Evaluation of mesoionic compound aromaticity using the HOMHED index†
Marcos Antonio Pinto
Martins
 *a,
Tainára
Orlando
*a,
Tainára
Orlando
 b,
Jéssica Maria Luis
Rosa
b,
Jéssica Maria Luis
Rosa
 a,
Priscila Santos Vieira de
Lima
a,
Priscila Santos Vieira de
Lima
 a and
Paulo Roberto dos Santos
Salbego
a and
Paulo Roberto dos Santos
Salbego
 c
c
aNúcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria (UFSM), 97105-900, Santa Maria, RS, Brazil. E-mail: martins.marcos@ufsm.br
bAcademic Department of Chemistry, Federal Technological University of Paraná (UTFPR), 85884-000 Medianeira Campus, PR, Brazil
cNúcleo de Química de Heterociclos (NUQUIMHE), Departamento de Engenharia e Tecnologia Ambiental (DETA), Federal University of Santa Maria (UFSM), 98400-000, Frederico Westphalen, RS, Brazil
First published on 8th May 2025
Abstract
Mesoionic compounds are a class of heterocyclic compounds whose aromaticity has ignited considerable debate within the scientific community. This study aims to systematically evaluate the aromaticity of 25 mesoionic compounds across eight structural classes, utilizing experimental X-ray diffraction data in conjunction with the HOMHED geometric index to clarify this issue. The findings reveal a wide range of aromaticity (0.494–0.976), influenced by the type of heteroatoms in the heterocycle and exocyclic positions. Compounds containing oxygen atoms typically exhibit lower aromaticity, with HOMHED values below 0.7. In contrast, those containing nitrogen and boron atoms, especially in exocyclic positions, show higher aromaticity, with HOMHED values exceeding 0.9. Remarkably, compounds featuring exocyclic boron atoms displayed the highest aromaticity, with HOMHED values between 0.951 and 0.976. Compound 25 stood out with the lowest aromaticity, having a HOMHED value of 0.494, arising as a missing link, representing an important factor in elucidating the boundaries of the continuum of aromaticity. The study found that, on average, HOMHED values increase in the order of heterocyclic atom (Y): O1 < S1 < N1 and exocyclic atom (X): O6 < S6 ≈ N6 < B6. The research compares two canonical forms, the aromatic structure (AS) and the betaine structure (BS), to assess their impact on mesoionic compounds' overall resonance and aromatic character. Compounds with higher aromaticity demonstrated increased resonance in the D-A-Y and D-E-C-Y fragments, indicating a predominant AS contribution, whereas those with lower aromaticity showed a stronger BS influence. By introducing the AS index (IAS) and the BS index (IBS), this study quantitatively distinguishes the contributions of each structure. The findings challenge the binary view of mesoionic compounds as merely aromatic or non-aromatic, suggesting instead that these compounds exhibit varying degrees of aromaticity, as a continuum. The study concludes that the appropriate question is not whether mesoionic compounds are aromatic, but rather, ‘How aromatic are mesoionic compounds?’.
Introduction
According to the International Union of Pure and Applied Chemistry (IUPAC), mesoionic compounds are dipolar heterocyclic compounds in which both the negative and positive charges are delocalized. These compounds, which cannot be adequately described by a totally covalent structure nor represented satisfactorily by any single polar structure,1 contain two or more heteroatoms. The formal positive charge is associated with the ring atoms, whereas the formal negative charge is linked to ring atoms or an exocyclic nitrogen or chalcogen atom.1The structure of mesoionic compounds has attracted significant attention in structural chemistry and has spurred numerous theoretical studies aimed at elucidating their electronic structures.2–5 Much of the theoretical work on mesoionic compounds focuses on discussing their aromaticity. While some researchers assert that mesoionic compounds are aromatic,6,7 others firmly contend that they are not.4,8
The debate over the aromaticity of mesoionic compounds stems from how these compounds are represented. The controversy began in 1955 when Katritzky urged the scientific community to refrain from using the term ‘mesoionic compounds’ and to instead refer to them as ‘mesomeric betaines’.9 This nomenclature persisted until 1985 when Ollis, Stanforth, and Ramsden introduced a new classification based on the different types of heterocycle conjugations (Scheme 1).2
Subsequently, mesoionic compounds were categorized into two types based on their heteroatoms' arrangement, with A and B serving as positional isomers. The tautomerism in structures A and B, showcasing the heterocycle as aromatic, has been utilized to explore, for example, the reactivity of some mesoionic compounds (Scheme 1).2,3
Although the representation of forms A and B has been widely recommended for years, it has been consistently questioned due to its suggestion of aromatization in the heterocycle. Despite the conjugated system adhering to Hückel's rule, the aromatic nature of these heterocyclic rings has always been questioned. For instance, the reactivity of mesoionic compounds towards electrophiles (SnAr) could suggest their aromatic character;10 however, their ability to react as 1,3-dipoles presents evidence against such aromatic behavior.11
In pioneering work, Simas et al.8 in 1998 concluded that mesoionic compounds are not aromatic, based on X-ray diffraction data and theoretical studies, which indicate clearly separated positive and negative regions functioning independently as electron-deficient and electron-rich areas. Following this, Champagne and Houk4 proposed structures C and D for mesoionic compounds, relying on theoretical data and referencing X-ray data from Simas,8,12–14 although they overlooked critical experimental observations about the presence of two electron-delocalized regions, calling into question the validity of structures C and D.
Conversely, M. Duvall in 20136 demonstrated through extensive theoretical analysis using Bird and nucleus independent chemical shift (NICS) aromaticity indices that mesoionic compounds, including sydnones and münchnones, are aromatic, exhibiting indices comparable to furans and pyrroles. Similarly, Kuroda et al.7 based on experimental X-ray diffraction data contributed to the general idea of aromaticity of mesoionic compounds, demonstrating that these compounds possess a moderate degree of aromaticity, showing that the X-ray diffraction data aligned consistently with previous computational predictions.
More recently, considering the research conducted by several scientists, including Champagne and Houk,4 it was determined that the bond, charge, and magnetic field data in the heterocyclic ring indicated a lack of aromatization in mesoionic compounds. Therefore, structures C and D (Fig. 1) were recommended for representing mesoionic compounds. Following Champagne and Houk's4 research, Porte et al. published a review in 2021,5 adopting and recommending the presentation of mesoionic compounds as structures C and D. Their recommendation was based on a theoretical study of cycloaddition reactions4 and did not include data on aromaticity indices, merely referencing the work by Simas et al.8 and Katritzky et al.15 as though these articles encompassed all known aromaticity data for mesoionic compounds.
From these controversial positions, it is clear that the aromaticity of mesoionic compounds remains a contested issue. In response, we embarked on this study to systematically investigate the aromaticity of mesoionic compounds and contribute to the ongoing debate: are mesoionic compounds aromatic? To this end, we employed experimental X-ray data alongside the HOMHED geometric index16 to evaluate the aromaticity of these compounds. Our study covers a collection of 25 mesoionic compounds across eight distinctive classes, offering diverse structures for analysis (Fig. 1).
Experimental
X-ray structures
The X-ray data for mesoionic compounds numbered 1–25 used in this study were collected from the CCDC database using the software Conquest (CSD version 5.45). The bond lengths (in Ångströms) are listed in Table S1.† The search involved drawing the specific nuclei of mesoionic structures. Subsequently, a manual search was conducted to identify the structures with the best crystallographic data, resulting in 25 structures. Crystallographic information files (CIF) for compounds 1–25 are deposited at the Cambridge Crystallographic Data Centre (CCDC) under the following identification refcodes: YOMWAA (1),17 WADNOG (2),18 JOMBUK (3),19 EWOXAP (4),20 SOLZOJ (5),21 BIJKEN (6),22 ETHBSD (7),23 XAZTOJ (8),24 XAZJEQ (9),25 XZBTZO (10),26 IJOWUB (11),27 DEWYIQ (12),28 TUFPIW (13),29 KUCYEN (14),30 OVIWAU (15),31 OVIWIC (16),31 OVIWEY (17),31 RIPROB (18),32 MAGTEV (19),33 MAGTIZ (20),33 KIXHUW (21),34 KIXJAE (22),34 KIXJEI (23),34 KIXJIM (24),34 and ZOCLAI (25).7HOMHED index
The HOMHED index was used to calculate the aromaticity of mesoionic compounds' structures.16 To calculate the HOMHED index, it was necessary to obtain the Ropt bond length and the normalized constant (α) for each type of bond. The Ropt bond length was calculated according to eqn (1), where Rs and Rd are the reference single and double bond lengths, respectively, taken from experimental X-ray data, respectively, and ω is the ratio of stretching force constants for pure double and single bonds. The ω ratio close to 2 was used for the C–C and C–X bonds. The normalized constant (α) for each type of bond was calculated using eqn (2). The HOMHED, a dimensionless parameter, is calculated for each compound using eqn (3), where n is the number of bonds considered in the resonance system, and Ri represents the bond lengths. Table 1 presents the data for Rs, Rd, and Ropt for C–C, C–N, C–O, C–S, N–N, N–O, and N–S bonds based on data from the literature.16 | (1) |
 | (2) |
 | (3) |
| Bond | C–C | C–N | C–O | C–S | N–N | N–O | N–S | C–B | C–N+ |
|---|---|---|---|---|---|---|---|---|---|
| a Data determined using eqn (1) and (2). | |||||||||
| R s (Å) | 1.530 | 1.474 | 1.426 | 1.819 | 1.454 | 1.463 | 1.765 | 1.600 | 1.494 |
| R d (Å) | 1.316 | 1.271 | 1.210 | 1.599 | 1.240 | 1.218 | 1.541 | 1.362 | 1.266 |
| R opt (Å) | 1.387 | 1.339 | 1.282 | 1.672 | 1.311 | 1.300 | 1.616 | 1.441 | 1.342 |
| α | 78.6 | 87.4 | 77.2 | 74.4 | 78.6 | 60.0 | 71.7 | 63.4 | 69.3 |
| Reference | 16 | 16 | 16 | 16 | 16 | 16 | 16 | [This study]a | [This study]a |
Bond lengths for HOMHED
The bond lengths for HOMHED are detailed in Table 1. Data for Rs, Rd, and Ropt, related to C–C, C–N, C–O, C–S, N–N, N–O, and N–S bonds, were derived from Frizzo and Martins.16 The C–N+ and C–B bond lengths were determined using eqn (1) and (2) based on the data below.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2Br− (LILLOH), Me2N+
CH2Br− (LILLOH), Me2N+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHO2Cl− (VAPREJ), and Et2N+
CHO2Cl− (VAPREJ), and Et2N+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2Cl− (TIHGOH).35–42
CH2Cl− (TIHGOH).35–42
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B (VARJED).45
B (VARJED).45
Results and discussion
Mesoionic compounds cannot be represented by a single contributing structure among several canonical forms described in various mesomeric structures and sometimes suggest a high level of aromaticity in their heterocycles. We propose that a mesoionic compound, regardless of the ring heteroatoms or the exocyclic atom, should be represented by two main canonical structures: the aromatic (AS) and betaine structures (BS), as shown in Scheme 2. In the AS, the formal positive charge of the mesoionic compound is associated with all the ring atoms (D-A-Y-C-E), and a formal negative charge is associated with the exocyclic atom (X). In the BS, the formal positive charge of mesoionic compounds is associated with the ring atoms (D-A-Y), and the formal negative charge is associated with both ring atoms and an exocyclic atom (E-C-X). Going forward, we aim to bridge the gap in research on mesoionic compounds' aromaticity by seeking evidence to determine which canonical structures (i.e., AS or BS) best represent the mesoionic compounds' structures.The HOMHED index
Initially, we applied the HOMHED index to evaluate the aromaticity of 25 mesoionic compounds. Introduced in 2012 by Frizzo and Martins,16 it builds on the original HOMA concept for assessing the aromaticity of heterocycles46 and utilizes experimental single-crystal X-ray diffraction data to estimate optimal bond lengths, classifying compounds based on how closely their values approach 1, with those closer to 1 being deemed more aromatic. This index has been used for a variety of five- and six-membered heterocycles with C–C, C–N, C–O, C–S, N–N, N–O, and N–S bonds.16 A comprehensive review on the geometric criteria for aromaticity and the development of HOMA-based indices was published by Krygowski et al.47Nevertheless, to encompass the full range of compounds in this study, it became necessary to determine the optimal bond length (Ropt) for C–B bonds; the Ropt(C–B) values were determined using the same methodology16 (Table 1). The optimal bond lengths Rs, Rd were derived from experimental X-ray data of compounds containing both C–B and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B bonds. Despite Zborowski et al.48 previous publication of Ropt for the C–B bond based on X-ray diffraction data, we observed that the authors used a Csp3–Bsp bond for Rs. This single bond is shorter than that of a ‘pure’ Csp3–Bsp2 bond by approximately 0.05 Å, leading to an underestimated Ropt (1.424 Å) and an overestimated α (104.5). Conversely, aiming to use the same criteria in our 2012 work,16 we used X-ray diffraction data from ‘pure’ single bonds of compounds containing Csp3–Bsp2 bond to determine the Ropt(C–B).
B bonds. Despite Zborowski et al.48 previous publication of Ropt for the C–B bond based on X-ray diffraction data, we observed that the authors used a Csp3–Bsp bond for Rs. This single bond is shorter than that of a ‘pure’ Csp3–Bsp2 bond by approximately 0.05 Å, leading to an underestimated Ropt (1.424 Å) and an overestimated α (104.5). Conversely, aiming to use the same criteria in our 2012 work,16 we used X-ray diffraction data from ‘pure’ single bonds of compounds containing Csp3–Bsp2 bond to determine the Ropt(C–B).
The HOMHED indices for compounds 1–25 were calculated using eqn (3), which considers the bond lengths (Ri) between atoms Y-A, A-D, D-E, E-C, C-Y in the heterocyclic ring and C-X (exocyclic bond) (Table S1 in ESI†). Compound 25 has the lowest aromaticity index (0.494), while compounds 21–24 show the highest aromaticity indices (0.951–0.976). Fig. 2 illustrates the distribution of HOMHED data for compounds 1–25.
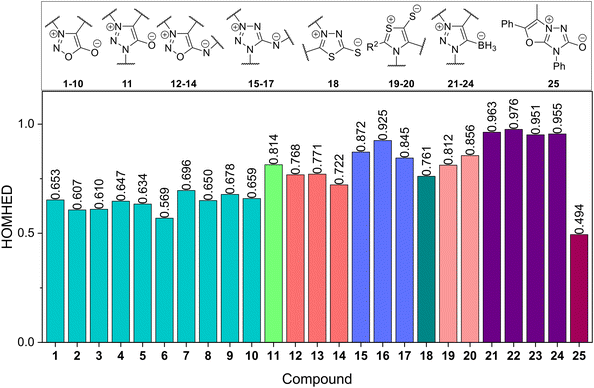 | ||
| Fig. 2 HOMHED values of compounds 1–25. The colored bars represent the different structures of mesoionic compounds. | ||
By observing the HOMHED values of the compounds, we see that compounds 1–10 exhibit low aromaticity (0.569–0.696), as expected for heterocycles containing an oxygen atom (O1) in the ring. This phenomenon, where a cyclic oxygen atom promotes lower aromaticity, has already been reported.49,50 Compound 11 displays an increase in aromaticity due to the substitution of O1 with N1 in the heterocyclic ring (HOMHED = 0.8). In compounds 12–14, the HOMHED values vary at 0.72–0.77; this is related to the replacement of the exocyclic oxygen atom with a nitrogen atom; however, unlike the previous example, the increase in aromaticity was very subtle. In both scenarios, substituting a chalcogen atom for a nitrogen atom results in an increase in HOMHED, suggesting that mesoionic compounds containing oxygen atoms, whether in the heterocycle or exocyclically, have lesser aromaticity.
Mesoionic tetrazolium derivatives (compounds 15–17) and thiazole derivatives (compounds 19–20) also exhibit HOMHED values above 0.8. Compound 18 has a lower HOMHED than compounds 15–17 and 19–20, likely due to the replacement of N1 with S1. Lastly, compounds 21–24 present HOMHED values around 0.95, suggesting an aromaticity similar to that of uncharged nitrogenous heterocyclic compounds.16 This can be attributed to the fact that the negative charge on the exocyclic boron atom does not resonate with the heterocyclic ring, unlike in compounds 1–20. In summary, the average HOMHED values increase in the order of heterocyclic atom (Y): O1 < S1 < N1; and for the exocyclic atom (X): O6 < S6 ≈ N6 < B6, as shown in Fig. 3.
 | ||
| Fig. 3 Effect of heterocyclic (Y) and exocyclic (X) atom on the aromaticity of mesoionic compounds 1–24. | ||
Although Kuroda et al.7 described compound 25 and calculated its HOMHED and NICS indices, they concluded that the synthesized mesoionic triazolone exhibits a moderate degree of aromatic character. The authors did not present a comparative study to demonstrate how this type of structure's HOMHED index compares to those of other mesoionic compounds with similar structures. Our comparative study indicates that compound 25 may be an exception, as it has a very low HOMHED value (0.494). Therefore, arising as a missing link, representing an important factor in elucidating the boundaries of the aromaticity continuum. For 5-membered heterocycles containing a sp3-nitrogen in the ring, HOMHED values greater than 0.800 are typically expected. This suggests that other factors may decrease the aromaticity of the heterocycle, with the most significant effect likely being the tension between the two condensed 5-membered rings that comprise the molecule.
The HOMHED vs. HOMHED+
The next issue to address concerns determining the HOMHED of aromatic compounds containing charged nitrogen, such as mesoionic or salts. More specifically, would it be more accurate to calculate the HOMHED value with the Ropt(C–N) bond or with the Ropt(C–N+) bond? To answer this question, this study presents, for the first time in the literature, the optimal bond (Ropt) values for C–N+. The HOMHED+ data for compounds 1–25, which utilize Ropt(C–N+) instead of Ropt(C–N), can be found in Table S2 (ESI†). Overall, the results show no significant differences between the HOMHED and HOMHED+ values, whether using the Ropt(C–N) or the Ropt(C–N+) bond (Fig. 4a). | ||
| Fig. 4 (a) Correlation between HOMHED and HOMHED+; R = 0.988 and (b) variation of the HOMHED index for compounds 1–25. Calculated by ((HOMHED+/HOMHED) − 1) × 100. | ||
In the series of compounds studied, the use of Ropt(C–N+) increases HOMHED+ value by a range of 0–5%, depending on the number of C–N bonds in the heterocycle and the presence of nitrogen-sp3 (π-excess heterocycles), as shown in Fig. 4b. A notable exception is the mesoionic compound 25, which consists of two fused 5-membered rings with 4 C–N bonds. For this compound, the HOMHED+ value is 18% higher than HOMHED. Furthermore, the application of Ropt(C–N+) bonds in certain tetrazolinium+, imidazolinium+, and pyridinium+ salts was evaluated. The results showed that the HOMHED+ value was over 2% higher for imidazolinium+ salts, while it was negligible for the other two series of salts.
Aromatic vs. betaine structures
Generally, researchers have posited that mesoionic compounds consist of a five-member heterocyclic ring in which the D-A-Y atoms carry the positive charge, while the E-C-X atoms carry the negative charge, similar to betaines. In contrast, another form presents the positive charge distributed across the D-A-Y-C-E atoms of the ring, with the negative charge localized on the exocyclic X atom, resembling aromatic compounds, as illustrated in Fig. 5. This distinction underpins the understanding that the lower aromaticity observed in mesoionic compounds is attributed to the betaine-like structure. This structure significantly reduces the resonance in the Y-C and D-E bonds, limiting the interaction between the positive and negative charge resonance systems.8In this context, considering the betaine and aromatic forms, it seems important to investigate the resonance (electronic density delocalization) in fragments that comprise the betaine structure: D-A-Y, E-C-X fragments, and the D-E, C-Y fragments that connect D-A-Y and E-C-X. Our preliminary understanding suggests that a mesoionic compound exhibits several canonical structures, notably the AS and BS, each contributing to varying extents to the mesoionic compound's properties (Fig. 5). Thus, we cannot prefer one canonical structure over another but rather need to understand the extent of each structure's contribution.
Using HOMHED, it is possible to calculate the resonance for selected fragments, that is, not to the entire molecule, but to specific fragments of the analyzed structure.16 This method allowed us to obtain the resonance data for the residues Res(ECX), Res(DAY), Res(DE,CY) in compounds 1–25 (Table 2). With this data, we aim to determine how the aromatic character of a mesoionic compound correlates with the resonance of its fragments.
| Compound | Res(ECX) | Res(DAY) | Res(DE,CY) | Compound | Res(ECX) | Res(DAY) | Res(DE,CY) |
|---|---|---|---|---|---|---|---|
| 1 | 0.836 | 0.824 | 0.317 | 14 | 0.902 | 0.788 | 0.537 |
| 2 | 0.820 | 0.801 | 0.232 | 15 | 0.864 | 0.951 | 0.797 |
| 3 | 0.715 | 0.851 | 0.241 | 16 | 0.979 | 0.968 | 0.855 |
| 4 | 0.790 | 0.816 | 0.317 | 17 | 0.794 | 0.956 | 0.745 |
| 5 | 0.814 | 0.806 | 0.306 | 18 | 0.978 | 0.950 | 0.473 |
| 6 | 0.715 | 0.799 | 0.150 | 19 | 0.953 | 0.947 | 0.589 |
| 7 | 0.818 | 0.829 | 0.414 | 20 | 0.938 | 0.960 | 0.680 |
| 8 | 0.736 | 0.856 | 0.322 | 21 | 0.135 | 0.980 | 0.929 |
| 9 | 0.761 | 0.821 | 0.414 | 22 | 0.093 | 0.989 | 0.953 |
| 10 | 0.662 | 0.702 | 0.498 | 23 | 0.147 | 0.975 | 0.904 |
| 11 | 0.619 | 0.940 | 0.627 | 24 | 0.196 | 0.963 | 0.927 |
| 12 | 0.965 | 0.793 | 0.634 | 25 | 0.895 | 0.949 | 0.000 |
| 13 | 0.969 | 0.816 | 0.610 | ||||
With resonance data available, analyzing the mesoionic compound's aromatic character—in terms of greater or lesser—based on the HOMHED geometric data,16 relies on correlating it with the resonances of the fragments (D-A-Y), (D-E, C-Y), and (E-C-X). In this context, we anticipate: (i) high resonance indices for the fragments (D-A-Y) and (D-E, C-Y) and low resonance indices for the fragment (E-C-X) in compounds with high aromaticity indices (HOMHED); and (ii) compounds exhibiting high resonance indices in the fragments (D-A-Y) and (E-C-X) will display characteristics typical of betaines. The resonance index in the fragment (D-E, C-Y) will determine the extent to which the resonances of the two fragments (D-A-Y) and (E-C-X) differ, predominantly contributing to the betaine characteristic. We associate the HOMHED index with fragment resonances to gather additional insights into mesoionic behavior (Fig. 6). The data points on the graph are categorized into three primary regions.
 | ||
| Fig. 6 Correlation of Res(DAY), Res(DE,CY), and Res(ECX) parameters with HOMHED. A region is in the orange box, B region is in the red box, and C region is in the blue box. | ||
As shown in Fig. 6, in region A, the resonance data Res(DAY), Res(DE,CY), and Res(ECX) for compounds 21–24 are presented, showcasing HOMHED values greater than 0.950. It is evident that Res(DAY) and Res(DE,CY) values exceed 0.9, while Res(ECX) values fall below 0.2. This indicates that compounds 21–24 possess a degree of aromaticity and highlight the minimal contribution of the canonical betaine structure. It is important to note that these compounds contain a boron atom carrying a negative charge, which does not resonate with the ring atoms, as reflected by the low Res(ECX) values.
Region B displays the highest values of Res(DAY) and Res(ECX), although the lowest of Res(DE,CY). Compound 25, the sole compound in this region, exhibits values of Res(DAY) = 0.985, Res(ECX) = 0.949, and Res(DE,CY) = 0, with a HOMHED of 0.494. These figures suggest compound 25 has a low aromaticity level and a significant contribution from the canonical betaine structure.
In region C, the resonance data Res(DAY) and Res(ECX) for most studied compounds presenting fragment resonance greater than 0.7 and HOMHED values ranging from 0.60 to 0.95 are found. This region may indicate a notable contribution of the canonical betaine structure, although the Res(DE,CY) data span a wide range (0.150–0.855), pointing out the need for additional data to more accurately quantify the contributions of each canonical aromatic or betaine structure.
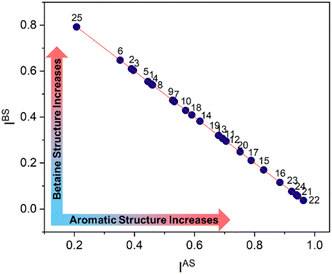
A critical observation is that the data Res(DE,CY) exhibits a strong linear correlation with the HOMHED data (r = 0.973; N = 25; equation: Res(DE,CY) = 1.958 HOMHED − 0.947), indicating the varying resonance in the D-E and C-Y bonds. High Res(DE,CY) values suggest a stronger aromatic character in the mesoionic compounds. This fact shows that the degree of aromaticity can be determined quantitatively from an error calculation between the HOMHED and Res(DE,CY) in relation to 1, as demonstrated by eqn 4, which provides the aromatic structure index (IAS). Eqn (5) provides the betaine structure index (IBS) of mesoionic compounds. The IAS and IBS data for the 25 compounds are listed in Table S3 (ESI†) and shown in Fig. 7.
 | (4) |
| IBS = 1 − IAS | (5) |
Considering the average HOMHED of each class of mesoionic compounds (1–10, 11, 12–14, 15–17, 18, 19–20, 21–24, 25), we compared them with the average HOMHED of a series of different heterocycles that have been previously published (Fig. 8).16 This comparison allows us to observe that the HOMHED increases from the least aromatic compounds, which are the 5-membered rings containing oxygen (e.g., oxazoles, furans, isoxazoles), to the sulfur-containing compounds (e.g., thiophenes, thiazoles), then to the nitrogenous compounds (e.g., imidazoles, pyrazoles, pyrroles), and finally to the nitrogenous compounds with 6-membered rings (e.g., pyrimidines, pyridines, pyrazines).
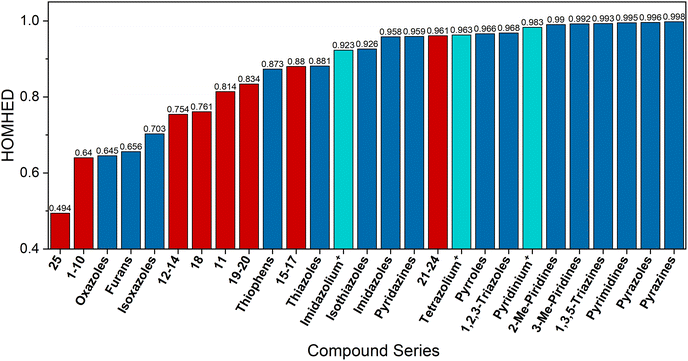 | ||
| Fig. 8 Comparison of the average HOMHED of the series of mesoionic compounds 1–25 (red), heterocyclic salts (cyan), and heterocycles (blue). | ||
In Fig. 8, one can observe that mesoionic compounds 1–24 are distributed based on HOMHED values in the same order as uncharged heterocycles with similar structures. The three series of charged heterocycles, namely, the imidazolium, tetrazolium, and pyrimidinium salts, follow the same order as the uncharged heterocycles. However, an exception was noted with compound 25, which exhibits a HOMHED value lower than expected compared to 5-membered rings containing an N-sp3, typically characterized by HOMHED values greater than 0.800.
Previous research on this topic of mesoionic aromatic order includes a study by Bird50 demonstrating that the introduction of an oxygen atom into the ring decreases the aromaticity of heterocycles. Frizzo and peers,16 in their studies on the aromaticity of heterocyclic compounds, found an increase in aromaticity in the sequence furan → thiophene → pyrrole. Morzherin et al.51 showed that mesoionic compounds, such as 1,2,3-triazoles and 1,2,3,4-tetrazoles, are more aromatic than other mesoionic compounds, considering them more as ‘masked dipoles’. Moreover, Frizzo et al.16 also showed that 5- and 6-membered heterocyclic compounds that contain a larger number of nitrogen atoms are more aromatic than their less nitrogenous counterparts.
Our preliminary study suggests that the aromaticity of heterocycles is minimally impacted by the presence of charges within the heterocycle, whether in mesoionic compounds (1–24) or salts (imidazolinium+, tetrazolinium+, pyridinium+), especially when evaluating the aromaticity order among 5- or 6-membered heterocycles. This phenomenon of ‘charges not mattering’ has recently been reported in a recent study by our group32 covering supramolecular behavior of mesoionic compounds 1–24 based on their X-ray diffraction data. The findings revealed that the mesoionic compounds exhibit behavior similar to that of uncharged compounds.32
Furthermore, the supramolecular data indicated that these compounds exhibit characteristics typical of aromatic compounds because (i) the molecular electrostatic potential predominantly shows positive potential in the ring and negative potential in the exocyclic heteroatom; (ii) there are numerous intermolecular interactions based on the stacking of mesoionic rings with the exocyclic atom; (iii) stackings between mesoionic rings and phenyls are also observed; and (iv) most of the crystallization mechanisms of the studied mesoionic compounds involve molecular stacking structures of the 1D nuclei and 1D-dimer types.32
Conclusions
This study thoroughly investigated the aromaticity of 25 mesoionic compounds, divided into eight structural categories, by utilizing the HOMHED geometric index and experimental X-ray diffraction data. The combination of experimental and theoretical approaches contributes significantly to the debate on the aromatic nature of mesoionic compounds and refines the understanding of their electronic structures. This understanding has implications for their reactivity, stability, and potential applications in supramolecular chemistry. The findings reveal a substantial variation in aromaticity among the compounds, primarily influenced by the nature of the heterocyclic and exocyclic atoms. This underscores the necessity for a nuanced approach to evaluating the electronic properties of mesoionic compounds beyond the oversimplified definition of to be or not to be aromatic.Our findings revealed that the degree of aromaticity in mesoionic compounds varies depending on the heteroatoms present. Compounds containing oxygen atoms in the ring generally exhibit lower aromaticity, whereas those with nitrogen atoms, particularly when nitrogen is part of an exocyclic element or sp3 bond, tend to have higher aromaticity. Compounds 21–24 showed the highest aromaticity due to the presence of a negatively charged boron atom that does not resonate with the ring atoms.
Furthermore, this study highlights the significance of the aromatic and betaine structures in their canonical forms in understanding the aromatic nature of mesoionic compounds. Analysis of resonance data across different fragments within the compounds revealed that high resonance in D-E and C-Y bonds correlates with increased aromatic character, whereas high resonance in D-A-Y and E-C-X fragments suggests a predominant betaine character. The introduction of the aromatic structure index and betaine structure index provides a quantitative method to assess the contributions of these canonical structures. Compounds such as 21–24 were found to predominantly exhibit aromatic character with minimal betaine contribution, while compound 25 displayed a dominant betaine structure, leading to its lower aromaticity. Additionally, our findings showed that mesoionic compounds 1–24 possess aromaticity comparable to uncharged heterocyclic compounds, indicating that their aromaticity is minimally impacted by the presence of charges within the heterocycle.
It is often mistakenly accepted that mesoionic compounds are not aromatic, a belief that has become widespread. This suggests the need for a critical perspective devoid of preconceived notions, as the binary question of “to be or not to be aromatic” may be oversimplifying the matter. Focusing on one canonical structure over another due to formal charges in mesoionic compounds can lead to a skewed view. While research on mesoionic compounds has contributed valuable insights into synthesis, reactivity, and structure, it often overlooks a detailed analysis of aromaticity. Many investigations into their non-aromatic nature are based solely on theoretical data, creating a potential risk. These results can contain circular data output; the data introduced into the systems and the response data are part of the same type of calculation, with no experimental data breaking this theoretical bubble created.
Nevertheless, from a systematic perspective, with the researcher suspending any preconceptions, it is possible to observe that mesoionic compounds are heterocycles with all Hückel's requirements to behave with some degree of aromaticity. Regardless of the index used for measuring, most mesoionic compounds in the literature display a wide range of aromaticity indices comparable to those of heterocycles such as furans and thiophenes. Hence, mesoionic compounds are aromatic, though the extent of their aromaticity still needs clarification. Therefore, supported by experimental data, we propose that the correct scientific question to be answered here is: how aromatic are mesoionic compounds?
Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Rio Grande do Sul Research Support Foundation (FAPERGS process no. 17/2551-0000981-9), the National Council for Scientific and Technological Development (CNPq process no. 405071/2016-7), and the Coordination for the Improvement of Higher Education Personnel (CAPES) for financial support. The fellowships from CNPq (MAPM) and CAPES (JMLR and PSVL) are also acknowledged.References
- Mesoionic compounds, in IUPAC Compendium of Chemical Terminology, International Union of Pure and Applied Chemistry, 5th edn, 2025, DOI:10.1351/goldbook.M03842.
- W. D. Ollis, S. P. Stanforth and C. A. Ramsden, Tetrahedron, 1985, 41, 2239–2329 CrossRef CAS.
- C. A. Ramsden, Tetrahedron, 2013, 69, 4146–4159 CrossRef CAS.
- P. A. Champagne and K. N. Houk, J. Org. Chem., 2017, 82, 10980–10988 CrossRef CAS PubMed.
- K. Porte, M. Riomet, C. Figliola, D. Audisio and F. Taran, Chem. Rev., 2021, 121, 6718–6743 CrossRef CAS PubMed.
- M. B. Duvall, Master of Science thesis, University of Georgia, 2013 Search PubMed.
- Y. Kuroda, M. Krell, K. Kurokawa and K. Takasu, Chem. Commun., 2024, 60, 1719–1722 RSC.
- A. M. Simas, J. Miller and P. F. de Athayade Filho, Can. J. Chem., 1998, 76, 869–872 CrossRef CAS.
- A. J. Boulton, Biogr. Mem. Fellows R. Soc., 2015, 61, 225–245 CrossRef.
- B. V. Badami, Resonance, 2006, 11, 40–48 CrossRef CAS.
- W. P. Oziminski and C. A. Ramsden, Tetrahedron, 2015, 71, 7191–7198 CrossRef CAS.
- K. K. Cheung, S. Galembeck, J. Miller, M. B. de Oliveira, A. B. Pereira and A. M. Simas, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 2630–2632 CrossRef.
- K. K. Cheung, S. Galembeck, J. Miller, M. B. de Oliveira, A. B. Pereira and A. M. Simas, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1992, 48, 523–525 CrossRef.
- K. K. Cheung, A. Echevarria, S. Galembeck, M. A. M. Maciel, J. Miller, V. M. Rumjanek and A. M. Simas, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1992, 48, 1471–1474 CrossRef.
- A. R. Katritzky, K. Jug and D. C. Oniciu, Chem. Rev., 2001, 101, 1421–1450 CrossRef CAS PubMed.
- C. P. Frizzo and M. A. P. Martins, Struct. Chem., 2012, 23, 375–380 CrossRef CAS.
- S. Wiechmann, T. Freese, M. H. H. Drafz, E. G. Hübner, J. C. Namyslo, M. Nieger and A. Schmidt, Chem. Commun., 2014, 50, 11822–11824 RSC.
- H. K. Fun, J. H. Goh, Nithinchandra and B. Kalluraya, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66(12), o3252 CrossRef CAS PubMed.
- D. Grossie, L. Harrison and K. Turnbull, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2014, 70, o1165–o1166 CrossRef CAS PubMed.
- G. B. Riddle, D. A. Grossie and K. Turnbull, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2004, 60, 2003–2004 Search PubMed.
- D. A. Grossie, K. Turnbull, S. Felix-Balderrama and S. Raghavapuram, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, o554–o555 CrossRef CAS PubMed.
- C. Favre and F. Friscourt, Org. Lett., 2018, 20, 4213–4217 CrossRef CAS PubMed.
- H. Hope and W. E. Thiessen, Acta Crystallogr., Sect. B, 1969, 25, 1237–1247 CrossRef CAS.
- H. K. Fun, T. S. Chia, Nithinchandra, B. Kalluraya and S. Shetty, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68(7), o2103 CrossRef CAS PubMed.
- Y. Dürüst, E. Yıldız, H. Karakuş and B. M. Kariuki, Synth. Commun., 2017, 47, 660–670 CrossRef.
- T. J. King, P. N. Preston, J. S. Suffolk and K. Turnbull, J. Chem. Soc., Perkin Trans. 2, 1979, 1751 RSC.
- I. S. Khazhieva, P. M. Demkin, J. INein, T. V. Glukhareva and Y. Y. Morzherin, CCDC 1030491: Experimental Crystal Structure Determination, 2016 Search PubMed.
- I. A. Cherepanov, A. S. Samarskaya, I. A. Godovikov, K. A. Lyssenko, A. A. Pankratova and V. N. Kalinin, Tetrahedron Lett., 2018, 59, 727–729 CrossRef CAS.
- M. Riomet, K. Porte, L. Madegard, P. Thuéry, D. Audisio and F. Taran, Org. Lett., 2020, 22, 2403–2408 CrossRef CAS PubMed.
- V. N. Kalinin, S. N. Lebedev, I. A. Cherepanov, I. A. Godovikov, K. A. Lyssenko and E. Hey-Hawkins, Polyhedron, 2009, 28, 2411–2417 CrossRef CAS.
- V. A. Budevich, S. V. Voitekhovich, A. V. Zuraev, V. E. Matulis, V. E. Matulis, A. S. Lyakhov, L. S. Ivashkevich and O. A. Ivashkevich, Beilstein J. Org. Chem., 2021, 17, 385–395 CrossRef CAS PubMed.
- P. S. V. Lima, G. H. Weimer, L. P. Oliveira, H. D. S. Souza, G. F. Fiss, H. G. Bonacorso and M. A. P. Martins, CrystEngComm, 2023, 25, 4976–4991 RSC.
- D. Cantillo, M. Ávalos, R. Babiano, P. Cintas, J. L. Jiménez, M. E. Light, J. C. Palacios and V. Rodríguez, Org. Biomol. Chem., 2010, 8, 5367–5374 RSC.
- L. B. de Oliveira Freitas, P. Eisenberger and C. M. Crudden, Organometallics, 2013, 32, 6635–6638 CrossRef CAS.
- G. Herrschaft and H. Hartl, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1989, 45, 1021–1024 CrossRef.
- H. Ishigami, M. Sumita, Y. Tsunashima, T. Hori and S. Sato, J. Korean Phys. Soc., 2003, 42, 1237–1239 Search PubMed.
- U. Böhme and M. Gerwig, CCDC 1874521: Experimental Crystal Structure Determination, 2018 Search PubMed.
- D. J. Evans and D. L. Hughes, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1990, 46, 1452–1454 CrossRef.
- J. K. Cockcroft, A. Shamsabadi, H. Wu and A. R. Rennie, Phys. Chem. Chem. Phys., 2019, 21, 25945–25951 RSC.
- G. R. Clark, G. L. Shaw, P. W. J. Surman, M. J. Taylor and D. Steele, J. Chem. Soc., Faraday Trans., 1994, 90, 3139 RSC.
- A. B. Burg, Inorg. Chem., 1989, 28, 1295–1300 CrossRef CAS.
- R. T. Boeré, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, o3581 CrossRef.
- A. R. Cowley, A. J. Downs, S. Marchant, V. A. Macrae, R. A. Taylor and S. Parsons, Organometallics, 2005, 24, 5702–5709 CrossRef CAS.
- K. L. Mears, M. A. Kutzleb, C. R. Stennett, J. C. Fettinger, D. C. Kaseman, P. Yu, P. Vasko and P. P. Power, Chem. Commun., 2022, 58, 9910–9913 RSC.
- R. Boese, P. Paetzold, A. Tapper and R. Ziembinski, Chem. Ber., 1989, 122, 1057–1060 CrossRef CAS.
- J. Kruszewski and T. M. Krygowski, Tetrahedron Lett., 1972, 13, 3839–3842 CrossRef.
- T. M. Krygowski, H. Szatylowicz, O. A. Stasyuk, J. Dominikowska and M. Palusiak, Chem. Rev., 2014, 114, 6383–6422 CrossRef CAS PubMed.
- K. K. Zborowski, I. Alkorta, J. Elguero and L. M. Proniewicz, Struct. Chem., 2012, 23, 595–600 CrossRef CAS.
- A. Huber, J. Dubbert, T. D. Scherz and J. Voskuhl, Chem. – Eur. J., 2023, 29, 1294 Search PubMed.
- C. W. Bird, Tetrahedron, 1985, 41, 1409–1414 CrossRef CAS.
- Y. I. Nein and Y. Y. Morzherin, Russ. Chem. Bull., 2012, 61, 1111–1116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ce00334b |
| This journal is © The Royal Society of Chemistry 2025 |