 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural evolution and bonding features of electron deficient copper chalcogenides
Sergei A.
Novikov
 ,
James
Casey
,
James
Casey
 ,
Hope A.
Long
and
Vladislav V.
Klepov
,
Hope A.
Long
and
Vladislav V.
Klepov
 *
*
Department of Chemistry, University of Georgia, Athens, Georgia 30602, USA. E-mail: klepov@uga.edu
First published on 11th June 2025
Abstract
A group of otherwise covalent ternary copper sulfides and selenides demonstrate metallic p-type conductivity and Pauli paramagnetism, which distinguishes them from semiconducting counterparts built up from the same elements. This difference originates from the electron deficiency delocalized over structural units of copper chalcogenides. Considering the significant technological importance of chalcogenide materials, we surveyed ternary copper sulfides and selenides with alkali and alkaline earth cations to provide insights into their crystal structures and transport properties. We found that the compositions and structures of many chalcogenides can be rationalized based on two 2D nets with honeycomb and square lattice net topologies, respectively. We have also shown how simple electron-orbital counts can be applied to metallic chalcogenides with formally charge unbalanced compositions and demonstrated that these compounds are indeed electron deficient. In agreement with experimental data and DFT calculations, such phases demonstrate metallic p-type conductivity.
Introduction
Electron-deficient covalent materials combine directional bonding and low coordination numbers typical for covalent phases with metallic type conductivity. These materials can be described by the term “covalent metals” that summarizes their structural and physical properties. The “covalent” part reflects on the nature of chemical bonding and local structure (i.e., a small difference in electronegativities), while the “metal” highlights the low electrical resistivity of the materials due to electron delocalization and deficiency. One common example of a compound with metallic properties and electronic delocalization is graphite, which is built of layers of covalently bonded carbon atoms with trigonal planar coordination and exhibits strong electron delocalization due to half-occupied bands with pz character. Although strong electron delocalization is usually associated with bonds between elements that have a small electronegativity difference, Δχ, some chalcogenide compounds exhibit a significant electron delocalization in the presence of a relatively large Δχ. One such example is copper sulfides and selenides, which structures show features of covalently bound materials (low coordination numbers for copper and well-defined geometry of the Cu coordination polyhedra), while demonstrating metallic conductivity.Numerous natural and synthetic copper chalcogenides with metallic conductivity and Pauli paramagnetism have been known for decades.1–4 Unlike graphite, materials such as covellite CuS, klockmannite CuSe, or umangite Cu3Se2, are p-type conductors. While the physical properties of many chalcogenides have been thoroughly studied, the theoretical basis of their physical behavior has been developed afterwards. The common trait of these phases is a deviation from the oxidation state formalism. It became widely acknowledged that the oxidation state of Cu in these peculiar phases is +1, while mixed +1/+2 states were ruled out,5–8 resulting in the deficit of the formal negative charge in binary (CuS, CuSe, Cu3Se2, CuS2, CuSe2) and ternary (NaCu4S3, NaCu4Se3, NaCu4S4, NaCu4Se4) phases. This distinguishes copper from other metals, demonstrating truly mixed oxidation states in chalcogenides.9,10 DFT study of covellite confirmed the presence of holes in the valence band and the absence of a bandgap in CuS (more precisely Cu6S6, as the structure is built on stacking of CuS and Cu2S2 layers and has S–S bonds), and thus, the observed p-type metallic conductivity aligned perfectly with the experiments.11 The holes originate from a mismatch between the number of molecular orbitals and the number of available valence electrons to fill them. The same study showed a significant level of hybridization between copper and sulfur valence orbitals near the Fermi level, indicating the delocalization of holes over Cu3S3 building blocks in the covellite structure. The delocalization of holes over copper chalcogenide slabs (hereinafter, slab is defined as a layer made by connecting the simplest layers) suggests slightly higher positive charges on both copper and chalcogenide atoms (in other words, more positive on copper, less negative on sulfur), which must not be confused with mixed valence on both copper and chalcogen atoms.12 The same phenomenon was confirmed in the recently reported paramagnetic NaCu4S3 phase13 and a series of related electron deficient phases.14–16
The unique electronic structure of covalent metals made them attractive for applications as a new class of catalyst for CO2 conversion into value-added products.17 Apart from catalysis, metal chalcogenides were studied as prospective materials for a wide range of vital applications, including rechargeable batteries,18–27 thermoelectric power generation,28–35 heterogeneous photo- and electrocatalysis,36–39 photovoltaics,40,41 solar cells with unmatched performance,42–46 radionuclides removal,47 neuromorphic engineering,48,49 cancer treatment,50,51 and others.52,53 Although most chalcogenide research focuses on the semiconductors, recent advances in understanding the electronic behavior of materials with an intermediate type of bonding challenged the traditional systematics of covalent/metallic/ionic bonding mechanisms.54–56 This intermediate type of bonding, which has also been termed “metavalent”, is responsible for unconventional properties of materials such as GeTe, SnTe, and PbTe,54–56 which have promising applications in thermoelectrics. Although these materials started attracting more interest, a detailed understanding of their electronic structures and crystal design principles is still missing.
The goal of this highlight is to illustrate electron deficiency in metallic covalent compounds by using the structural and electronic features of ternary copper sulfides and selenides with alkali and alkaline metals as an example. These materials are divided into two major groups: formally charge balanced and formally charge unbalanced (i.e. electron deficient). A common and most apparent indication of a potential chalcogenide covalent metal is the difficulty of assigning oxidation states in the composition using the common oxidation states formalism, which provides a starting point for their systematic analysis. Following a traditional crystallographic approach, we start with the local coordination of copper and chalcogenide atoms and consider building blocks in the structures to show how they can be arranged in more complex structures. While many hypothetical building unit stackings are possible, few of them have been realized in real materials, leaving this field awaiting the synthesis of many new compositions.
Synthesis of copper chalcogenides
Before discussing the electronic and structural properties of chalcogenide materials, we first briefly describe the synthetic methods for their preparation (Fig. 1). Ternary copper sulfides and selenides are very commonly synthesized by employing alkali polychalcogenide fluxes.57 We use the term “flux” here to highlight the excess of polychalcogenide reagents compared to the stoichiometry of a product and the subsequent necessity of flux removal after reaction completion. Alkali polychalcogenide fluxes offer numerous advantages, including compositional versatility and low melting temperatures.16,58–63 The major drawback of these compounds is high moisture sensitivity, as well as challenges associated with the tedious synthesis of these starting materials by reacting alkali metals with chalcogens in liquid ammonia. However, this disadvantage can be overcome by using metal chalcogenides (Na2Q, K2Q, etc., Q = S or Se) in combination with the respective chalcogens to generate polychalcogenide fluxes in situ. Copper can be introduced in the reaction in the elemental form or as a pre-made binary chalcogenide. Copper(II) oxide, used for the preparation of NaCu4Se4 phase is a rather unusual starting material.16 In most cases, the reagents are typically mixed in an inert atmosphere and sealed in a glass ampule (quartz or Pyrex, depending on reaction temperatures) under vacuum, then melted and slowly cooled for crystallization. To melt and react the starting materials, a wide temperature range of 350–1100 °C is employed.16,58–63 Excess flux is removed by washing the reaction products with DI water and organic solvents, typically DMF. Some sulfides were synthesized using binary precursors combined with elemental sulfur in exact stoichiometric ratios to target certain compositions.58,64,65 Overall, this approach employs the same apparatus as flux reactions (i.e., slow cooling of reactions in sealed tubes).Besides polychalcogenides, other sources of alkali cations, including carbonates, thiocyanates, and hydroxide–halide mixtures, can be employed to create fluxes. To avoid pressure buildup during the synthesis, reactions with carbonates are performed in open crucibles under an inert atmosphere.14,66–71 Mixed alkali hydroxides-sodium iodide flux was successfully employed for the synthesis of charge unbalanced Na3Cu4Se4 phase isostructural with K3Cu4Se4.72 Such flux, however, is highly reactive and cannot be used for synthesis in a glass ampule or alumina crucible. Therefore, preparations of Na3Cu4Se4 and some other phases were done in a glassy carbon boat under nitrogen flow in a tube furnace.72 Thiocyanate fluxes were used to prepare BaCu2S2 and CsCu5S3 phases in two-step processes.73,74 Potassium thiocyanate, used for the synthesis of Ba phase, acted more like a solvent, as the resulting structure does not contain potassium. On the contrary, the synthesis of CsCu5S3 was assisted by cesium thiocyanate flux.
Although, in most cases reported so far, elemental starting materials are used as primary reagents, one can employ boron–chalcogen mixtures (BCM) to reduce oxides and form chalcogenide phases. This method is generally utilized for the synthesis of copper chalcogenides with elements that have high oxygen affinity and, thus, tend to oxidize in air. For example, NaCuUS3 phase can be formed by reacting U3O8, Cu, Na2CO3, B, and S.75 In this reaction, B binds oxygen in the system and S reacts with the metals, providing both metal chalcogenides to form the final phase and the polychalcogenide flux, NaSn, to grow its single crystals. Similarly, the CsCu4Q3 phase has been prepared using BCM.76 Unlike the previous report on the synthesis of CsCu4S3 phase, where the reaction was performed under an Ar atmosphere,70 the BCM reaction was performed in a sealed silica tube. Alternatively, the CsCu4Se3 phase can be obtained by using a hydrothermal route, which is relatively less common for chalcogenides.77 This phase formed in the reaction between ≈250 mg of a mixture of K2Se4, Cu, and CsCl in a sealed quartz ampoule with 0.5 ml of DI water at 120 and 170 °C.77 These examples show interchangeability between the synthetic techniques. Additionally, new phases can be obtained by solid state postsynthetic modifications of the known phases. For example, a rare case of utilizing ternary sulfide as a starting material was the preparation of the CsCu5S3 phase via solid state reaction of the CsCu4S3 phase with extra copper.78 Overall, rich variability of synthetic techniques offers a convenient playground for the synthesis of new copper chalcogenide phases with desired compositions and properties.
Naturally, the formation of solid solutions between isostructural sulfides and selenides can be assumed. As a recent report on this matter demonstrated, the mismatches of chalcogenide sizes and covalency result in the pronounced structural ordering in mixed chalcogenides.79 This ordering was rationalized based on crystallographic data and hard–soft acid–base principles, which allows for the avoidance of costly DFT calculations. The results were confirmed experimentally, and the new KCuZrTe2S ordered mixed anionic phase was prepared.
Copper chalcogenides building blocks and the electron count
In the structures of binary and ternary chalcogenides, copper atoms coordinate two to four Q atoms (Table 1), with the most common coordination numbers being 3 and 4, corresponding to trigonal planar and tetrahedral coordination, respectively (Fig. 2). By sharing Q atoms, CuQn units form discrete 0D ions, infinite 1D chains, 2D layers and slabs, or 3D frameworks. The reduction of structural dimensionality is commonly caused by additional cations, which divide copper chalcogenide units, and can be employed to decrease the thermal conductivity of materials.80,81 Some structures of layered and quasi-layered chalcogenides can be described with two common 2D motifs. From the point of view of the structural topology, they are variations of honeycomb (hcb) and square lattice (sql) nets, with trigonally coordinated copper atoms predominantly forming basic hcb nets and tetrahedrally coordinated copper atoms – sql ones (Fig. 2). Both types of nets have the same CuQ stoichiometry of a basic single net; however, the formation of more complex structures through node connecting and sharing results in a variety of compositions in sulfides and selenides.| Composition | Orbital count | Electron count | Electron-orbital mismatch | Type | sql phases | hcb phases |
|---|---|---|---|---|---|---|
| A(CuQ)n, n = 1 | ||||||
| A+(CuQ)− | 18/2 | 17 + 1 | 0 | Balanced | LiCuSe (ref. 82), NaCuSe (ref. 83) | KCuSe (ref. 83) |
| n = 2 | ||||||
| A+(Cu2Q2)− | 36/2 | 34 + 1 | 1 | Unbalanced | KCu2Se2 (ref. 84) | — |
| A2+(Cu2Q2)2− | 36/2 | 34 + 2 | 0 | Balanced | t-BaCu2Q2 (ref. 73) | CaCu2S2 (ref. 85) |
| A+(Cu3Q3)− | 54/2 | 51 + 1 | 2 | Unbalanced | Likely unstable | |
| A2+(Cu3Q3)2− | 54/2 | 51 + 2 | 1 | Unbalanced | — | — |
| n = 4 | ||||||
| A+(Cu4Q4)− | 72/2 | 68 + 1 | 3 | Unbalanced | Likely unstable | |
| A2+(Cu4Q4)2− | 72/2 | 68 + 2 | 2 | Unbalanced | Likely unstable | |
| A+(Cu4Q2(Q2))− | 70/2 | 68 + 1 | 1 | Unbalanced | — | NaCu4Q4 (ref. 16 and 63) |
| A+(Cu4Q3)− | 64/2 | 62 + 1 | 1 | Unbalanced | ACu4Q3 (ref. 60, 71, 76 and 86) | NaCu4Q3 (ref. 13 and 15) |
| n = 6 | ||||||
| A+(Cu6Q4)− | 92/2 | 90 + 1 | 1 | Unbalanced | — | NaCu6Se4 (ref. 61) |
 | ||
| Fig. 2 (a) hcb copper selenide net with trigonal planar Cu coordination in KCuSe phase; (b) sql copper selenide net with tetrahedral Cu coordination in LiCuSe structure. | ||
Basic single CuQ nets can be found in charge balanced A+CuSe phases (A+ = Li–K). The tetragonal structures of LiCuSe and NaCuSe phases are built of sql nets, whereas the hexagonal KCuSe is comprised of hcb nets (Fig. 2, Table 1). By doubling the basic structural units, one can derive the structures with [Cu2Q2]n− stoichiometries. Depending on the counter cation (either alkali or alkaline earth metal), these compounds can represent either charge balanced (such as BaCu2Q2 series) or charge unbalanced compositions (for example, orthorhombic KCu2Se2 phase). Nevertheless, both examples feature isolated sql nets separated by the cations. Multiplying of the layers can also occur by connecting atoms between them and forming a slab. For example, the structure of charge balanced trigonal CaCu2S2 phase is built by connecting some nodes of two hcb nets to build Cu2S22− slabs (Fig. 3a–c). Interactions between two flat hcb layers change the local environment of both Cu and S atoms, leading to their distorted tetrahedral coordination environment. Ca atoms in this structure also have octahedral sulfur coordination and occupy the interlayer space. Two neighboring sql nets with common nodes form a Cu4S3− slab in KCu4S3 phase (Fig. 3d–f).
 | ||
| Fig. 3 (a–c) Double hcb and (d–e) double sql slabs in the structures of charge-balanced CaCuS2 and charge-unbalanced KCu4S3 phases, respectively. | ||
The electron deficiency of copper chalcogenide phases can be easily calculated from the chemical composition and the knowledge about Q–Q bonds in the structure (Table 1). For a simple NaCuSe case, the total number of valence orbitals is given as a sum of five 3d orbitals from the Cu atoms, one 4s and three 4p orbitals from the Se atoms, resulting in a total of nine. In these orbital count calculations, we assume that Cu 4s and 4p orbitals have significantly higher energy and have only a marginal (yet, very important in some cases) contribution to the top of the valence band. For the electrons, each Cu atom provides 11 electrons, and each S atom offers six electrons, resulting in 17 electrons per (CuS) unit. For A+CuQ phases (A = Li–K), an additional electron is coming from the alkali metals, charge balancing the layers with one electron to result in nine orbitals with 18 electrons. Doubling the (CuS) units yields 18 orbitals with only 34 electrons, requiring an alkaline earth metal to charge balance it, as in BaCu2Q2. On the other hand, if only one alkali cation is balancing the layers in an A+Cu2Q2 composition, such as KCu2Se2, the layers are one electron short of the charge balanced state, resulting in a one-electron-deficient structure. Further increase in the number of stacking layers increases the electron deficiency. The copper chalcogenide phases reported to date exhibit a maximum of one electron deficiency per formula unit (or 0.5 electron per single CuS unit in NaCu2S2), allowing one to surmise that the structures with two or more electron deficiency sites per formula unit are unstable. Therefore, ternaries with A+Cu3Q3 stoichiometry have not been reported to date, with (CuQ)3− 2D units being likely unstable due to high electron deficiency, 2/3 electrons per CuS unit.
It is easy to see that the higher homologues of the (CuS)n family should also be unstable. There are, however, ways to alleviate the electron deficiency in compositions with high copper content, by either forming dichalcogenide bonds or sharing atoms between layer units. A good example of the former is NaCu4Q4 compounds, which formulas can also be written as Na+[Cu4Q2(Q2)]−. Forming a Q–Q bond effectively eliminates one orbital from the orbital count by shifting Q–Q antibonding state significantly above the Fermi level,13 resulting in 9 × 4 − 1 = 35 orbitals with 17 × 4 + 1 = 69 electrons. Such units are present in the NaCu4Q4 phases, which contain a slab of four hcb layers with a dichalcogenide bond connecting two inner ones. This dichalcogenide bond binds the layers into a double layer with a kagome dual net topology (Fig. 4, Table 1). The former approach to reducing orbital count achieves the stabilization of the structure by sharing atoms from different layers, typically a chalcogen atom. One common example is A+(Cu4Q3)− and A2+(Cu4Q3)2− compositions. In A+(Cu4Q3)−, 4 × 5 + 3 × 4 = 32 orbitals host 11 × 4 + 3 × 6 + 1 = 63 electrons, having a deficiency of one hole per formula unit. In the ACu4Q3 (A = K–Cs) series of charge unbalanced tetragonal phases, Cu4Q3− slabs are formed by two sql nets with shared Q atoms. The slabs are separated by alkali cations in the structures. When a smaller Na+ cation occupies the A site, the resulting structure contains vertex-sharing hcb layers combined into Cu4S3− slabs. Similar to the CaCu2S2 phase, the copper coordination number in the Cu4S3− slabs is increased to four and the hcb layers are visibly corrugated. The way two inner hcb layers of a Cu4S3− slab are connected also matches the topology of kagome dual net. Finally, the highest homologue reported so far has a NaCu6Se4 composition. In NaCu6Se4, two CuSe hcb nets are divided not by one but by two Cu2Se double hcb, forming slabs with a Cu6Se4 composition. The formula unit contains 46 orbitals and 91 electrons, showing the same one hole per formula unit electron deficiency. To the best of our knowledge, no ternary phase built on more single and double hcb layers has been reported; however, a slab made of two single and three double layers can be found in the structure of Cu9S5 binary. Overall, shared atoms and dichalcogenide bonds are an efficient way to stabilize higher homologues of copper chalcogenide ternaries, enabling the formation of phases with lower hole concentration per Cu atom. Since higher homologues require using small A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratios, their synthesis may be challenging in the presence of commonly used polychalcogenide fluxes. Alternative synthetic routes using fluxes or solvents that dissolve the starting materials but do not incorporate into the final products are therefore required to stabilize those phases.
Cu molar ratios, their synthesis may be challenging in the presence of commonly used polychalcogenide fluxes. Alternative synthetic routes using fluxes or solvents that dissolve the starting materials but do not incorporate into the final products are therefore required to stabilize those phases.
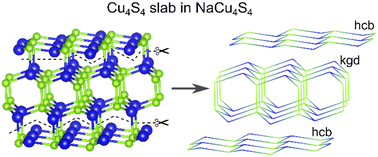 | ||
| Fig. 4 Cu4S4 slab in the NaCu4S4 structure made from four hcb layers. Two central layers are connected in kagome dual net motif (kgd). | ||
The remaining ternary copper sulfide and selenide structures are not based on hcb and sql nets (Table 2), and their dimensionality varies from 0D to 3D. For example, unlike the A+(Cu4Q3)− series, the charge balanced Na2Cu4S3 phase, as well as both the orthorhombic primitive (oP) and base centered (oC) modifications of the BaCu4S3 phase, feature complex 2D (Na, oC Ba) and 3D (oP Ba) structures. Another example of isomerism among copper chalcogenide units appears in A3+Cu4S4 phases (A+ = Na+ or K+) containing 1D Cu4S4 units with decreased dimensionality due to a high content of alkali cations. Besides oP BaCu4S3, anionic frameworks can be found in the structures of o-BaCu2Q2, ACu5Q3 (A = Na, Cs), and ACu7S4 (A = K, Rb) series with alkali and alkaline earth cations occupying channels of frameworks. 2D anionic slabs and layers are quite common among chalcogenides with various stoichiometries. Usually, alkali and alkaline cations separate 2D anionic units, however, in the recently reported K3BaCu7S6 phase Ba2+ cations are inserted in Cu7S6 double layers, while K+ cations separate such layers.72 Interstitial sites in copper sulfides and selenides layers are usually vacant. However, in mixed anion ACu4.2TeS2 series of compounds isostructural to KCu4S3, there are additional copper atoms partially occupying the voids of the CuTeS2 layers.91 The volume of the Voronoi polyhedron92 for the interstitial copper atom in the KCu4.2TeS2 structure is about 3.6 Å3 larger than the void in the KCu4S3 phases. This discrepancy volume is due to the larger size of the Te atom, which allows for the accommodation of additional Cu atoms. High content of cations per formula unit results in chalcogenides dimensionality reduction, while the presence of polychalcogenide anions facilitates the formation of 1D anionic chains: for example, CuQ4 and CuS6 chains contain Q42− and S62− anions, respectively (Table 2). Finally, the charge balanced Na4Cu2S3 phase appears to be the only compound with discrete Cu2S34− 0D anions. As can be seen, the complexity of anionic units exceeds structures solely built on sql and hcb motifs in many chalcogenides. Nevertheless, the same principles of orbital and electron count can be applied to them (Table 2). Similar to phases built on CuQ fragments, the electron deficiency in phases without such fragments does not exceed one electron per formula unit.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Q ratio in formula unit
Q ratio in formula unit
| Composition | Orbitals count | Electron count | Electron-orbital count mismatch | Type | Dimensionality of CuxQy unit |
|---|---|---|---|---|---|
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 1/6 Q = 1/6
|
|||||
| CsCu((S6)2−) (ref. 93) | 58/2 − 10/2 | 47 + 1 | 0 | Balanced | 1D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 1/4 Q = 1/4
|
|||||
| A+Cu(Q42−) (ref. 77, 94–96) | 42/2 − 6/2 | 35 + 1 | 0 | Balanced | 1D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 8/13 Q = 8/13
|
|||||
| Ba4Cu8Se2(Se22−)4(Se34−) (ref. 97) | 184/2 − 10/2 | 166 + 8 | 0 | Balanced | 2D |
| Na4Cu2S3 (ref. 64) | 44/2 | 40 + 4 | 0 | Balanced | 0D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 1 Q = 1
|
|||||
| o-BaCu2Q2 (ref. 73) | 36/2 | 34 + 2 | 0 | Balanced | 3D |
| KCuS (ref. 65) | 18/2 | 17 +1 | 0 | Balanced | 1D |
| A3Cu4S4, A = Na, K (ref. 59 and 67) | 72/2 | 68 + 3 | 1 | Unbalanced | 1D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 7/6 Q = 7/6
|
|||||
| K3BaCu7S6 (ref. 72) | 118/2 | 113 + 5 | 0 | Balanced | 2D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 6/5 Q = 6/5
|
|||||
| Na7Cu12S10 (ref. 58) | 200/2 | 192 + 7 | 1 | Unbalanced | 1D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 5/4 Q = 5/4
|
|||||
| Cs2Cu5Se4 (ref. 69) | 82/2 | 79 + 2 | 1 | Unbalanced | 2D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 4/3 Q = 4/3
|
|||||
| BaCu4S3 (ref. 98), Na2Cu4S3 (ref. 99) | 64/2 | 62 + 2 | 0 | Balanced | 3D (Ba), 2D (Na) |
| A3Cu8Q6, A = K–Cs (ref. 62, 66 and 68) | 128/2 | 124 + 3 | 1 | Unbalanced | 2D |
| ACu3Q2, A = K–Cs (ref. 70 and 100–102) | 46/2 | 45 + 1 | 0 | Balanced | 2D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 5/3 Q = 5/3
|
|||||
| ACu5Q3, A = Na, Cs (ref. 78 and 87–90) | 74/2 | 73 + 1 | 0 | Balanced | 3D and 2D |
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Q = 7/4 Q = 7/4
|
|||||
| ACu7S4, A = K, Rb (ref. 103) | 102/2 | 101 + 1 | 0 | Balanced | 3D |
Electronic structures of copper chalcogenides
DFT calculations and physical properties measurements of the copper chalcogenide phases can provide a more nuanced view on the electron deficiency than that derived from simple electron count. X-ray photoelectron spectroscopy, bond valence method, and structural data for a series of chalcogenides showed a +1 oxidation state for copper atoms exclusively, implying the formal oxidation states above −2 for chalcogen atoms.5–7,104–106 This evidence suggests the electron deficit being distributed over chalcogen np valence orbitals, resulting in the presence of holes on the chalcogenide atoms and ultimately in the metallic p-type conductivity. More detailed electronic structure calculations, however, show that the holes are distributed over bands with Cu 3d and S 3p character, indicating that the positive charge is distributed over both Cu+ and S2− ions. For example, Fig. 5 shows the results of DFT calculations of electronic band structure and densities of states (DOS) for CuS,11 NaCu4S3,13 NaCu4S4,13 and NaCu4Se4 (ref. 16) phases. | ||
| Fig. 5 Electronic band structure and DOS for (a and b) CuS, (c) NaCu4S3, (d) NaCu4S4, and (e) NaCu4Se4 phases. Adapted with permission from ref. 11, 13 and 16. Copyright: American Chemical Society. | ||
DFT calculations show crucial common features of all four phases under consideration: first, the upper bands in the band structure cross the Fermi level in the proximity of the Γ point. This accounts for the metallic properties of the chalcogenides and agrees with the experimental data on electrical conductivity and magnetism. Second, the dispersion in the upper bands significantly depends on the direction. Bands contributed by Cu 3dx2–y2, 3dxy, and S 3px, 3py orbitals are highly dispersed in directions parallel to CuxSy slabs. This agrees with the structural data, as orbitals forming these bands participate in the copper–chalcogen bonding within the CuxSy slabs. On the contrary, bands with predominant contributions from Cu 3dxz and 3dyz orbitals are weakly dispersed in all directions, as the corresponding orbitals do not participate in the bonding. Notable differences in the band structures of CuS,9 NaCu4S3,11 NaCu4S4,11 and NaCu4Se4 (ref. 14) phases are observed in the direction perpendicular to the slabs. Cu 3dz2 and Q npz orbitals are oriented in this direction. In NaCu4S3, the overlap of these orbitals results in the bands crossing the Fermi level and their dispersion in the c direction in the crystal structure. In CuS, NaCu4S4 and NaCu4Se4 Q–Q bonds form, and the overlap of npz orbitals significantly surpasses the overlap of 3dz2 and npz orbitals. The resulting antibonding band has much higher energy and lies entirely above the Fermi level. DFT calculations confirm strong covalency of the Cu–Q bonds and the metallic nature of the electron deficient chalcogenides. It is important to note that this view agrees well with the XPS results, which only show that Cu atoms in a selected structure have the same oxidation state, which is different from the +2 oxidation state. Having a slight hole delocalized over Cu 3d orbitals only marginally increases the formal charge on Cu atoms.
Conclusions
Ternary copper sulfides and selenides can be divided into two broad groups, charge-balanced and charge unbalanced compounds. The combination of metallic p-type conductivities and covalent bonding makes charge unbalanced phases “covalent metals”. While the compositions of new phases vary significantly, the same square lattice and honeycomb 2D topological motifs persist in the structures of many layered and quasi-layered copper chalcogenides. The metallic nature of charge-unbalanced compounds originates from the presence of holes on the bands with 3d Cu and 3p S character, indicating hole distribution between them. Carrier concentration in the electron deficient phases can be estimated with a simple vacancy count approach, which shows good agreement with the experimental data. Since copper chalcogenides show a significant variability in their compositions, one can expect that more phases can be discovered when using a reaction mixture with an alkali metal deficit, leading to homologous series with higher Cu content. To date, no phases with Cu3Q3 stoichiometry of copper chalcogenide units have been reported. While Cu3Q3− slabs are likely unstable due to high electron deficiency, the formation of Cu3Q32− units in the reactions with alkaline earth metals is worth investigating. Overall, electron count can be used to specifically target either metallic or semiconducting phases in the synthesis based on the charge balance. Moreover, conversion of p-type metal into a semiconductor can be done with compositional tuning, as it was demonstrated by charge unbalanced CsCu4S3 phase transition to charge balanced CsCu5S3.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Sergei A. Novikov: investigation, methodology, validation, writing – original draft, writing – review & editing; James Casey: writing – original draft, writing – review & editing; Hope A. Long: writing – original draft, writing – review & editing; Vladislav V. Klepov: conceptualization, funding acquisition, supervision, investigation, methodology, validation, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts of interest to report.Acknowledgements
This work was supported by the University of Georgia Department of Chemistry, Franklin College of Arts and Sciences, and the Office of Provost.References
- K. Okamoto, S. Kawai and R. Kiriyama, Jpn. J. Appl. Phys., 1969, 8, 718 CrossRef CAS.
- F. Gautier, G. Krill, P. Panissod and C. Robert, J. Phys. C: Solid State Phys., 1974, 7, L170 CrossRef CAS.
- T. Kanashiro, T. Ohno, M. Satoh, K. Okamoto, A. Kojima and F. Akao, Solid State Ionics, 1981, 3–4, 327–330 CrossRef CAS.
- R. Berger and C. F. Van Bruggen, J. Less-Common Met., 1984, 99, 113–123 CrossRef CAS.
- J. C. W. Folmer and F. Jellinek, J. Less-Common Met., 1980, 76, 153–162 CrossRef CAS.
- G. van der Laan, R. A. D. Pattrick, C. M. B. Henderson and D. J. Vaughan, J. Phys. Chem. Solids, 1992, 53, 1185–1190 CrossRef CAS.
- S. W. Goh, A. N. Buckley and R. N. Lamb, Miner. Eng., 2006, 19, 204–208 CrossRef CAS.
- B. Raveau, Phys. C, 2023, 611, 1354295 CrossRef CAS.
- A. Mesbah and J. A. Ibers, J. Solid State Chem., 2013, 199, 253–257 CrossRef CAS.
- A. Mesbah, J. Prakash, J. C. Beard, E. A. Pozzi, M. S. Tarasenko, S. Lebègue, C. D. Malliakas, R. P. Van Duyne and J. A. Ibers, Inorg. Chem., 2015, 54, 2851–2857 CrossRef CAS PubMed.
- S. Conejeros, I. de P. R. Moreira, P. Alemany and E. Canadell, Inorg. Chem., 2014, 53, 12402–12406 CrossRef CAS PubMed.
- V. A. Starodub, Russ. Chem. Rev., 1999, 68, 801–820 CrossRef CAS.
- S. A. Novikov, J. Casey, H. A. Long, J. C. Bledsoe, J. J. Locklin and V. V. Klepov, Cryst. Growth Des., 2023, 23, 7243–7251 CrossRef CAS.
- Z. Peplinski, D. B. Brown, T. Watt, W. E. Hatfield and P. Day, Inorg. Chem., 1982, 21, 1752–1755 CrossRef CAS.
- M. Sturza, D. E. Bugaris, C. D. Malliakas, F. Han, D. Y. Chung and M. G. Kanatzidis, Inorg. Chem., 2016, 55, 4884–4890 CrossRef CAS PubMed.
- H. Chen, J. N. B. Rodrigues, A. J. E. Rettie, T.-B. Song, D. G. Chica, X. Su, J.-K. Bao, D. Y. Chung, W.-K. Kwok, L. K. Wagner and M. G. Kanatzidis, J. Am. Chem. Soc., 2019, 141, 635–642 CrossRef CAS PubMed.
- H. Shin, Y. Ha and H. Kim, J. Phys. Chem. Lett., 2016, 7, 4124–4129 CrossRef CAS PubMed.
- E. Levi, G. Gershinsky, D. Aurbach, O. Isnard and G. Ceder, Chem. Mater., 2009, 21, 1390–1399 CrossRef CAS.
- E. Levi, M. D. Levi, O. Chasid and D. Aurbach, J. Electroceram., 2009, 22, 13–19 CrossRef CAS.
- H. S. Kim, T. S. Arthur, G. D. Allred, J. Zajicek, J. G. Newman, A. E. Rodnyansky, A. G. Oliver, W. C. Boggess and J. Muldoon, Nat. Commun., 2011, 2, 427 CrossRef PubMed.
- A. Manthiram, Y. Fu and Y.-S. Su, Acc. Chem. Res., 2013, 46, 1125–1134 CrossRef CAS PubMed.
- Y. Seino, T. Ota, K. Takada, A. Hayashi and M. Tatsumisago, Energy Environ. Sci., 2014, 7, 627–631 RSC.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 1–7 Search PubMed.
- P. Canepa, G. Sai Gautam, D. C. Hannah, R. Malik, M. Liu, K. G. Gallagher, K. A. Persson and G. Ceder, Chem. Rev., 2017, 117, 4287–4341 CrossRef CAS PubMed.
- Z. Hu, Q. Liu, S.-L. Chou and S.-X. Dou, Adv. Mater., 2017, 29, 1700606 CrossRef PubMed.
- M. D. Regulacio, D.-T. Nguyen, R. Horia and Z. W. Seh, Small, 2021, 17, 2007683 CrossRef CAS PubMed.
- C.-B. Chang and H.-Y. Tuan, Chem. – Asian J., 2022, 17, e202200170 CrossRef CAS PubMed.
- W. Liu, K. C. Lukas, K. McEnaney, S. Lee, Q. Zhang, C. P. Opeil, G. Chen and Z. Ren, Energy Environ. Sci., 2013, 6, 552–560 RSC.
- M. Ohta, D. Y. Chung, M. Kunii and M. G. Kanatzidis, J. Mater. Chem. A, 2014, 2, 20048–20058 RSC.
- M. M. Kubenova, K. A. Kuterbekov, M. K. Balapanov, R. K. Ishembetov, A. M. Kabyshev and K. Z. Bekmyrza, Nanomaterials, 2021, 11, 2238 CrossRef CAS PubMed.
- G. Dennler, R. Chmielowski, S. Jacob, F. Capet, P. Roussel, S. Zastrow, K. Nielsch, I. Opahle and G. K. H. Madsen, Adv. Energy Mater., 2014, 4, 1301581 CrossRef.
- M. M. Alsalama, H. Hamoudi, A. Abdala, Z. K. Ghouri and K. M. Youssef, Rev. Adv. Mater. Sci., 2020, 59, 371–398 CAS.
- R. Mulla and M. H. K. Rabinal, Energy Technol., 2019, 7, 1800850 CrossRef.
- Z. Yang, E. Smith, Y.-C. Tseng, K. Ciesielski, S. Novikov, T. Kalab, Y. Huang, E. Toberer and Y. Mozharivskyj, J. Mater. Chem. A, 2024, 12, 5357–5365 RSC.
- Y. Shi, C. Sturm and H. Kleinke, J. Solid State Chem., 2019, 270, 273–279 CrossRef CAS.
- C. G. Morales-Guio, L.-A. Stern and X. Hu, Chem. Soc. Rev., 2014, 43, 6555–6569 RSC.
- J. N. Hausmann and P. W. Menezes, Curr. Opin. Electrochem., 2022, 34, 100991 CrossRef CAS.
- K. Sharma, A. Kumar, T. Ahamad, Q. V. Le, P. Raizada, A. Singh, L. H. Nguyen, S. Thakur, V.-H. Nguyen and P. Singh, J. Mater. Sci. Technol., 2023, 152, 50–64 CrossRef CAS.
- Z. Chen, W. Wei and B.-J. Ni, Curr. Opin. Electrochem., 2022, 31, 100888 CrossRef CAS.
- A. Dey, J. Ye, A. De, E. Debroye, S. K. Ha, E. Bladt, A. S. Kshirsagar, Z. Wang, J. Yin, Y. Wang, L. N. Quan, F. Yan, M. Gao, X. Li, J. Shamsi, T. Debnath, M. Cao, M. A. Scheel, S. Kumar, J. A. Steele, M. Gerhard, L. Chouhan, K. Xu, X. Wu, Y. Li, Y. Zhang, A. Dutta, C. Han, I. Vincon, A. L. Rogach, A. Nag, A. Samanta, B. A. Korgel, C.-J. Shih, D. R. Gamelin, D. H. Son, H. Zeng, H. Zhong, H. Sun, H. V. Demir, I. G. Scheblykin, I. Mora-Seró, J. K. Stolarczyk, J. Z. Zhang, J. Feldmann, J. Hofkens, J. M. Luther, J. Pérez-Prieto, L. Li, L. Manna, M. I. Bodnarchuk, M. V. Kovalenko, M. B. J. Roeffaers, N. Pradhan, O. F. Mohammed, O. M. Bakr, P. Yang, P. Müller-Buschbaum, P. V. Kamat, Q. Bao, Q. Zhang, R. Krahne, R. E. Galian, S. D. Stranks, S. Bals, V. Biju, W. A. Tisdale, Y. Yan, R. L. Z. Hoye and L. Polavarapu, ACS Nano, 2021, 15, 10775–10981 CrossRef CAS PubMed.
- K. Srivastava, A. K. Ray, S. Yadav, M. Deepa and J. Prakash, New J. Chem., 2024, 48, 16869–16876 RSC.
- P. Myagmarsereejid, M. Ingram, M. Batmunkh and Y. L. Zhong, Small, 2021, 17, 2100241 CrossRef CAS PubMed.
- R. S. Hall, D. Lamb and S. J. C. Irvine, Energy Sci. Eng., 2021, 9, 606–632 CrossRef CAS.
- X. Jin, Y. Fang, T. Salim, M. Feng, Z. Yuan, S. Hadke, T. C. Sum and L. H. Wong, Adv. Mater., 2021, 33, 2104346 CrossRef CAS PubMed.
- J. Kangsabanik, M. K. Svendsen, A. Taghizadeh, A. Crovetto and K. S. Thygesen, J. Am. Chem. Soc., 2022, 144, 19872–19883 CrossRef CAS PubMed.
- M. A. Scarpulla, B. McCandless, A. B. Phillips, Y. Yan, M. J. Heben, C. Wolden, G. Xiong, W. K. Metzger, D. Mao, D. Krasikov, I. Sankin, S. Grover, A. Munshi, W. Sampath, J. R. Sites, A. Bothwell, D. Albin, M. O. Reese, A. Romeo, M. Nardone, R. Klie, J. M. Walls, T. Fiducia, A. Abbas and S. M. Hayes, Sol. Energy Mater. Sol. Cells, 2023, 255, 112289 CrossRef CAS.
- S. Ma, L. Huang, L. Ma, Y. Shim, S. M. Islam, P. Wang, L.-D. Zhao, S. Wang, G. Sun, X. Yang and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 3670–3677 CrossRef CAS PubMed.
- S. R. Bauers, M. B. Tellekamp, D. M. Roberts, B. Hammett, S. Lany, A. J. Ferguson, A. Zakutayev and S. U. Nanayakkara, Nanotechnology, 2021, 32, 372001 CrossRef CAS PubMed.
- K. C. Kwon, J. H. Baek, K. Hong, S. Y. Kim and H. W. Jang, Nano-Micro Lett., 2022, 14, 58 Search PubMed.
- F. Olawale, O. Oladimeji, M. Ariatti and M. Singh, J. Nanotechnol., 2022, 2022, 6176610 Search PubMed.
- M. Liu, Y. Liu, B. Gu, X. Wei, G. Xu, X. Wang, M. T. Swihart and K.-T. Yong, Chem. Soc. Rev., 2019, 48, 4950–4965 RSC.
- T. Lu, S. Dong, C. Zhang, L. Zhang and G. Cui, Coord. Chem. Rev., 2017, 332, 75–99 CrossRef CAS.
- Y. Zhou, D. Wu, Y. Zhu, Y. Cho, Q. He, X. Yang, K. Herrera, Z. Chu, Y. Han, M. C. Downer, H. Peng and K. Lai, Nano Lett., 2017, 17, 5508–5513 CrossRef CAS PubMed.
- M. Wuttig, V. L. Deringer, X. Gonze, C. Bichara and J.-Y. Raty, Adv. Mater., 2018, 30, 1803777 CrossRef PubMed.
- J.-Y. Raty, M. Schumacher, P. Golub, V. L. Deringer, C. Gatti and M. Wuttig, Adv. Mater., 2019, 31, 1806280 CrossRef PubMed.
- M. Wuttig, C.-F. Schön, J. Lötfering, P. Golub, C. Gatti and J.-Y. Raty, Adv. Mater., 2023, 35, 2208485 CrossRef CAS PubMed.
- M. G. Kanatzidis, Chem. Mater., 1990, 2, 353–363 Search PubMed.
- K. O. Klepp, M. Sing and H. Boller, J. Alloys Compd., 1993, 198, 25–30 Search PubMed.
- D. P. Shoemaker, Y.-J. Hu, D. Y. Chung, G. J. Halder, P. J. Chupas, L. Soderholm, J. F. Mitchell and M. G. Kanatzidis, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 10922–10927 CrossRef CAS PubMed.
- P. Stoll, C. Näther, I. Jeß and W. Bensch, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 286–288 CrossRef.
- M. Sturza, C. D. Malliakas, D. E. Bugaris, F. Han, D. Y. Chung and M. G. Kanatzidis, Inorg. Chem., 2014, 53, 12191–12198 Search PubMed.
- C. Näther, D. Röhnert and W. Bensch, Eur. J. Solid State Inorg. Chem., 1998, 35, 565–577 CrossRef.
- X. Zhang, M. G. Kanatzidis, T. Hogan and C. R. Kannewurf, J. Am. Chem. Soc., 1996, 118, 693–694 CrossRef.
- K. O. Klepp, M. Sing and H. Boller, J. Alloys Compd., 1992, 184, 265–273 CrossRef CAS.
- G. Savelsberg and H. Schäfer, Z. Naturforsch., B: J. Chem. Sci., 1978, 33, 711–713 CrossRef.
- C. Burschka, Z. Naturforsch., B: J. Chem. Sci., 1979, 34, 675–677 CrossRef.
- C. Burschka, Z. Naturforsch., B: J. Chem. Sci., 1979, 34, 396–397 CrossRef.
- H. Schils and W. Bronger, Z. Anorg. Allg. Chem., 1979, 456, 187–193 CrossRef CAS.
- W. Bronger and H. Schils, J. Less-Common Met., 1982, 83, 279–285 CrossRef CAS.
- C. H. Burschka, Z. Anorg. Allg. Chem., 1980, 463, 65–71 CrossRef CAS.
- W. Rüdorff, H. G. Schwarz and M. Walter, Z. Anorg. Allg. Chem., 1952, 269, 141–152 CrossRef.
- X. Zhou, V. S. C. Kolluru, W. Xu, L. Wang, T. Chang, Y.-S. Chen, L. Yu, J. Wen, M. K. Y. Chan, D. Y. Chung and M. G. Kanatzidis, Nature, 2022, 612, 72–77 CrossRef CAS PubMed.
- J. Huster and W. Bronger, Z. Anorg. Allg. Chem., 1999, 625, 2033–2040 CrossRef CAS.
- J. Huster and W. Bronger, Z. Anorg. Allg. Chem., 2001, 627, 1395–1400 CrossRef CAS.
- A. A. Berseneva, V. V. Klepov, K. Pal, K. Seeley, D. Koury, J. Schaeperkoetter, J. T. Wright, S. T. Misture, M. G. Kanatzidis, C. Wolverton, A. V. Gelis and H.-C. zur Loye, J. Am. Chem. Soc., 2022, 144, 13773–13786 CrossRef CAS PubMed.
- N. Ma, F. Li, J.-G. Li, X. Liu, D.-B. Zhang, Y.-Y. Li, L. Chen and L.-M. Wu, J. Am. Chem. Soc., 2021, 143, 18490–18501 CrossRef CAS PubMed.
- N. S. Hartig, P. K. Dorhout and S. M. Miller, J. Solid State Chem., 1994, 113, 88–93 CrossRef CAS.
- N. Ma, F. Jia, L. Xiong, L. Chen, Y.-Y. Li and L.-M. Wu, Inorg. Chem., 2019, 58, 1371–1376 CrossRef CAS PubMed.
- A. Tassanov, H. Lee, Y. Xia and J. M. Hodges, J. Am. Chem. Soc., 2024, 146, 32627–32639 Search PubMed.
- A. J. E. Rettie, C. D. Malliakas, A. S. Botana, J. M. Hodges, F. Han, R. Huang, D. Y. Chung and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 9193–9202 CrossRef CAS PubMed.
- E. Muzaffar, S. Azam, A. I. Bashir and M. Irfan, Mater. Sci. Eng., B, 2023, 292, 116448 Search PubMed.
- M. Valldor, D. Mikhailova, L. Giebeler, K. T. Lai, L. Spillecke, H.-J. Grafe and B. Büchner, Inorg. Chem., 2018, 57, 7201–7207 CrossRef CAS PubMed.
- G. Savelsberg, Z. Naturforsch., B: J. Chem. Sci., 1978, 33, 370–373 CrossRef.
- O. Tiedje, E. E. Krasovskii, W. Schattke, P. Stoll, C. Näther and W. Bensch, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 134105 CrossRef.
- A. P. Purdy, Chem. Mater., 1998, 10, 692–694 CrossRef CAS.
- D. B. Brown, J. A. Zubieta, P. A. Vella, J. T. Wrobleski, T. Watt, W. E. Hatfield and P. Day, Inorg. Chem., 1980, 19, 1945–1950 Search PubMed.
- H. Effenberger and F. Pertlik, Monatsh. Chem., 1985, 116, 921–926 Search PubMed.
- F. Li, X. Liu, N. Ma, Y.-C. Yang, J.-P. Yin, L. Chen and L.-M. Wu, J. Am. Chem. Soc., 2023, 145, 14981–14993 CrossRef CAS PubMed.
- Z. Xia, H. Fang, X. Zhang, M. S. Molokeev, R. Gautier, Q. Yan, S.-H. Wei and K. R. Poeppelmeier, Chem. Mater., 2018, 30, 1121–1126 CrossRef CAS.
- N. Ma, Y.-Y. Li, L. Chen and L.-M. Wu, J. Am. Chem. Soc., 2020, 142, 5293–5303 CrossRef CAS PubMed.
- J. M. Hodges, Y. Xia, C. D. Malliakas, T. J. Slade, C. Wolverton and M. G. Kanatzidis, Chem. Mater., 2020, 32, 10146–10154 Search PubMed.
- V. A. Blatov, A. P. Shevchenko and V. N. Serezhkin, J. Appl. Crystallogr., 2000, 33, 1193–1193 CrossRef CAS.
- T. J. McCarthy, X. Zhang and M. G. Kanatzidis, Inorg. Chem., 1993, 32, 2944–2948 Search PubMed.
- P. K. Dorhout, C. C. Raymond and S. M. Miller, Z. Kristallogr., 1995, 210, 776–776 Search PubMed.
- K. O. Klepp and C. Weithaler, Z. Naturforsch., B: J. Chem. Sci., 1995, 50, 1791–1793 Search PubMed.
- M. G. Kanatzidis and Y. Park, J. Am. Chem. Soc., 1989, 111, 3767–3769 Search PubMed.
- S. Maier, O. Perez, D. Pelloquin, D. Berthebaud, S. Hébert and F. Gascoin, Inorg. Chem., 2017, 56, 9209–9218 Search PubMed.
- J. E. Iglesias, K. E. Pachali and H. Steinfink, Mater. Res. Bull., 1972, 7, 1247–1258 CrossRef CAS.
- G. Savelsberg and H. Schäfer, Mater. Res. Bull., 1981, 16, 1291–1297 Search PubMed.
- C. Burschka and W. Bronger, Z. Naturforsch., B: J. Chem. Sci., 1977, 32, 11–14 Search PubMed.
- K. O. Klepp and M. Sing, Z. Kristallogr. - New Cryst. Struct., 2002, 217, 474–474 Search PubMed.
- A. J. E. Rettie, M. Sturza, C. D. Malliakas, A. S. Botana, D. Y. Chung and M. G. Kanatzidis, Chem. Mater., 2017, 29, 6114–6121 Search PubMed.
- T. Ohtani, J. Ogura, H. Yoshihara and Y. Yokota, J. Solid State Chem., 1995, 115, 379–389 Search PubMed.
- C. D. Malliakas, J. Yao, D. M. Wells, G. B. Jin, S. Skanthakumar, E. S. Choi, M. Balasubramanian, L. Soderholm, D. E. Ellis, M. G. Kanatzidis and J. A. Ibers, Inorg. Chem., 2012, 51, 6153–6163 Search PubMed.
- A. N. Buckley, W. M. Skinner, S. L. Harmer, A. Pring, R. N. Lamb, L.-J. Fan and Y. Yang, Can. J. Chem., 2007, 85, 767–781 Search PubMed.
- Y. Moëlo, A. F. Popa and V. Dubost, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2022, 78, 627–636 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

