0, 2) with β = −1. For molecular orbitals (MOs), the interpretation of the β parameter is more nuanced, but trends in β can still yield qualitative information about the orbital character of the electron being detached.
2.3. Computational methods
Theoretical calculations were conducted to help interpret the experimental observations. All calculations were performed using density functional theory (DFT) as implemented in Gaussian 09.63 Ground-state geometries and harmonic vibrational frequencies of both the anion and the neutral species were calculated at the B3LYP/aug-cc-pVTZ level. Vertical detachment energies (VDEs) were computed using time-dependent DFT (TD-DFT) based on the optimized anion geometries. Franck–Condon (FC) factors were calculated with the FC-Lab2 program.643. Results
3.1. Non-resonant photoelectron imaging and spectroscopy
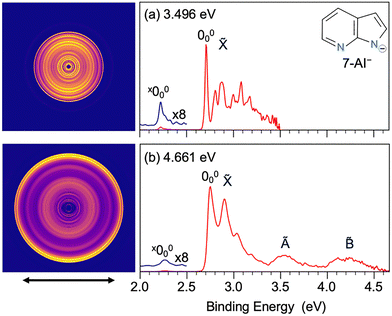
We first performed non-resonant PE imaging of the 7-AI− anion at 3.496 eV and 4.661 eV, as presented in Fig. 1. The 4.661 eV spectrum revealed three broad bands,![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) , Ã, and
, Ã, and ![[B with combining tilde]](https://www.rsc.org/images/entities/char_0042_0303.gif) . The 3.496 eV spectrum is dominated by vibrational features resolved for the
. The 3.496 eV spectrum is dominated by vibrational features resolved for the ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) band up to approximately 3.4 eV binding energy. The
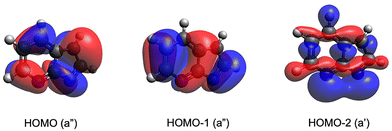
band up to approximately 3.4 eV binding energy. The ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) band arises from photodetachment transition to the ground electronic state of neutral 7-AI by removing an electron from the highest occupied molecular orbital (HOMO). Analysis of the PAD associated with band
band arises from photodetachment transition to the ground electronic state of neutral 7-AI by removing an electron from the highest occupied molecular orbital (HOMO). Analysis of the PAD associated with band ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) yields an anisotropy parameter of β = −0.40 ± 0.05, indicative of partial wave contributions beyond pure s-character, suggesting detachment from a π-type orbital, in agreement with the HOMO of 7-AI−, as illustrated in Fig. 2. The most prominent transition at 2.7 eV defines the vibrational origin (000). The à band at ∼3.5 eV should be due to detachment from the HOMO−1 of 7-AI−, representing the first excited electronic state of neutral 7-AI. The HOMO−1 is also a π orbital, consistent with the β value (−0.28 ± 0.05) obtained from the PAD of band Ã. The broad band
yields an anisotropy parameter of β = −0.40 ± 0.05, indicative of partial wave contributions beyond pure s-character, suggesting detachment from a π-type orbital, in agreement with the HOMO of 7-AI−, as illustrated in Fig. 2. The most prominent transition at 2.7 eV defines the vibrational origin (000). The à band at ∼3.5 eV should be due to detachment from the HOMO−1 of 7-AI−, representing the first excited electronic state of neutral 7-AI. The HOMO−1 is also a π orbital, consistent with the β value (−0.28 ± 0.05) obtained from the PAD of band Ã. The broad band ![[B with combining tilde]](https://www.rsc.org/images/entities/char_0042_0303.gif) at ∼4.1 eV should come from detachment from the HOMO−2 to the second excited state of the neutral molecule with a β value of –0.19 ± 0.05. The HOMO−2 is a delocalized σ orbital primarily formed by the in-plane p orbitals of C and N (Fig. 2). The PAD is clearly more isotropic and the β value is less conclusive in informing the electronic nature of the HOMO−2. We also computed the excitation energies of 7-AI, as compared with the experimental data in Table S1. We should note that a very weak feature is discernible on the low binding energy side at ∼2.2 eV (x000). As will be shown later, this signal arises from an isomer of 7-AI− (denoted as x7-AI−), which is shown in Fig. S1.
at ∼4.1 eV should come from detachment from the HOMO−2 to the second excited state of the neutral molecule with a β value of –0.19 ± 0.05. The HOMO−2 is a delocalized σ orbital primarily formed by the in-plane p orbitals of C and N (Fig. 2). The PAD is clearly more isotropic and the β value is less conclusive in informing the electronic nature of the HOMO−2. We also computed the excitation energies of 7-AI, as compared with the experimental data in Table S1. We should note that a very weak feature is discernible on the low binding energy side at ∼2.2 eV (x000). As will be shown later, this signal arises from an isomer of 7-AI− (denoted as x7-AI−), which is shown in Fig. S1.
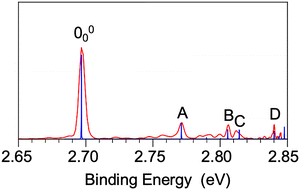
We also obtained high-resolution PE images and spectra for the ground state detachment transition of 7-AI− at lower photon energies, as shown in Fig. 3. The near-threshold spectrum at the 2.7025 eV photon energy (Fig. 3(a)) yields a very accurate adiabatic detachment energy (ADE) or the electron affinity (EA) of 2.6967 ± 0.0008 eV (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 ± 6 cm−1) for the 7-AI radical. The 2.8458 eV spectrum (Fig. 3(b)) resolves a number of weak vibrational peaks, labeled A through D. The binding energies and shifts relative to the 0–0 transition are given in Table S2, where they are compared with the computed vibrational frequencies for the 7-AI radical. The very weak x000 signal due to the x7-AI− isomer is also observed in Fig. 3.
751 ± 6 cm−1) for the 7-AI radical. The 2.8458 eV spectrum (Fig. 3(b)) resolves a number of weak vibrational peaks, labeled A through D. The binding energies and shifts relative to the 0–0 transition are given in Table S2, where they are compared with the computed vibrational frequencies for the 7-AI radical. The very weak x000 signal due to the x7-AI− isomer is also observed in Fig. 3.
3.2. Photodetachment spectroscopy
The previous PDS study of 7-AI− by Noble et al. revealed a DBS, because the 7-AI radical possesses a dipole moment of 4.64 D,28 higher than the 2.5 D critical value.65 They observed a photodetachment threshold of 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 ± 10 cm−1 (2.697 eV), in excellent agreement with the EA of 21
750 ± 10 cm−1 (2.697 eV), in excellent agreement with the EA of 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 ± 6 cm−1 (2.6967 eV) for the 7-AI radical measured in our PES experiment (Fig. 3). The binding energy of the DBS was measured to be 160 ± 10 cm−1 by Noble et al. In addition, they observed seven vibrational Feshbach resonances of the DBS in their reported spectral range ending at 22
751 ± 6 cm−1 (2.6967 eV) for the 7-AI radical measured in our PES experiment (Fig. 3). The binding energy of the DBS was measured to be 160 ± 10 cm−1 by Noble et al. In addition, they observed seven vibrational Feshbach resonances of the DBS in their reported spectral range ending at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cm−1. However, assignments of the vibrational features were challenging. We have shown that resonant PES via the DBS can be used to assign the vibrational features on the basis of the Δv = −1 propensity rule for vibrational autodetachment.66,67 Furthermore, resonant PES yields much richer vibrational information than conventional non-resonant PES.39
700 cm−1. However, assignments of the vibrational features were challenging. We have shown that resonant PES via the DBS can be used to assign the vibrational features on the basis of the Δv = −1 propensity rule for vibrational autodetachment.66,67 Furthermore, resonant PES yields much richer vibrational information than conventional non-resonant PES.39
In the current work, we conducted a PDS study of 7-AI− with higher spectral resolution using a 0.1 nm scanning step size over a broader spectral range extending to 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 cm−1, as shown in Fig. 4. In the same spectral range up to 22
520 cm−1, as shown in Fig. 4. In the same spectral range up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cm−1, the current spectrum agrees with that reported by Noble et al.:27 the seven Feshbach resonances, labeled as 1, 4–6, 9, 10, and 13, were observed previously. Note that peak 13 is the most prominent resonance in the current PDS, whereas it was a very weak peak in the spectrum reported by Noble et al. We were also able to identify six weak resonances (peaks 2, 3, 7, 8, 11, 12) in this spectral range, which were not recognized previously. In addition, we observed three new Feshbach resonances, labeled as 14, 15, 16, at higher excitation energies beyond the spectral range reported by Noble et al. A sharp threshold is observed at 21
700 cm−1, the current spectrum agrees with that reported by Noble et al.:27 the seven Feshbach resonances, labeled as 1, 4–6, 9, 10, and 13, were observed previously. Note that peak 13 is the most prominent resonance in the current PDS, whereas it was a very weak peak in the spectrum reported by Noble et al. We were also able to identify six weak resonances (peaks 2, 3, 7, 8, 11, 12) in this spectral range, which were not recognized previously. In addition, we observed three new Feshbach resonances, labeled as 14, 15, 16, at higher excitation energies beyond the spectral range reported by Noble et al. A sharp threshold is observed at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 cm−1, matching exactly the EA of 7-AI determined from the high resolution PES (Fig. 3). The prompt onset at threshold suggests s-wave detachment according to the Wigner threshold law,68 consistent with detachment from the π HOMO (Fig. 2). Below the detachment threshold, a distinct feature (peak 0) appears at 21
751 cm−1, matching exactly the EA of 7-AI determined from the high resolution PES (Fig. 3). The prompt onset at threshold suggests s-wave detachment according to the Wigner threshold law,68 consistent with detachment from the π HOMO (Fig. 2). Below the detachment threshold, a distinct feature (peak 0) appears at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 cm−1 (2.6774 eV), corresponding to the zero-point vibrational level of the DBS. The DBS binding energy is measured as 156 ± 5 cm−1 (0.0193 ± 0.0006 eV), slightly more accurate than the value of 160 ± 10 cm−1 reported by Noble et al.27 The excitation energies and vibrational assignments of the DBS vibrational levels are summarized in Table S3.
595 cm−1 (2.6774 eV), corresponding to the zero-point vibrational level of the DBS. The DBS binding energy is measured as 156 ± 5 cm−1 (0.0193 ± 0.0006 eV), slightly more accurate than the value of 160 ± 10 cm−1 reported by Noble et al.27 The excitation energies and vibrational assignments of the DBS vibrational levels are summarized in Table S3.
Owing to improved signal-to-noise ratios, the current photodetachment spectrum reveals a broad background near peak 0, which disappears below ∼21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1. Within this broad background, two weak features (α and γ) are discernible (inset of Fig. 4). As will be discussed later, these signals arise from isomeric forms of 7-AI−, as already hinted in the PE spectra (Fig. 1 and 3).
200 cm−1. Within this broad background, two weak features (α and γ) are discernible (inset of Fig. 4). As will be discussed later, these signals arise from isomeric forms of 7-AI−, as already hinted in the PE spectra (Fig. 1 and 3).
As we have shown previously, the DBS vibrational levels in general mirror the FC envelope of the ground state transition in non-resonant PES.39 This is because the highly diffuse dipole-bound electron has little effect on the structure of the neutral core and the potential energy surface of the DBS is nearly identical to that of the neutral ground state. Fig. 1 shows that the FC envelope for the ground state transition (the ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) band) spans an energy range of ∼0.6 eV. However, the Feshbach resonances in the photodetachment spectrum abruptly end at peak 16, only about 0.16 eV above the DBS zero-point level. According to the FC envelope of the PE spectra, we expected to observe extensive DBS vibrational peaks above peak 16 in the photodetachment spectrum, extending to at least 4800 cm−1. We will show later that the abrupt loss of Feshbach resonances above peak 16 is likely due to the competition between autodetachment and relaxation to a nearby electronic excited state (shape resonance) of the 7-AI− anion.
band) spans an energy range of ∼0.6 eV. However, the Feshbach resonances in the photodetachment spectrum abruptly end at peak 16, only about 0.16 eV above the DBS zero-point level. According to the FC envelope of the PE spectra, we expected to observe extensive DBS vibrational peaks above peak 16 in the photodetachment spectrum, extending to at least 4800 cm−1. We will show later that the abrupt loss of Feshbach resonances above peak 16 is likely due to the competition between autodetachment and relaxation to a nearby electronic excited state (shape resonance) of the 7-AI− anion.
3.3. R2PD photoelectron imaging
The bound zero-point level of the DBS (peak 0 in Fig. 4) is observed through the single-color R2PD process, where the first photon excites the anion to the DBS zero-point level and the second photon within the same 5 ns laser pulse detaches the diffuse DBS electron.39,49 R2PD photoelectron imaging probes the nature of the DBS and relaxation processes that may occur within the duration of the laser pulse. Fig. 5 displays the single-color R2PD photoelectron image and spectrum at peak 0 in the PDS. Two major signals are observed, one at the low binding energy side (labeled as “DBS”) and another at the high binding energy side (labeled as “S0”). The “DBS” band at the low binding energy side originates from the expected sequential two-photon process. The low binding energy of this band, which could not be measured accurately due to its high electron kinetic energy, is consistent with the accurate measurement of 156 cm−1 (0.0193 eV) from the PDS (Fig. 4). The angular distribution of this band (the outermost ring in the PE image in Fig. 5) exhibits a distinct p-wave character with a β parameter of 1.33 ± 0.05, as expected from the s-like dipole-bound orbital. The signals at the high binding energy side (“S0”) should be due to detachment from vibrational levels of the ground state (S0) of 7-AI−, populated due to relaxation from the DBS zero-point level following the absorption of the first photon.48,51,53 Four vibrational peaks (Aa–Da) are resolved within the “S0” signals; their binding energies, shifts from the 0–0 transition in the PE spectra of 7-AI−, and their assignments are summarized in Table S4. The assignments are assisted with the computed frequencies of the 7-AI− anion (Fig. S2 and Table S5). The weak x000 peak due to isomer x7-AI− is also observed in Fig. 5, similar to that in the PE spectra shown in Fig. 1 and 3. In addition, an even weaker feature is observed at ∼1.9 eV (peak y000), which is attributed to a different isomer y7-AI− (Fig. S1). | ||
| Fig. 5 One-color R2PD photoelectron image and spectrum of 7-AI− obtained at 2.6774 eV (corresponding to peak 0 in Fig. 4). The double arrow below the image indicates the laser polarization. The inset shows an expanded view of the high binding energy region. | ||
We took PE spectra by tuning the detachment laser to photon energies corresponding to the weak signals observed in the PDS around peak 0, i.e. at positions of α and γ, as shown in Fig. S3. Notably, the low binding energy “DBS” signal observed in Fig. 5 disappeared, suggesting that the weak signals in the PDS around peak 0 are not related to the main isomer of 7-AI−. However, the x000 and y000 signals are the same as those in Fig. 5, confirming that those weak signals in the PDS should come from different isomers of 7-AI−, i.e., most likely corresponding to above-threshold excited states (i.e., shape resonances) of the x7-AI− and y7-AI− isomers. The sharp peaks at the high binding energy side are probably due to autodetachment from the shape resonances from these isomers. We should emphasize that the x7-AI− and y7-AI− isomers were very weakly populated, only ∼1% compared to the main isomer. The resolved sharp peaks in the two spectra in Fig. S3 are different, indicating that the weak signals in the PDS probably consist of contributions from both isomers. The binding energies of the resolved features are given in Table S6. The ADEs of x7-AI− and y7-AI− measured from peaks x000 and y000 are 2.200 ± 0.003 eV and 1.918 ± 0.005 eV, respectively, which are compared with the calculated values in Table S7.
3.4. Resonant photoelectron imaging
Resonant PES via the Feshbach resonances yields highly non-Franck–Condon spectra due to the Δv = −1 propensity rule for vibrational autodetachment, allowing vibrational peaks with low FC factors or even FC-inactive vibrational modes in conventional PES to be significantly enhanced. Thus, rPES provides not only much richer spectroscopic information, but also a powerful means to assign the vibrational levels of the DBS. By tuning the detachment laser to the 16 Feshbach resonances observed in the PDS (Fig. 4), we obtained 16 resonant PE spectra, as shown in Fig. 6 and 7. Depending on the DBS levels accessed, certain vibrational peaks in the rPES become selectively enhanced, in comparison to the non-resonant PES shown in Fig. 3. More importantly, many new vibrational features are observed and they are labeled by lower case letters from a to j in Fig. 6 and 7. These newly observed vibrational features, along with their corresponding electron binding energies, relative shifts from the vibrational origin (000), and their assignments, are also given in Table S2. | ||
Fig. 6 Resonant PE images and spectra of 7-AI− at peaks 1–8 in the PDS (Fig. 4 and Table S3). (a) 2.7048 eV (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 cm−1), (b) 2.7279 eV (22 816 cm−1), (b) 2.7279 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 002 cm−1), (c) 2.7298 eV (22 002 cm−1), (c) 2.7298 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 018 cm−1), (d) 2.7395 eV (22 018 cm−1), (d) 2.7395 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096 cm−1), (e) 2.7510 eV (22 096 cm−1), (e) 2.7510 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 188 cm−1), (f) 2.7665 eV (22 188 cm−1), (f) 2.7665 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 314 cm−1), (g) 2.7701 eV (22 314 cm−1), (g) 2.7701 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 cm−1), (h) 2.7814 eV (22 343 cm−1), (h) 2.7814 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 cm−1). The double arrow below the images represents the polarization direction of the laser. 434 cm−1). The double arrow below the images represents the polarization direction of the laser. | ||
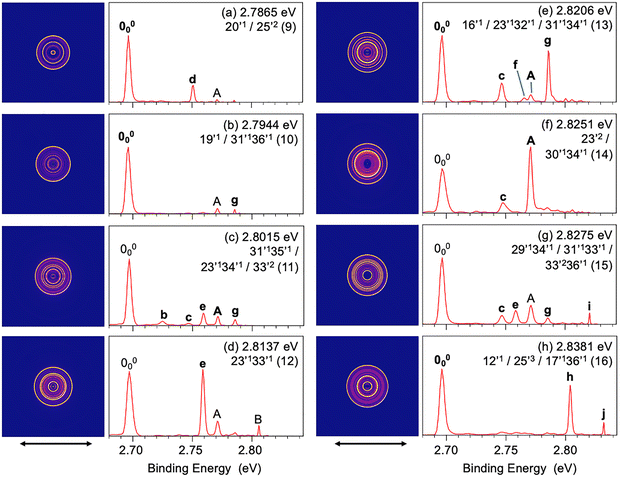 | ||
Fig. 7 Resonant PE images and spectra of 7-AI− at peaks 9–16 in the PDS (Fig. 4 and Table S3). (a) 2.7865 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475 cm−1), (b) 2.7944 eV (22 475 cm−1), (b) 2.7944 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539 cm−1), (c) 2.8015 eV (22 539 cm−1), (c) 2.8015 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 596 cm−1), (d) 2.8137 eV (22 596 cm−1), (d) 2.8137 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694 cm−1), (e) 2.8206 eV (22 694 cm−1), (e) 2.8206 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 cm−1), (f) 2.8251 eV (22 750 cm−1), (f) 2.8251 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 cm−1), (g) 2.8275 eV (22 786 cm−1), (g) 2.8275 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806 cm−1), (h) 2.8381 eV (22 806 cm−1), (h) 2.8381 eV (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 891 cm−1). The double arrow below the images represents the polarization direction of the laser. 891 cm−1). The double arrow below the images represents the polarization direction of the laser. | ||
4. Discussion
4.1. Non-resonant PES
![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) band in the 3.496 eV spectrum (Fig. 1(a)). Vibrational features are partially resolved for the
band in the 3.496 eV spectrum (Fig. 1(a)). Vibrational features are partially resolved for the ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) band even in the 4.661 eV spectrum. However, no discernible vibrational features are resolved for the two weaker excited state bands, Ã and
band even in the 4.661 eV spectrum. However, no discernible vibrational features are resolved for the two weaker excited state bands, Ã and ![[B with combining tilde]](https://www.rsc.org/images/entities/char_0042_0303.gif) . This observation suggests strong vibronic couplings among the low-lying excited states of the 7-AI radical, as observed previously in the smaller pyrrolyl radical or the isoelectronic indolyl radical.47,69 In fact, the 4.661 eV spectrum of 7-AI− exhibits some similarities to that of pyrrolide at 4.3446 eV or in particular that of indolide. We also find that the valence MOs of 7-AI− are similar to those of the indolide anion.47 The strong vibronic coupling among the low-lying states in 7-AI is probably one of the reasons that the computed excitation energies using TD-DFT are overestimated in comparison to the experimental observations (Table S1).
. This observation suggests strong vibronic couplings among the low-lying excited states of the 7-AI radical, as observed previously in the smaller pyrrolyl radical or the isoelectronic indolyl radical.47,69 In fact, the 4.661 eV spectrum of 7-AI− exhibits some similarities to that of pyrrolide at 4.3446 eV or in particular that of indolide. We also find that the valence MOs of 7-AI− are similar to those of the indolide anion.47 The strong vibronic coupling among the low-lying states in 7-AI is probably one of the reasons that the computed excitation energies using TD-DFT are overestimated in comparison to the experimental observations (Table S1).
![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) band shape at 4.661 eV is reasonable, although the FC profile of the 3.496 eV spectrum is anomalous with increasing intensity on the higher binding energy side. As will be shown below, the 7-AI− anion has a valence excited state near this photon energy, which may distort the PE spectrum due to autodetachment. Owing to the planar geometry (Cs) of both the 7-AI− anion and the 7-AI neutral radical, only in-plane vibrational modes (A′ symmetry) are symmetry-allowed. The calculated FC factors agree well with the observed vibrational features in the low binding energy region probed at the 2.8458 eV photon energy (Fig. 8). Peaks A, B, C, and D are due to the ν23, ν20, ν19, and ν16 modes (Table S2), respectively, on the basis of the computed harmonic frequencies. The good agreement is expected because the low photon energy at 2.8458 eV only probes the potential energy surface near the equilibrium of the 7-AI ground state. The higher energy part accessed at high photon energies (Fig. S5) is expected to deviate from the FC calculations due to the vibronic coupling of the ground state with the excited states, as observed in similar molecular systems previously.47,69
band shape at 4.661 eV is reasonable, although the FC profile of the 3.496 eV spectrum is anomalous with increasing intensity on the higher binding energy side. As will be shown below, the 7-AI− anion has a valence excited state near this photon energy, which may distort the PE spectrum due to autodetachment. Owing to the planar geometry (Cs) of both the 7-AI− anion and the 7-AI neutral radical, only in-plane vibrational modes (A′ symmetry) are symmetry-allowed. The calculated FC factors agree well with the observed vibrational features in the low binding energy region probed at the 2.8458 eV photon energy (Fig. 8). Peaks A, B, C, and D are due to the ν23, ν20, ν19, and ν16 modes (Table S2), respectively, on the basis of the computed harmonic frequencies. The good agreement is expected because the low photon energy at 2.8458 eV only probes the potential energy surface near the equilibrium of the 7-AI ground state. The higher energy part accessed at high photon energies (Fig. S5) is expected to deviate from the FC calculations due to the vibronic coupling of the ground state with the excited states, as observed in similar molecular systems previously.47,69
4.2. Comparison between PDS and non-resonant PES
The photodetachment spectrum of 7-AI− (Fig. 4) exhibits a series of resonances associated with the vibrational levels of the DBS. The weak below-threshold feature at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 cm−1 (2.6774 eV) (peak 0) is the zero-point level of the DBS with a binding energy of 156 ± 5 cm−1. Because the 156 cm−1 DBS binding energy is lower than the lowest vibrational frequency of neutral 7-AI (Fig. S4 and Table S8), any vibrational excitation of the DBS would be above the detachment threshold, which can result in vibrational autodetachment. The DBS origin is observed through R2PD processes, whereas the Feshbach resonances (peaks 1–16) are observed due to single photon excitation, followed by vibrational autodetachment, during which vibrational energy is transferred to the dipole-bound electron. The diffuse dipole-bound electron has negligible perturbation to the geometry of neutral 7-AI, such that the vibrational structures of the DBS mirror those of the neutral final state observed in the PES.39 This similarity is evident in Fig. S6, where the PD spectrum is overlaid on the high resolution PE spectrum at 2.8458 eV by aligning peak 0 (the DBS origin) with the 0–0 transition in the PE spectrum. The vibrational transitions observed in the PE spectrum have clear counterparts in the PD spectrum, allowing those DBS vibrational levels to be readily assigned. In general, much more vibrational features are observed in PDS because of the much higher cross sections in the resonant excitation. Thus, PDS can be viewed as “electron-tagging” vibrational spectroscopy of the neutral radicals via the DBS,49 with the caveat that the observed DBS features are governed by vibrational autodetachment.
595 cm−1 (2.6774 eV) (peak 0) is the zero-point level of the DBS with a binding energy of 156 ± 5 cm−1. Because the 156 cm−1 DBS binding energy is lower than the lowest vibrational frequency of neutral 7-AI (Fig. S4 and Table S8), any vibrational excitation of the DBS would be above the detachment threshold, which can result in vibrational autodetachment. The DBS origin is observed through R2PD processes, whereas the Feshbach resonances (peaks 1–16) are observed due to single photon excitation, followed by vibrational autodetachment, during which vibrational energy is transferred to the dipole-bound electron. The diffuse dipole-bound electron has negligible perturbation to the geometry of neutral 7-AI, such that the vibrational structures of the DBS mirror those of the neutral final state observed in the PES.39 This similarity is evident in Fig. S6, where the PD spectrum is overlaid on the high resolution PE spectrum at 2.8458 eV by aligning peak 0 (the DBS origin) with the 0–0 transition in the PE spectrum. The vibrational transitions observed in the PE spectrum have clear counterparts in the PD spectrum, allowing those DBS vibrational levels to be readily assigned. In general, much more vibrational features are observed in PDS because of the much higher cross sections in the resonant excitation. Thus, PDS can be viewed as “electron-tagging” vibrational spectroscopy of the neutral radicals via the DBS,49 with the caveat that the observed DBS features are governed by vibrational autodetachment.
The comparison between the PES and PDS (Fig. S6) shows that the PES peaks A, B, C, and D align with the PDS peaks 5, 9, 10, and 13, respectively. Following the assignment of the PES peaks from the FC simulation (Fig. 8 and Table S2), the PDS peaks can be immediately assigned to 23′1, 20′1, 19′1, and 16′1,1 respectively (the ′ sign is used to designate DBS levels). Notably, more vibrational resonances are present in the PD spectrum, e.g. the intense peaks 4 and 6, as well as several very weak peaks (i.e., 2, 3, 7, 8, 11, and 12), which do not have counterparts in the PES. These resonances likely involve vibrational modes with weak FC factors or symmetry-forbidden transitions. They become accessible under resonant excitation conditions in PDS, illustrating the sensitivity of PDS and its power to yield richer vibrational information. Assignment of these DBS resonances can be done using rPES on the basis of the Δv = −1 vibrational autodetachment propensity rule,66,67 and the computed vibrational frequencies for neutral 7-AI (Table S8). Not only is the rPES powerful to reveal the nature of the observed DBS vibrational peaks, but it also often uncovers overlapping DBS vibrational levels which give rise to complicated PES features with new vibrational final states not accessible in non-resonant PES.
The FC simulation (Fig. S5) confirms the broad FC profile for the ground state detachment transition. However, the DBS vibrational features appear only in a narrow energy range above threshold and end abruptly at peak 16 (Fig. 4), suggesting other processes may compete with vibrational autodetachment beyond peak 16. We computed the first excited state for the 7-AI− anion at the B3LYP level and found that both S1 and T1 occur around 2.81 eV (Table S9), near peak 16, which has an excitation energy of 2.8381 eV (Table S3). Our computed excitation energy for S1 is consistent with the 2.98 eV excitation energy calculated by Noble et al. at the CAM-B3LYP level.28 The S1 of 7-AI− should be a broad shape resonance. Thus, two competing processes may happen for the Feshbach resonances above peak 16: (1) autodetachment; and (2) relaxation to S1. The autodetachment lifetime is known to be on the order of a few to few tens of picosecond,70 whereas the relaxation to the S1 could occur on much faster time scale,71 which would prevent the Feshbach resonances from being observed. While not explicitly recognized, such a competition between the Feshbach resonances and shape resonance was actually present in the 1-pyrenolate anion reported previously,43 resulting in a short DBS vibrational profile in its PDS, compared to the PES data.
4.3. Resonant photoelectron images and spectra
The rPES images and spectra in Fig. 6 and 7 consist of two distinct electron detachment processes: (1) direct photodetachment from the anion ground state to that of the neutral final states and (2) autodetachment from specific vibrational levels of the DBS. Because of the structural similarity between the DBS of 7-AI− and the neutral 7-AI, autodetachment follows the Δv = –1 propensity rule under the harmonic approximation.66,67 If a vibrational level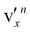 of the DBS is excited, the νn−1x vibrational level of the neutral will be enhanced due to the coupling of one vibrational quantum to the weakly-bound electron during autodetachment. For combinational levels involving multiple vibrational modes, e.g.,
of the DBS is excited, the νn−1x vibrational level of the neutral will be enhanced due to the coupling of one vibrational quantum to the weakly-bound electron during autodetachment. For combinational levels involving multiple vibrational modes, e.g., 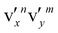 , multiple neutral vibrational levels, i.e, νn−1xνmy or νnxνm−1y may be enhanced. Thus, rPES is highly non-Franck–Condon, because vibrational final states with weak FC factors or even FC-inactive modes can be significantly enhanced. Thus, in addition to providing rich vibrational information for the neutral final state, rPES serves as a powerful tool to assist the assignment of the Feshbach resonances observed in PDS. The normal modes of vibration for neutral 7-AI are shown in Fig. S4. The computed frequencies scaled by a factor of 0.98 are given in Table S8.
, multiple neutral vibrational levels, i.e, νn−1xνmy or νnxνm−1y may be enhanced. Thus, rPES is highly non-Franck–Condon, because vibrational final states with weak FC factors or even FC-inactive modes can be significantly enhanced. Thus, in addition to providing rich vibrational information for the neutral final state, rPES serves as a powerful tool to assist the assignment of the Feshbach resonances observed in PDS. The normal modes of vibration for neutral 7-AI are shown in Fig. S4. The computed frequencies scaled by a factor of 0.98 are given in Table S8.
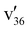 mode couples more strongly with the dipole-bound electron, causing the appearance of peak b (351). Such mode selectivity was observed in the first rPES done on the phenoxide anion33 and was quantitatively investigated using pump–probe experiment.70 The strong DBS peak 4 at an excitation energy of 501 cm−1 (Table S3) corresponds to 33′1, resulting in the enhanced 000 transition (Fig. 6(d)). However, peak a unexpectedly appeared, corresponding to excitation of the lowest frequency mode of the neutral 7-AI final state (361, Table S2). The appearance of this peak could be due to inelastic scattering or threshold effect as a result of vibronic coupling.72,73 The strong resonant peak 6 is from excitation to the 33′136′1 combinational level of the DBS, yielding two new PES peaks a (361) and e (331) (Fig. 6(f)), as expected from Δv = –1 autodetachment processes. The weak peak f (321) right at threshold is likely due to a threshold effect and vibronic coupling. Fig. 6(g) comes from excitation of an overlapping DBS level: 22′1, resulting in the enhanced 000 transition and the 33′135′1 combinational level, giving rise to the weak b (351) and e (331) final states (Fig. 6(g)). The appearance of peak f in Fig. 6(g) is probably due to the same reason as that in Fig. 6(f) because the resonances 6 and 7 are very close to each other.
mode couples more strongly with the dipole-bound electron, causing the appearance of peak b (351). Such mode selectivity was observed in the first rPES done on the phenoxide anion33 and was quantitatively investigated using pump–probe experiment.70 The strong DBS peak 4 at an excitation energy of 501 cm−1 (Table S3) corresponds to 33′1, resulting in the enhanced 000 transition (Fig. 6(d)). However, peak a unexpectedly appeared, corresponding to excitation of the lowest frequency mode of the neutral 7-AI final state (361, Table S2). The appearance of this peak could be due to inelastic scattering or threshold effect as a result of vibronic coupling.72,73 The strong resonant peak 6 is from excitation to the 33′136′1 combinational level of the DBS, yielding two new PES peaks a (361) and e (331) (Fig. 6(f)), as expected from Δv = –1 autodetachment processes. The weak peak f (321) right at threshold is likely due to a threshold effect and vibronic coupling. Fig. 6(g) comes from excitation of an overlapping DBS level: 22′1, resulting in the enhanced 000 transition and the 33′135′1 combinational level, giving rise to the weak b (351) and e (331) final states (Fig. 6(g)). The appearance of peak f in Fig. 6(g) is probably due to the same reason as that in Fig. 6(f) because the resonances 6 and 7 are very close to each other.
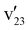 mode is selectively coupled with the dipole-bound electron to induce autodetachment, whereas the coupling of the
mode is selectively coupled with the dipole-bound electron to induce autodetachment, whereas the coupling of the 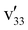 mode is weak. The resonant peak 13 represents the strongest transition in the PDS (Fig. 4), corresponding to the FC-active
mode is weak. The resonant peak 13 represents the strongest transition in the PDS (Fig. 4), corresponding to the FC-active 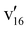 mode (Fig. S5). Thus, we expect a strongly enhanced 0–0 transition in the rPES (Fig. 7(e)). However, three new peaks are observed in the rPES, c (341), f (321), and g (311). The intense peaks c and g indicate that excitation to the 31′134′1 combinational DBS level makes a strong contribution to peak 13. The observation of weak peak f suggests that excitation to the 23′132′1 combinational DBS level also contributes slightly to peak 13.
mode (Fig. S5). Thus, we expect a strongly enhanced 0–0 transition in the rPES (Fig. 7(e)). However, three new peaks are observed in the rPES, c (341), f (321), and g (311). The intense peaks c and g indicate that excitation to the 31′134′1 combinational DBS level makes a strong contribution to peak 13. The observation of weak peak f suggests that excitation to the 23′132′1 combinational DBS level also contributes slightly to peak 13.
The weak resonant peak 14 also produces a remarkable resonant PE spectrum with a hugely enhanced peak A (Fig. 7(f)), suggesting that peak 14 is mainly due to excitation to the v = 2 level of the FC-active 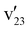 mode in the DBS. The appearance of peak c (341) suggests that the 30′134′1 combinational DBS level also contributes to peak 14. The resonant peak 15 gives rise to a complicated resonant PE spectrum with four new peaks, c (341), e (331), g (311), and i (332) (Fig. 7(g) and Table S2). These new rPES features come from autodetachment from three overlapping vibrational levels contained in peak 15: 29′134′1/31′133′1/33′236′1, which all have excitation energies around 1200 cm−1 above the DBS zero-point level (Table S3). Finally, a simple resonant PE spectrum (Fig. 7(h)) is observed at the resonant peak 16 with a strongly enhanced 000 transition due to excitation of the 12′1 DBS level. The strong peak h (252) has to come from an overlapping 25′3 DBS level, which has an excitation energy nearly identical as that of 12′1 (Table S3). The relatively weak peak j (171) in Fig. 7(h) suggests that excitation of the 17′136′1 DBS level also contributes to the resonant peak 16.
mode in the DBS. The appearance of peak c (341) suggests that the 30′134′1 combinational DBS level also contributes to peak 14. The resonant peak 15 gives rise to a complicated resonant PE spectrum with four new peaks, c (341), e (331), g (311), and i (332) (Fig. 7(g) and Table S2). These new rPES features come from autodetachment from three overlapping vibrational levels contained in peak 15: 29′134′1/31′133′1/33′236′1, which all have excitation energies around 1200 cm−1 above the DBS zero-point level (Table S3). Finally, a simple resonant PE spectrum (Fig. 7(h)) is observed at the resonant peak 16 with a strongly enhanced 000 transition due to excitation of the 12′1 DBS level. The strong peak h (252) has to come from an overlapping 25′3 DBS level, which has an excitation energy nearly identical as that of 12′1 (Table S3). The relatively weak peak j (171) in Fig. 7(h) suggests that excitation of the 17′136′1 DBS level also contributes to the resonant peak 16.
4.4. Vibrational information obtained for the 7-AI radical
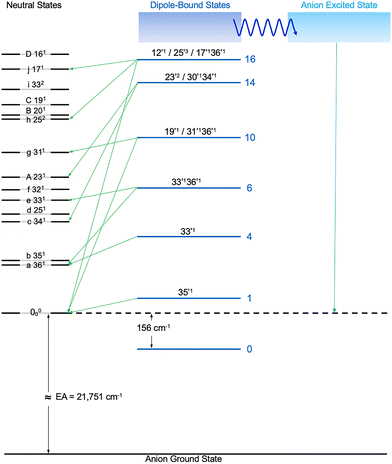
Fig. 9 displays the vibrational levels observed for neutral 7-AI and autodetachment from selected DBS vibrational levels. Note that many more vibrational features (a–j) come from rPES. The relaxation from the DBS to the anion excited state (shape resonance) at higher excitation energies is also schematically shown, explaining why the DBS resonances end abruptly above peak 16 in the PDS (Fig. 4).The rich vibrational information obtained from the resonant PE spectra shown in Fig. 6 and 7 illustrates the potential of rPES, in comparison with conventional PES. Not only can vibrational transitions with weak FC factors be significantly enhanced, even symmetry-forbidden transitions or transitions with negligible FC factors can be observed in rPES. Furthermore, PDS provides additional and complementary vibrational information about the neutral final states, because the dipole-bound electron has negligible influence on the neutral geometry. In fact, PDS often yields more accurate vibrational information, because the resolution of PEI depends on the kinetic energies of the photoelectrons. For the 7-AI radical, the high resolution PES in Fig. 3 only reveal four vibrational peaks, A, B, C, D, corresponding to the FC-active ν23, ν20, ν19, and ν16 modes of 7-AI, respectively. However, the rPES data yield 10 additional vibrational peaks, a to j (Table S2), most of which are symmetry-forbidden bending modes. Combining the PES, rPES, and PDS data, we obtain 16 experimental vibrational frequencies for the 7-AI radical, as given in Table 1, along with the calculated frequencies. We found that the computed frequencies at the B3LYP level are in good agreement with the experimental data with a scaling factor of 0.98.74
| Vibrational mode | Symmetry | Experimental frequencya (cm−1) | Theoretical frequencyb (cm−1) |
|---|---|---|---|
| a The numbers in parentheses denote the uncertainty associated with the last digit. b The calculated shift is scaled by a factor of 0.98. | |||
| ν12 | A′ | 1296(5) | 1304 |
| ν16 | 1155(5) | 1156 | |
| ν17 | 1092(6) | 1089 | |
| ν19 | 944(5) | 947 | |
| ν20 | 880(5) | 878 | |
| ν21 | 839(5) | 836 | |
| ν22 | 748(5) | 751 | |
| ν23 | 593(5) | 600 | |
| ν25 | 423(5) | 435 | |
| ν26 | A′′ | 1000(6) | 975 |
| ν31 | 717(8) | 728 | |
| ν32 | 550(6) | 561 | |
| ν33 | 501(5) | 502 | |
| ν34 | 407(5) | 413 | |
| ν35 | 221(5) | 232 | |
| ν36 | 210(9) | 219 | |
5. Conclusion
In conclusion, we report an investigation of the cryogenically-cooled 7-azaindolide anion (7-AI−) using high-resolution photoelectron imaging in combination with photodetachment spectroscopy and resonant photoelectron spectroscopy. The electron affinity of the 7-AI radical is measured accurately to be 2.6967(8) eV (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 ± 6 cm−1). Two low-lying electronic excited states of the neutral radical are identified at ∼0.8 eV and ∼1.4 eV above the ground state with evidence of strong vibronic coupling. Two minor isomers of the 7-AI− anions are also observed with lower adiabatic detachment energies measured at 2.200(3) eV and 1.918(5) eV, corresponding to deprotonation at the α- and β-carbon of 7-AI−, respectively. A dipole-bound state is observed at 156 cm−1 below the detachment threshold of 7-AI−, along with 16 vibrational Feshbach resonances. The resonant two-photon photoelectron spectrum obtained via the zero-point level reveals a relatively long-lived DBS, while relaxation to the ground state of the anion is also observed within the 5 ns duration of the detachment laser. Resonant photoelectron spectroscopy was conducted via the 16 vibrational levels of the DBS, revealing rich spectral features not accessible in conventional PES. The resonant PES was further used to confirm the assignment of the Feshbach resonances based on the Δν = −1 autodetachment propensity rule. The combination of PES, PDS, and rPES establishes vibrational frequencies for sixteen fundamental modes of the 7-AI radical. The electronic and vibrational spectroscopic information provides new benchmarks for further theoretical studies of this important radical species.
751 ± 6 cm−1). Two low-lying electronic excited states of the neutral radical are identified at ∼0.8 eV and ∼1.4 eV above the ground state with evidence of strong vibronic coupling. Two minor isomers of the 7-AI− anions are also observed with lower adiabatic detachment energies measured at 2.200(3) eV and 1.918(5) eV, corresponding to deprotonation at the α- and β-carbon of 7-AI−, respectively. A dipole-bound state is observed at 156 cm−1 below the detachment threshold of 7-AI−, along with 16 vibrational Feshbach resonances. The resonant two-photon photoelectron spectrum obtained via the zero-point level reveals a relatively long-lived DBS, while relaxation to the ground state of the anion is also observed within the 5 ns duration of the detachment laser. Resonant photoelectron spectroscopy was conducted via the 16 vibrational levels of the DBS, revealing rich spectral features not accessible in conventional PES. The resonant PES was further used to confirm the assignment of the Feshbach resonances based on the Δν = −1 autodetachment propensity rule. The combination of PES, PDS, and rPES establishes vibrational frequencies for sixteen fundamental modes of the 7-AI radical. The electronic and vibrational spectroscopic information provides new benchmarks for further theoretical studies of this important radical species.
Author contributions
J. K. did the experiment, analyzed the data and wrote the first draft of the manuscript. E. I. B. assisted with the experiment. L. S. W. guided and advised the project and revised and finalized the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data that supports the findings of this study is available from the corresponding author upon request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp04123f.
Acknowledgements
We would like to thank Dr Yue-Rou Zhang and Dr Dao-Fu Yuan for their invaluable experimental expertise. This work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division under Grant No. DE-SC0018679. The calculations were conducted using computational resources and services at the Center for Computation and Visualization (CCV), Brown University.References
- Y. Chen, R. L. Rich, F. Gai and J. W. Petrich, J. Phys. Chem., 1993, 97, 1770–1778 CrossRef.
- Y. Huang, S. Arnold and M. Sulkes, J. Phys. Chem., 1996, 100, 4734–4738 CrossRef.
- A. V. Smirnov, D. S. English, R. L. Rich, J. Lane, L. Teyton, A. W. Schwabacher, S. Luo, R. W. Thornburg and J. W. Petrich, J. Phys. Chem. B, 1997, 101, 2758–2769 CrossRef.
- A. Nakajima, M. Hirano, R. Hasumi, K. Kaya, H. Watanabe, C. C. Carter, J. M. Williamson and T. A. Miller, J. Phys. Chem. A, 1997, 101, 392–398 CrossRef.
- A. Nakajima, Y. Negishi, R. Hasumi and K. Kaya, Eur. Phys. J. D, 1999, 9, 303–307 CrossRef.
- L. Serrano-Andres, M. Merchan, A. C. Borin and J. Stalring, Int. J. Quant. Chem., 2001, 84, 181–191 CrossRef.
- C. Kang, J. T. Yi and D. W. Pratt, J. Chem. Phys., 2005, 123, 094306 CrossRef PubMed.
- Y. Koizumi, C. Jouvet, T. Norihiro, S. Ishiuchi, C. Dedonder-Lardeux and M. Fujii, J. Chem. Phys., 2008, 129, 104311 CrossRef.
- K. Sakota, N. Komure, W. Ishikawa and H. Sekiya, J. Chem. Phys., 2009, 130, 224307 CrossRef.
- M. Mukherjee, B. Bandyopadhyay and T. Chakraborty, Chem. Phys. Lett., 2012, 546, 74–79 CrossRef.
- B. Marchetti, T. N. V. Karsili, M. N. R. Ashfold and W. Domcke, Phys. Chem. Chem. Phys., 2016, 18, 20007–20027 RSC.
- I. Lamas, R. Montero, V. Martínez-Martínez, A. Longarte and L. Blancafort, Phys. Chem. Chem. Phys., 2020, 22, 18639–18645 RSC.
- D. P. Chong, Molecules, 2021, 26, 1947 CrossRef PubMed.
- I. Lamas, R. Montero, V. Martínez-Martínez and A. Longarte, Phys. Chem. Chem. Phys., 2024, 26, 3240–3252 RSC.
- C. A. Taylor, M. A. El-Bayoumi and M. Kasha, Proc. Natl. Acad. Sci. U. S. A., 1969, 63, 253–260 CrossRef.
- A. Douhal, S. K. Kim and A. H. Zewail, Nature, 1995, 378, 260–263 CrossRef PubMed.
- P. T. Chou, W. S. Yu, Y. C. Chen, C. Y. Wei and S. S. Martinez, J. Am. Chem. Soc., 1998, 120, 12927–12934 CrossRef.
- M. Chachisvilis, T. Fiebig, A. Douhal and A. H. Zewail, J. Phys. Chem. A, 1998, 102, 669–673 CrossRef.
- D. E. Folmer, E. S. Wisniewski, S. M. Hurley and A. W. Castleman, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 12980–12986 CrossRef.
- T. Fiebig, M. Chachisvilis, M. Manger, A. H. Zewail, A. Douhal, I. Garcia-Ochoa and A. de La Hoz Ayuso, J. Phys. Chem. A, 1999, 103, 7419–7431 CrossRef.
- H. Y. Chen and I. Chao, ChemPhysChem, 2004, 5, 1855–1863 CrossRef.
- J. Catalan, P. Perez, J. C. del Valle, J. L. G. de Paz and M. Kasha, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 419–422 CrossRef PubMed.
- O. H. Kwon and A. H. Zewail, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8703–8708 CrossRef PubMed.
- H. Sekiya and K. Sakota, J. Photochem. Photobiol., C, 2008, 9, 81–91 CrossRef.
- K. Fuke and H. Ishikawa, Chem. Phys. Lett., 2015, 623, 117–129 CrossRef.
- W. C. Lineberger, Ann. Rev. Phys. Chem., 2013, 64, 21–36 CrossRef.
- A. M. de Oliveira, PhD thesis, University of Colorado, 2018.
- J. A. Noble, E. Marceca, C. Dedonder and C. Jouvet, Phys. Chem. Chem. Phys., 2020, 22, 27290–27299 RSC.
- C. Hock, J. B. Kim, M. L. Weichman, T. I. Yacovitch and D. M. Neumark, J. Chem. Phys., 2012, 137, 244201 CrossRef PubMed.
- I. Leon, Z. Yang and L. S. Wang, J. Chem. Phys., 2013, 138, 184304 CrossRef.
- M. L. Weichman and D. M. Neumark, Annu. Rev. Phys. Chem., 2018, 69, 101–124 CrossRef PubMed.
- D. L. Huang, P. D. Dau, H. T. Liu and L. S. Wang, J. Chem. Phys., 2014, 140, 224315 CrossRef PubMed.
- H. T. Liu, C. G. Ning, D. L. Huang, P. D. Dau and L. S. Wang, Angew. Chem., Int. Ed., 2013, 52, 8976–8979 CrossRef PubMed.
- H. T. Liu, C. G. Ning, D. L. Huang and L. S. Wang, Angew. Chem., Int. Ed., 2014, 53, 2464–2468 CrossRef PubMed.
- G. Z. Zhu, C. H. Qian and L. S. Wang, J. Chem. Phys., 2018, 149, 164301 CrossRef PubMed.
- D. L. Huang, H. T. Liu, C. G. Ning and L. S. Wang, J. Chem. Phys., 2015, 142, 124309 CrossRef.
- D. L. Huang, C. G. Ning, H. T. Liu and L. S. Wang, J. Phys. Chem. Lett., 2015, 6, 2153–2157 CrossRef.
- G. Z. Zhu, D. H. Huang and L. S. Wang, J. Chem. Phys., 2017, 147, 013910 CrossRef.
- G. Z. Zhu and L. S. Wang, Chem. Sci., 2019, 10, 9409–9423 RSC.
- C. H. Qian, G. Z. Zhu, Y. R. Zhang and L. S. Wang, J. Chem. Phys., 2020, 152, 214307 CrossRef PubMed.
- D. F. Yuan, Y. Liu, C. H. Qian, Y. R. Zhang, B. M. Rubenstein and L. S. Wang, Phys. Rev. Lett., 2020, 125, 073003 CrossRef PubMed.
- D. F. Yuan, Y. Liu, C. H. Qian, G. S. Kocheril, Y. R. Zhang, B. M. Rubenstein and L. S. Wang, J. Phys. Chem. Lett., 2020, 11, 7914–7919 CrossRef PubMed.
- C. H. Qian, Y. R. Zhang, D. F. Yuan and L. S. Wang, J. Chem. Phys., 2021, 154, 094308 CrossRef PubMed.
- D. F. Yuan, Y. R. Zhang, C. H. Qian, Y. Liu and L. S. Wang, J. Phys. Chem. A, 2021, 125, 2967–2976 CrossRef PubMed.
- Y. R. Zhang, D. F. Yuan and L. S. Wang, J. Am. Chem. Soc., 2022, 144, 16620–16630 CrossRef PubMed.
- Y. R. Zhang, D. F. Yuan and L. S. Wang, J. Phys. Chem. Lett., 2022, 13, 11481–11488 CrossRef PubMed.
- D. F. Yuan, Y. Liu, Y. R. Zhang and L. S. Wang, J. Am. Chem. Soc., 2023, 145, 5512–5522 CrossRef PubMed.
- Y. R. Zhang, D. F. Yuan, C. H. Qian, G. Z. Zhu and L. S. Wang, J. Am. Chem. Soc., 2023, 145, 14952–14962 CrossRef PubMed.
- Y. R. Zhang, D. F. Yuan and L. S. Wang, J. Phys. Chem. Lett., 2023, 14, 7368–7381 CrossRef PubMed.
- J. Kang, E. I. Brewer, D. F. Yuan, Y. R. Zhang and L. S. Wang, J. Phys. Chem. A, 2025, 129, 1060–1067 CrossRef PubMed.
- J. Kang and L. S. Wang, J. Phys. Chem. A, 2025, 129, 8828–8836 CrossRef PubMed.
- G. Z. Zhu, L. F. Cheung, Y. Liu, C. H. Qian and L. S. Wang, J. Phys. Chem. Lett., 2019, 10, 4339–4344 CrossRef PubMed.
- Y. R. Zhang, D. F. Yuan, C. H. Qian and L. S. Wang, J. Chem. Phys., 2021, 155, 124305 CrossRef PubMed.
- D. F. Yuan, Y. R. Zhang, C. H. Qian and L. S. Wang, Phys. Chem. Chem. Phys., 2022, 24, 1380–1389 RSC.
- J. Kang, E. I. Brewer, Y. R. Zhang, D. F. Yuan, G. S. Kocheril and L. S. Wang, J. Chem. Phys., 2024, 160, 184301 CrossRef PubMed.
- J. Kang, E. I. Brewer, Y. R. Zhang, D. F. Yuan, S. C. Tian, W. Roberts and L. S. Wang, J. Chem. Phys., 2025, 162, 194302 CrossRef CAS PubMed.
- L. S. Wang, J. Chem. Phys., 2015, 143, 040901 CrossRef.
- L. S. Wang, C. F. Ding, X. B. Wang and S. E. Barlow, Rev. Sci. Instrum., 1999, 70, 1957–1966 CrossRef CAS.
- X. B. Wang and L. S. Wang, Rev. Sci. Instrum., 2008, 79, 073108 CrossRef PubMed.
- I. Leon, Z. Yang, H. T. Liu and L. S. Wang, Rev. Sci. Instrum., 2014, 85, 083106 CrossRef PubMed.
- B. Dick, Phys. Chem. Chem. Phys., 2019, 21, 19499–19512 RSC.
- J. Cooper and R. N. Zare, J. Chem. Phys., 1968, 48, 942–943 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- I. Pugliesi and K. Müller-Dethlefs, J. Phys. Chem. A, 2006, 110, 4657–4667 CrossRef CAS PubMed.
- C. H. Qian, G. Z. Zhu and L. S. Wang, J. Phys. Chem. Lett., 2019, 10, 6472–6477 CrossRef CAS.
- R. S. Berry, J. Chem. Phys., 1966, 45, 1228–1245 CrossRef CAS.
- J. Simons, J. Am. Chem. Soc., 1981, 103, 3971–3976 CrossRef CAS.
- E. P. Wigner, Phys. Rev., 1948, 73, 1002–1009 CrossRef CAS.
- Y. R. Zhang, D. F. Yuan and L. S. Wang, Phys. Chem. Chem. Phys., 2022, 24, 6505–6514 RSC.
- D. H. Kang, S. An and S. K. Kim, Phys. Rev. Lett., 2020, 125, 093001 CrossRef CAS.
- C. J. Clarke and J. R. R. Verlet, Annu. Rev. Phys. Chem., 2024, 75, 89–110 CrossRef CAS PubMed.
- D. L. Huang, H. T. Liu, C. G. Ning, P. D. Dau and L. S. Wang, Chem. Phys., 2017, 482, 374–383 CrossRef CAS.
- H. W. Gao, J. Hui and L. S. Wang, J. Phys. Chem. Lett., 2025, 16, 2039–2046 CrossRef CAS.
- P. Sinha, S. E. Boesch, C. Gu, R. A. Wheeler and A. K. Wilson, J. Phys. Chem. A, 2004, 108, 9213–9217 CrossRef CAS.
| This journal is © the Owner Societies 2025 |