 Open Access Article
Open Access ArticlePrediction of NMR parameters and geometry in 133Cs-containing compounds using density functional theory†
N.
Manukovsky
 a,
N.
Vaisleib
a,
M.
Arbel-Haddad
a,
N.
Vaisleib
a,
M.
Arbel-Haddad
 b and
A.
Goldbourt
b and
A.
Goldbourt
 *a
*a
aSchool of Chemistry, Tel Aviv University, Ramat Aviv 6997801, Tel Aviv, Israel. E-mail: amirgo@tauex.tau.ac.il
bNuclear Research Center Negev, PO Box 9001, Beer Sheva 84901, Israel
First published on 29th January 2025
Abstract
The need to immobilize low-level nuclear waste, in particular 137Cs-bearing waste, has led to a growing interest in geopolymer-based waste matrices, in addition to optimization attempts of cement matrix compositions for this specific application. Although the overall phase composition and structure of these matrices are well characterized, the binding sites of Cs in these materials have not been clearly identified. Recent studies have suggested that combining the sensitivity of solid-state nuclear magnetic resonance (SSNMR) to the local atomic structure with other structural techniques provides insights into the mode of Cs binding and release. Density functional theory (DFT) can provide the connection between spectroscopic parameters and geometric properties. However, the reliability of DFT results strongly relies on the choice of a suitable exchange–correlation functional, which for 133Cs, the NMR surrogate for such studies, is not well-established. In this work we benchmark various functionals against their performance in predicting the geometry of various simple Cs compounds, their NMR quadrupolar coupling constants, and their chemical shift values, while prioritizing the ability to incorporate dispersion interactions and maintaining low computational cost. We examined Cs salts, Cs oxides, perovskites, caged materials, a borate glass and a cesium fluoroscandate. While no single functional performs equally well for all parameters, the results show rev-vdW-DF2 and PBEsol+D3 to be leading candidates for these systems, in particular with respect to geometry and chemical shifts, which are of high importance for Cs-immobilization matrices.
Introduction
Cesium is an alkali metal whose ionic form plays a key role in various materials and applications, including chemical catalysis,1 purification of biological macromolecules,2 and optoelectronic devices.3 Radioisotopes of Cs, specifically 137Cs, a β-emitter with a half life time of ∼30 years, are found in radioactive waste streams. The long term immobilization of Cs-bearing radioactive waste is a challenging task due to the high solubility of Cs ions in aqueous media. Whereas vitrification is the method of choice for high-level nuclear waste,4,5 cementitious matrices are usually used for low-level and intermediate-level waste immobilization due to their low cost and ease of preparation, combined with good mechanical properties and durability under irradiation. However, cesium is not efficiently immobilized by the alkaline environment afforded by cement. Geopolymers have been proposed as an alternative for Cs-bearing low-level nuclear waste due to their ability to specifically bind Cs ions.6 Examples from recent years are the many studies regarding immobilization of incineration ashes from the Fukushima region, which have a high content of radioactive 134Cs and 137Cs following the nuclear accident in 2011. Alkaline activation of the ashes leads to the formation of geopolymeric wasteforms7–9 consisting of various crystalline and amorphous phases. The ultimate Cs immobilizing matrix should be characterized by high Cs binding, and consequently minimal leaching to the environment under relevant environmental conditions. High-resolution structural characterization of Cs-bearing matrices, clarifying properties such as the binding site(s) geometry, the binding phase where several phases coexist, and the equilibrium distribution between competing sites, can contribute to the rational design of improved immobilization matrices.Parameters provided by solid-state NMR (SSNMR), such as chemical shift, dipolar coupling, and quadrupolar coupling, are sensitive to the local geometry of the studied nuclei, including its binding atoms, bond lengths and angles, and its non-covalent interactions with the environment. Thus, they contain a wealth of local geometric and electronic structure information. However, only few examples show how 133Cs SSNMR can be utilized to characterize Cs binding sites in nuclear waste matrices.4,10–17
In general, there is no simple, straightforward method to directly infer structural properties from the NMR parameters, unless the dipolar interaction, which is proportional to the inverse cube of the distance, is directly measured. First-principles calculations are known to bridge the gap between spectroscopy and structure, thus allowing to utilize the information content of SSNMR to its fullest. Density functional theory (DFT) has been in vast use in the physics and chemistry communities for decades due to its ability to predict diverse properties such as ground state structure, excitation energies, spectroscopic properties, reaction energies and more, for molecules and solids, with high accuracy and at a relatively modest computational cost. Specifically, the use of DFT calculations side by side with NMR measurements has become widespread since the introduction of the GIPAW (Gauge Including Projector Augmented Wave) method,18–20 which provides the ability to compute NMR tensors in solid systems with all-electron accuracy. Although DFT has been helpful in the structural characterization of zeolites,21,22133Cs has not been extensively studied using DFT-GIPAW. Relevant examples of the combination of 133Cs SSNMR and DFT are the study of Cs adsorption on Montmorillonite clay,12 and their use to study Cs-containing perovskites23 and cryptands.24
Prior to the use of DFT-GIPAW for probing Cs binding in complex systems such as cements and geopolymers, which are often a conglomerate of several crystalline and non-crystalline phases, it is instructive to screen the suitability of different DFT functionals to correctly predict 133Cs NMR parameters using simpler, well characterized Cs-containing systems. Cs-halides (CsF, CsCl, CsBr, and CsI) are the obvious place to start. Experimentally, it is well known that under ambient temperature and pressure, CsF crystallizes in the B1 phase (Fig. 1(a)), forming the so called NaCl or rock-salt structure, as most alkali halides do. However, CsCl, CsBr, and CsI crystalize in the B2 phase (Fig. 1(b)), being the only alkali halides known to crystallize in the so-called CsCl structure under these conditions25 (the discussion omits Fr and At, about which no data is available).26 The reason for this difference is dispersion interactions which favor the phase with the shorter distances between ions of the same kind, that is, the CsCl phase. These interactions depend on the ion polarizability, and are therefore more substantial in the mentioned systems consisting of heavy ions.27,28 Van der Waals forces make only a minor contribution to the total lattice energy of an ionic lattice (1–5% of the lattice energy), but they are the factor determining the phase in this case.28 Zhang et al.29 have shown that despite the simplicity of cesium halide structures, the popular Perdew–Burke–Ernzerhof (PBE)30 functional, commonly used for the prediction of NMR parameters for 133Cs12,23 and other nuclei,31–34 fails to predict the correct phase for CsCl. The same also occurs when using other GGA-type functionals such as the solid-state optimized PBEsol,29 PW91 and revPBE,35 as well as for various meta-GGA type functionals (MS0, MS1, MS2, TPSS and rTPSS).35 A plausible explanation is that pure DFT functionals do not include terms describing dispersion forces.29,36 Pure DFT functionals are semilocal, meaning that the energy density at any point depends only on properties at that point. Consequently, these functionals are by definition unable to model dispersion, which is an interaction between instantaneous dipoles at two different sites. Indeed, the correct CsCl structure is obtained using calculation schemes incorporating dispersion.29,37
 | ||
| Fig. 1 (a) B1 (NaCl) phase vs. (b) B2 (CsCl) phase unit cells.25 The large spheres represent the metal, whereas the small ones represent the halide. At room temperature, only CsF adopts the B1 phase. | ||
Dispersive interactions can be introduced into DFT by various approaches.36 A common approach is the addition of asymptotic dispersion corrections to the energy obtained by a standard DFT calculation, in the manner of an interatomic potential.38–45 Notable examples of dispersion corrections are the Grimme schemes DFT+D,38 DFT+D239 and DFT+D3;46 Tkatchenko-Scheffler (TS) dispersion correction40 and the subsequent many-body dispersion (MBD) correction,47 and exchange-hole dipole-moment model (XDM).48–50 Asymptotic dispersion corrections can attain good accuracy at a relatively low computational cost, and can be applied in conjunction with various, well-tested functionals. Another approach is the development of explicitly non-local density functionals,51–58 whereby the energy depends on the density and its gradient at two points in space simultaneously. Notable examples of non-local functionals are vdW-DF,53 vdW-DF2,56 vdW-DF3,59 and rVV10.54,57 Additional methods include dispersion-correcting potentials,60–63 and parameterized exchange–correlation functionals.64–66 Finally, meta-GGA functionals67,68 can incorporate short-range, but not long-range, dispersion interactions through their dependence on the kinetic energy.
For Cs halides, forcing the right phase upon the popular PBE functional yields a good prediction of the chemical shift,69 but does not solve the problem of the accurate geometry prediction. It is currently unknown which functional can correctly capture both the geometry and the NMR parameters of Cs-compounds, ranging from Cs halides to Cs-bearing zeolites.
This work aims to identify the most suitable DFT scheme for the prediction of the correct geometry and NMR parameters of various Cs-containing systems. First, we screen various functionals for their ability to predict the correct phase in Cs halides. We then compare their performance in predicting the crystal unit cell volume, chemical shift and quadrupolar coupling constants in Cs halides, Cs oxyanion compounds, and Cs-containing perovskites. Keeping in mind our interest in more computationally-demanding Cs-bearing materials, we have restricted this study to schemes of a reasonable computational cost. Differences in the performance and accuracy of various functionals, as well as their performance compared with experimental results available in the literature, are discussed, and recommendations for functional selection for the prediction of 133Cs NMR parameters are proposed.
Computational methods
DFT calculations were carried out using the Quantum Espresso 7.170,71 software package, which uses the gauge-including projector augmented waves (GIPAW) method for computing magnetic resonance properties of crystalline structures. We used scalar relativistic PAW PBE or PBEsol pseudopotentials,72 downloaded from the Quantum Espresso website. Spin–orbit coupling (SOC) effects on the geometry were found to be sufficiently small to be neglected at this stage and were not further considered (see ESI,† Table S13). The plane wave cutoff energies for eighteen cesium-containing systems, determined by convergence tests, are listed in Table S1 in the ESI.† The kinetic energy cutoff was four times that of the wave function. Monkhorst–Pack grids were used to sample the Brillouin zone (see Table S1, ESI† for their dimensions), and the initial structures were taken to be the reported crystal structures (Table S2, ESI†).Various functionals and dispersion corrections were used for the calculations of geometry and NMR parameters. PBE,30 known to fail in predicting the correct phase in cesium halides, is used as a reference, being a very common functional. Other GGA-type functionals examined here are revPBE,73 a one-parameter modification of PBE; its further modification rPBE,74 which performs well for the calculation of NMR parameters in solids containing elements of the third and fourth periods;75,76 PBEsol,77 tailored to solids; and B86bPBE,30,78 the recommended functional to be paired with XDM for solid state calculations.44 These five semilocal functionals are used as base functionals in conjunction with three dispersion corrections – DFT-D2,39 DFT-D3,46 and XDM.50,79 PBE+MBD was not used as it is known to fail to converge for many cesium halides, and neither was PBE+TS which is known to yield the correct phase but provide incorrect lattice constants.80 Non-local functionals considered are rVV10,58 vdW-DF1,53 vdW-DF2,56 vdW-DF3,59 vdW-DF-C6,81 rev-vdW-DF2 (also called vdW-DF2-B86R).82
Asymptotic dispersion corrections (such as D2, D3, and XDM) are implemented post-self-consistently, meaning they do not affect the electron density. Therefore, for a given geometric structure, the NMR properties, which strongly depend on the electronic structure, will be unaffected by these corrections. In contrast, nonlocal functionals are formulated self-consistently, directly influencing the electron density. However, all dispersion corrections of any type can indirectly affect any molecular property via their effect on the geometric structure. For this reason, we found it instructive to compare the NMR parameters obtained by different functionals using either the experimental (crystallographic) geometry or the DFT-optimized geometry, allowing us to distinguish between geometry-mediated effects and electronic-structure-mediated effects. In the cases where structural relaxation was implemented, both the lattice constants and the atomic positions were optimized using the BFGS algorithm, until the residual forces acting on each atom were below 1.0 × 10−3 Ry a.u.−1.
Results and discussions
Cesium halide polymorphs
Pure DFT functionals are known to fail in the prediction of the ground state structure of heavy Cs halides, owing to the absence of dispersion in the energy calculation. Therefore, the first criterion for DFT scheme screening was the ability to correctly predict the ground state phase, which is B1 for CsF and B2 for heavier Cs halides. Unfortunately, the comparison to experiment is only qualitative, as the experimental values of the energy gap between Cs halides polymorphs are unavailable.The results confirm that semilocal functionals erroneously assign the B1 phase to all Cs halides (Fig. 2(a)). Interestingly, the slope of the energy gap curve is the same for all five GGA-type functionals tested here, with the exception of PBEsol for the CsF–CsCl segment. The addition of an asymptotic dispersion correction leads to the correct phase prediction in most, yet not all, combinations of base functionals (PBE, rPBE, revPBE, PBEsol, B86bPBE) and dispersion corrections (D2, D3, and XDM) tested here. The D2 correction (Fig. 2(b)) using the default parameters leads to a non-physical trend where the energy gap is non-monotonous versus the halide size and was therefore not further used. Using Zhang's parameterization for Cs  29 rectifies this. Using Schurko's D2* reparameterization of the damping function83 (d = 3.25 instead of the default 20), originally implemented using rPBE, on its own leads to a nearly flat curve and a wrong phase in CsF. Using Zhang's parameters together with D2* does not settle the phase issue and does not reproduce the trend with respect to the halide size. We note that D2* was parametrized using the EFG tensors of three elements, and should in principle be transferrable to all elements. However, it was designed to provide a good fit of the EFG, not necessarily of the phase stability, and was trained on small organic molecules, very different from the inorganic salt structures studied here.
29 rectifies this. Using Schurko's D2* reparameterization of the damping function83 (d = 3.25 instead of the default 20), originally implemented using rPBE, on its own leads to a nearly flat curve and a wrong phase in CsF. Using Zhang's parameters together with D2* does not settle the phase issue and does not reproduce the trend with respect to the halide size. We note that D2* was parametrized using the EFG tensors of three elements, and should in principle be transferrable to all elements. However, it was designed to provide a good fit of the EFG, not necessarily of the phase stability, and was trained on small organic molecules, very different from the inorganic salt structures studied here.
The D3 and XDM corrections (Fig. 2(c)) lead in most cases tested here to the correct phase, although the trend is violated for PBE+D3, PBEsol+D3, and B86bPBE+XDM (the curves go slightly up from CsBr to CsI). A similar behavior was described for PBE+D3 with Li halides.84 The energetic gap between the phases is larger for PBE (or PBEsol)+D2 than for PBE (or PBEsol)+D3. This can be explained by the fact that D2 describes dispersion using only two-body attractive interactions, whereas D3 also incorporates local environment effects and three-body repulsive interactions.85
All vdW-DF functionals tested (Fig. 2(d)) yield curves of the same slope. Most of them yield the correct phase, as does rVV10. The hybrid functionals tested here do not capture the correct phase.
The reliable structure prediction of Cs halides is challenging, since the energy gap between competing phases, caused by the typically weak dispersion forces,28 is small, and is therefore very sensitive to errors arising in various approximations of the exchange–correlation energy. The energy gaps predicted here are similar to those calculated by Pyper (0.078 eV f.u.−1),86 Aguado (0.14 eV f.u.−1),87 and Zhang (0.16 eV f.u.−1).29 As shown by others,29 GGA and hybrid functionals fail to predict the correct phase of Cs halides. We therefore chose to proceed with non-local and dispersion-corrected semilocal functionals.
Unit cell volume
In the next step of this study the ability of different functionals to predict the unit cell volume of Cs compounds was tested. The panel of Cs-compounds was expanded to include additional systems, namely four Cs oxyanion salts and three CsGeX3 perovskites in addition to the four Cs halides studied thus far. Fig. 3 features unit-cell volumes obtained from X-ray diffraction (literature values) and calculated unit cell volumes obtained using various DFT schemes, along with the mean error (ME) for each functional. We note that the DFT results refer to 0 K, while the experimental structures were determined at or around room temperature. | ||
| Fig. 3 Unit-cell volume of various Cs-containing compounds as found experimentally by X-ray crystallography (asterisk) or by calculation using various DFT schemes. Some of the points are slightly horizontally shifted to avoid overlap. See Table S2 (ESI†) for references of the experimental data. The bottom figure show the mean error (ME) of each functional. The error bars represent the standard deviation (1 SD above and below the mean). The mean relative error (MRE) and the coordinates root-mean-square-error (RMSE) are found in Fig. S1 (ESI†). | ||
Most schemes give a reasonable prediction of the unit cell volume, and correctly capture the trend among different compounds. Quantitatively, however, there are differences between various schemes. As expected, PBE overestimates the unit-cell volume. Adding a dispersion correction to PBE results, as expected, in a contraction of the calculated unit cell, to an extent that depends on the type of dispersion correction used. D2 is superior to XDM and D3 in this context, with XDM leading to an underestimation of the cell volume. The functionals revPBE+D3 and B86bPBE+XDM are outperformed even by uncorrected PBE. This is in contrast to the good performance of B86bPBE-XDM in predicting the lattice constant of the entire series of alkali halides.88 This may be related to the fact that unlike the former study,88 the current work includes compounds containing polyatomic anions. Good performance is also displayed by the non-local functionals, as well as by rPBE+D2* Zhang parametrization and PBEsol+D3. The tested non-local functionals are the best performers in this task, as manifested by both their small mean error and their small standard deviation values.
133Cs NMR parameters: The quadrupolar coupling constant
The calculation of the 133Cs quadrupolar coupling constant Cq, using GIPAW and the same DFT schemes, is shown in Fig. 4. A great difference is noticeable between different compounds: some are very sensitive to the calculation scheme (e.g. CsClO4, CsVO3), whereas some are not (e.g. CsGeX3, Cs2CrO4 site 2, Cs2SO4 site 1). This observation highlights the importance of screening a large sample of materials before choosing which calculation scheme to use.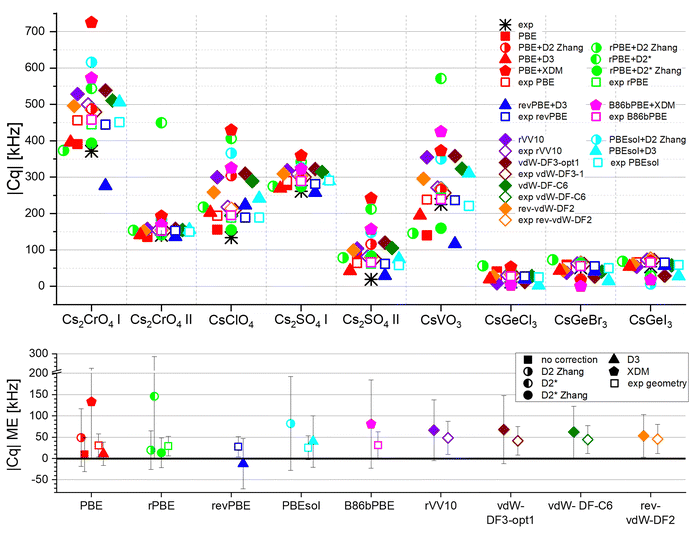 | ||
| Fig. 4 Quadrupolar coupling constant Cq of various Cs compounds. The black asterisks correspond to the experimental values (Table S3, ESI†), whereas the colored symbols correspond to computational results (Tables S4–S6, ESI†). Solid symbols represent calculations where the geometry was optimized, whereas open symbols correspond to calculations employing the experimental (XRD) geometry. The bottom plot shows the mean error in Cq. Additional error metrics are given in Fig. S1 (ESI†). The error bars represent the standard deviation. | ||
PBE, though not accounting for dispersion and not providing an accurate unit cell volume (Fig. 3), provides a very good prediction of the Cq parameter, slightly outperformed only by PBE+D3 and rPBE+D2* Zhang. Adding D2 or XDM to PBE leads to an overestimation of |Cq|, although PBE+D2 yields good geometry (Fig. 3). The other combinations of a GGA functional with an asymptotic dispersion correction are not better in terms of absolute error, but adding Grimme's D3 correction improves the relative error. All four non-local functionals yield similar results and are all inferior to PBE and to PBE+D3, although their unit cell volume prediction was better (Fig. 3).
Interestingly, when using the experimental geometry without geometry optimization (empty squares), all schemes tested here provide nearly identical results. It is not surprising that the semilocal functionals provide results similar to each other, as do the non-local functionals. However, it is not obvious that these two different families of functionals provide results similar to each other, despite the fact that dispersion is inherent in the non-local functionals, while this is not the case in the corrected ones. We therefore conclude that the main effect determining the accuracy of Cq here is the geometric structure, not the difference in the functional itself. This is in agreement with other studies.89 However, remarkably, the functionals excelling at geometry prediction (e.g. rVV10, rev-vdW-DF2) do not excel at Cq prediction, showing that the structure most similar to the experimental one is not necessarily the one leading to the best Cq, at least for the type of Cs compounds studied here. Moreover, geometry optimization often results in a higher deviation in the Cq predicted value from that of the experimental value, with the exception being uncorrected PBE, PBE+D3, and rPBE+D2* Zhang parameterization. We note that the small sample of materials used here, owing to the scarcity of experimental 133Cs NMR results in the literature, results in large standard deviation values. Still, a fairly linear correlation is maintained between calculated and experimental Cq values (Fig. S2 and Table S7, ESI†).
An important aspect in the analysis of 133Cs, is that in many materials of interest the value of its quadrupolar coupling constant is small, and its experimental measurement is therefore difficult, also due to its high spin (S = 7/2). The chemical shift, which has a relatively broad ppm range, becomes the more informative 133Cs NMR observable. We therefore turn to calculations of 133Cs shifts.
133Cs NMR parameters: Chemical shifts
We have used DFT calculations to predict the 133Cs chemical shielding tensor. To convert the absolute shielding to the chemical shift, we plotted in Fig. 5(a) the calculated isotropic shieldings versus the experimental isotropic shifts. Experimental values have been obtained from various literature sources, as shown and referenced in Table S8 (ESI†), as well as experiments performed in our lab. A literature review revealed that for the simple Cs salts we analyzed, different reference compounds have been used and occasionally spectral referencing was not clearly stated. 133Cs chemical shifts are known to be concentration dependent,90 and the use of a solution containing Cs+ as an external reference must be done with care, in particular when using hygroscopic compounds such as CsNO3. The commonly cited manuscript by Haase91 contains data from experiments performed on static samples and at a low field (2.1T), and reports that a solution of 0.5 m CsCl resonates at +6.1 ppm with respect to infinite dilution, and that solid CsCl is at 228.1 ppm. A later study by Mooibroek92 reports solid CsCl to be +223.2 ppm with respect to 0.5 m CsCl, and this value is more commonly used in the literature, although various studies also use a 0.5 M or 1 M CsCl solution as the reference value of 0 ppm. Our experimental data, measured under 5 kHz magic-angle spinning at a field of 14.1T, shown in Fig. S4 (ESI†), allowed us to reconcile the seemingly contradicting literature results, setting a uniform reference scale. We used solid CsCl as a reference at +223.2 ppm, obtained that a 1 M CsCl solution has a chemical shift of 5.0 ppm, and from several solutions of CsCl the infinite dilution chemical shift value is projected to −6.1 ppm, in agreement with Haase and the correction by Mooibroek for solid samples. All experimental values obtained from the literature were therefore re-referenced using the corrected scale. To demonstrate the usefulness of using solid CsCl as a reference, we also show in Fig. S4 (ESI†) that its chemical shift is independent of temperature, whereas for 0.1 M CsNO3 in D2O (IUPAC recommendation), a shift of 1.9 ppm is observed over 15 °C. | ||
| Fig. 5 (a) Linearization plots of the isotropic shielding of various schemes and (b) their corresponding slopes. The error bars of the slopes represent standard deviations. δiso are averaged experimental results collected from the literature (re-referenced) and from our own measurements (Table S8 and Fig. S4, ESI†). | ||
The correlations between the re-referenced experimental chemical shifts and calculated chemical shielding, presented in Fig. 5(a), reveal that most calculation schemes are well fitted by linear curves, with the exception being rPBE+D2*. However, the slopes differ from the ideal value of −1 (Fig. 5). The greatest deviations from the ideal slope are those of XDM corrected functionals and of rPBE+D2*, the latter being greatly improved by adding Zhang's parametrization along with that of D2*. The slopes closest to −1 are those of revPBE+D3 (−0.90 ± 0.03) and PBE+D3 (−1.18 ± 0.04). Most other functionals feature slopes in the range [−1.45, −1.2], the differences between most functionals being insignificant, even when comparing, for example, between uncorrected semilocal functionals using the experimental geometry and non-local ones including geometry optimization.
We used the fitted correlations to convert the calculated chemical shieldings to shifts. The results, plotted in Fig. 6, show that all functionals capture the general trend in the chemical shifts of different systems. However, quantitative accuracy differs between different schemes. When using the experimental geometry, the shift is nearly independent of functional, as reported in other studies,93 and as we observed in the prediction of Cq. Geometry optimization improves the accuracy for most schemes, in contrast to the case of Cq calculation. While for experimental geometry the mean absolute error (MAE) of δiso values is mostly between 15–20 ppm (empty symbols), the best accuracy after optimization is achieved by PBEsol+D3 (MAE = 6.2 ppm), rev-vdW-DF2 (8.9), PBE+D2 (9.7), revPBE+D3 (10.4), PBE+D3 (10.5), rVV10 (10.8), and vdW-DF-C6 (10.9). These MAE's amount to 3% of the total shift span (372 ppm). Interestingly, it has been shown that PBE+D3 does not perform well in the calculation of 13C chemical shift in zeolites,93 whereas PBEsol+D3 is usually not used for chemical shift calculations.
 | ||
| Fig. 6 Computational and experimental values of the isotropic chemical shifts for various Cs compounds. Experimental values (Table S8, ESI†) were partly measured by us (spectra shown on Fig. S4, ESI†) and partly by others. The bottom plot shows the mean absolute error in δiso. Error bars represent standard deviation. An additional error metric is found in Fig. S1 (ESI†). The calculated shifts, after linearization, are explicitly given in Tables S9–S12 (ESI†). | ||
Again, the limited availability of experimental 133Cs NMR results manifests itself in broad error margins, precluding a definite choice of the best-performing functional. However, the functionals displaying a small mean absolute error also tend to display a lower standard deviation compared to the others, supporting their identification as good performers. Another comment regarding the variability of the average error, is that this variability is small for the unit cell volume (Fig. 3), but large for the NMR parameters (Fig. 4 and 6), although the sample size is nearly the same for both (not identically the same due to two of the systems containing two Cs-sites each). This suggests that for many functionals, the performance in geometry optimization is quite the same for different materials, but the performance of GIPAW for NMR parameters calculation is more sensitive to the system.
To distinguish between a systematic tendency rather than a random error in the estimation of the chemical shift, we also show the mean signed error in Fig. S1 (ESI†). For all tested functionals, the MSE is practically zero, indicating a random error.
When zooming in on a more subtle trend, that of the isotropic chemical shift versus the halide size (Fig. 7), and observing the apparently best functionals for the chemical shift prediction, it can be seen that revPBE+D3 fails to predict the trend in CsI, whereas rev-vdW-DF2 and PBEsol+D3, which were successful for the Cs halides, fail in CsGeX3 (note that the parabolic shape, where the maximum is at Br, is kept). Only PBE+D3 qualitatively captures the halide trends in both series.
Chemical shift anisotropy (CSA) values have also been calculated for the various materials, and are shown in Fig. S5 in the ESI.†
Overall performance of functionals – Cs halides, oxyanions, perovskites
The mean errors of all functionals (including geometry optimization) for the unit cell volume, isotropic shift, and quadrupolar coupling constant are summarized in Fig. 8.PBE characteristically overestimates the unit cell volume, while providing good (yet not the best) NMR parameters. Adding the D2 correction leads to an underestimation of the cell volume while marginally improving the accuracy of the chemical shift. Adding the D3 correction to PBE leads to very good overall performance, among the best in each of the three observables. XDM correction to PBE or B86bPBE, leads to a major underestimation of the cell volume, as well as to poor chemical shift prediction. rPBE+D2, using Zhang's parametrization for Cs, provides good accuracy in the geometry, but not in the NMR parameters. Schurko's D2* parametrization has a marginal effect only. Interestingly, PBEsol+D2 fails in the geometry, but performs well for the NMR parameters (but with larger than typical standard deviations). Likewise, revPBE+D3 fails in the geometry, but surprisingly excels in Cq prediction, while performing well for the shift. PBEsol+D3 performs very well, among the best, for the volume and the quadrupolar coupling, and excels in chemical shift. Its overall performance is among the best.
rVV10, vdW-DF-C6 and rev-vdW-DF2 excel at the unit cell prediction, while performing among the best in the chemical shift, and provide reasonably good quadrupolar coupling constants. vdW-DF3-opt1 is slightly inferior to them.
To sum up, this panel shows PBE+D3, PBEsol+D3, rVV10, vdW-DF-C6 and rev-vdW-DF2 to display good overall performance in predicting the geometry and NMR parameters of Cs salts, oxyanions and perovskites.
Extension to additional systems
To examine whether the results obtained using Cs salts, oxyanions and perovskites are transferrable to different Cs-containing systems, we applied the best performing functionals to very different systems, the structures of which are shown in Fig. 9.94 These systems differ from those in the set used in the previous section in several ways. CsCd(SCN)3 (Fig. 9(a)) is again a perovskite, but it contains a heavier element, Cd; in CsBPh4 (9b) Cs interacts with induced dipoles in the aromatic rings, unlike the ionic systems studied above; in [2.2.2] cryptand (9c) Cs interacts with electronegative atoms (O, N) in the altogether neutral complex via ion–dipole interactions, forming a stable coordination complex encapsulating the metal in a spheroidal cavity, somewhat analogously to Cs binding to zeolites, although with shorter Cs–O distances; CsB3O5 (9d) is a glassy system; in CsSc3F10 (9e) the coordination number of Cs is uncharacteristically high (18); CsPbI3 (9f–g) is challenging due to the presence of the heavy Pb atom, in addition to Cs. Hence, we also compared in Fig. S6 (ESI†) our calculations to those obtained including SOC showing that improvement is system dependent.95 | ||
| Fig. 9 Structures of (a) CsCd(SCN)3,96 (b) CsBPh4,97 (c) [Cs+(Cryptand[2.2.2])]I−,94 (d) CsB3O5,98 (e) CsSc3F10,99 (f) hexagonal CsPbI3,100 (g) tetragonal CsPbI3.101 | ||
Here again we examine the prediction of unit cell volume, the quadrupolar coupling constant, and the isotropic chemical shift. The results are shown in Fig. 10.
 | ||
| Fig. 10 (a) Unit cell volume, (b) Cq, (c) δiso and (d)–(f) error analysis. Symbols represent mean values; error bars represent standard deviation. See ESI,† Table S8 for references of experimental values. | ||
As shown in Fig. 10, here again the PBE functional overestimates the unit cell volume, while performing reasonably well (but not the best) in Cq. Its chemical shift performance is similar to that of most other functionals considered at this stage. PBE+D3 excels in the geometry, while being on-par with others in chemical shift, in line with the previous results (Fig. 4 and 6). RevPBE+D3 shows the largest error in chemical shift, despite a relatively good geometry prediction, unlike the previous results. PBEsol+D3, which was the best performer in chemical shift for the simple systems, followed closely by rev-vdW-DF2, is again comparable with the latter in all three observables. All non-local functionals tested here excel at geometry prediction. Rev-vdW-DF282 has previously been shown to successfully predict the structural, mechanical, cohesive and vibrational properties of both weakly and strongly bound solids,102 as well as the interlayer binding energy of multilayered solids103 and the adsorption energy of adsorbed molecules.104,105 It is however not yet widely used for chemical shift prediction,106 and we are unaware of Cq calculations using this functional. We are also unaware of studies using PBEsol+D3 for the calculation of NMR parameters.
To conclude, switching from small systems to more complicated and diverse compounds, PBEsol+D3 and rev-vdW-DF2 maintain their overall good performance and relative advantage. While we cannot determine if this conclusion holds for other materials due to the relatively small (20) set of distinct Cs sites studied here, these functionals appear to be a promising choice for the determination of Cs geometry and NMR parameters, in particular chemical shifts that are more useful for distinguishing between the chemical environments of Cs.
Summary & conclusions
A series of DFT functionals were screened for their performance and accuracy in the prediction of the geometry, quadrupolar coupling constants, and chemical shifts of cesium-133 in Cs-based halides, oxyanions and perovskites. The functionals chosen were those considering dispersive interactions, in order to faithfully predict the phase of Cs halides, while keeping a low computational cost. The best performing functionals, chosen mainly based on their performance with respect to geometry and isotropic chemical shift predictions, were then tested on seven additional materials, which exhibit chemical environments, bonding and coordination that are different than the above-mentioned inorganic compounds, including interactions of the Cs cation with polar groups resembling to some extent Cs caged in zeolites. Overall, we examined 20 different Cs sites from 18 different compounds. While no single functional performs best for all parameters (geometry, chemical shift, quadrupolar coupling), the results suggest that two of the functionals tested, rev-vdW-DF2 and PBEsol+D3, maintained their advantage in predicting both geometry and isotropic shifts in these seven additional diverse materials.Further work should be dedicated to the implementation of these functionals to the more challenging systems such as zeolites or geopolymers, used for the sequestration and immobilization of radioactive Cs species. More advanced (and computationally more expensive) meta-GGA and various hybrid functionals, possibly in combination with dispersion corrections may also be considered for these structurally complex systems.
As this work was the first stage of functional screening, it did not incorporate SOC, whose computational cost would have limited the number of tested systems and functionals. Further studies will have to consider the contribution of SOC to the accuracy and precision in predicting geometry and NMR parameters.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was partially funded by the Pazy Foundation grant ID 701-2025. We thank Orr Simon Lusky for preparing the CsNO3 solution.References
- K. Jagadeeswaraiah, M. Balaraju, P. S. Prasad and N. Lingaiah, Selective Esterification of Glycerol to Bioadditives over Heteropoly Tungstate Supported on Cs-Containing Zirconia Catalysts, Appl. Catal., A, 2010, 386(1–2), 166–170 CrossRef CAS
.
- U. Bachrach and A. Friedmann, Practical Procedures for the Purification of Bacterial Viruses, Appl. Microbiol., 1971, 22(4), 706–715 CrossRef CAS PubMed
.
- F. Bella, P. Renzi, C. Cavallo and C. Gerbaldi, Caesium for Perovskite Solar Cells: An Overview, Chem. – Eur. J., 2018, 24(47), 12183–12205 CrossRef CAS PubMed
.
- S. Kroeker, S. Schuller, J. E. Wren, B. J. Greer and A. Mesbah,
133Cs and 23 Na MAS NMR Spectroscopy of Molybdate Crystallization in Model Nuclear Glasses, J. Am. Ceram. Soc., 2016, 99(5), 1557–1564 CrossRef CAS
.
- S. Gin, P. Jollivet, M. Tribet, S. Peuget and S. Schuller, Radionuclides Containment in Nuclear Glasses: An Overview, Radiochim. Acta, 2017, 105(11), 927–959 CrossRef CAS
.
- M. Khalil and E. Merz, Immobilization of Intermediate-Level Wastes in Geopolymers, J. Nucl. Mater., 1994, 211(2), 141–148 CrossRef CAS
.
- S.-Y. Hong and F. P. Glasser, Alkali Binding in Cement Pastes, Cem. Concr. Res., 1999, 29(12), 1893–1903, DOI:10.1016/S0008-8846(99)00187-8
.
- H. Xu and J. S. J. van Deventer, Microstructural Characterisation of Geopolymers Synthesized from Kaolinite/Stilbite Mixtures Using XRD, MAS-NMR, SEM/EDX, TEM/EDX and HREM, Cem. Concr. Res., 2002, 32, 1705–1716 CrossRef CAS
.
- K. Komnitsas and D. Zaharaki, Geopolymerisation: A Review and Prospects for the Minerals Industry, Miner. Eng., 2007, 20(14), 1261–1277, DOI:10.1016/j.mineng.2007.07.011
.
- M. K. Ahn and L. E. Iton, Solid-State Cesium-133 NMR Studies of Cations in Cesium/Lithium/Sodium Zeolite A: An Example of Cation Dynamics Involving Three Sites, J. Phys. Chem., 1991, 95(11), 4496–4500, DOI:10.1021/j100164a059
.
- S. E. Ashbrook, K. R. Whittle, L. Le Polles and I. Farnan, Disorder and Dynamics in Pollucite from 133Cs and 27Al NMR, J. Am. Ceram. Soc., 2005, 88(6), 1575–1583, DOI:10.1111/j.1551-2916.2005.00294.x
.
- T. Ohkubo, T. Okamoto, K. Kawamura, R. Guégan, K. Deguchi, S. Ohki, T. Shimizu, Y. Tachi and Y. Iwadate, New Insights into the Cs Adsorption on Montmorillonite Clay from 133Cs Solid-State NMR and Density Functional Theory Calculations, J. Phys. Chem. A, 2018, 122(48), 9326–9337, DOI:10.1021/acs.jpca.8b07276
.
- M. Arbel-Haddad, Y. Harnik, Y. Schlosser and A. Goldbourt, Cesium Immobilization in Metakaolin-Based Geopolymers Elucidated by 133Cs Solid State NMR Spectroscopy, J. Nucl. Mater., 2022, 562, 153570, DOI:10.1016/j.jnucmat.2022.153570
.
- M. J. Lambregts and S. M. Frank, Characterization of Cesium Containing Glass-Bonded Ceramic Waste Forms, Microporous Mesoporous Mater., 2003, 64(1–3), 1–9, DOI:10.1016/S1387-1811(03)00486-4
.
- I. A. Ibarra, E. Lima, S. Loera, P. Bosch, S. Bulbulian and V. Lara, II. Cesium Leaching in CsA and CsX Zeolites: Use of Blocking Agents to Inhibit the Cesium Cation Mobility, J. Phys. Chem. B, 2006, 110(42), 21086–21091, DOI:10.1021/jp061926h
.
- P. Norby, F. I. Poshni, A. F. Gualtieri, J. C. Hanson and C. P. Grey, Cation Migration in Zeolites: An in Situ Powder Diffraction and MAS NMR Study of the Structure of Zeolite Cs(Na)−Y during Dehydration, J. Phys. Chem. B, 1998, 102(5), 839–856, DOI:10.1021/jp9730398
.
- B. J. Greer and S. Kroeker, Characterisation of Heterogeneous Molybdate and Chromate Phase Assemblages in Model Nuclear Waste Glasses by Multinuclear Magnetic Resonance Spectroscopy, Phys. Chem. Chem. Phys., 2012, 14(20), 7375–7383 RSC
.
- C. J. Pickard and F. Mauri, All-Electron Magnetic Response with Pseudopotentials: NMR Chemical Shifts, Phys. Rev. B: Condens. Matter Mater.
Phys., 2001, 63(24), 245101 CrossRef
.
- T. Charpentier, The PAW/GIPAW Approach for Computing NMR Parameters: A New Dimension Added to NMR Study of Solids, Solid State Nucl. Magn. Reson., 2011, 40(1), 1–20 Search PubMed
.
- C. Bonhomme, C. Gervais, F. Babonneau, C. Coelho, F. Pourpoint, T. Azais, S. E. Ashbrook, J. M. Griffin, J. R. Yates and F. Mauri, First-Principles Calculation of NMR Parameters Using the Gauge Including Projector Augmented Wave Method: A Chemist's Point of View, Chem. Rev., 2012, 112(11), 5733–5779 Search PubMed
.
- C. Schroeder, M. R. Hansen and H. Koller, Spatial Proximities between Brønsted Acid Sites, AlOH Groups, and Residual NH4+ Cations in Zeolites Mordenite and Ferrierite, J. Phys. Chem. C, 2023, 127(1), 736–745 CrossRef CAS
.
- E. Dib, S. Mintova, G. N. Vayssilov, H. A. Aleksandrov and M. Carravetta, Chemical Shift Anisotropy: A Promising Parameter to Distinguish the 29Si NMR Peaks in Zeolites, J. Phys. Chem. C, 2023, 127(22), 10792–10796 CrossRef CAS
.
- R. W. Hooper, C. Ni, D. G. Tkachuk, Y. He, V. V. Terskikh, J. G. Veinot and V. K. Michaelis, Exploring Structural Nuances in Germanium Halide Perovskites Using Solid-State 73Ge and 133Cs NMR Spectroscopy, J. Phys. Chem. Lett., 2022, 13(7), 1687–1696 CrossRef CAS PubMed
.
- C. J. Moon, J. Park, H. Im, H. Ryu, M. Y. Choi, T. H. Kim and J. Kim, Chemical Shift and Second-Order Quadrupolar Effects in the Solid-State 133Cs NMR Spectra of [Cs+ (Cryptand [2.2. 2])] X (X = I−, SCN−·H2O), Bull. Korean Chem. Soc., 2020, 41(7), 702–708 CrossRef CAS
.
- P. Toledano, K. Knorr, L. Ehm and W. Depmeier, Phenomenological Theory of the Reconstructive Phase Transition between the NaCl and CsCl Structure Types, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67(14), 144106 CrossRef
.
- E. Posnjak and R. W. Wyckoff, The Crystal Structures of the Alkali Halides. II., J. Wash. Acad. Sci., 1922, 12(10), 248–251 CAS
.
- J. E. Mayer, Dispersion and Polarizability and the van der Waals Potential in the Alkali Halides, J. Chem. Phys., 1933, 1(4), 270–279 CrossRef CAS
.
- F. London, The General Theory of Molecular Forces, Trans. Faraday Soc., 1937, 33, 8b–26 RSC
.
- F. Zhang, J. Gale, B. Uberuaga, C. Stanek and N. Marks, Importance of Dispersion in Density Functional Calculations of Cesium Chloride and Its Related Halides, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88(5), 054112 CrossRef
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77(18), 3865 CrossRef CAS PubMed
.
- S. S. Köcher, P. Schleker, M. Graf, R.-A. Eichel, K. Reuter, J. Granwehr and C. Scheurer, Chemical Shift Reference Scale for Li Solid State NMR Derived by First-Principles DFT Calculations, J. Magn. Reson., 2018, 297, 33–41 CrossRef PubMed
.
- F. Iwase,
Ab Initio Calculations of Nuclear Quadrupole Resonance Frequencies in Trichloroacetyl Halides: A Comparison of DFT and Experimental Data, Mater. Res. Express, 2020, 7(2), 025104 CrossRef CAS
.
- J. V. Reina, F. Civaia, A. F. Harper, C. Scheurer and S. Köcher, The EFG Rosetta Stone: Translating between DFT Calculations and Solid State NMR Experiments, Faraday Discuss, 2025, 255, 266–287 RSC
.
- J. D. Hartman and D. Capistran, Predicting 51V Nuclear Magnetic Resonance Observables in Molecular Crystals, Magn. Reson. Chem., 2024, 62(6), 416–428 CrossRef CAS PubMed
.
- J.-S. Park, Comparison Study of Exchange-Correlation Functionals on Prediction of Ground States and Structural Properties, Curr. Appl. Phys., 2021, 22, 61–64 CrossRef
.
- G. A. DiLabio and A. Otero-de-la-Roza, Noncovalent Interactions in Density Functional Theory. Rev, Comput. Chem., 2016, 29, 1–97 CAS
.
- T. B. E. Grønbech, K. Tolborg, D. Ceresoli and B. B. Iversen, Anharmonic Motion and Aspherical Nuclear Probability Density Functions in Cesium Halides, Phys. Rev. B, 2022, 105(10), 104113 CrossRef
.
- S. Grimme, Accurate Description of van der Waals Complexes by Density Functional Theory Including Empirical Corrections, J. Comput. Chem., 2004, 25(12), 1463–1473 CrossRef CAS PubMed
.
- S. Grimme, Semiempirical GGA-type Density Functional Constructed with a Long-range Dispersion Correction, J. Comput. Chem., 2006, 27(15), 1787–1799 CrossRef CAS PubMed
.
- A. Tkatchenko and M. Scheffler, Accurate Molecular van der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data, Phys. Rev. Lett., 2009, 102(7), 073005 CrossRef PubMed
.
- S. N. Steinmann, G. Csonka and C. Corminboeuf, Unified Inter-and Intramolecular Dispersion Correction Formula for Generalized Gradient Approximation Density Functional Theory, J. Chem. Theory Comput., 2009, 5(11), 2950–2958 CrossRef CAS PubMed
.
- S. N. Steinmann and C. Corminboeuf, Comprehensive Benchmarking of a Density-Dependent Dispersion Correction, J. Chem. Theory Comput., 2011, 7(11), 3567–3577 CrossRef CAS PubMed
.
- Y. Sun, Z. Zhang, V. M. Grigoryants, W. K. Myers, F. Liu, K. A. Earle, J. H. Freed and C. P. Scholes, The Internal Dynamics of Mini c TAR DNA Probed by Electron Paramagnetic Resonance of Nitroxide Spin-Labels at the Lower Stem, the Loop, and the Bulge, Biochemistry, 2012, 51(43), 8530–8541 CrossRef CAS PubMed
.
-
E. R. Johnson, The Exchange-Hole Dipole Moment Dispersion Model, in Non-covalent Interactions in Quantum Chemistry and Physics Theory and Applications, ed. A. O. de la Roza and G. A. DiLabio, Elsevier, 2017, pp 169–194 Search PubMed
.
- E. Caldeweyher, S. Ehlert, A. Hansen, H. Neugebauer, S. Spicher, C. Bannwarth and S. Grimme, A Generally Applicable Atomic-Charge Dependent London Dispersion Correction, J. Chem. Phys., 2019, 150(15), 154122 CrossRef PubMed
.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu, J. Chem. Phys., 2010, 132(15), 154104 CrossRef PubMed
.
- A. Ambrosetti, A. M. Reilly, R. A. DiStasio and A. Tkatchenko, Long-Range Correlation Energy Calculated from Coupled Atomic Response Functions, J. Chem. Phys., 2014, 140, 18A508 CrossRef PubMed
.
- A. D. Becke and E. R. Johnson, Exchange-Hole Dipole Moment and the Dispersion Interaction, J. Chem. Phys., 2005, 122(15), 154104 CrossRef PubMed
.
- A. D. Becke and E. R. Johnson, A Density-Functional Model of the Dispersion Interaction, J. Chem. Phys., 2005, 123(15), 154101 CrossRef PubMed
.
- A. Otero-De-La-Roza and E. R. Johnson, van der Waals Interactions in Solids Using the Exchange-Hole Dipole Moment Model, J. Chem. Phys., 2012, 136, 174109 CrossRef CAS PubMed
.
- Y. Andersson, D. C. Langreth and B. I. Lundqvist, van der Waals Interactions in Density-Functional Theory, Phys. Rev. Lett., 1996, 76(1), 102–105, DOI:10.1103/PhysRevLett.76.102
.
- J. F. Dobson and B. P. Dinte, Constraint Satisfaction in Local and Gradient Susceptibility Approximations: Application to a van der Waals Density Functional, Phys. Rev. Lett., 1996, 76(11), 1780 CrossRef CAS PubMed
.
- M. Dion, H. Rydberg, E. Schröder, D. C. Langreth and B. I. Lundqvist, van der Waals Density Functional for General Geometries, Phys. Rev. Lett., 2004, 92(24), 246401 CrossRef CAS PubMed
.
- O. A. Vydrov and T. Van Voorhis, Nonlocal van der Waals Density Functional Made Simple, Phys. Rev. Lett., 2009, 103(6), 063004 CrossRef PubMed
.
- D. Langreth, B. I. Lundqvist, S. D. Chakarova-Käck, V. Cooper, M. Dion, P. Hyldgaard, A. Kelkkanen, J. Kleis, L. Kong and S. Li, A Density Functional for Sparse Matter, J. Phys.: Condens. Matter, 2009, 21(8), 084203 CrossRef CAS PubMed
.
- K. Lee, É. D. Murray, L. Kong, B. I. Lundqvist and D. C. Langreth, Higher-Accuracy van der Waals Density Functional, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82(8), 081101 CrossRef
.
- O. A. Vydrov and T. Van Voorhis, Nonlocal van der Waals Density Functional: The Simpler the Better, J. Chem. Phys., 2010, 133(24), 244103 CrossRef PubMed
.
- R. Sabatini, T. Gorni and S. De Gironcoli, Nonlocal van der Waals Density Functional Made Simple and Efficient, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87(4), 041108 CrossRef
.
- D. Chakraborty, K. Berland and T. Thonhauser, Next-Generation Nonlocal van der Waals Density Functional, J. Chem. Theory Comput., 2020, 16(9), 5893–5911 CrossRef CAS PubMed
.
- I.-C. Lin, M. D. Coutinho-Neto, C. Felsenheimer, O. A. von Lilienfeld, I. Tavernelli and U. Rothlisberger, Library of Dispersion-Corrected Atom-Centered Potentials for Generalized Gradient Approximation Functionals: Elements H, C, N, O, He, Ne, Ar, and Kr, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75(20), 205131 CrossRef
.
- I. D. Mackie and G. A. DiLabio, Interactions in Large, Polyaromatic Hydrocarbon Dimers: Application of Density Functional Theory with Dispersion Corrections, J. Phys. Chem. A, 2008, 112(43), 10968–10976 CrossRef CAS PubMed
.
- G. A. DiLabio, Accurate Treatment of van der Waals Interactions Using Standard Density Functional Theory Methods with Effective Core-Type Potentials: Application to Carbon-Containing Dimers, Chem. Phys. Lett., 2008, 455(4–6), 348–353 CrossRef CAS
.
- E. Torres and G. A. DiLabio, A (Nearly) Universally Applicable Method for Modeling Noncovalent Interactions Using B3LYP, J. Phys. Chem. Lett., 2012, 3(13), 1738–1744 CrossRef CAS PubMed
.
- Y. Zhao, N. E. Schultz and D. G. Truhlar, Exchange-Correlation Functional with Broad Accuracy for Metallic and Nonmetallic Compounds, Kinetics, and Noncovalent Interactions, J. Chem. Phys., 2005, 123(16), 161103 CrossRef PubMed
.
- Y. Zhao and D. G. Truhlar, The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals, Theor. Chem. Acc., 2008, 120(1), 215–241 Search PubMed
.
- R. Peverati and D. G. Truhlar, Exchange–Correlation Functional with Good Accuracy for Both Structural and Energetic Properties While Depending Only on the Density and Its Gradient, J. Chem. Theory Comput., 2012, 8(7), 2310–2319 CrossRef CAS PubMed
.
- J. P. Perdew, S. Kurth, A. Zupan and P. Blaha, Accurate Density Functional with Correct Formal Properties: A Step beyond the Generalized Gradient Approximation, Phys. Rev. Lett., 1999, 82(12), 2544 CrossRef CAS
.
- J. Sun, A. Ruzsinszky and J. P. Perdew, Strongly Constrained and Appropriately Normed Semilocal Density Functional, Phys. Rev. Lett., 2015, 115(3), 036402 CrossRef PubMed
.
- T. Ohkubo, A. Takei, Y. Tachi, Y. Fukatsu, K. Deguchi, S. Ohki and T. Shimizu, New Approach To Understanding the Experimental 133Cs NMR Chemical Shift of Clay Minerals via Machine Learning and DFT-GIPAW Calculations, J. Phys. Chem. A, 2023, 127(4), 973–986 CrossRef CAS PubMed
.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni and I. Dabo, QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials, J. Phys.: Condens. Matter, 2009, 21(39), 395502 CrossRef PubMed
.
- P. Giannozzi, O. Andreussi, T. Brumme, O. Bunau, M. B. Nardelli, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli and M. Cococcioni, Advanced Capabilities for Materials Modelling with Quantum ESPRESSO, J. Phys.: Condens. Matter, 2017, 29(46), 465901 CrossRef CAS PubMed
.
- A. Dal Corso, Pseudopotentials Periodic Table: From H to Pu, Comput. Mater. Sci., 2014, 95, 337–350 CrossRef CAS
.
- B. Hammer, L. B. Hansen and J. K. Nørskov, Improved Adsorption Energetics within Density-Functional
Theory Using Revised Perdew-Burke-Ernzerhof Functionals, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59(11), 7413 CrossRef
.
- Y. Zhang and W. Yang, Comment on “Generalized Gradient Approximation Made Simple.”, Phys. Rev. Lett., 1998, 80(4), 890 CrossRef CAS
.
- S. T. Holmes, F. Alkan, R. J. Iuliucci, K. T. Mueller and C. Dybowski, Analysis of the Bond-valence Method for Calculating 29Si and 31P Magnetic Shielding in Covalent Network Solids, J. Comput. Chem., 2016, 37(18), 1704–1710 CrossRef CAS PubMed
.
- S. T. Holmes, S. Bai, R. J. Iuliucci, K. T. Mueller and C. Dybowski, Calculations of Solid-state 43Ca NMR Parameters: A Comparison of Periodic and Cluster Approaches and an Evaluation of DFT Functionals, J. Comput. Chem., 2017, 38(13), 949–956 CrossRef CAS PubMed
.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces, Phys. Rev. Lett., 2008, 100(13), 136406 CrossRef PubMed
.
- A. D. Becke, On the Large-gradient Behavior of the Density Functional Exchange Energy, J. Chem. Phys., 1986, 85(12), 7184–7187, DOI:10.1063/1.451353
.
- A. D. Becke and E. R. Johnson, Exchange-Hole Dipole Moment and the Dispersion Interaction Revisited, J. Chem. Phys., 2007, 127(15), 154108 CrossRef PubMed
.
- J. Tao, F. Zheng, J. Gebhardt, J. P. Perdew and A. M. Rappe, Screened van der Waals Correction to Density Functional Theory for Solids, Phys. Rev. Mater., 2017, 1(2), 020802 CrossRef
.
- K. Berland, D. Chakraborty and T. Thonhauser, van der Waals Density Functional with Corrected C 6 Coefficients, Phys. Rev. B, 2019, 99(19), 195418 CrossRef CAS
.
- I. Hamada, van der Waals Density Functional Made Accurate, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89(12), 121103 CrossRef
.
- S. T. Holmes and R. W. Schurko, Refining Crystal Structures with Quadrupolar NMR and Dispersion-Corrected Density Functional Theory, J. Phys. Chem. C, 2018, 122(3), 1809–1820 CrossRef CAS
.
- J. H. Yang, D. A. Kitchaev and G. Ceder, Rationalizing Accurate Structure Prediction in the Meta-GGA SCAN Functional, Phys. Rev. B, 2019, 100(3), 035132 CrossRef CAS
.
- O. Anatole von Lilienfeld and A. Tkatchenko, Two-and Three-Body Interatomic Dispersion Energy Contributions to Binding in Molecules and Solids, J. Chem. Phys., 2010, 132(23), 234109 CrossRef PubMed
.
- N. Pyper, The Cohesive Energetics of Solid Cesium Chloride, J. Chem. Phys., 2003, 118(5), 2308–2324 CrossRef CAS
.
- A. Aguado, Comment on “The Cohesive Energetics of Solid Cesium Chloride”[J. Chem. Phys. 118, 2308 (2003)], J. Chem. Phys., 2003, 119(16), 8765–8766 CrossRef CAS
.
- A. Otero-de-la-Roza and E. R. Johnson, Application of XDM to Ionic Solids: The Importance of Dispersion for Bulk Moduli and Crystal Geometries, J. Chem. Phys., 2020, 153(5), 054121 CrossRef CAS PubMed
.
- J. Czernek and J. Brus, Describing the Anisotropic 133Cs Solid State NMR Interactions in Cesium Chromate, Chem. Phys. Lett., 2017, 684, 8–13 CrossRef CAS
.
- J. Halliday, H. Hill and R. Richards, Solvent Isotope Shifts of Caesium Resonances in Dilute Salt Solutions, J. Chem. Soc., Chem. Commun., 1969, 5, 219–220 RSC
.
- A. Haase, M. Kerber, D. Kessler, J. Kronenbitter, H. Krüger, O. Lutz, M. Müller and A. Nolle, Nuclear Magnetic Shielding and Quadrupole Coupling of 133Cs in Cesium Salt Powders, Z. Naturforsch., A, 1977, 32(9), 952–956 Search PubMed
.
- S. Mooibroek, R. E. Wasylishen, R. Dickson, G. Facey and B. A. Pettitt, Simultaneous Observation of Shielding Anisotropies and Quadrupolar Splittings in Solid State 133Cs NMR Spectra, J. Magn. Reson., 1969, 66(3), 542–545 Search PubMed
.
- A. A. Kolganov, A. A. Gabrienko, I. Y. Chernyshov, A. G. Stepanov and E. A. Pidko, The Accuracy Challenge of the DFT-Based Molecular Assignment of 13 C MAS NMR Characterization of Surface Intermediates in Zeolite Catalysis, Phys. Chem. Chem. Phys., 2020, 22(41), 24004–24013 RSC
.
- B. Dietrich, J. M. Lehn and J. P. Sauvage, Les Cryptates, Tetrahedron Lett., 1969, 10(34), 2889–2892 CrossRef
.
- D. J. Kubicki, D. Prochowicz, A. Hofstetter, S. M. Zakeeruddin, M. Grätzel and L. Emsley, Phase Segregation in Cs-, Rb-and K-Doped Mixed-Cation (MA)x(FA)1–x PbI3 Hybrid Perovskites from Solid-State NMR, J. Am. Chem. Soc., 2017, 139(40), 14173–14180 CrossRef CAS PubMed
.
- G. Thiele and D. Messer, S-Thiocyanato-und N-Isothiocyanato-Bindungsisomerie in Den Kristallstrukturen von RbCd (SCN)3 Und CsCd (SCN)3, Z. Anorg. Allg. Chem., 1980, 464(1), 255–267 CrossRef CAS
.
- U. Behrens, F. Hoffmann and F. Olbrich, Solid-State Structures of Base-Free Lithium and Sodium Tetraphenylborates at Room and Low Temperature: Comparison with the Higher Homologues MB (C6H5)4 (M = K, Rb, Cs), Organometallics, 2012, 31(3), 905–913 CrossRef CAS
.
- J. Krogh-Moe, The Crystal Structure of Cesium Triborate, Cs2O. 3B2O3, Acta Crystallogr., 1960, 13(11), 889–892 CrossRef CAS
.
- A. Rakhmatullin, M. Allix, I. B. Polovov, D. Maltsev, A. V. Chukin, R. Bakirov and C. Bessada, Combining Solid State NMR, Powder X-Ray Diffraction, and DFT Calculations for CsSc3F10 Structure Determination, J. Alloys Compd., 2019, 787, 1349–1355 CrossRef CAS
.
- D. Trots and S. Myagkota, High-Temperature Structural Evolution of Caesium and Rubidium Triiodoplumbates, J. Phys. Chem. Solids, 2008, 69(10), 2520–2526 CrossRef CAS
.
- M. A. Fadla, B. Bentria, T. Dahame and A. Benghia, First-Principles Investigation on the Stability and Material Properties of All-Inorganic Cesium Lead Iodide Perovskites CsPbI3 Polymorphs, Phys. B, 2020, 585, 412118 CrossRef CAS
.
- F. Tran, L. Kalantari, B. Traoré, X. Rocquefelte and P. Blaha, Nonlocal van der Waals Functionals for Solids: Choosing an Appropriate One, Phys. Rev. Mater., 2019, 3(6), 063602 CrossRef CAS
.
- R. R. Del Grande, M. G. Menezes and R. B. Capaz, Layer Breathing and Shear Modes in Multilayer Graphene: A DFT-vdW Study, J. Phys.: Condens. Matter, 2019, 31(29), 295301 CrossRef CAS PubMed
.
- Y. Takano, N. Kobayashi and Y. Morikawa, Computational Study on Atomic Structures, Electronic Properties, and Chemical Reactions at Surfaces and Interfaces and in Biomaterials, J. Phys. Soc. Jpn., 2018, 87(6), 061013 CrossRef
.
- A. F. Z. Abidin and I. Hamada, Interaction of Water with Nitrogen-Doped Graphene, Phys. Rev. B, 2022, 105(7), 075416 CrossRef CAS
.
- D. Bernasconi, S. Bordignon, F. Rossi, E. Priola, C. Nervi, R. Gobetto, D. Voinovich, D. Hasa, N. T. Duong and Y. Nishiyama, Selective Synthesis of a Salt and a Cocrystal of the Ethionamide–Salicylic Acid System, Cryst. Growth Des., 2019, 20(2), 906–915 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp03451a |
| This journal is © the Owner Societies 2025 |


![[pentagon filled]](https://www.rsc.org/images/entities/char_e13d.gif) ) dispersion correction; (d) non-local (◆) and hybrid (★) functionals.
) dispersion correction; (d) non-local (◆) and hybrid (★) functionals.
