 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Calix[4]arene-supported trigonal and square prisms of Mn and Na†
Lucinda R. B.
Wilson
 a,
Scott J.
Dalgarno
a,
Scott J.
Dalgarno
 *b and
Euan K.
Brechin
*b and
Euan K.
Brechin
 *a
*a
aEastCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, Scotland EH9 3FJ, UK. E-mail: ebrechin@ed.ac.uk
bInstitute of Chemical Sciences, Heriot-Watt University, Riccarton, Edinburgh, Scotland EH14 4AS, UK. E-mail: S.J.Dalgarno@hw.ac.uk
First published on 9th April 2025
Abstract
The heterometallic tricapped trigonal prism [MnIII3Na6(TBC[4])3(CO3)(Cl)(dmso)7]·5MeCN ([Mn3Na6]; 1·5MeCN) and the related heterometallic tetracapped square prism [MnIII4MnII2Na6(bisTBC[4])2(CO3)2(Cl)2(dmf)8(MeOH)1.2(H2O)0.8]·6MeCN ([Mn6Na6]; 2·6MeCN), are isolated using p-tBu-calix[4]arene (H4TBC[4]) and 2,2′-bis-p-tBu-calix[4]arene (H8-bisTBC[4]), respectively. In the former the MnIII ions sit in the TBC[4] polyphenolic pockets, forming three Mn-TBC[4] metalloligands that cap the square faces of the prism, whose vertices contain the six Na ions. Introduction of the bis-calix[4]arene enables expansion of the main building block from three Mn-TBC[4] metalloligands in 1 to four Mn-TBC[4] metalloligands in 2. The result is the formation of a square prism of Na/MnII ions, with four of the square faces capped with MnIII ions. Dc magnetic susceptibility and magnetisation measurements reveal paramagnetism in 1 and weak antiferromagnetic exchange interactions in 2.
Introduction
The synthetic versatility and accessibility of p-tert-butylcalix[n]arenes have seen them employed in an incredibly diverse range of chemistry.1–8 In coordination chemistry, the tetraphenolic pocket of p-tert-butylcalix[4]arene (H4TBC[4], Fig. 1) presents an attractive ligand motif for the construction of transition metal (TM) and lanthanide metal (LnMIII) metalloligands that can self-assemble into larger, polymetallic cages.9 Structurally, this means the TM-TBC[4]/LnM-TBC[4] moiety acts as a capping fragment in the resulting metallic skeleton, with some or all of the phenolato O-atoms further bridging to other metal ions. When these metal ions are paramagnetic, the clusters can display fascinating magnetic behaviour including, for example, single-molecule magnetism,10 spin frustration11 or an enhanced magnetocaloric effect.12 The dominant structure-directing role of the calix[4]arene also means that, in some cases, analogous structural topologies can be made with different metals and with metals in a variety of oxidation states, allowing detailed magneto-structural studies.13The tetraphenolato pocket of TBC[4]4− is particularly well suited to bonding Jahn–Teller (JT) distorted ions such as octahedral MnIII because it will preferentially coordinate metals possessing four short equatorial bonds.14 Indeed, this ability has been exploited to isolate a number of polymetallic Mn clusters, including [MnIIIMnII],15 [MnIIIMnII]2,16 [MnIII2MnII2],17 [MnIII3MnII2],18 [MnIII7MnII],19 and [MnIII8MnII4].20 In each case the MnIII ions sits in the TBC[4] pocket, and the MnIII-TBC[4] metalloligand further bridges to additional metal ions, often encapsulating a polymetallic metal-oxo/hydroxo cluster.
Extension of the chemistry to the related to 2,2′-bis-p-tBu-calix[4]arene21 (H8-bisTBC[4], Fig. 1) in which two H4TBC[4]s are linked via a methylene bridge allows expansion from a single organic scaffold possessing one to two MnIII-TBC[4] metalloligands. Indeed, the conformational flexibility (ring inversion) of H8-bisTBC[4], provides an organic skeleton with eight proximal phenolic O-atoms, and this potentially offers a route to larger polymetallic cages. Indeed, in Mn chemistry this has led to the isolation of [MnIII4MnII6],22 [MnIII6MnII4],23 [MnIII4MnII4],24 [MnIV2MnIII10MnII8],25 and [MnIII10MnII4].26 Here we extend this family of compounds to include heterometallic species by reporting the synthesis, structure and magnetic properties of [MnIII3Na6(TBC[4])3(CO3)(Cl)(dmso)7]·5MeCN ([Mn3Na6]; 1·5MeCN) and [MnIII4MnII2Na6(bisTBC[4])2(CO3)2(Cl)2(dmf)8(MeOH)1.2(H2O)0.8]·6MeCN ([Mn6Na6]; 2·6MeCN). The former describes a tricapped trigonal prism, and the latter a tetracapped square prism. Both structure types are thus far unknown in Mn-based calix[n]arene chemistry.
Experimental
Synthesis
MnCl2·4H2O, NaOH, NaOPh and NEt3 were purchased from commercial suppliers and used without further purification. p-tBu-calix[4]arene (H4TBC[4]) and 2,2′-bis-p-tBu-calix[4]arene (H8-bisTBC[4]) were prepared as previously described.1,21[MnIII3Na6(TBC[4])3(CO3)(Cl)(dmso)7]·5MeCN ([Mn3Na6]; 1·5MeCN)
H4TBC[4] (0.200 g, 0.308 mmol) and MnCl2·4H2O (0.061 g, 0.308 mmol) were dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dmso/MeOH mixture (20 mL) and stirred for 10 minutes. NaOH (0.025 g, 0.616 mmol) was added and the resulting dark purple solution stirred for 3 hours. After filtration, MeCN was diffused into the mother liquor, affording crystals of 1·5MeCN in ∼15% yield after 5 days. Addition of Na2CO3 (0.065 g, 0.616 mmol) or NaHCO3 (0.052 g, 0.616 mmol) increases the yield to ∼30%. Elemental analysis (%) calculated for 1 (without any solvent of crystallisation): C, 61.31%; H, 6.93%; N, 0.00%. Found: C, 61.03%; H, 6.81%, N, 0.08%.
1 dmso/MeOH mixture (20 mL) and stirred for 10 minutes. NaOH (0.025 g, 0.616 mmol) was added and the resulting dark purple solution stirred for 3 hours. After filtration, MeCN was diffused into the mother liquor, affording crystals of 1·5MeCN in ∼15% yield after 5 days. Addition of Na2CO3 (0.065 g, 0.616 mmol) or NaHCO3 (0.052 g, 0.616 mmol) increases the yield to ∼30%. Elemental analysis (%) calculated for 1 (without any solvent of crystallisation): C, 61.31%; H, 6.93%; N, 0.00%. Found: C, 61.03%; H, 6.81%, N, 0.08%.
[MnIII4MnII2Na6(bisTBC[4])2(CO3)2(Cl)2(dmf)8(MeOH)1.2(H2O)0.8]·6MeCN ([Mn6Na6]; 2·6MeCN)
H8-bisTBC[4] (0.100 g, 0.077 mmol), MnCl2·4H2O (0.091 g, 0.462 mmol) and NaOPh (0.054 g, 0.462 mmol) were dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dmf/MeOH mixture (24 mL) and stirred for 10 minutes. NEt3 (0.4 mL, 2.87 mmol) was added and the resulting dark purple solution stirred for 2 hours. After filtration, MeCN was diffused into the mother liquor, affording crystals of 2 in ∼8% yield after 5 days. Addition of Na2CO3 (0.073 g, 0.693 mmol) or NaHCO3 (0.058 g, 0.693 mmol) increases the yield to ∼30%. Elemental analysis (%) calculated for 2 (without any solvent of crystallisation): C, 63.58%; H, 6.93%; N, 2.95%. Found: C, 63.21%; H, 6.84%, N, 2.57%.
1 dmf/MeOH mixture (24 mL) and stirred for 10 minutes. NEt3 (0.4 mL, 2.87 mmol) was added and the resulting dark purple solution stirred for 2 hours. After filtration, MeCN was diffused into the mother liquor, affording crystals of 2 in ∼8% yield after 5 days. Addition of Na2CO3 (0.073 g, 0.693 mmol) or NaHCO3 (0.058 g, 0.693 mmol) increases the yield to ∼30%. Elemental analysis (%) calculated for 2 (without any solvent of crystallisation): C, 63.58%; H, 6.93%; N, 2.95%. Found: C, 63.21%; H, 6.84%, N, 2.57%.
Note the change from dmso to dmf in the reactions used to produce 1 and 2, respectfully. No crystalline material was isolated when dmf was employed in the former or dmso in the latter.
X-ray diffraction
Single crystal X-ray diffraction data for 1–2 were collected on a Bruker D8 Venture diffractometer operating with CuKα radiation (1.54178 Å) at 100(2) K. Structures were solved using ShelXT/ShelXL and refined with version 2018/3 of ShelXL interfaced through Olex2.27–29 All non-hydrogen atoms were refined anisotropically. Hydrogen atom positions were calculated geometrically and refined using the riding model. Disorder was handled using partial occupancies and appropriate restraints. Crystal data for 1 (CCDC 2430896): C318H432Cl2Mn6N12Na12O44S14, M = 6251.99 g mol−1, monoclinic, space group C2/c (no. 15), a = 34.6511(7) Å, b = 20.6506(5) Å, c = 24.2274(6) Å, β = 100.104(2)°, V = 17067.4(7) Å3, Z = 2, T = 100(2) K, μ(CuKα) = 3.371 mm−1, Dcalc = 1.217 g cm−3, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 reflections measured (5.948° ≤ 2θ ≤ 145.022°), 16
571 reflections measured (5.948° ≤ 2θ ≤ 145.022°), 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 unique (Rsigma = 0.0903) which were used in all calculations. The final R1 was 0.1094 (I > 2σ(I)) and wR2 was 0.2844 (all data). Crystal data for 2 (CCDC 2430897): C212.2H274.6Cl2Mn6N14Na6O32, M = 4071.92 g mol−1, triclinic, space group P
571 unique (Rsigma = 0.0903) which were used in all calculations. The final R1 was 0.1094 (I > 2σ(I)) and wR2 was 0.2844 (all data). Crystal data for 2 (CCDC 2430897): C212.2H274.6Cl2Mn6N14Na6O32, M = 4071.92 g mol−1, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 17.5535(4) Å, b = 18.1099(4) Å, c = 20.5110(5) Å, α = 91.090(2)°, β = 110.5190(10)°, γ = 109.8950(10)°, V = 5670.9(2) Å3, Z = 1, T = 100(2) K, μ(CuKα) = 3.497 mm−1, Dcalc = 1.192 g cm−3, 223
(no. 2), a = 17.5535(4) Å, b = 18.1099(4) Å, c = 20.5110(5) Å, α = 91.090(2)°, β = 110.5190(10)°, γ = 109.8950(10)°, V = 5670.9(2) Å3, Z = 1, T = 100(2) K, μ(CuKα) = 3.497 mm−1, Dcalc = 1.192 g cm−3, 223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893 reflections measured (8.552° ≤ 2θ ≤ 148.976°), 23
893 reflections measured (8.552° ≤ 2θ ≤ 148.976°), 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 unique (Rint = 0.0697, Rsigma = 0.0542) which were used in all calculations. The final R1 was 0.1178 (I > 2σ(I)) and wR2 was 0.4061 (all data).
081 unique (Rint = 0.0697, Rsigma = 0.0542) which were used in all calculations. The final R1 was 0.1178 (I > 2σ(I)) and wR2 was 0.4061 (all data).
Powder X-ray diffraction data for 1–2 were collected on polycrystalline powders using a Bruker D8 ADVANCE with Cu radiation at 40 kV, 40 mA and a Johansson monochromator, 2 mm divergence slit, 2.5 degree Soller slits on the incident beam side, LynxEye detector and Bruker DIFFRAC software. Diffraction data were measured from 2θ = 2.5–20°; step size, 0.0101°. Freshly prepared crystalline powders were loaded into borosilicate capillaries with a 0.7 mm inside diameter and measured while spinning (Fig. S1†).
Magnetic data
Magnetic susceptibility and magnetisation data were collected on powdered microcrystalline samples of 1–2 using a Quantum Design PPMS Dynacool. Susceptibility data were collected in the T = 2–300 K range under an applied magnetic field, B = 0.1 T. Magnetisation data were collected in the T = 3–10 K and B = 0.5–9.0 T ranges. A unit cell check was performed prior to measurement. Powdered samples were restrained in eicosane.Results and discussion
Reaction of MnCl2·4H2O with H4TBC[4] in a basic dmso/MeOH mixture affords single crystals of formula [MnIII3Na6(TBC[4])3(CO3)(Cl)(dmso)7]·5MeCN ([Mn3Na6]; 1·5MeCN, Fig. 2 and S2†) following diffusion of MeCN into the mother liquor. The crystals were found to be in a monoclinic cell and structure solution was carried out in the space group C2/c with the asymmetric unit (ASU) comprising half of the formula.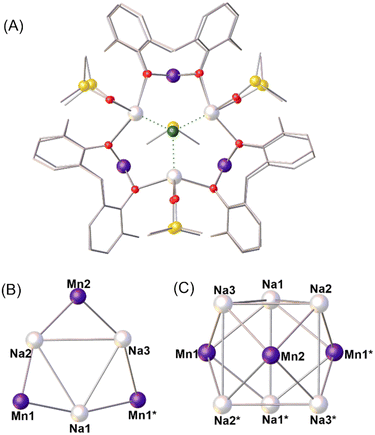 | ||
| Fig. 2 A) The molecular structure of complex 1 ([Mn3Na6]) viewed down the c-axis of the cell. B) and C) Orthogonal views of the metallic skeleton/tricapped trigonal prism, with the Na ions sitting on the six vertices of the trigonal prism and the three Mn ions capping the square faces. Colour code: MnIII = dark purple, Na = white, O = red, C = grey, S = yellow, Cl = green. H atoms, tBu groups of TBC[4], the disordered carbonate ion and solvent molecules are omitted for clarity. Additional figures are provided in the ESI.† * = symmetry equivalent. | ||
The metallic skeleton describes a [MnIII3Na6] tricapped trigonal prism with the Na ions sitting on the vertices and the Mn ions capping the square faces. A disordered μ9-[CO3]2− ion sits in the centre of the cage, bonding to all nine metal ions (Mn–O, ∼2.14–2.21 Å; Na-O, ∼2.43–2.48 Å). The square pyramidal Mn ions sit in the phenolic TBC[4] pockets and thus have [MnO5] coordination geometries with a sixth, longer contact to a MeCN molecule of crystallisation that sits in the TBC[4] cavity (∼4.1 Å). The Mn–O(carbonate) bond (∼2.14–2.21 Å) is significantly longer than the four Mn–O (phenoxide) bonds (∼1.92–1.93 Å). The TBC[4] ions are all in their maximal μ5-bridging mode, each O-arm bonded to a Mn ion and further bridging to a Na ion, thus directing the formation of square pyramidal [MnNa4(TBC[4])] building blocks (for example as shown by Mn2, Na2, Na3, Na2* and Na3* in Fig. 2C, * = symmetry equivalent). The triangular [Na3] faces of the prism are capped by a disordered mixture of one μ3-Cl− ion (Na⋯Cl, ∼2.6–2.7 Å) and one dmso molecule (Na⋯O, ∼2.4–2.6 Å), both present at 50% occupancy. The latter is asymmetrically situated with one Na–O distance of ∼2.58 Å and two Na–O distances in the range ∼2.42–2.48 Å. The remaining site on each 5-coordinate, distorted square pyramidal Na ion is occupied by a dmso molecule. The Na ions in 1 originate from the NaOH employed to deprotonate the H4TBC[4] ligands, and the [CO3]2− ion from the fixation of atmospheric CO2. Deliberate addition of carbonate in the form of Na2CO3 or NaHCO3 increases the yield of 1 approximately two-fold (see the experimental section for details). We note the structure of 1 is rather similar to the TBC[4]-supported tricapped trigonal prisms [CuII9(OH)3(TBC[4])3Cl2(dmso)5.5(EtOH)0.5][CuICl2] and [CuII9(OH)3(TBC[4])3(NO3)2(dmso)6](NO3).30 However, the CuII-based cages do not contain a [CO3]2− ion and neighbouring vertex CuII ions in the trigonal prism are connected to each other with μ-OH− ions. Alongside the larger anionic radius of Na, the result is that the dimensions of the prisms are rather different, with [Mn3Na6] being “taller and thinner” than the “shorter and wider” [Cu9] (Fig. S3†). Comparison of these [Cu9] and [Mn3Na6] cluster motifs shows remarkable similarity despite the marked change in the anions present in the centre of the cores, 3 × μ-OH− or 1 × μ9-CO32− respectively. This warrants further investigation into the nature of the anions in this general tricapped trigonal prismatic cluster topology, results of which will be reported in due course.
Examination of the extended structure found in 1 reveals similar packing behaviour to that observed for [Cu9] analogues.16,31 As in those examples, the overall triangular shape of 1 drives packing of symmetry equivalents to form layers in the ab plane (Fig. S4†) in which TBC[4] cavities are arranged opposite regions in clusters that are reminiscent of H4TBC[4] itself.31 Although it is impossible for such species to form bi-layers that are preferred by solvates of TBC[4], these assemblies do display bi-layer type behaviour within layers. Inspection of the extended structure reveals the closest inter-cluster MnIII⋯MnIII distance to be ∼15.7 Å, and between layers this is shorter at ∼12.7 Å. In both cases these are well-isolated by the TBC[4] ligands and packing of the assemblies.
Extension of this chemistry to the related to 2,2′-bis-p-tBu-calix[4]arene (H8-bisTBC[4], Fig. 1) affords a tetracapped square prism. Reaction of MnCl2·4H2O with H8-bisTBC[4] and NaOPh in a basic dmf/MeOH mixture affords single crystals of formula [MnIII4MnII2Na6(BisTBC[4])2(CO3)2(Cl)2(dmf)8(MeOH)1.2(H2O)0.8]·6MeCN ([Mn6Na6]; 2·6MeCN) following diffusion of MeCN into the mother liquor (Fig. 3 and S5†). The crystals were found to be in a triclinic cell and structure solution was carried out in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with the ASU comprising half of the formula. The metallic skeleton describes a [MnIII4MnII2Na6] tetracapped square prism with the MnII/Na ions sitting on the vertices of the square prism and the MnIII ions capping the square faces. Disordered μ6-[CO3]2− ions occupy the inside of the cage, bridging between four vertex metal ions (Na1–3, Mn3) and two capping metal ions (Mn1*, Mn2). The four face-capping MnIII ions are housed in the polyphenolic pockets of the two fully deprotonated bis-calixarene ligands whose O-atoms further bridge to the MnII/NaI ions in the square prism. The fully deprotonated bisTBC[4]8− ligands adopt their maximal μ8-bridging mode and thus wrap around the four square faces of the prism, completely encapsulating the [MnIII4MnII2Na6(CO3)2] moiety.
with the ASU comprising half of the formula. The metallic skeleton describes a [MnIII4MnII2Na6] tetracapped square prism with the MnII/Na ions sitting on the vertices of the square prism and the MnIII ions capping the square faces. Disordered μ6-[CO3]2− ions occupy the inside of the cage, bridging between four vertex metal ions (Na1–3, Mn3) and two capping metal ions (Mn1*, Mn2). The four face-capping MnIII ions are housed in the polyphenolic pockets of the two fully deprotonated bis-calixarene ligands whose O-atoms further bridge to the MnII/NaI ions in the square prism. The fully deprotonated bisTBC[4]8− ligands adopt their maximal μ8-bridging mode and thus wrap around the four square faces of the prism, completely encapsulating the [MnIII4MnII2Na6(CO3)2] moiety.
 | ||
| Fig. 3 A) The molecular structure of complex 2 ([Mn6Na6]) viewed down the c-axis of the cell. B) and C) Orthogonal views of the metallic skeleton of 2. Colour code: MnIII = dark purple, MnII = light purple, Na = white, O = red, C = grey, N = blue, Cl = green. H atoms, tBu groups of H8-bisTBC[4], carbonate ions and solvent molecules are omitted for clarity. Additional figures are included in the ESI.† * = symmetry equivalent. | ||
The ‘upper’ and ‘lower’ square faces of the prism (as drawn in Fig. 3A and S5†) are each asymmetrically capped by a μ3-Cl− ion (Mn⋯Cl, ∼2.38 Å, Na⋯Cl, ∼2.94–3.04 Å). The MnIII ions in the calix[4]arene pockets have square pyramidal [MnO5] coordination spheres, with the bonds to the four phenolic O-atoms (∼1.90–2.00 Å) significantly shorter than that to the carbonate O-atom (∼2.07–2.30 Å), with a sixth, longer contact to a MeCN molecule of crystallisation that sits in the TBC[4] cavity (∼4 Å). The coordination spheres of the distorted square pyramidal MnII ions ([MnO5]) and distorted pentagonal bipyramidal Na ions are completed by solvent molecules (MeOH, H2O) of partial occupancy.
The packing found in the extended structure of 2 is markedly different to that outlined above for 1. In this case the clusters are arranged diagonally side-on in the ab plane and form a bi-layer type arrangement as shown in Fig. S6.† The TBC[4] sub-units in the bisTBC[4]s shrouding the cluster cores are arranged such that cavities are arranged approximately opposite those of symmetry equivalent assemblies in adjacent bi-layers, behaviour that is also reminiscent of a range of H4TBC[4] solvates.16,31 Inspection of the structure reveals that the closest inter-cluster MnIII⋯MnIII distance to be ∼13.1 Å, again confirming that this approach of shrouding the clusters with bulky ligands has been successful in isolating cluster cores from one another.
As for 1, deliberate addition of carbonate in the form of Na2CO3 or NaHCO3 increases the yield of 2, in this case approximately four-fold (see experimental section for details). Inspection of related chemistry with CuII shows a similar structural expansion relationship upon moving from H4TBC[4] to H8-bisTBC[4]. The structure of 2 is reminiscent of the biscalix[4]arene-supported cluster [CuII13(bisTBC[4])2(NO3)(μ-OH)8(dmf)7][OH],24 whose metallic skeleton also describes a (centred) tetra-capped square prism (Fig. S7†). Interestingly the latter contains an encapsulated and disordered CuII ion at its centre. Like the [Cu9] cages, neighbouring vertices in the square prism in [Cu13] are linked to each other through μ-OH− bridges, making [Mn6Na6] (2) “taller and thinner” than the “shorter and wider” [Cu13]. Attempts to make the ‘exact’ [MnII9]/[MnII13] analogues of [CuII9]/[CuII13] in our laboratory have failed thus far, presumably (or at least in part) due to the very strong preference for the metal ion to be MnIII when housed in the TBC[4] (or C[4]) polyphenolic pocket. Indeed, there are no examples of Mn-based TBC[4] structures in the CCDC where MnII ions are coordinated in the TBC[4] pocket, and all reactions between H4TBC[4] and MnII salts in basic solutions under standard conditions reported thus far lead to the isolation of [MnIII2MnII2(OH)2(TBC[4])2(solvent)6] or analogues thereof.17 The structure of the latter describes a [MnIII2MnII2] butterfly (or near planar diamond) in which the two ‘body’ MnII ions are encapsulated by two ‘wing’ MnIII-TBC[4] metalloligands. The addition of Group I metal ions to the reaction (here Na), and the in situ generation of carbonate, clearly alters the reaction pathway, leading to the isolation of [Mn3Na6] and [Mn6Na6] multiply capped trigonal and square prisms. The average metal oxidation state in 1 and 2 is 1.67 and 2.00, respectively, perhaps suggesting that these prisms are the preferred topology for TBC[4]-supported clusters of metal ions in lower oxidation states (≤2). Interestingly, there are zero TBC[4]-supported homometallic clusters of FeII, CoII or ZnII reported in the literature, so it will be intriguing to discover the coordination chemistry of these metal ions. Likewise, it will be interesting to see if the incorporation of other Group I metal ions (Li, K, Cs) leads to the same structure types, and how this might change through the use of Group II metal ions (Mg–Ba). Similarly, the TBC[4]-based coordination chemistry of other combinations of MIII–MI ions remains to be explored, as does the deliberate and systematic employment of the carbonate ion as a bridging ligand.
Magnetic measurements
Direct current magnetic susceptibility (χ) and magnetisation (M) studies were performed on polycrystalline samples of 1–2 in the temperature and field ranges T = 2–300 K, B = 0.1 T and T = 3–10 K, B = 0.5–9.0 T, respectively (Fig. 4 and 5). At 300 K, the χT value of ∼9.0 cm3 K mol−1 for 1 matches the Curie constant expected for three uncorrelated MnIII ions with g = 2.0. Upon cooling, the χT product is invariant with temperature until approximately T = 8 K where it rapidly decreases to a minimum value of ∼5.7 cm3 mol−1 K at T = 2 K. The high temperature data are therefore suggestive of paramagnetism, with the low temperature decline assigned to a combination of zero-field splitting effects and/or very weak intra- and inter-molecular antiferromagnetic exchange interactions. Note that at these temperatures all three are likely to be correlated. The magnetisation data rise rapidly with increasing field, reaching a value of M = ∼10.4 μB at T = 3 K and B = 9.0 T. The zero, or very weak exchange, is not surprising given that the three Mn ions are only connected to each other via the single, disordered [CO3]2− ion lying in the centre of the cage (Fig. S2;† Mn⋯Mn ∼5.31–5.43 Å). A simultaneous fit of the susceptibility and magnetisation data using spin-Hamiltonian (1) (without the exchange term) afforded the best fit parameters |DMn(III)| = 3.7 cm−1 and zJ = 0.01 cm−1. The value of D is line with that expected for a Jahn–Teller distorted MnIII ion with a [MnO5] coordination sphere.32 | (1) |
 | ||
| Fig. 4 Magnetic susceptibility and magnetisation (inset) data for 1. The solid lines are a simultaneous fit of the data to spin-Hamiltonian (1). See text for full details. | ||
 | ||
| Fig. 5 Magnetic susceptibility and magnetisation (inset) data for 2. The solid lines are a simultaneous fit of the data to spin-Hamiltonian (1). See text for full details. | ||
Conclusions
Employment of p-tBu-calix[4]arene (H4TBC[4]) and 2,2′-bis-p-tBu-calix[4]arene (H8-bisTBC[4]) in Mn–Na chemistry leads to the isolation of [MnIII3Na6(TBC[4])3(CO3)(Cl)(dmso)7]·5MeCN ([Mn3Na6]; 1·5MeCN) and [MnIII4MnII2Na6(BisTBC[4])2(CO3)2(Cl)2(dmf)8(MeOH)1.2(H2O)0.8]·6MeCN ([Mn6Na6]; 2·6MeCN), respectively. The metallic skeleton of 1 describes a tricapped trigonal prism, and that of 2 a tetracapped square prism. The change from a calix[4]arene to a bis-calix[4]arene enables expansion from three MnIII-TBC[4] metalloligands in 1 to four MnIII-TBC[4] metalloligands in 2 and thus the self-assembly of a larger species. In each case the MnIII ions sit in the TBC[4] polyphenolic pockets and act as capping atoms in the prism/metallic skeleton, with the Na and Na/MnII ions defining the vertices of the prism. The carbonate present in both structures results from CO2 fixation, with yields considerably improved through deliberate addition of Na2CO3/NaHCO3. The formation of 1 and 2 follows established binding rules in calix[4]arene coordination chemistry in which (under ambient conditions) the ligand preferentially binds MnIII ions over MnII and NaI ions.9 Alongside the preferential electrostatic interaction, the tetraphenolic ligand pocket is particularly suited to accommodating four short equatorial bonds and thus the axially elongated (Jahn–Teller distorted), octahedral or square pyramidal MnIII ion. The structural similarities of 1 to [CuII9] and of 2 to [CuII13] highlights the dominant structural role played by the calix[4]arene ligands, and their ability to form both heterometallic and heterovalent cages whilst maintaining analogous topologies. Magnetic susceptibility and magnetisation measurements reveal that 1 behaves as a paramagnet – the long distances between MnIII ions connected by a single, disordered [CO3]2− ion resulting in negligible magnetic exchange. Magnetic data for 2 show weak antiferromagnetic exchange in the ‘linear’ [MnIII–MnII–MnIII] trimers present, with values in the range 0.10 cm−1 < J < −2.52 cm−1, consistent with previous TBC[4]-supported MnIII/II clusters with similar MnIII–O–MnII bridging angles.Data availability
Crystallographic data for compounds 1–2 have been deposited at the CCDC under numbers 2430896 and 2430897. Further data supporting this manuscript have been included as part of the ESI.†Author contributions
LRBW performed the synthesis, measured the PXRD data, collected and fitted magnetic data. SJD collected and solved the single crystal XRD data. EKB and SJD conceived the idea. All authors contributed to writing/editing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
EKB thanks the Leverhulme Trust (RPG-2021-176).Notes and references
- C. D. Gutsche, in Calixarenes: An Introduction, The Royal Society of Chemistry, Cambridge, 2nd edn, 2008, ch. 2, pp. 27–160 Search PubMed.
- Calixarenes and Beyond, ed. P. Neri, J. L. Sessler and M.-X. Wang, Springer International Publishing, Switzerland, 2016 Search PubMed.
- J. Vicens and J. Harrowfield, Calixarenes in the nanoworld, Springer, Dordrecht, Netherlands, 2006 Search PubMed.
- L. Mandolini and R. Ungaro, Calixarenes in action, Imperial College Press, London, 2000 Search PubMed.
- G. Sachdeva, D. Vaya, C. M. Srivastava, A. Kumar, V. Rawat, M. Singh, M. Verma, P. Rawat and G. K. Rao, Coord. Chem. Rev., 2022, 472, 214791 CrossRef CAS.
- O. Santoro and C. Redshaw, Coord. Chem. Rev., 2021, 448, 214173 CrossRef CAS.
- J. B. Simões, D. L. da Silva, S. A. Fernandes and Â. de Fátima, Eur. J. Org. Chem., 2022, e202200532 CrossRef.
- A. R. Kongor, V. A. Mehta, K. M. Modi, M. K. Panchal, S. A. Dey, U. S. Panchal and V. K. Jain, Calix-Based Nanoparticles: A Review, Top. Curr. Chem., 2016, 374, 28 Search PubMed.
- L. R. B. Wilson, M. Coletta, M. Evangelisti, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Dalton Trans., 2022, 51, 4213 RSC.
- G. Karotsis, S. J. Teat, W. Wernsdorfer, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 8285 CrossRef CAS PubMed.
- L. R. B. Wilson, A. B. Canaj, D. J. Cutler, L. J. McCormick McPherson, S. J. Coles, H. Nojiri, M. Evangelisti, J. Schnack, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2024, 63, e202405666 CrossRef CAS PubMed.
- G. Karotsis, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 9928 CrossRef CAS PubMed.
- M. A. Palacios, R. McLellan, C. M. Beavers, S. J. Teat, H. Weihe, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. – Eur. J., 2015, 21, 11212 CrossRef CAS PubMed.
- M. Coletta, S. Sanz, D. J. Cutler, S. J. Teat, K. J. Gagnon, M. K. Singh, E. K. Brechin and S. J. Dalgarno, Dalton Trans., 2020, 49, 14790 RSC.
- S. M. Taylor, J. M. Frost, R. McLellan, R. D. McIntosh, E. K. Brechin and S. J. Dalgarno, CrystEngComm, 2014, 16, 8098 RSC.
- S. M. Taylor, R. D. McIntosh, C. M. Beavers, S. J. Teat, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2011, 47, 1440 RSC.
- S. M. Taylor, G. Karotsis, R. D. McIntosh, S. Kennedy, S. J. Teat, C. M. Beavers, W. Wernsdorfer, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. – Eur. J., 2011, 17, 7521 CrossRef CAS PubMed.
- S. M. Taylor, R. D. McIntosh, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2012, 48, 11190 RSC.
- R. McLellan, M. A. Palacios, S. Sanz, E. K. Brechin and S. J. Dalgarno, Inorg. Chem., 2017, 56, 10044 CrossRef CAS PubMed.
- M. Coletta, M. A. Palacios, E. K. Brechin and S. J. Dalgarno, Chemistry, 2020, 2, 253 CrossRef CAS.
- L. T. Carroll, P. Aru Hill, C. Q. Ngo, K. P. Klatt and J. L. Fantini, Tetrahedron, 2013, 69, 5002 Search PubMed.
- L. R. B. Wilson, M. Coletta, R. Jose, G. Rajaraman, S. J. Dalgarno and E. K. Brechin, Dalton Trans., 2021, 50, 17566 RSC.
- M. Coletta, R. McLellan, A. Waddington, S. Sanz, K. J. Gagnon, S. J. Teat, E. K. Brechin and S. J. Dalgarno, Chem. Commun., 2016, 52, 14246 RSC.
- R. McLellan, M. A. Palacios, C. M. Beavers, S. J. Teat, S. Piligkos, E. K. Brechin and S. J. Dalgarno, Chem. – Eur. J., 2015, 21, 2804 CrossRef CAS PubMed.
- M. Coletta, S. Sanz, L. J. McCormick, S. J. Teat, E. K. Brechin and S. J. Dalgarno, Dalton Trans., 2017, 46, 16807 RSC.
- M. Coletta, S. Sanz, E. K. Brechin and S. J. Dalgarno, Dalton Trans., 2020, 49, 9882 RSC.
- G. M. Sheldrick, Acta Crystallogr., Sect. C:Struct. Chem., 2015, 71, 3 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A, 2015, 71, 3 CrossRef PubMed.
- G. Karotsis, S. Kennedy, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2010, 46, 3884 RSC.
- G. D. Andreetti, R. Ungaro and A. Pochini, J. Chem. Soc., Chem. Commun., 1979, 1005–1007 Search PubMed; E. B. Brouwer, G. D. Enright and J. A. Ripmeester, J. Am. Chem. Soc., 1997, 119, 5404–5412 Search PubMed; J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000–1002 Search PubMed.
- A. Abragam and B. Bleaney, Electron Paramagnetic Resonance of Transition Ions, Clarendon Press, Oxford, 1970 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD data, additional figures. CCDC 2430896 and 2430897. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ce00285k |
| This journal is © The Royal Society of Chemistry 2025 |

