Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Revealing SO2 and CO2 adsorption features on forsterite via IR spectroscopy and automated computational approaches†
Eric
Mates-Torres‡
 *a,
Guillermo
Escolano Casado‡§
*a,
Guillermo
Escolano Casado‡§
 b,
Lorenzo
Mino
b,
Lorenzo
Mino
 *b,
Nadia
Balucani
*b,
Nadia
Balucani
 c,
Piero
Ugliengo
c,
Piero
Ugliengo
 b and
Albert
Rimola
b and
Albert
Rimola
 *a
*a
aDepartament de Química, Universitat Autònoma de Barcelona, 08193 Bellaterra, Catalonia, Spain. E-mail: eric.mates@uab.cat; albert.rimola@uab.cat
bDipartimento di Chimica and Nanomaterials for Industry and Sustainability (NIS) Centre, Università degli Studi di Torino, via P. Giuria 7, I-10125, Torino, Italy. E-mail: lorenzo.mino@unito.it
cDipartimento di Chimica, Biologia e Biotecnologie, Università degli Studi di Perugia, Via Elce di Sotto 8, I-06123, Perugia, Italy
First published on 3rd June 2025
Abstract
The interaction between interstellar molecules and silicate dust plays a critical role in the chemical evolution of interstellar and circumstellar environments. In this work, we combine in situ infrared (IR) spectroscopy with automated density functional theory (DFT) calculations to investigate the adsorption and vibrational signatures of CO2 and SO2 on forsterite surfaces. Experimental IR spectra collected under cryogenic conditions reveal coverage- and temperature-dependent features that evolve from physisorbed to chemisorbed regimes. To interpret these observations, we construct theoretical spectra from a large ensemble of adsorption configurations across multiple surface terminations, weighted by their Boltzmann distributions at 100 K and by a per-surface abundance factor. The resulting spectra reproduce key experimental features, enabling the identification of binding trends. For CO2, we predict the transition from weakly bound species to carbonate-like modes at lower frequencies. For SO2, our simulations identify the dominant bands due to bidentate and tridentate chemisorption. This integrative approach highlights the importance of surface morphology and thermodynamic weighting in reconciling theory and experiments providing a framework for the spectroscopic analysis of molecular adsorption on interstellar dust analogs.
Introduction
Silicates are ubiquitous in cosmic and early-Earth environments. This family of minerals, particularly forsterite (Mg2SiO4) and enstatite (MgSiO3), have been observed in protoplanetary disks, around young stellar objects (YSOs) and conforming dust shells around evolved stars, both in their crystalline and amorphous forms.1–3 While dust grains grown in the interstellar medium (ISM) are expected to contain purely amorphous silicates,4 the upper limit for the fraction of crystalline silicate grains injected into the ISM is up to 20%,1 yielding a total fraction of crystalline to amorphous mineral of 10% in active starburst regions.5 Silicates are also abundant in chondritic meteorite samples recovered on Earth,6,7 in interplanetary dust particles (IDPs) collected in the stratosphere8,9 and in the nuclei of the 81P/Wild (trapped within the aerogel of the Stardust mission)10 and 67P/CG comets.11 Thus, assessing the interactions between common interstellar molecules and forsterite is key to understand the primary steps of the ice growth on interstellar dust particles, to rationalize molecular abundance in diffuse and molecular clouds, and to decipher the formation of interstellar compounds, including those categorized as complex organic molecules (iCOMs). For instance, quantum chemical computations based on a cluster approach to model forsterite grain particles have shown that simple S-bearing species strongly bind on the silicate surfaces, potentially accounting for the missing sulfur in the gas phase in observational spectra.12 Calculations on crystalline forsterite surfaces have also elucidated how H2 and H2O may form from the adsorption, diffusion and reaction of two H atoms and H addition to O, respectively.13,14 Combined theoretical and synthetic laboratory experiments showcasing the reactivity of HCN and H2CO on forsterite surfaces have also shown promising results to rationalize the catalytic potential of this mineral for the formation of nucleobases and sugars, respectively.15–18To realistically model the interactions between silicates and key interstellar molecules in a way that can be extrapolated to astronomical observations, quantum chemical calculations and spectroscopic laboratory measurements must work in tandem. In this regard, recent work by some of us has been devoted to understanding the true nature of the interactions between forsterite and HCN by fitting calculated infrared (IR) spectra to that obtained in low-temperature laboratory experiments, revealing a mainly dissociative mechanism undergoing on the forsterite interface, catalyzed by the Lewis acid–base behavior of surface O2− and Mg2+ sites.19 Similarly, calculations have shown that the spectroscopic features of the molecular species upon interaction with forsterite are heavily influenced by its complex surface morphology.20 Thus, modeling atomistically a sufficiently large pool of adsorption structures in different coordination environments on the silicate surfaces is key to obtain an accurate representation of the potential vibrational modes in the laboratory and, ultimately, to unravel the true nature of the interaction between molecules and these mineral surfaces in the interstellar and circumstellar environments.
Among the most spectroscopically relevant astrochemical molecules, CO2 stands out due to its observed abundance in interstellar ices, ranging from 10 to 23% relative to H2O in dense molecular clouds and forming segregated CO2-rich ice phases upon thermal processing of the grain.21 Given the affinity of CO2 towards forsterite surfaces,22 the adsorption of CO2 and incorporation into the mineral parent body of interstellar dust particles as carbonates has been postulated to account for the anomalous oxygen depletion in the denser ISM.23,24 Furthermore, the interaction of CO2 and silicate-bearing meteoritic and volcanic particles has shown to be essential for the formation of key precursors in the abiotic synthesis of relevant biomolecules on early-Earth environments.25
In a similar context, SO2 is a key sulfur-bearing molecule in the ISM. Jointly with OCS, they are both major carriers of gaseous sulfur and are the only sulfurated molecules detected in interstellar ices to date.26,27 SO2 adsorption and subsequent incorporation into silicate samples is of most interest given the long-standing problem of sulfur depletion in the gas phase.12,28 Previous analyses on the adsorption of SO2 on MgO surfaces have shown an efficient activation of SO2 onto terminal lattice O2− sites,29,30 hinting at the potential role of acid and basic sites of forsterite in similar mechanisms.
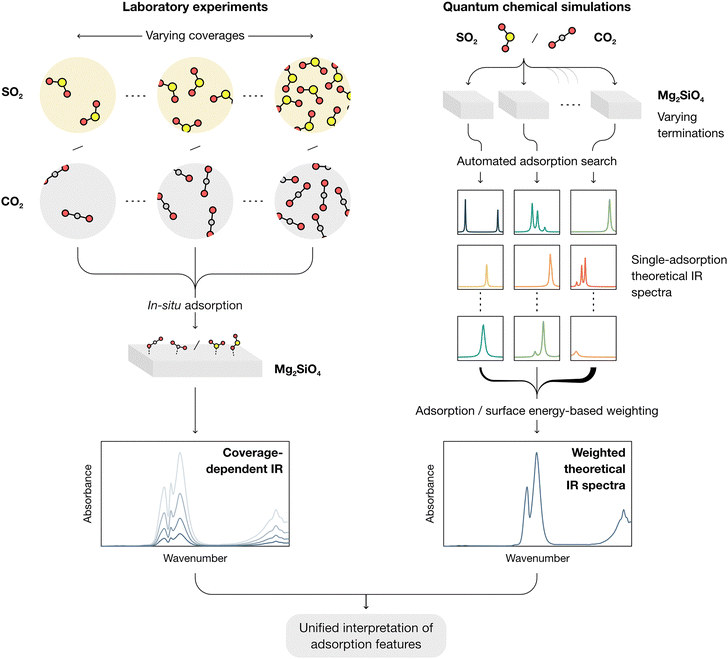
In this work, a combined framework for a comprehensive study of the interactions of CO2 and SO2 with forsterite surfaces (here used as a test case for cosmic silicates) is showcased, as schematized in Fig. 1. From the experimental side, molecular adsorption is studied by in situ IR spectroscopy at progressively declining coverages both on amorphous (AMS) and crystalline (CMS) forsterite at 100 K, akin to the conditions found in collapsing dense molecular clouds and cometary grains. From the modelling side, an automated computational approach is followed to obtain all potential adsorption structures of both molecules onto all theorized forsterite terminations, aimed at representing the local chemical environments found both in crystalline and amorphous surfaces. This approach simplifies the treatment of the amorphous forsterite, whose atomistic structure is unknown and would require modelling large and amorphized unit cell as recently studied by some of us.31 A termination-factored adsorption energy-based Boltzmann distribution is then utilized to obtain total weighed spectra of the interaction of SO2 and CO2 and forsterite (ensuring that the final theoretical IR spectra reflect the complexity of dust grain interfaces under astrophysical conditions), which are compared with the experimental measurements.
Methods
Experimental details
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K) and following quenching.32 Its specific surface area was 37 m2 g−1.
000 K) and following quenching.32 Its specific surface area was 37 m2 g−1.
Crystalline magnesium silicate (Mg2SiO4, CMS) was prepared from AMS by heating it at 1073 K for 24 h in air. Its specific surface area was 26 m2 g−1.
The spectrum of the sample before the adsorbate admission was subtracted to the whole batch of subsequent spectra. The spectra were also normalized by the optical density (weight of the of pellet in mg/area of the pellet in cm2) to normalize for the differences in the thickness of the pellets.
Computational details
Surface models of forsterite were built using PolyCleaver,37 an in-house algorithm that generates charge-neutral and non-polar slabs from crystalline bulks of ionic compounds with polyatomic anions. Hence, our base systems were modelled as slabs with thicknesses of ca. 15 Å (value above which surface energy became invariable) and containing the most energetically favorable Miller indices predicted for forsterite,17,37,38i.e., (001), (010), (021), (101), (110), (111) and (120) (note that indices in the frequently used Pbnm space group can be converted to the standard Pnma space group by exchanging the last two indices). A vacuum of at least 15 Å was included in the c axis to avoid spurious interactions within the direction perpendicular to the plane. Atomic positions were optimized by considering a free-standing slab model, where all atoms were allowed to relax while keeping the unit cell parameters fixed at the bulk values. Surface energies, as well as the fraction of each termination in the equilibrium shape of forsterite using a Wulff construction method, were taken from previous work;37 these values are reported in Table S1 (ESI†).
The adsorption of CO2 and SO2 was investigated on the unit cell of the modelled slabs of forsterite on supercells with p(1 × 2) or p(2 × 1) multiplicity to ensure all slabs were close in size for consistency and avoid spurious interactions of the adsorbates with neighboring images. Then, molecule–surface interaction was assessed using a procedural approach: first, for each predicted adsorption site point, the molecule was positioned on the xy coordinates of the point and 3 Å away from the surface along the z coordinate. Then, the molecule was rotated and approached to the surface through a given anchor point until contact (defined as overlapping of the covalent radii of the atomic species of the molecule and of the surface, at which point was then separated by 0.5 Å to remove any steric hindrance and allow any diffusion to a neighboring stable position). This was carried out for one anchor point per molecule (O in both CO2 and SO2). The initial guess geometry of the approached molecule on the slab was then optimized using the semiempirical GFN1-xTB tight binding method implemented for periodic systems in the tblite package,40 keeping the atoms of the slab fixed to limit the drawbacks of the GFN1-xTB method with highly ionic surfaces. The Broyden–Fletcher–Goldfarb–Shanno (BFGS) algorithm implemented in the atomic simulation environment package (ASE)41 was used for structural optimization with a convergence criterion of 0.001 eV Å−1. This method allows us to rapidly screen how the molecules interact with the surface at each adsorption site and potentially migrate to a neighboring site, yielding geometries akin to those obtainable using DFT. Nonetheless, an initial assessment of the method revealed that GFN1-xTB often encountered structural global minima corresponding to bidentately chemisorbed molecules which are then found to be higher in energy than their respective monodentate counterparts using DFT. To ensure that our initial screening did not fall in spurious energy pitfalls induced by this method, an additional per-atom constraint was introduced where an external Hookean-type repulsive force of 15 eV Å−2 was applied between surface and adsorbate atoms within a distance cut-off of 2.4 Å. To account for structures converging to the same highly stable adsorption site, final geometries were then passed through a filter to remove similar structures within a root-mean-square deviation (RMSD) threshold of 2 Å. After careful inspection of the final optimized structures, this automated pipeline allows us to obtain geometrically accurate initial candidates with minimal user intervention, upon which high-precision optimizations at the DFT level of theory will be subsequently applied, as detailed below.
Finally, the positions of all atoms in the filtered geometries were optimized at the PBE level using the residual minimization method with direct inversion in the iterative subspace (RMM-DIIS) algorithm42 to ensure geometrical accuracy and replicability. Long-range interactions were considered by using Grimme's D2 dispersion model43 for all atoms, including the modified parameters for Mg2+ outlined in the revised D*(N) model to take into account its highly ionic character.44 At variance with surface relaxation, optimizations of the molecule adsorptions were performed at Γ-point only; this method yielded sufficiently accurate geometries while limiting the computational cost, given the large amount of molecular adsorptions modelled. To prevent spurious molecular dissociation of some SO2 molecules observed in preliminary DFT calculations (where the GFN1-xTB method yielded adsorbates too close to the surface), the z distance of the adsorbate atoms in the filtered geometries that showed that behavior with respect to the surface was increased by 1.5 to 2.5 Å prior to relaxation with DFT. In these latter cases, a blocked-Davidson iteration scheme was used for optimization.
Adsorption energies (ΔEB) of the optimized molecule–surface systems were calculated as:
| ΔEB = Ei − Emslab − Emol |
where fm is the surface factor for the surface with Miller index m, Ii and
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) i are the unweighted intensity and the wavenumber (in cm−1) of the theoretical IR peak of adsorption mode i, respectively, Γ is the Lorentzian broadening parameter (which was conservatively set to 15 cm−1), and wi is the weighted Boltzmann factor, defined as:
i are the unweighted intensity and the wavenumber (in cm−1) of the theoretical IR peak of adsorption mode i, respectively, Γ is the Lorentzian broadening parameter (which was conservatively set to 15 cm−1), and wi is the weighted Boltzmann factor, defined as:where Emin,m is the energy of the most stable adsorption on each surface termination with Miller index m and kB is the Boltzmann constant. The temperature T is set to the experimental value of 100 K. Final spectra are normalized.
Results and discussion
IR spectroscopy
Fig. 2 reports the experimental IR spectra of CO2 adsorption on the dehydrated Mg-silicates. In both samples, in the initial stages of the adsorption at low temperature (blue spectra), the most intense peak is centered at 2345 cm−1 and can be assigned to weakly adsorbed CO2.49,50 During the temperature increase (see experimental section), we observe the progressive desorption of the physisorbed CO2 and the parallel growth of new bands in the 1700–1200 cm−1 spectral region, which are due to the formation of surface carbonates.49,50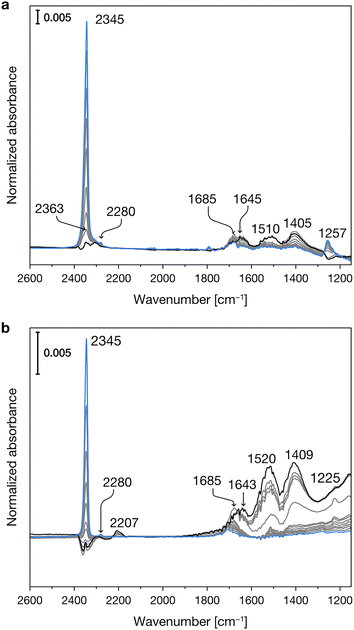 | ||
| Fig. 2 Experimental IR spectra of CO2 adsorbed at progressively increasing temperatures (from blue to black spectra) on crystalline (a) and amorphous (b) Mg-silicates previously outgassed at 673 K. | ||
Fig. 3 shows the experimental IR spectra of SO2 adsorption on the dehydrated Mg-silicates. For both amorphous and crystalline materials, the main band is centered at ca. 1340 cm−1 and can be ascribed to the asymmetric stretching mode of adsorbed SO2. The slightly higher wavenumber observed for the amorphous sample (1341 vs. 1338 cm−1) can be related to its higher surface basicity.51 Around 1150 cm−1, we can note the presence of the corresponding symmetric stretching mode. Finally, we can observe signals between 1375 and 1360 cm−1, which have been attributed to the coexistence of SO2 in both the liquid-like and gas phases.52 The different intensities of these signals in the crystalline and amorphous Mg-silicates are likely due to their differing porosity.
 | ||
| Fig. 3 Experimental IR spectra of SO2 adsorbed at progressively increasing temperatures (from blue to black spectra) on crystalline (a) and amorphous (b) Mg-silicates previously outgassed at 673 K. | ||
Computational results
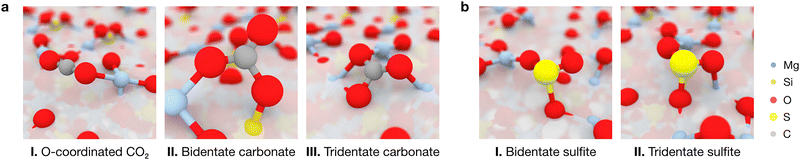
To obtain a complete picture of the binding modes of CO2 and SO2 on the forsterite terminations, we assessed the interactions of both molecules with the seven characteristic forsterite terminations present in the crystalline shape of the crystal at the PBE-D*(N) level (see Methods section). Fig. 4 shows all studied adsorption configurations based on their computed adsorption energy, arranged from left to right by increasing surface energy of their corresponding forsterite termination; the position of the corresponding optimized adsorbates on the surfaces is displayed in Fig. S1 (ESI†). Generally, this analysis revealed that the surface energy of the bare surface is inversely correlated with their binding potential: more stable terminations—those with lower surface energies, such as the (010) or the (120) surfaces—have a lower adsorption energy towards both CO2 (middle panel) and SO2 (lower panel) than high surface energy terminations such as (110) or (021), whose adsorbates possess more favorable adsorption energy values. This is, in part, due to the nature of both CO2 and SO2, and the surface atoms of every surface; CO2 has been shown to spontaneously activate on the surface basic O2− sites of forsterite,22 effectively forming carbonate units with surface O ions. In our analysis, this predominantly occurs on higher surface energy terminations, accounting for the low adsorption energy structures presented in the middle panel of Fig. 4. This results in two main energy differentiated regimes for CO2 adsorption: it either adsorbs through a terminal O atom on acidic Mg2+ sites with adsorption energies of up to −100 kcal mol−1, or it activates on the surface upon interaction of its C and O atoms with an exposed O2− ion and either one or two Mg2+ sites (depending on the steric availability), forming bidentate or tridentate bound carbonates with adsorption energies of −100 to −200 kcal mol−1 (Fig. 5a). The former configuration conforms the largest population of adsorbates on all surfaces, as shown in the kernel density distribution (see Fig. 4).In contrast to the non-polar CO2, the polar nature of SO2 as a bent geometry facilitates its interaction with surface O2− sites which, sterically, are unavailable for CO2 activation. Hence, SO2 adsorbates on the studied forsterite terminations display strikingly more favorable adsorption energies, up to −340 kcal mol−1; more importantly, we see that the energetic distribution of the adsorbates is spread out across all adsorption energy values, yielding a broader kernel density distribution. This is partly due to SO2 interacting similarly in all simulations, i.e., S interacts with a surface O2− site and one or both of the O atoms of SO2 bind to surface Mg2+ sites, forming bidentate or tridentate bound sulfites, analogous to its behavior on stepped MgO surfaces29 (Fig. 5b).
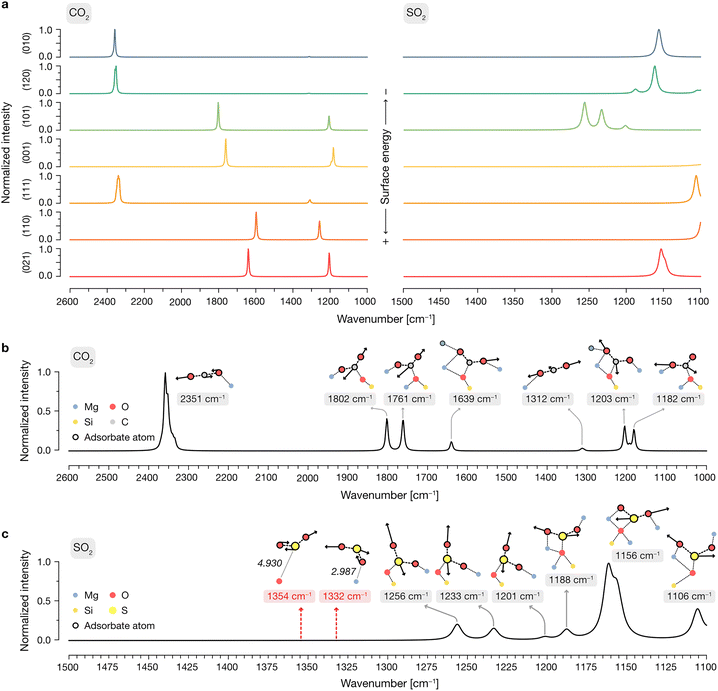
To elucidate the vibrational modes governing the spectroscopic features observed experimentally, we then generated per-surface IR spectra including all adsorptions of SO2 and CO2, as displayed in Fig. 6a. In these spectra, a weight was applied to the calculated IR intensities of each configuration following a Boltzmann distribution based on their relative adsorption energies, as detailed in the Methods section. This procedure is aimed at representing a low-coverage situation in which the contribution of each adsorbate structure to the simulated total IR spectra is determined by the probability of an adsorbate to bind to that respective site in each orientation.
In the case of CO2, this approach yields very different features depending on the surface: given that the low surface energy terminations cannot activate CO2 due to their peculiar exposed O2−/Mg2+ site distribution (see above), the predominant features present in the adsorbate spectra of the (010), (120) and (111) are those of the gas-phase CO2, arising from physisorbed molecules on the surface with relatively low favorable adsorption energies. In contrast, given the ability of the (101), (001), (110) and (021) surfaces to activate CO2 with lower adsorption energies than its physisorbed counterpart, any contributions of the latter to the global IR are wiped away by the Boltzmann factor by those of newly conformed carbonate structures on the surface.
After determining the predominant IR features on each forsterite termination, we then proceeded to build a global spectrum where the contribution of each surface to the crystalline equilibrium shape (Table S1, ESI†) is computed. These global spectra are depicted in Fig. 6b and c for CO2 and SO2, respectively, displaying representative vibrational modes contributing to each theoretical peak.
Our simulations show that the IR features arising from low surface energy terminations such as (010) and (120) dominate the spectrum: we predict the characteristic peak corresponding to the asymmetric stretching of CO2 to appear prominently at 2351 cm−1, indicative of weakly physisorbed CO2. The slight redshift of this mode relative to gas-phase CO2 (2349 cm−1) is due to the interaction of a terminal O with a surface Mg2+ site; this interaction breaks the symmetry of the adsorbed molecule, rendering the normally IR-inactive symmetric stretching mode weakly activated at a frequency of 1312 cm−1. We assign all other calculated features to vibrational modes associated with the formation of surface-bound carbonates, with frequencies of 1802, 1761, 1639, 1203 and 1182 cm−1. In these configurations, CO2 binds either in a bidentate fashion, with its C and one O atom interacting with surface O2− and Mg2+ sites, respectively, or in a tridentate mode, where the second terminal oxygen of CO2 also coordinates to a nearby Mg2+ site.
For SO2, our simulations do not predict the existence of gas-phase features in the most stable adsorption configurations on each termination, as any peaks arising from weakly physisorbed molecules have a negligible impact in the computed Boltzmann distribution due to their high adsorption energies compared to those coming from the chemisorbed SO2. Upon building the global IR spectrum, our calculations predict the modes of these weakly adsorbed molecules to be redshifted relative to gas-phase SO2 due to their interaction with the surfaces, as exemplified in the representative red-labeled modes in Fig. 6c. Thus, all modes present in the global IR spectrum are assigned to chemisorbed SO2 which, akin to CO2, binds to surface O2− and Mg2+ sites in a bidentate fashion through its S and a terminal O atom, respectively, or in a tridentate fashion where the remaining O binds to a neighboring Mg2+ site. The resulting features in the IR spectrum span a range of frequencies, with increasing coordination strength leading to more pronounced redshifts: while bidentate bound molecules yield peaks at 1256, 1233 and 1201 cm−1, the strongest redshifts are associated with tridentate binding, producing peaks at 1188, 1156, and 1106 cm−1. Notably, the peak at 1156 cm−1, corresponding to the “symmetric” stretching of the tridentate SO2, dominates the spectrum due to its association with the most thermodynamically favored adsorption configurations on the low surface energy (010) and (120) terminations.
Integrating experiments and theory
A comparison between experimental results and the simulated global IR spectrum for CO2 and SO2 reveals good agreement in both the position and evolution of key spectral features (as shown in Fig. S2 (ESI†) for the case of CMS as an illustrative case). In the case of CO2, low temperature experimental IR spectra are dominated by a peak at 2345 cm−1, progressively diminishing with increasing temperature and giving rise to new absorptions between 1700 and 1200 cm−1, consistent with a predominance of carbonates at low coverages. In the experimental spectra, the carbonate signals become more evident at higher temperatures because their formation requires overcoming a relatively high activation barrier to bend the CO2 molecule. This aspect is not considered in the theoretical part, in which the adsorbed species are already prepared to be ready for carbonate formation. These trends are present in the theoretical spectrum: while the contributions from low-energy terminations—particularly (010) and (120)—account for the physisorbed CO2 signal, higher surface energy terminations contain peaks ranging from approximately 1800 to 1200 cm−1. While our calculations do not account for the formation of additional carbonate layers at higher CO2 coverages, nor for the interaction of CO2 with traces of co-adsorbed water—which may contribute to the observed discrepancies in carbonate peak positions—the theoretical results suggest that as the most favorable adsorption sites become occupied, configurations with higher adsorption energies begin to dominate. This leads to an increased contribution from carbonate-like modes, which progressively overshadow the spectral features associated with physisorbed CO2 in the high temperature regimes.In the case of SO2, our simulations accurately predict the presence of chemisorbed structures, characterized by the peak located around 1150 cm−1 both in theory and experiments. Our calculations predict this peak to be prominent under both high and low coverage conditions: at high coverages (low temperature), we expect it to arise from the symmetric stretching mode of weakly physisorbed SO2 and from the modes of surface-bound species, whereas at low coverages (high temperature), it originates exclusively from chemisorbed SO2 molecules predicted by the simulations. Additionally, both the experimental broad bands and the peak observed at 1255 cm−1 in the CMS samples are consistent with the simulated results, which suggest a diverse landscape of interaction modes that drastically affect the spectroscopic features. Illustrative representations of the atomic configurations conforming the most stable adsorptions of CO2 and SO2 on each surface termination are shown in Fig. S3–S16 (ESI†).
Conclusions
In this work, we have combined in situ IR spectroscopy and an automated computational framework to systematically investigate the adsorption behavior of CO2 and SO2 on amorphous and crystalline forsterite surfaces under astrophysical relevant conditions. Our results reveal that both molecules exhibit distinct spectroscopic signatures depending on their interaction strength with the surface, the surface termination, and the coverage regime.By explicitly accounting for the relative stability of adsorption modes via Boltzmann statistics and the morphological distribution of surface terminations in the equilibrium shape of the crystal, our theoretical approach achieves good agreement with experimental IR spectra. This methodology not only enables the assignment of vibrational features to specific surface configurations, but also provides a framework to study the spectroscopic behavior of other interstellar molecules on mineral surfaces of cosmic relevance.
Data availability
The data supporting this article, including supporting figures and tables, have been included as part of the ESI.† All computational data presented herein, including the Jupyter notebooks for the generation of weighed theoretical IR spectra and scripts for the automatic optimization of adsorbates on the silicate surfaces, are shared with a GNU General Public License v4.0 license through a Zenodo dataset with the following DOI: https://doi.org/10.5281/zenodo.15341156.Conflicts of interest
There are no conflicts to declare.Acknowledgements
E. M-.T. acknowledges the support from the Spanish Ministry of Universities and the European Union's Next Generation EU fund. This project received funding from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Program (Grant Agreement 865657) for Project “Quantum Chemistry on Interstellar Grains” (QUANTUMGRAIN). MICIN is also acknowledged for financing the projects PID2021-126427NBI00 and CNS2023-144902. The Italian MUR (PRIN 2020, “Astrochemistry beyond the second period elements”, Prot. 2020AFB3FX) and the Italian Space Agency (Bando ASI Prot. n. DC-DSR-UVS-2022-231, Grant no. 2023-10-U.0 “Modeling Chemical Complexity: all'Origine di questa e di altre Vite per una visione aggiornata delle missioni spaziali (MIGLIORA)” Codice Unico di Progetto (CUP) F83C23000800005) are gratefully acknowledged. This research has received funding from the Project CH4.0 under the MUR program “Dipartimenti di Eccellenza 2023–2027” (CUP: D13C22003520001). We also thank the the EuroHPC Joint Undertaking for the provision of computational resources through the Regular Access call program, project no. 2023R01-112, hosted by the Ministry of Education, Youth and Sports of the Czech Republic through the e-INFRA CZ (ID: 9025). A. R. gratefully acknowledges support through a 2023 ICREA Award.Notes and references
- T. Do-Duy, C. M. Wright, T. Fujiyoshi, A. Glasse, R. Siebenmorgen, R. Smith, B. Stecklum and M. Sterzik, Mon. Not. R. Astron. Soc., 2020, 493, 4463–4517 CrossRef CAS.
- A. Takigawa, S. Tachibana, H. Nagahara, K. Ozawa and M. Yokoyama, Adv. Pharm. J., 2009, 707, L97–L101 CAS.
- B. L. De Vries, M. Min, L. B. F. M. Waters, J. A. D. L. Blommaert and F. Kemper, Astron. Astrophys., 2010, 516, A86 CrossRef.
- B. T. Draine, in Cosmic Dust - Near and Far, ed. Th. Henning, E. Grün and J. Steinacker, ASP Conference Series, 2009, vol. 414, pp. 453–472.
- H. W. W. Spoon, A. G. G. M. Tielens, L. Armus, G. C. Sloan, B. Sargent, J. Cami, V. Charmandaris, J. R. Houck and B. T. Soifer, Adv. Pharm. J., 2006, 638, 759–765 CAS.
- A. L. Graham, A. J. Easton and R. Hutchison, Mineral. Mag., 1977, 41, 201–210 CrossRef CAS.
- M. Kołodziej, D. Michalska, K. Załęski, I. Iatsunskyi, A. Muszyński and E. Coy, Sci. Rep., 2025, 15, 6866 CrossRef PubMed.
- S. Messenger, L. P. Keller, F. J. Stadermann, R. M. Walker and E. Zinner, Science, 2003, 300, 105–108 CrossRef CAS PubMed.
- J. Bradley, in Astromineralogy, ed T. Henning, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, vol. 815, pp. 259–276 Search PubMed.
- K. Nakamura-Messenger, L. P. Keller, S. J. Clemett, S. Messenger and M. Ito, Meteorit. Planet. Sci., 2011, 46, 1033–1051 CrossRef CAS.
- H. Sierks, C. Barbieri, P. L. Lamy, R. Rodrigo, D. Koschny, H. Rickman, H. U. Keller, J. Agarwal, M. F. A’Hearn, F. Angrilli, A.-T. Auger, M. A. Barucci, J.-L. Bertaux, I. Bertini, S. Besse, D. Bodewits, C. Capanna, G. Cremonese, V. Da Deppo, B. Davidsson, S. Debei, M. De Cecco, F. Ferri, S. Fornasier, M. Fulle, R. Gaskell, L. Giacomini, O. Groussin, P. Gutierrez-Marques, P. J. Gutiérrez, C. Güttler, N. Hoekzema, S. F. Hviid, W.-H. Ip, L. Jorda, J. Knollenberg, G. Kovacs, J. R. Kramm, E. Kührt, M. Küppers, F. La Forgia, L. M. Lara, M. Lazzarin, C. Leyrat, J. J. Lopez Moreno, S. Magrin, S. Marchi, F. Marzari, M. Massironi, H. Michalik, R. Moissl, S. Mottola, G. Naletto, N. Oklay, M. Pajola, M. Pertile, F. Preusker, L. Sabau, F. Scholten, C. Snodgrass, N. Thomas, C. Tubiana, J.-B. Vincent, K.-P. Wenzel, M. Zaccariotto and M. Pätzold, Science, 2015, 347, aaa1044 CrossRef PubMed.
- J. Perrero, L. Beitia-Antero, A. Fuente, P. Ugliengo and A. Rimola, Mon. Not. R. Astron. Soc., 2023, 527, 10697–10704 CrossRef.
- G. Molpeceres, A. Rimola, C. Ceccarelli, J. Kästner, P. Ugliengo and B. Maté, Mon. Not. R. Astron. Soc., 2019, 482, 5389–5400 CrossRef CAS PubMed.
- J. Navarro-Ruiz, M. Sodupe, P. Ugliengo and A. Rimola, Phys. Chem. Chem. Phys., 2014, 16, 17447–17457 RSC.
- R. Santalucia, M. Pazzi, F. Bonino, M. Signorile, D. Scarano, P. Ugliengo, G. Spoto and L. Mino, Phys. Chem. Chem. Phys., 2022, 24, 7224–7230 RSC.
- V. Vinogradoff, V. Leyva, E. Mates-Torres, R. Pepino, G. Danger, A. Rimola, L. Cazals, C. Serra, R. Pascal and C. Meinert, Earth Planet. Sci. Lett., 2024, 626, 118558 CrossRef CAS.
- N. Bancone, S. Pantaleone, P. Ugliengo, A. Rimola and M. Corno, Phys. Chem. Chem. Phys., 2023, 25, 26797–26812 RSC.
- N. Bancone, S. Pantaleone, P. Ugliengo, A. Rimola and M. Corno, ACS Earth Space Chem., 2025, 9, 303–313 CrossRef CAS PubMed.
- N. Bancone, R. Santalucia, S. Pantaleone, P. Ugliengo, L. Mino, A. Rimola and M. Corno, J. Phys. Chem. C, 2024, 128, 15171–15178 CrossRef CAS.
- L. Zamirri, S. Pantaleone and P. Ugliengo, J. Chem. Phys., 2019, 150, 064702 CrossRef PubMed.
- P. A. Gerakines, D. C. B. Whittet, P. Ehrenfreund, A. C. A. Boogert, A. G. G. M. Tielens, W. A. Schutte, J. E. Chiar, E. F. Van Dishoeck, T. Prusti, F. P. Helmich and T. De Graauw, Adv. Pharm. J., 1999, 522, 357–377 CAS.
- Y. Ermolov, A. Vasilchenko and G. Lazorenko, Int. J. Mater. Sci., 2024, 25, 12639 CAS.
- A. P. Jones and N. Ysard, Astron. Astrophys., 2019, 627, A38 CrossRef CAS.
- F. Kemper, C. Jäger, L. B. F. M. Waters, Th Henning, F. J. Molster, M. J. Barlow, T. Lim and A. De Koter, Nature, 2002, 415, 295–297 CrossRef CAS PubMed.
- S. Peters, D. A. Semenov, R. Hochleitner and O. Trapp, Sci. Rep., 2023, 13, 6843 CrossRef CAS PubMed.
- M. K. McClure, W. R. M. Rocha, K. M. Pontoppidan, N. Crouzet, L. E. U. Chu, E. Dartois, T. Lamberts, J. A. Noble, Y. J. Pendleton, G. Perotti, D. Qasim, M. G. Rachid, Z. L. Smith, F. Sun, T. L. Beck, A. C. A. Boogert, W. A. Brown, P. Caselli, S. B. Charnley, H. M. Cuppen, H. Dickinson, M. N. Drozdovskaya, E. Egami, J. Erkal, H. Fraser, R. T. Garrod, D. Harsono, S. Ioppolo, I. Jiménez-Serra, M. Jin, J. K. Jørgensen, L. E. Kristensen, D. C. Lis, M. R. S. McCoustra, B. A. McGuire, G. J. Melnick, K. I. Öberg, M. E. Palumbo, T. Shimonishi, J. A. Sturm, E. F. van Dishoeck and H. Linnartz, Nat. Astron., 2023, 7, 431–443 CrossRef.
- W. R. M. Rocha, E. F. Van Dishoeck, M. E. Ressler, M. L. Van Gelder, K. Slavicinska, N. G. C. Brunken, H. Linnartz, T. P. Ray, H. Beuther, A. Caratti, O. Garatti, V. Geers, P. J. Kavanagh, P. D. Klaassen, K. Justtanont, Y. Chen, L. Francis, C. Gieser, G. Perotti, Ł. Tychoniec, M. Barsony, L. Majumdar, V. J. M. Le Gouellec, L. E. U. Chu, B. W. P. Lew, Th Henning and G. Wright, Astron. Astrophys., 2024, 683, A124 CrossRef CAS.
- J. C. Laas and P. Caselli, Astron. Astrophys., 2019, 624, A108 CrossRef CAS.
- G. Pacchioni, A. Clotet and J. M. Ricart, Surf. Sci., 1994, 315, 337–350 CrossRef CAS.
- J. A. Rodriguez, T. Jirsak, A. Freitag, J. Z. Larese and A. Maiti, J. Phys. Chem. B, 2000, 104, 7439–7448 CrossRef CAS.
- J. Martínez-González, J. Navarro-Ruiz and A. Rimola, Minerals, 2018, 8, 353 CrossRef.
- D. Yamamoto and S. Tachibana, ACS Earth Space Chem., 2018, 2, 778–786 CrossRef CAS.
- C. Pavan, R. Santalucia, G. Escolano-Casado, P. Ugliengo, L. Mino and F. Turci, Int. J. Mater. Sci., 2023, 24, 11482 CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, APL Mater., 2013, 1, 011002 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- E. Mates-Torres and A. Rimola, J. Appl. Crystallogr., 2024, 57, 503–508 CrossRef CAS PubMed.
- M. Bruno, F. R. Massaro, M. Prencipe, R. Demichelis, M. De La Pierre and F. Nestola, J. Phys. Chem. C, 2014, 118, 2498–2506 CrossRef CAS.
- J. H. Montoya and K. A. Persson, npj Comput. Mater., 2017, 3, 14 Search PubMed.
- S. Grimme, C. Bannwarth and P. Shushkov, J. Chem. Theory Comput., 2017, 13, 1989–2009 CrossRef CAS PubMed.
- A. Hjorth Larsen, J. Jørgen Mortensen, J. Blomqvist, I. E. Castelli, R. Christensen, M. Dułak, J. Friis, M. N. Groves, B. Hammer, C. Hargus, E. D. Hermes, P. C. Jennings, P. Bjerre Jensen, J. Kermode, J. R. Kitchin, E. Leonhard Kolsbjerg, J. Kubal, K. Kaasbjerg, S. Lysgaard, J. Bergmann Maronsson, T. Maxson, T. Olsen, L. Pastewka, A. Peterson, C. Rostgaard, J. Schiøtz, O. Schütt, M. Strange, K. S. Thygesen, T. Vegge, L. Vilhelmsen, M. Walter, Z. Zeng and K. W. Jacobsen, J. Phys.: Condens. Matter, 2017, 29, 273002 Search PubMed.
- P. Pulay, Chem. Phys. Lett., 1980, 73, 393–398 CrossRef CAS.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- M. Cutini, L. Maschio and P. Ugliengo, J. Chem. Theory Comput., 2020, 16, 5244–5252 Search PubMed.
- A. Stukowski, Modelling Simul. Mater. Sci. Eng., 2010, 18, 015012 CrossRef.
- S. Baroni, S. De Gironcoli, A. Dal Corso and P. Giannozzi, Rev. Mod. Phys., 2001, 73, 515–562 CrossRef CAS.
- D. Karhánek, Dakarhanek/VASP-infrared-intensities (version v1.0) Zenodo, 2020.
- T. Shimanouchi, Tables of molecular vibrational frequencies, consolidated volume i, National Bureau of Standards, Gaithersburg, MD, 1972 Search PubMed.
- F. X. L. I. Xamena and A. Zecchina, Phys. Chem. Chem. Phys., 2002, 4, 1978–1982 RSC.
- L. Mino, F. Cesano, D. Scarano, G. Spoto and G. Martra, Res. Chem. Intermed., 2019, 45, 5801–5829 CrossRef CAS.
- M. Waqif, A. M. Saad, M. Bensitel, J. Bachelier, O. Saur and J.-C. Lavalley, Faraday Trans., 1992, 88, 2931 RSC.
- D. B. Nash and B. H. Betts, Icarus, 1995, 117, 402–419 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cp01699a |
| ‡ These authors contributed equally to this work. |
| § Present address: Department of Chemistry and Biochemistry, Mendel University in Brno, Zemedelska 1, CZ-613 00, Brno, Czech Republic. |
| This journal is © the Owner Societies 2025 |