Open Access Article
Open Access ArticleLadder-type 1D coordination polymer of Dy(III): synthesis and investigation of magnetic behaviour†
Naushad Ahmed *a,
Rizwan Nabib,
Xiaoguang Zhang
*a,
Rizwan Nabib,
Xiaoguang Zhang b and
Donald J. Darensbourg
b and
Donald J. Darensbourg *a
*a
aDepartment of Chemistry, Texas A&M University, College Station, TX 77843, USA. E-mail: naushad.ahmed@tamu.edu; djdarens@chem.tamu.edu
bDepartment of Physics, Center for Molecular Magnetic Quantum Materials and Quantum Theory Project, University of Florida, Gainesville, Florida 32611, USA. E-mail: xgz@ufl.edu
First published on 8th June 2025
Abstract
Herein, we report the construction of a H-bonded ladder-type Dy(III) based 1D coordination polymer 1-Dypoly using a 2,6-diacetylpyridine bis-salicyl hydrazone (H4L) ligand system under thermal condition. The X-ray diffraction studies on the reported coordination polymer revealed that it crystallizes in a monoclinic system having the P21/c space group. The static and dynamic magnetic behavior of 1-Dypoly was investigated using combined experimental and theoretical tools. The zero-field out-of-phase signals for 1-Dypoly were observed, but with fast under-barrier magnetic relaxation processes. CASSCF-based ab initio and BS-DFT calculations were used to rationalize our experimental findings.
Introduction
In the construction of 1D, 2D coordination polymers (CPs), including metal–organic frameworks, MOFs architecture, the ligands' design plays a vital role in polymeric structure propagation and defining the geometry around the metal ions for various studies.1–6 There are several examples of multidentate multi-arm ligand systems used for the construction of transition and lanthanide-based CPs and MOFs.7–9 The crystal field imposed by ligating atoms around the metal ions is reported to governs the spin Hamiltonian SH parameters, which in turn governs the magnetic behaviour of d- and f-block metal ions.10–13 The fine-tuning in the crystal field around d- and f-block metal ions under different ligand architectures can affect their magnetic and photophysical properties.14–19 The last few decades have witnessed probing the magnetic properties of lanthanide and transition metal discrete complexes and coordination polymers at the molecular level as single-molecule magnets SMMs that have the feature of slow magnetic relaxations.20–22 The research area of SMMs has garnered significant attention due to their proposed technological applications in high-density storage devices, molecular spintronics, and quantum computations.23,24 Due to the unquenched orbital angular momentum and strong spin–orbit coupling, lanthanides exhibit large magnetic anisotropy and are widely used in SMM construction.25–29 Among the several symmetries, D5h (for pentagonal bipyramidal geometry)30,31 and D6h (for hexagonal bipyramidal geometry)32–34 are more popular as in these geometries Dy(III) ions stabilize oblate ground state mJ = 15/2, and have been reported to show high-performance SMMs with an energy barrier up to ∼1600 K that are next to metallocene Dy(III) complexes35,36 having D5d symmetry and an energy barrier up to ∼2200 K. The hydrazone ligands derived from Schiff base condensation of 2,6-diacetylpyridine (or 2,6-diformylpyridine) and hydrazide or phenyl-substituted hydrazides are widely explored in constructing the f-block as well as d-block metal coordination complexes. This is due to their ability to provide an equatorial rigid pentagonal plane through N3O2 donor atoms around metal ions.37–39 Interestingly, the presence of phenolic –OH on the phenyl ring of 2,6-diacetylpyridine bis-salicylhydrazone ligands opens further coordination opportunities and assembles clusters of multinuclear lanthanide(III) and 3d-lanthanide(III) complexes, including CPs (Fig. 1).40–44 Several coordination polymers have been reported to date with various applications such as luminescence,45 proton conduction,2 gas sorption,46 and magnetism.47–49 Since lanthanide metal ions exhibit high coordination numbers and are flexible to variable geometries, the construction of 1D, 2D, and MOFs coordination polymers is challenging to rational design and synthesis. Not only a synthetic challenge, but these polymeric materials also face the challenge to show the expected magnetic property, such as slow magnetic relaxations. Recently, we and others have reported that the intra- and intermolecular dipolar magnetic interactions through hydrogen bonding and short contacts may trigger the under-barrier magnetic relaxations such as QTM, and Raman, which may hamper the SMM behaviour.7,50 This is not surprising in the cases of coordination polymers CPs having extensive hydrogen bonding, π–π, and CH–π stacking interactions that provide structural stability and, on the other hand, may also promote dipolar magnetic interactions, which can suppress their SMM properties.In this work, we report the construction of a 1D ladder-type coordination polymer of Dy(III) utilizing the phenolic –OH of 2,6-bis(1-salicyloylhydrazonoethyl)-pyridine (H4L) ligand under thermal conditions. Our several efforts to synthesize Y(III) and Gd(III) coordination polymers under similar conditions as for 1-Dypoly were not successful. We have investigated the static and dynamic magnetic properties of the reported CPs using experimental and theoretical tools.
Results and discussion
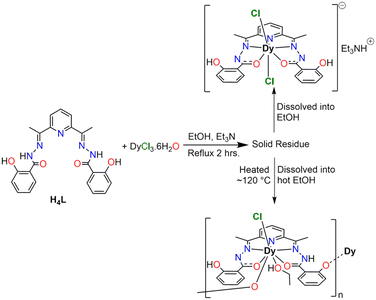
The reaction of 2,6-diacetylpyridine bis-salicyl hydrazone H4L ligand having N3O2 equatorial donor atoms with hydrated Dy(III) chloride salts in ethanol has led to the isolation of a 1D coordination polymer chain 1-Dypoly upon heating the solid residue to ∼120 °C temperature and dissolving it in hot ethanol solvent (Scheme 1). The dissolution of solid residue directly (as obtained from the reaction) in ethanol solvent produces the [H2LDy(Cl)2]Et3NH complex, having Dy(III) in pentagonal bipyramidal geometry, which has been reported elsewhere.37The formation of 1-Dypoly is proposed to occur via tautomerism at higher temperatures; that is, a proton from one of the phenolic –OH permanently shifts to a neighbour deprotonated nitrogen, making the phenolate oxygen available to coordinate, which acts as a linker in this 1-Dypoly. Simultaneously, removing one of the axial Cl− from other molecules invites phenolate and ethanol ligands to coordinate with Dy(III) from the same side. In this way, the 1D structure of 1-Dypoly propagates using the phenolate ligating oxygen atoms (Scheme 2).
X-ray diffraction studies revealed that 1-Dypoly crystallized in a monoclinic crystal system having the P21/c space group. From the crystal structure analysis and elucidation, it is found that the asymmetric unit (ASU) of 1-Dypoly consists of one doubly deprotonated H2L2− ligand, which uses its N3O2 donor atoms to create a distorted pentagonal plane around the Dy(III) metal ion from the equatorial positions. Ethanol solvent was coordinated to one of the axial sites of the Dy(III) ion. One of the phenolic oxygens (O1, O1#) present in the ligand was found to be deprotonated and coordinated with the neighbouring Dy(III) metal ion from the same axial side where ethanol is present. This deprotonated phenolic oxygen is responsible for the 1D growth of the ladder-type coordination polymer chain in 1-Dypoly (see Fig. 2).
The Cl− ligand occupies the other axial position. The Dy(III) ion is surrounded by eight coordinating atoms with an N3O5 environment in 1-Dypoly. The continuous shape measurement (CShM)51 analysis confirms that the distorted triangular dodecahedron geometry (CShM = 4.724) has D2d symmetry around each Dy(III) ion in the chain.
Crystal structure description
The crystal structure analysis of 1-Dypoly confirms the bond distance of 2.524 Å for Dy–N1 (N1 belongs to pyridine) and the bond distances of 2.543 Å and 2.456 Å, respectively, for Dy–N3 and Dy–N4 (N3 and N4 belong to the hydrazides unit). The shorter bond for Dy–N4 indicates that N4 is deprotonated while N3 is not. Furthermore, the equatorial Dy–O2 and Dy–O4 bond distances are 2.273 Å and 2.381 Å, respectively, and show a shorter distance for the deprotonated side of the hydrazone ligand. Along with the bond distances, we also observed a significant difference in the bond angles: ∠N2–N3–C = 115.7°, while ∠N4–N5–C = 110.5°. The bond angle around 111° indicates the absence of H on nitrogen N5.52The axial Dy–Cl and Dy–O1 (O1 from phenolic OH) bond distances were 2.695 Å and 2.306 Å, respectively, while the axial Dy–O5 (O5 from ethanol) bond distance was 2.393 Å.
Primarily the phenolate oxygen donor atoms of the hydrazone ligand play a vital role in propagating the 1D structure in 1-Dypoly, while the π-electron clouds of the pyridine and benzene rings of the neighboring unit further support the structure by π–π stacking interactions within the range of 3.65 Å distance (Fig. 2). The intrachain Dy⋯Dy distance was found to be 7.475 Å. The packing structure of 1-Dypoly shows the shortest interchain Dy⋯Dy distance of 10.37 Å, while the interchain distance grown through the hydrogen bonding network (Fig. S2 in ESI†) shows the shortest Dy⋯Dy distance of 7.546 Å. The shorter interchain Dy⋯Dy is due to the extensive interchain hydrogen bonding through the axially coordinated ethanol and Cl− ligands (Fig. 3 and S2 in ESI†).
 | ||
| Fig. 3 Intra (sky blue dotted bonds) and intermolecular (light green dotted bonds) hydrogen bonding network in 1-Dypoly. | ||
Magnetic properties
Direct current magnetic susceptibility measurements were performed on the polycrystalline powder sample of 1-Dypoly in the temperature range of 2–300 K under 1 kOe magnetic field (Fig. 4). The collected data was processed considering the dinuclear molecule having two Dy(III) ions and two full hydrazone ligands along with coordinated Cl− and ethanol ligands. The observed room temperature χMT value of 27.68 cm−1 K mol−1 for 1-Dypoly was found to be slightly lower but in close agreement with the theoretical value of 28.34 cm−1 K mol−1 for two uncoupled DyIII (S = 5/2, g = 4/3, 6H15/2). Upon decreasing the temperature, a gradual decrease in the χMT value was observed up to 20 K and below this temperature, a sudden drop in the χMT value was observed and reached the minimum value of 20 cm−1 K mol−1 at 2 K. This can be attributed to the depopulation of the excited Stark or ±mJ levels of DyIII ion, while the low temperature χMT profile of 1-Dypoly is due to the intra- and interchain antiferromagnetic interactions. As stated in the crystal structure description section, the shortest Dy⋯Dy inter- and intrachain distances were found to be ∼7 Å, suggesting dipolar magnetic interactions are highly possible in 1-Dypoly. Field-dependent magnetization data were collected at 2–8 K temperature and magnetic field from zero to 70 kOe (Fig. S6 in ESI†). | ||
| Fig. 4 DC magnetic susceptibility under 1 kOe field. The green solid curve represents the simulated data for 1-Dypoly. | ||
To estimate the inter- and intrachain magnetic exchange between Dy(III) ions, we have utilized the computed crystal field axial parameters Bqk = −1.33 (where k = 2 and q = 0) and gDy value of 1.33 to simulate the magnetic susceptibility data using the PHI program53 (Fig. 4). In this case, since the overall exchange energy for intra- or interchain Dy–Dy magnetic interactions can be represented as J or zJ, during simulation, we considered the zJ, which reproduced the experimental data well with zJ = −0.02 cm−1.
Dynamic magnetic susceptibility measurements were carried out under zero and applied field in the frequency range of 1–103 Hz and temperature range 1.8 to 4 K. The field-dependent ac magnetic susceptibility measurements at 1.8 K and applied field up to 3 kOe indicate no clear maxima for the out-of-phase susceptibility 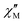 signals in the instrumental frequency range, while the zero field broad
signals in the instrumental frequency range, while the zero field broad  signals appeared at higher frequency above 102 Hz, which suggest that 1-Dypoly behaves as zero-field single-molecule magnets but with faster under barrier magnetic relaxations such as quantum tunnelling (QTM) and Raman processes. The application of a dc magnetic field is reported to suppress the QTM processes between the degenerate ±mJ states and supports arriving and or resolving the
signals appeared at higher frequency above 102 Hz, which suggest that 1-Dypoly behaves as zero-field single-molecule magnets but with faster under barrier magnetic relaxations such as quantum tunnelling (QTM) and Raman processes. The application of a dc magnetic field is reported to suppress the QTM processes between the degenerate ±mJ states and supports arriving and or resolving the  signals maximum.54 On the contrary, we observed the intensity of the applied field
signals maximum.54 On the contrary, we observed the intensity of the applied field  signals decrease with increasing field strength; this seems to be a direct magnetic relaxation process that becomes active and faster, and for this reason, 1-Dypoly fails to show improved SMM behaviour.
signals decrease with increasing field strength; this seems to be a direct magnetic relaxation process that becomes active and faster, and for this reason, 1-Dypoly fails to show improved SMM behaviour.
Despite the broad 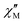 signals observed at zero field, we were interested in understanding the slow-magnetic relaxation behavior of 1-Dypoly and the presence of under-barrier magnetic relaxation at higher temperatures. To this end, we collected detailed ac data at zero-field in the temperature range of 1.8 K to 4 K. We observed
signals observed at zero field, we were interested in understanding the slow-magnetic relaxation behavior of 1-Dypoly and the presence of under-barrier magnetic relaxation at higher temperatures. To this end, we collected detailed ac data at zero-field in the temperature range of 1.8 K to 4 K. We observed 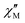 signals maximum centred at 210 Hz, which were not significantly shifted with temperature (Fig. 5). We constructed Cole–Cole plots
signals maximum centred at 210 Hz, which were not significantly shifted with temperature (Fig. 5). We constructed Cole–Cole plots  but were unable to fit the curves to extract relaxation times (τ) and relaxation distribution parameter (α).
but were unable to fit the curves to extract relaxation times (τ) and relaxation distribution parameter (α).
 | ||
| Fig. 5 Zero-field out-of-phase magnetic susceptibility (top A) and Cole–Cole plot (bottom B) for 1-Dypoly. | ||
However, the ab initio CASSCF/SO-RASSI/SINGLE_ANISO calculations performed on the single crystal X-ray structure of 1-Dypoly indicate the axial nature of Dy(III) ion with ground state gzz = 19.47 and the first excited state Kramers' doublet KD2 located at 115 cm−1 from the ground state KD1 (see the computational studies). These observations suggest that the extensive hydrogen bonding (inter- and intrachain) may enforce dipolar interactions and suppress the SMM properties in 1-Dypoly by triggering the underbarrier magnetic relaxation processes.
Computational studies
The CASSCF-based ab initio calculations were performed on the X-ray structure of 1-Dypoly using OpenMolcas v24.02 software.55 Since the asymmetric unit of 1-Dypoly consists of one metal ion, we have considered all the ligands present in the ASU when performing our calculations. We also performed DFT calculations to understand the magnetic exchange between intrachain as well as interchain Dy(III) ions. The detailed ab initio and DFT calculation methods were discussed in the Experimental section. The CASSCF computed spin-free and spin–orbit states are provided in Tables S3 and S4 in the ESI.† The computed g-tensors associated with the ground state Kramers doublet KD1 (arising from 6H15/2) of Dy(III) ion (gxx = 0.104, gyy = 0.271, and gzz = 19.470) suggest that 1-Dypoly has a moderately strong axial anisotropic nature. Moreover, as we move further from the ground state, the transversal anisotropy is increased as gxx = 3.052, gyy = 5.669, and gzz = 12.611 at the first excited state (Table 1). The LoProp charge densities on the coordinated atoms are listed in Table S5.† The ground state main magnetization axis (gzz) was found perpendicular to N3O2 equatorial plane around Dy(III) while parallel to the axially coordinated ligands. The ground state gzz axis is oriented along the Dy–Cl (charge density on Cl = −0.87) bond and between the Dy–O5 and Dy–O1 (charge density on O5 = −0.48 and O1 = −0.80) bonds. The gzz axis deviates about 13.63° from the Dy–Cl axis (Fig. 6). Further, computed wave function analysis (Table S6†) confirms the ground state mJ = ±15/2 (KD1) with purity up to ∼95% and the transition magnetic moment 6.25 × 10−2 μB between KD1 indicates magnetization relaxation through a tunnelling process is slow enough to observe zero-field SMM behavior for 1-Dypoly. It should have an ab initio energy barrier of ∼115 cm−1. To shed light on this observation, we also computed crystal parameters using the Hamiltonian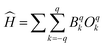 where the terms Bqk and Oqk are the crystal field parameters and Stevens' operator, respectively. The computed crystal field parameters are listed in Table S7 in the ESI.† The axial term B02 found to be negative (B02 = −1.33) that further suggests easy axis of anisotropy associated Dy(III) ion. Despite the strong axiality associated with Dy(III) ion, the observed experimental dynamic magnetic behavior of 1-Dypoly exhibits faster under-barrier magnetic relaxations, such as QTM and or Raman processes. The presence of extensive H-bonding is expected to trigger these under-barrier magnetic relaxation processes by promoting dipolar magnetic interactions.
where the terms Bqk and Oqk are the crystal field parameters and Stevens' operator, respectively. The computed crystal field parameters are listed in Table S7 in the ESI.† The axial term B02 found to be negative (B02 = −1.33) that further suggests easy axis of anisotropy associated Dy(III) ion. Despite the strong axiality associated with Dy(III) ion, the observed experimental dynamic magnetic behavior of 1-Dypoly exhibits faster under-barrier magnetic relaxations, such as QTM and or Raman processes. The presence of extensive H-bonding is expected to trigger these under-barrier magnetic relaxation processes by promoting dipolar magnetic interactions.
| Mult. | E (cm−1) | gx | gy | gz |
|---|---|---|---|---|
| 1 | 0.0 | 0.104 | 0.271 | 19.470 |
| 2 | 115.1 | 3.052 | 5.670 | 12.611 |
| 3 | 153.7 | 8.246 | 5.439 | 0.768 |
| 4 | 179.6 | 1.575 | 6.337 | 11.830 |
| 5 | 216.6 | 0.365 | 2.325 | 10.974 |
| 6 | 301.5 | 1.040 | 2.113 | 14.921 |
| 7 | 392.8 | 0.247 | 2.282 | 14.249 |
| 8 | 415.9 | 0.319 | 2.164 | 15.432 |
Further to understand the presence of inter and intrachain magnetic exchange (if any) in 1-Dypoly, we modelled dimeric Gd2mod-1 (intrachain) and Gd2mod-2 (interchain) models respectively by replacing the Dy with Gd from the grown structures and performed BS-DFT calculations using B3LYP56 density-functional. The computed spin densities on Gd(III) ions for high-spin and broken symmetry are listed in Table S8 in the ESI.† The spin density plots are given in Fig. 7. We have used the H = −2JS1S2 formalism to calculate the magnetic exchange parameter, leading to the weak antiferromagnetic coupling parameter of JGd–Gd = −0.0003 cm−1 between two Gd ions in Gd2mod-1 while for Gd2mod-2 this is −0.0001 cm−1. Upon rescaling the J values to the spin of Dy(III) (S = 5/2) we obtained JDy–Dy = −0.0002 cm−1 and −0.00007 cm−1 for the intra and interchain Dy–Dy magnetic exchange interactions respectively. The magnetic exchange is found to be very weak but it seems influence the dynamic properties strongly.
Experimental
Ligand H4L was synthesized following the synthetic procedure57 and used to isolate the coordination polymer 1-Dypoly. A Bruker D8 Venture APEX-II CCD diffractometer was employed for single-crystal X-ray diffraction data collection. SHELXTL and Olex2.4 software were used for X-ray data analysis and structural solutions. The Quantum Design MPMS3 SQUID magnetometer was used for magnetic measurements. A straw was utilized as the sample holder, and the crushed powder sample was tightly wrapped in Teflon tape and inserted into the straw.Synthesis of 1-Dypoly
In a round-bottom flask, 50 mg of H4L ligand (0.1159 mmol) was suspended in 50 mL of ethanol. At 60 °C, the dropwise addition of an ethanolic solution of DyCl3·6H2O (43.68 mg, 0.1159 mmol) turns the reaction mixture a transparent yellow color. After 1 hour of stirring at the same temperature, the reaction mixture was brought to room temperature, and two equivalents of triethylamine (0.032 mL, 0.2317 mmol) were added. The slightly turbid reaction mixture was further stirred at 60 °C for two hours, and the solvent was evaporated to dryness. The yellow solid residue was heated up to 120 °C for 2–3 hours. During this period of heating, the solid residue turned dark yellow, which was dissolved in 2 mL of hot ethanol solvent, and hexane was diffused into this solution. The X-ray quality yellow-colored single crystals were grown within a week in good yield, 35 mg (45% based on H4L ligand). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds characteristic bands appeared at ν(C
O bonds characteristic bands appeared at ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) = 1612 cm−1 and ν(C
N) = 1612 cm−1 and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1593 cm−1 respectively in FT-IR spectrum (Fig. S3†). Powder X-ray diffraction data collected in a 2θ range of 5 to 40° and plotted over the pattern obtained from single-crystal X-ray diffraction (Fig. S4†). The thermogravimetric analysis revealed that 1-Dypoly is thermally stable up to 300 °C (Fig. S5†) with almost no weight loss.
O) = 1593 cm−1 respectively in FT-IR spectrum (Fig. S3†). Powder X-ray diffraction data collected in a 2θ range of 5 to 40° and plotted over the pattern obtained from single-crystal X-ray diffraction (Fig. S4†). The thermogravimetric analysis revealed that 1-Dypoly is thermally stable up to 300 °C (Fig. S5†) with almost no weight loss.
Computational details
Conclusions
In summary, we have utilized the phenolic –OH of 2,6-diacetylpyridine bis-salicyl hydrazone (H4L) ligand system to construct a 1D ladder-type Dy(III) coordination polymer. The proton of the phenolic –OH shifted to one of the deprotonated N atoms at around 120 °C and facilitated the 1D structure propagation through a phenolate linker. Dynamic magnetic measurement of reported polymer indicates zero-field SMM but has faster QTM and or Raman magnetic relaxation processes. CASSCF-based ab initio calculation revealed that the Dy(III) ion experiences a moderate axial nature, but due to extensive inter- and intrachain hydrogen bonding, particularly through the axially coordinated ethanol and Cl− ligands, triggers the under-barrier magnetic relaxation and suppresses SMM behavior.Data availability
We have provided the data in the ESI.† X-ray data for the crystal structure can be accessed through the CCDC 2433773.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
DJD gratefully acknowledges the Robert A. Welch Foundations (A-0923) for financial support. NA thanks the Department of Chemistry, Texas A&M University, for accessing the research facilities. RN and Zhang thank UF HiPerGator for the computational facilities. This research was supported as part of the Center for Molecular Magnetic Quantum Materials, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award No. DE-SC0019330.References
- C.-L. Chen, C. Wang, X.-Y. Zheng, R. Zhang, Y. Xu, G.-L. Zhuang, L.-S. Long, L.-S. Zheng, X.-J. Kong and Y. Cao, J. Am. Chem. Soc., 2023, 145, 16983–16987 CrossRef CAS PubMed.
- S. C. Pal, D. Mukherjee, Y. Oruganti, B. G. Lee, D.-W. Lim, B. Pramanik, A. K. Manna and M. C. Das, J. Am. Chem. Soc., 2024, 146, 14546–14557 CrossRef CAS PubMed.
- M.-X. Hong, Z.-H. Wu, C. Chen, L. Cao, H. Tian and X.-Y. Zheng, Polyhedron, 2024, 251, 116847 CrossRef CAS.
- Z.-P. Zhao, K. Zheng, H.-R. Li, C.-H. Zeng, S. Zhong, S. W. Ng, Y. Zheng and Y. Chen, Inorg. Chim. Acta, 2018, 482, 340–346 CrossRef CAS.
- F. Li, L. Xu, B. Bi, X. Liu and L. Fan, CrystEngComm, 2008, 10, 693–698 RSC.
- G. Mínguez Espallargas and E. Coronado, Chem. Soc. Rev., 2018, 47, 533–557 RSC.
- G. A. Ramakant, N. Ahmed, I. Tarannum, S. Mehta, M. Nandeshwar, A. Mondal, S. K. Singh and G. Prabusankar, Cryst. Growth Des., 2022, 22, 6046–6055 CrossRef CAS.
- C. Theppitak, S. Jiajaroen, N. Chongboriboon, S. Chanthee, F. Kielar, W. Dungkaew, M. Sukwattanasinitt and K. Chainok, Molecules, 2021, 26, 4428 CrossRef CAS PubMed.
- M. Yadav, A. Bhunia, S. K. Jana and P. W. Roesky, Inorg. Chem., 2016, 55, 2701–2708 CrossRef CAS PubMed.
- A. K. Bar, C. Pichon and J.-P. Sutter, Coord. Chem. Rev., 2016, 308, 346–380 CrossRef CAS.
- G. Charron, F. Bellot, F. Cisnetti, G. Pelosi, J.-N. Rebilly, E. Rivière, A.-L. Barra, T. Mallah and C. Policar, Chem. – Eur. J., 2007, 13, 2774–2782 CrossRef CAS PubMed.
- T. Goswami and A. Misra, J. Phys. Chem. A., 2012, 116, 5207–5215 CrossRef CAS PubMed.
- E. M. Manohar, T. Guizouarn, N. Ahmed, R. Herchel, F. Pointillart, S. Das and A. Dey, Appl. Organomet. Chem., 2024, 38, e7441 CrossRef CAS.
- J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078–2085 RSC.
- C. Wegeberg, D. Häussinger, S. Kupfer and O. S. Wenger, J. Am. Chem. Soc., 2024, 146, 4605–4619 CrossRef CAS PubMed.
- M. Kong, X. Feng, J. Wang, Y.-Q. Zhang and Y. Song, Dalton Trans., 2021, 50, 568–577 RSC.
- A. Raza and M. Perfetti, Coord. Chem. Rev., 2023, 490, 215213 CrossRef CAS.
- H. Wu, M. Li, B. Yin, Z. Xia, H. Ke, Q. Wei, G. Xie, S. Chen and S. Gao, Dalton Trans., 2019, 48, 16384–16394 RSC.
- S. Roy, P. Shukla, N. Ahmed, M.-H. Du, I. Tarannum, X.-J. Kong, T. Gupta, S. K. Singh and S. Das, Dalton Trans., 2022, 51, 18187–18202 RSC.
- A. Zabala-Lekuona, J. M. Seco and E. Colacio, Coord. Chem. Rev., 2021, 441, 213984 CrossRef CAS.
- A. B. Ruiz-Muelle, A. García-García, A. A. García-Valdivia, I. Oyarzabal, J. Cepeda, J. M. Seco, E. Colacio, A. Rodríguez-Diéguez and I. Fernández, Dalton Trans., 2018, 47, 12783–12794 RSC.
- L. Rigamonti, M. Vaccari, F. Roncaglia, C. Baschieri and A. Forni, Magnetochemistry, 2018, 4(4), 43 CrossRef CAS.
- J. J. Baldoví, L. E. Rosaleny, V. Ramachandran, J. Christian, N. S. Dalal, J. M. Clemente-Juan, P. Yang, U. Kortz, A. Gaita-Ariño and E. Coronado, Inorg. Chem. Front., 2015, 2, 893–897 RSC.
- J. J. Baldoví, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño and A. Palii, Inorg. Chem., 2012, 51, 12565–12574 CrossRef PubMed.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed.
- N. Ahmed and D. J. Darensbourg, Cryst. Growth Des., 2025, 25, 1992–2001 CrossRef CAS.
- A. Dey, P. Kalita and V. Chandrasekhar, ACS Omega, 2018, 3, 9462–9475 CrossRef CAS PubMed.
- N. Ahmed and K. U. Ansari, Dalton Trans., 2022, 51, 8766–8776 RSC.
- N. Ahmed and K. Uddin Ansari, Dalton Trans., 2022, 51, 4122–4134 RSC.
- J. Liu, Y.-C. Chen, J.-L. Liu, V. Vieru, L. Ungur, J.-H. Jia, L. F. Chibotaru, Y. Lan, W. Wernsdorfer, S. Gao, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 5441–5450 CrossRef CAS PubMed.
- Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829–2837 CrossRef CAS PubMed.
- S. Jia, X. Zhu, B. Yin, Y. Dong, A. Sun and D.-F. Li, Cryst. Growth Des., 2023, 23, 6967–6973 CrossRef CAS.
- J. Li, S. Gómez-Coca, B. S. Dolinar, L. Yang, F. Yu, M. Kong, Y.-Q. Zhang, Y. Song and K. R. Dunbar, Inorg. Chem., 2019, 58, 2610–2617 CrossRef CAS PubMed.
- A. B. Canaj, S. Dey, E. R. Martí, C. Wilson, G. Rajaraman and M. Murrie, Angew. Chem., Int. Ed., 2019, 58, 14146–14151 CrossRef CAS PubMed.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400–1403 CrossRef CAS PubMed.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Angew. Chem., Int. Ed., 2017, 56, 11445–11449 CrossRef CAS PubMed.
- A. K. Bar, P. Kalita, J.-P. Sutter and V. Chandrasekhar, Inorg. Chem., 2018, 57, 2398–2401 CrossRef CAS PubMed.
- P. Kalita, N. Ahmed, A. K. Bar, S. Dey, A. Jana, G. Rajaraman, J.-P. Sutter and V. Chandrasekhar, Inorg. Chem., 2020, 59, 6603–6612 CrossRef CAS PubMed.
- A. K. Bar, N. Gogoi, C. Pichon, V. M. L. D. P. Goli, M. Thlijeni, C. Duhayon, N. Suaud, N. Guihéry, A.-L. Barra, S. Ramasesha and J.-P. Sutter, Chem. – Eur. J., 2017, 23, 4380–4396 CrossRef CAS PubMed.
- P. Kalita, K. Kumari, P. Kumar, V. Kumar, S. K. Singh, G. Rogez and V. Chandrasekhar, Dalton Trans., 2024, 53, 10521–10535 RSC.
- H.-Q. Li, G.-L. Wang, Y.-C. Sun, Y.-Q. Zhang and X.-Y. Wang, Inorg. Chem., 2022, 61, 17537–17549 CrossRef CAS PubMed.
- H.-Q. Li, Y.-C. Sun, L. Shi, F.-L. Chen, F.-X. Shen, Y. Zhao and X.-Y. Wang, Inorg. Chem., 2022, 61, 2272–2283 CrossRef CAS PubMed.
- V. Singh, L. T. Suresh, J.-P. Sutter and A. K. Bar, Dalton Trans., 2024, 53, 7436–7449 RSC.
- R. Wang, H. Wang, J. Wang, F. Bai, Y. Ma, L. Li, Q. Wang, B. Zhao and P. Cheng, CrystEngComm, 2020, 22, 2998–3004 RSC.
- P. Chen, Q. Li, S. Grindy and N. Holten-Andersen, J. Am. Chem. Soc., 2015, 137, 11590–11593 CrossRef CAS PubMed.
- M. Lippi and M. Cametti, Coord. Chem. Rev., 2021, 430, 213661 CrossRef CAS.
- T. Grancha, J. Ferrando-Soria, M. Castellano, M. Julve, J. Pasán, D. Armentano and E. Pardo, Chem. Commun., 2014, 50, 7569–7585 RSC.
- X.-T. Dong, M.-Q. Yu, Y.-B. Peng, G.-X. Zhou, G. Peng and X.-M. Ren, Dalton Trans., 2023, 52, 12686–12694 RSC.
- C.-M. Liu, D.-Q. Zhang, J.-B. Su, Y.-Q. Zhang and D.-B. Zhu, Inorg. Chem., 2018, 57, 11077–11086 CrossRef CAS PubMed.
- S. K. Gupta, S. Dey, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Chem. – Eur. J., 2022, 28, e202103585 CrossRef CAS PubMed.
- M. Pinsky and D. Avnir, Inorg. Chem., 1998, 37, 5575–5582 CrossRef CAS PubMed.
- J.-P. Sutter, V. Béreau, V. Jubault, K. Bretosh, C. Pichon and C. Duhayon, Chem. Soc. Rev., 2022, 51, 3280–3313 RSC.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164–1175 CrossRef CAS PubMed.
- N. Malinová, J. Juráková, B. Brachňaková, J. D. Midlíková, E. Čižmár, V. T. Santana, R. Herchel, M. Orlita, I. Mohelský, J. Moncol, P. Neugebauer and I. Šalitroš, Cryst. Growth Des., 2023, 23, 2430–2441 CrossRef.
- I. F. Galván, M. Vacher, A. Alavi, C. Angeli, F. Aquilante, J. Autschbach, J. J. Bao, S. I. Bokarev, N. A. Bogdanov, R. K. Carlson, L. F. Chibotaru, J. Creutzberg, N. Dattani, M. G. Delcey, S. S. Dong, A. Dreuw, L. Freitag, L. M. Frutos, L. Gagliardi, F. Gendron, A. Giussani, L. González, G. Grell, M. Guo, C. E. Hoyer, M. Johansson, S. Keller, S. Knecht, G. Kovačević, E. Källman, G. Li Manni, M. Lundberg, Y. Ma, S. Mai, J. P. Malhado, P. Å. Malmqvist, P. Marquetand, S. A. Mewes, J. Norell, M. Olivucci, M. Oppel, Q. M. Phung, K. Pierloot, F. Plasser, M. Reiher, A. M. Sand, I. Schapiro, P. Sharma, C. J. Stein, L. K. Sørensen, D. G. Truhlar, M. Ugandi, L. Ungur, A. Valentini, S. Vancoillie, V. Veryazov, O. Weser, T. A. Wesołowski, P.-O. Widmark, S. Wouters, A. Zech, J. P. Zobel and R. Lindh, J. Chem. Theory Comput., 2019, 15, 5925–5964 CrossRef PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Pelizzi and G. Pelizzi, J. Chem. Soc., Dalton Trans., 1980, 1970–1973 RSC.
- M. Reiher, Theor. Chem. Acc., 2006, 116, 241–252 Search PubMed.
- R. Ditchfield, W. J. Hehre and J. A. Pople, J. Chem. Phys., 1971, 54, 724–728 CrossRef CAS.
- T. R. Cundari and W. J. Stevens, J. Chem. Phys., 1993, 98, 5555–5565 CrossRef CAS.
- L. Noodleman, D. A. Case and A. Aizman, J. Am. Chem. Soc., 1988, 110, 1001–1005 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting single crystal data, bond parameters, and magnetic data are provided in ESI. CCDC 2433773. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ce00491h |
| This journal is © The Royal Society of Chemistry 2025 |