The impact of crystal habit on the pharmaceutical properties of active pharmaceutical ingredients
Niranj H.
Ram†
a,
Madhukiran R.
Dhondale†
 a,
Maan
Singh
a,
Maan
Singh
 a,
Brahmeshwar
Mishra
a,
Brahmeshwar
Mishra
 a,
Ashish Kumar
Agrawal
a,
Anne Marie
Healy
b and
Dinesh
Kumar
a,
Ashish Kumar
Agrawal
a,
Anne Marie
Healy
b and
Dinesh
Kumar
 *a
*a
aDepartment of Pharmaceutical Engineering and Technology, Indian Institute of Technology (Banaras Hindu University), Varanasi, India-221005. E-mail: dinesh.phe@iitbhu.ac.in
bSchool of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin, Dublin 2, Ireland
First published on 29th May 2025
Abstract
Crystal habit modification potentially improves the pharmaceutical and biopharmaceutical properties related to active pharmaceutical ingredients (APIs) and is an important aspect of crystal engineering. It has been demonstrated that filterability, compaction properties, flow behavior, and dissolution performance of APIs are dependent on the crystal habit of the compound, which ultimately depends upon the combination of factors including but not limited to the nature of solvent, additives, supersaturation, the environment provided during crystallization, etc. Crystal habit modification may be considered as an economically viable approach to mitigate pharmaceutical manufacturing challenges. This tutorial provides a step-by-step approach to crystal habit modification using different crystallization methods. Also, the factors that affect crystallization experiments at each stage of the different methods are explained in detail. The effects of supersaturation, solute–solvent interactions, additives, rate of crystallization, etc., are described, with examples. The impact of crystal habits on pharmaceutical properties such as filtration, punch sticking, compressibility, dissolution rate, etc., is also explained in detail with case studies. Finally, the analytical methods that are useful in the characterization of crystalline materials (morphological, physicochemical, rheological, and surface characterization) are explained, along with the experimental procedures involved.
 Niranj H. Ram | Niranj H. Ram is an Integrated Dual Degree student in the PSSRL, IIT(BHU), Varanasi, India. |
 Madhukiran R. Dhondale | Madhukiran R. Dhondale is a PMRF (Prime Minister Research Fellow) in the PSSRL, IIT(BHU), Varanasi, India. |
 Maan Singh | Maan Singh is a Ph.D. research scholar in the PSSRL, IIT(BHU), Varanasi, India. |
Key learning points• Understanding API crystal habit: gain insights about crystal habit, about different types of habits, changes in habit during different stages of crystallization and its critical role in determining API performance.• Importance of crystal habit modification: learn the importance of crystal habit modification for APIs and the strategic approaches for improving the drug's processability and performance. • Factors affecting crystal habit growth: know the intrinsic and extrinsic factors such as temperature, agitation, and understand how changes in these factors leads to changes in API crystal habit and decide the final habit of the crystal. • Impact on pharmaceutical properties: what is the magnitude of effect of crystal habit on properties of the API like dissolution, flowability, filtration. • Post-modification characterization techniques: understand the different techniques to characterize morphology, to analyse dissolution rates, powder flow, surface characteristics and compaction behaviour of the API after crystal habit modification. |
Introduction
Recrystallization from solutions using solvents is the most employed crystallization method in the pharmaceutical and chemical industry. However, during the process, the crystallizing compound may assume different shapes depending upon factors such as solute–solvent interactions, solution properties, crystallization parameters, and the environmental conditions under which the crystallization is being carried out. The geometrical shape of the crystal or its outer appearance is commonly termed as the “Crystal Habit”.1 One of the important parameters for defining crystal habits is the aspect ratio which is defined as the ratio of the length of the crystal to the width of the same crystal. The crystal habit of a compound is dependent upon the crystal facets and their relative growth rates during crystallization from solution. Accordingly, crystal habits like rod-shaped, plate-like, needle-like, acicular, and many more with different aspect ratios are formed during crystallization.2 In solid-state pharmaceutics, crystal habit is of extensive importance since it has a crucial impact in determining the physicochemical properties of the active pharmaceutical ingredients (APIs) such as filterability, flowability, compaction, dissolution rate, and so on.3 Further, sufficient care must be taken to design the crystallization process such that only the habit is modified without any changes in the polymorphic form of the API.Depending upon the solute–solvent interactions, rate of crystallization, supersaturation etc., the crystals may exhibit rapid or stunted growth in a particular direction. For instance, the rapid growth of prismatic type crystals can lead to its elongation thereby forming needle-like crystals. On the other hand, the stunted growth gives rise to flat-shaped crystals such as plates and tabular crystals.4 It is evident that the unit cell of crystals also influences its habit/shape. For instance, block-shaped crystals are formed from cubic unit cell type and unit cells with two or single short axis tend to form needle/plate-shaped crystals, respectively.5 Further, the unit cell parameters such as the edges (a, b, & c), and angles between them (α, β, & γ) area useful parameters to differentiate between changes in polymorphism or crystal habit. Any changes in the unit cell parameters during habit modification experiments is undesired and must be controlled.
The commonly encountered crystal habits in the pharmaceutical manufacturing industry, along with their pros and cons in the context of downstream processability (post crystallization), are provided in Table 1 and are pictorially represented in Fig. 1.
| Crystal habit | Aspect ratio | Advantages | Disadvantages |
|---|---|---|---|
| Acicular/needle-like6,87 | >10 (Since length is much greater than width and thickness) | • Good compressibility profile | • Prone to aggregation and breakage |
| • Poor powder flow | |||
| • Causes considerable filterability issues | |||
| • Difficult to disintegrate compacts | |||
| Rods7,57 | 3–10 (Not as long as needles but still length > width and thickness) | • Good compressibility profile | • Poor powder flow |
| • Prone to breakage | |||
| • Causes filterability issues | |||
| Spherical8–10,94,99 | ∼1 (Dimensions are almost equal in all directions) | • Best powder flow properties | • Lowest surface area per volume of crystals |
| • Does not cause issues during filtration | • Moderate compressibility profile | ||
| Blocks11,12 | Between 1–1.5 (Dimensions are almost similar) | • Better physical stability and plasticity than plates | • Intermediate powder flowability |
| Plates12,13 | 2–3 (Length is greater than width, but thickness is smaller than width) | • Exhibit good compaction properties and physical stability | • Poor powder flow properties |
| Fibrous14,53 | >10 | • Good compressibility profile | • They tend to agglomerate and cause breakage |
| • Poor powder flow properties |
 | ||
| Fig. 1 Scanning electron microscopy images of some commonly encountered crystal habits A) needles, B) plates, C) tabular, D) rods, E) rhombohedral/cubic, F) aggregated blades, G) dendritic crystals. Reprinted (adapted) with permission from ref. 15–19. Copyright American Chemical Society. | ||
The description of the crystal habits are as follows:
• Acicular: slender, long and pointed crystals. e.g. needle-shaped crystals.
• Cubic: crystals with similar length, width, and thickness.
• Plate: flat crystals with similar length and width.
• Tabular: crystals thicker than a plate but with similar length and width.
• Blade: long, thin flat crystals.
• Dendritic: tree-like pattern formed by tiny crystallites.
• Rod: cylindrical crystal elongated along one-axis.
• Prismatic: hexagonal crystals having width and thickness great than acicular crystals but with smaller length. The facets are parallel to the growth axis.
These downstream processability aspects (filterability, flowability, compaction, etc.) are of great importance in the case of oral solid dosage form manufacturing, especially for tablets. Tablet manufacturing involves granulation, drying, compaction, etc., which requires the API under consideration to possess optimum stability and compaction properties to enable tableting. The compaction properties of the API are defined by compressibility and compactability, and together they determine the ease with which tableting can be undertaken. Powder compressibility is the measure of how readily the material undergoes a change in volume when compressed and is characterized by both tablet porosity and compression stress, while compactability relates the tablet tensile strength to tablet porosity.20 Similarly, during API synthesis and purification, filtration is an important step in isolating crystals from the mother liquor and the filtration efficiency depends on the size and morphology of the crystals. The relationship between filterability and the crystal habit has been extensively studied, and it is generally the case that needle-like crystals have poor filterability.21 Powders possessing poor flowability often pose issues with respect to weight variation and content uniformity during tablet compression and capsule filling. The flowability of crystals with habits closer to spherical or rhombohedral tend to be higher, while that of needle-like crystals tends to be very poor.22,23
Similarly, dissolution rate is an important aspect during tablet development. The dissolution rate of an API and thus, of the tablet is significantly influenced by the changes in the crystal habit, due to anisotropy of the crystal facets. Modification of crystal habit to increase the surface area of a particular functional group bearing facet is also a feasible solution to improve dissolution rate of APIs.24–26
Various researchers have reported the advantages of different crystal habit modification techniques to improve the processability or dissolution performance of APIs. Through thorough knowledge of the factors affecting the crystallization process, crystallization scientists can control and alter the crystal habits of compounds. A common practice to modify crystal habits involves changing the solvent system (choosing different polarity solvents), adding crystal growth inhibitors (additives), changing the supersaturation extent, or using ultrasound during crystallization. In addition, changing the method of crystallization (anti-solvent crystallization, cooling crystallization, and evaporative crystallization) can also result in modification of the crystal habit.
The present tutorial outlines the procedures that can be followed along with the process parameters that can affect the crystal habit during crystallization. Also, characterization methods, along with their associated procedures and requirements, are summarized and tabulated.
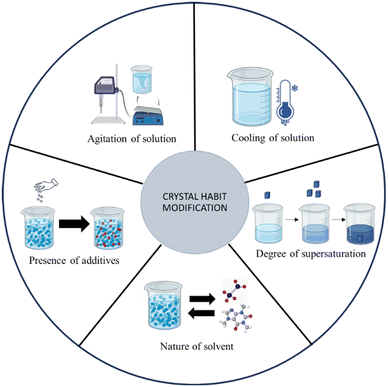
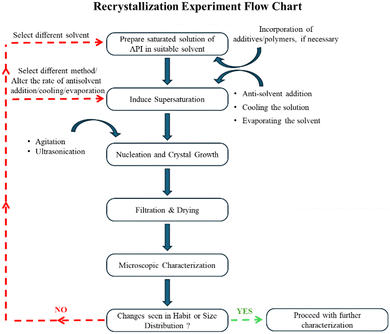
Crystal habit modification
Crystal habit has an immense impact on the processability and manufacturing process of crystalline pharmaceutical solids. At times, active ingredients may not have the required physical characteristics, and the existing properties must be modified to improve the quality of the product. Precise control over the crystal growth allows researchers to crystallize materials with tailored structures and properties.27 Several factors will determine the resultant habit of the crystals. The factors are summarized in Fig. 2 and explained in detail in Table 2. Fig. 3 depicts the experimental trial-and-error based experimental protocol for modifying the habit of pharmaceutical substances. Alternatively, molecular dynamics studies may be employed to screen solvents and additives for modification of crystal habits. The simulation-based software uses various models such as the Bravais–Friedel–Donnay–Harker (BFDH) model, attachment energy and modified attachment energy models, etc. to compute the habit of crystals grown under different environments (due to change of solvents, presence of additives, etc.). These simulation-based studies can be conducted using software packages such as Mercury by Cambridge Crystallographic Data Centre (CCDC), BIOVIA Materials Studio, etc. Such simulation-based predictions of crystal habit are out of the scope of the current tutorial. However, the interested readers are directed towards the works of Clercq et al.,28 Gu et al.,29 Kumar et al.30 for in-depth understanding of the in-silico crystal habit prediction methods. The theoretical aspects of the molecular modelling is explained in the chapter by Chadwick et al.31| Steps | Factors that influence the shape/habit of a crystal |
|---|---|
| Preparation of saturated solution of API in a suitable solvent32,33,40 |
• The degree of supersaturation affects the crystal habit of APIs. Nucleation kinetics can be controlled by altering the super-saturation levels. Different habits are formed at different levels of supersaturation
○ High degree of supersaturation: Leads to the formation of smaller crystals of a smaller size ○ Low degree of supersaturation: leads to the formation of a small number of large size crystals |
| • Choice of solvent: depending upon the chemistry of the solute, polarity, and hydrogen bonding capacity of the solvent, there is a differential interaction between the solvent and different crystal facets. This difference in the interaction at the solute–solvent interface leads to changes in the habit of a crystal. Hence, one can expect a different crystal habit by selecting different polarity solvents for a compound | |
| • Use of seed crystals: using seed crystals prevents uncontrolled nucleation, enabling us to obtain crystals of desired habit and size (similar to that of seed crystals used) | |
| Crystallization by: | • Additives: surfactants, impurities, and polymers at low concentration can selectively adsorb onto a particular crystal facet or modify the interfacial energy, thereby altering its growth rate and resulting in a different habit |
| Cooling | • Control of cooling/antisolvent addition/evaporation: control of the solvent's cooling/antisolvent addition rate/rate of evaporation can affect the crystal growth kinetics and lead to changes in the habit of crystals |
| Anti-solvent addition | • Ultrasound: the application of high-energy ultrasound can induce stress, influence the shape of primary crystals, and modify the crystal habit |
| Evaporation | • Degree of agitation: it is impossible to change the habit per se, but small size and uniform size distribution of crystals can be obtained by controlling the agitation rate |
| Additive mediated34–37,40 | • Impinging jet crystallization: similar to the effects of agitation, the use of the jet impinging crystallization method can produce crystals of smaller size and uniform size distribution |
| Filtration/separation of crystals87,89 | • Attrition during filtration (such as when using a glass filter dryer) must be avoided, as it leads to crystal breakage leading to changes in the aspect ratio of crystals after filtration process (e.g., breakage of needle-shaped crystals into smaller fragments) |
| • Use of pressure during filtration can cause the formation of a cake and breakage of crystals under its own weight | |
| Washing38 | • During washing with anti-solvent care must be taken to ensure the residual solvent does not contain dissolved API, which otherwise changes the shape of crystals (often forms irregular crystals) |
| • Washing is a critical step in additive-mediated crystallization, wherein the removal of additives (polymers, surfactants, etc.) is carried out. So, one must ensure that the solvent selected can dissolve the additive/impurity while the API is not soluble in the washing solvent. Also, it must be miscible with the solvent used and must be sufficiently volatile to ensure its easy removal (during drying) after washing | |
| • Sometimes, using a mixture of solvents is preferable to avoid API precipitation during the solvent-washing phase | |
| Drying39,87 | • The drying step ensures that the crystallized API is free of any residual solvent that otherwise might cause harm to patients. Drying must be carried out in conditions that ensure the product stability and should not cause degradation of the API under consideration |
| • Drying at higher temperatures can induce solid-state transformations in APIs | |
| • The rate of drying has an impact on particle size distribution of the crystals. Low drying rate for a sample with high capillary force strength can promote solid bridge formation leading to lumping |
Factors influencing the crystal habit
As mentioned in Table 2, various factors play an important role in determining the crystal habit of a compound during the crystallization process. Understanding and achieving precise control over these factors can result in the formation of desired habits and improved characteristics. In this section, the factors influencing crystal habits are briefly discussed. However, for detailed information, the readers are directed towards the review work by Pu and Hadinoto.40The polarity of solvents43 and hydrogen bonding between solute and solvent44 can also impact the shape of the crystals formed. The molecular composition at each facet of a crystal is different. For example, oxygen and nitrogen are polar (hydrophilic) in nature, while carbon and halogens are non-polar (hydrophobic). Polar solvents such as water, ethanol, methanol, acetonitrile, etc. interact more with the polar surfaces, while non-polar solvents such as dichloromethane, chloroform, n-hexane, etc. interact with the non-polar surfaces. Hence, different crystal habits are formed if different polarity solvents are used to crystallize a particular compound. However, the solubility of the compound in the selected solvent must be ensured prior to crystallization. The use of a mixture of solvents (in definite volumetric ratios) will also yield crystals with modified habits. For example, lovastatin gives prismatic-type crystals in hexane and methyl-cyclohexane (non-polar solvents), while in the water-acetone mixture (polar solvent system), it forms needle-shape crystals.45,46
High supersaturation levels lead to the formation of a higher number of nuclei in a solution. Hence, the driving force (i.e., super-saturation) is consumed in the formation of the nuclei, and less solute is available for the crystal growth phase. As a result, a large number of smaller crystals are formed at a high degree of supersaturation. In contrast, at lower super-saturation levels, relatively fewer nuclei are formed, and the crystals grow to a larger size. Also, a high degree of supersaturation leads to the formation of isotropic crystals due to higher crystallization rates and less facet discrimination. For example, the aspect ratio of benzoic acid crystals at a super-saturation level of 1.029 was ∼20.5, while at a super-saturation level of 2.941, the aspect ratio decreased to 4.9, with the shape-changing from needle to elongated rectangular plates at low and high supersaturation levels, respectively.47 Furthermore, the number of kink sites (sites where the growth takes place in a crystal) is also influenced by the super-saturation level.48
More precise control can be exercised by combining two or three crystallization methods. Crystallization can be carried out using both cooling and antisolvent addition methods. A combination of different methods can also yield different crystal habits compared to a single method.49 Also, during the cooling crystallization method, a few instances have been reported wherein temperature cycling was performed, resulting in the formation and dissolution of crystals to modify the growth facets. During the re-dissolution of crystals, there is a chance for the formation of new growth facets on the crystal, thereby modifying the final crystal shape.50
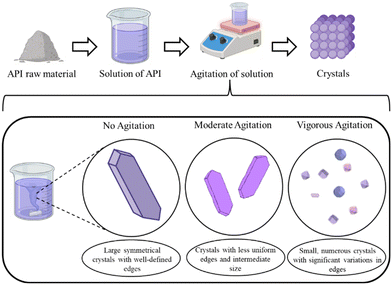
Generally, high agitation results in the formation of smaller, finer crystals, and moderate to low levels result in bigger crystals (depicted in Fig. 4). However, if the stirring is not rapid enough, it may lead to the accumulation of particles. Particle agglomeration caused the typical shape of the facets of paracetamol crystals to disappear at moderate stirring speeds, and well-defined crystals formed when the agitation level was raised.51 Aspirin crystals also showed evidence of agglomeration at lower agitation and the formation of single crystals with increasing stirring speed.52 This phenomenon can change when agitation is performed along with drying. Kougoulos et al. reported the transformation of fibrous crystals into irregular granules and fine particles after removing the mother liquor completely followed by agitated drying.53
On the other hand, the application of high energy ultrasound waves to the sample during crystallization can result in the breakage of the crystals along a particular axis and cause a change in the habit of the crystals. Also, due to the application of ultrasound energy, mass transfer is enhanced, thereby modulating the crystal facet growth rates. Hence, it is possible to modify the crystal habit of APIs using sonocrystallization. Cavitation and acoustic streaming phenomena are observed during the sonocrystallization. The phenomenon of inducing fluid flow by acoustic waves is called acoustic streaming. This enhances nucleation by increasing agitation and nucleation sites. The compression and rarefaction cycles during the application of ultrasound lead to bubbles forming and collapsing, a phenomenon known as cavitation (acoustic cavitation). The cavitation process results in the breakage of crystals, leading to the generation of additional growth facets and nucleation sites.54 A cavitating bubble symmetrically collapses, thus generating a very high local liquid velocity (>100 m s−1). As a result, surface pitting and localized erosion takes place due to this “jet impinging” process. The collapse of the cavitation bubble also generates shockwaves releasing a large amount of energy into the solution thereby inducing crystal breakage.55 Cavitation induces nucleation at much lower supersaturation levels by increasing the intermolecular collisions during crystallization. Due to the cavitation, the nucleation induction time is also reduced.56 Therefore, through sonocrystallization, it is possible to control the nucleation process precisely. Control of the nucleation process directly correlates to the control over the crystal size distribution of the powder API sample. The application of ultrasound energy during the crystallization process generally increases the nucleation rate, thereby leading to a higher number of nuclei in solution. Higher nucleation rates usually result in the formation of small-size crystals, and the uniform super-saturation provided due to the application of ultrasound energy results in a uniform and narrow size distribution of crystals. Therefore, through sonocrystallization not only can one modify the crystal habit of APIs but also obtain crystals of narrow crystal size distribution with control over the crystal size. A few research works have also reported the possibility of avoiding API agglomeration by using a sonocrystallization technique. During sonocrystallization, the process parameters such as the run time, ultrasound on/off cycles, ultrasound power rate, super-saturation, and rate of cooling/evaporation/antisolvent addition must be optimized so as to obtain desired results.57,58
| API | Resulting crystal habit | Polymer used | Changes seen |
|---|---|---|---|
| Nifedipine | Columnar and plate-like | HPMC (0–0.6% w/v) | Decrease in dissolution rate64 |
| Metformin HCl | • Needle | • PVP | Improvement in flow property was speculated. Changes not studied65 |
| • Thick rods | • PEG | ||
| • Prismatic | • HPMC | ||
| Paracetamol | • Subrounded-subangular polyhedral | • PEG-6000 & Brij 58 PVA | Improved flow, compressibility, and dissolution rate66 |
| Elongated | N-Hydroxyphenyl methacrylamide | Changes were not studied67 | |
| • Triangular-winged | • Agar | Rectangular blocks exhibited faster dissolution rates, while triangular-winged shape particles exhibited better tabletability profiles68 | |
| • Rectangular blocks | • Gelatin | ||
| • Rods | • HPMC, PVP | ||
| Naproxen | Needle-like | PVP and HPMC | Changes were not studied69 |
| Erythromycin A dihydrate | Irregular to acicular and plate-like | HPMC | Better tableting properties70 |
| Plates | HPC | Enhanced compaction27 | |
| Mebendazole | Plates and rods | SLS and PVP | Improved dissolution71 |
| Nitrofurantoin | Dendritic clusters | Poly(N-isopropyl acrylamide) | Increased solubility14 |
| Ethyl(hydroxy ethyl cellulose) and HPMC | Changes were not studied72 | ||
| Ropivacaine | Block-like | PVP | Uniform crystal size distribution73 |
| Succinic acid | Plates, blocks and needle-shaped | Pluronic P123 | Changes were not studied74 |
| Fenofibrate | Irregular plates and elongated plates/rods | HPMC and SDS | Changes were not studied75 |
| Tolazamide | Plates | PEG-b-PLA | Improved flow76 |
| Triacylglycerols | Spherulites, needle-like | Poly(stearyl methacrylate) homopolymer | Changes were not studied77 |
| Ethyl vanillin | Block-like | PVP | Better compaction78 |
| p-HMBA (hexamethyl benzoic acid) | Block-like | PVP | Changes were not studied79 |
| Indomethacin | • Acicular or needle-shaped | • Eudragit E100 | Enhanced dissolution80 |
| • Columnar-shaped | • PVP K90 | ||
| L-Alanine | Rods and blocks | Glycine-based oligopeptides | Changes were not studied81 |
| o-Amino benzoic acid | Irregular needles and prismatic shape | HPMC | Changes were not studied82 |
| Salbutamol sulphate | Block-like | PVP K25 | Better flow83 |
Impact of particle size reduction on crystal habit
Particle size reduction has a significant role in pharmaceutical manufacturing since the control over particle size distribution (PSD) improves formulation characteristics. Milling involves impact, attrition, and shear forces for the mechanical communition of API particles to reduce their size. Based on the use of solvents, milling can be classified as either wet or dry milling. The choice of milling method also influences the outcome. During dry milling, breakage usually occurs along crystallographic planes, which changes the aspect ratio and habit of crystals. This happens due to vigorous collisions between particles and the mill surface. This fracture naturally results in changes in the external geometric shape of the crystals, for instance, needle shaped crystals might become plate-like structures. The shear stress that occurs when particles slide against each other or against milling surfaces can also change the shape of crystal faces. This gradual surface removal of material usually observed in techniques like ball milling. Solvent-mediated effects might cause habit modifications during wet milling. Usually, needle-like and platy crystals are more prone to breakage. Crystals with anisotropic growth and strong intermolecular bond strength like the cubes are likely to undergo lesser surface changes than others. A possible explanation is that the applied mechanical stress is more evenly distributed in such cases and uneven fracture of crystals is reduced. Such crystals with weakly formed layers might also suffer more loss from milling shear.84Effect of crystal habit on pharmaceutical properties
In pharmaceutical manufacturing and development, the correlation between API properties and crystal habit must be explored and studied to optimize the formulation process. Numerous methods have been developed to control crystal habit to achieve the desired pharmaceutical properties.85,86 Here, we have reviewed the impact of crystal habits on manufacturing (filtration, flowability, compaction and punch sticking) and formulation performance characteristics (dissolution rate and bioavailability).Impact of crystal habit on filtration
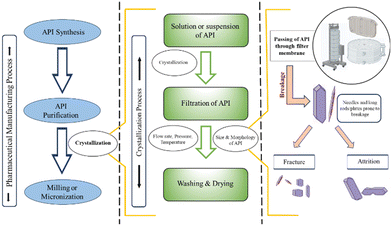
Filtration refers to the process in which the solid particulate matter is removed from the fluid portion using a porous membrane (filter). During API manufacturing in the pharmaceutical industry, filtration is frequently employed for crystal product isolation post crystallization. Various types of filtration methods are employed, such as surface, crossflow, ultra-filtration, cake filtration, etc. In the manufacturing industry, the filtration process must be uninterrupted so that there are no delays or compromises in product quality. The crystals are collected on top of the filter medium during filtration. This continues as the crystals stack up on top of one another to create the filter cake, which can be referred to as a cluster of particles. The crystals' size and shape determine the cake's filtration performance. Comprehending particle shape is essential because many APIs have a needle-like morphology, which is prone to breakage and impedes manufacturing by filter clogging and filter integrity breach (physical breakage/tear in filter membrane), as shown in Fig. 6. Breaking occurs by two mechanisms, namely, attrition and fracture. During fracture, particles break into two main fragments along with smaller fragments. During attrition, smaller particles are chipped off from the edges of the larger particles.87The habit of the crystals can significantly impact the filtration process. Needles are prone to attrition, and under stress, they break down into finer particles, filling up the particle bed's voids and reducing filtrate flow.88 However, optimization of the crystallization process, which includes processes like continuous filtration coupled with continuous product removal, can solve filterability-related problems to a significant level. For instance, Acevedo et al. designed a robust continuous crystallization process for cooling crystallization of acetaminophen and anti-solvent crystallization of benzoic acid using the mixed suspension mixed product removal crystallizer with continuous filtration system.89
Impact of crystal habit on flow properties
The flowability of pharmaceutical powders is critical in determining their processability, particularly during the solid dosage form manufacturing including tablets and capsules. Poor powder flowability poses significant manufacturing problems such as content uniformity and weight variation issues, obstruction of manufacturing, etc., leading to defects in final products or batch failures. The flow properties of the crystals depend on several factors, such as particle size distribution, crystal habit, particle–particle interaction, particle–machinery interaction etc.Crystals with a high aspect ratio in particular are found to have poor flow characteristics and often high cohesiveness. In this regard, needles are poorly flowable due to the high aspect ratio (surface energy also contributes to poor powder flow).90 Podczeck and Mia studied the powder flowability of eight different powders including, acetaminophen, acetyl salicylic acid, microcrystalline cellulose, etc. They found out that particles with needle-like habits, with higher aspect ratios, showed higher angles of internal friction which explains their poor powder flowability profiles.91 Modifying needles to allow for much more free-flowing habits is important for facilitating downstream API processing.92,93 Spherical particles, on the other hand, display superior flow properties, as seen in the case of cycloserine, metformin hydrochloride, ethambutol dihydrochloride, etc.94–98 This could be due to the low surface area of the spheres (which reduces the impact of cohesive forces) and uniform packing. Also, spheres with smooth surfaces experience low friction and tend to flow very easily. A comparative study was carried out to assess the processability of different crystal habits of 5-aminosalicylic acid (5-ASA). The needle-shaped crystals of 5-ASA had poor powder flowability, while the plate-shaped crystals exhibited moderate flowability, and spheroids and long hexagons were freely flowing.99 Thus, habit modification can serve as an essential tool to improve the powder flow properties of APIs.
Impact of crystal habit on compaction
Compressibility, tabletability, and compactability collectively define the compaction properties of pharmaceutical powders. These factors are critical in designing and developing solid dosage forms, like tablets, in filling hard-shell gelatin capsules, and in handling powder formulations.100Compressibility is the ability of a pharmaceutical formulation to deform under pressure. Tabletability reflects the ability of a powder to form a tablet of adequate strength at different compaction pressures. Compactability represents the ability of a material to be manufactured into tablets of the required strength under the effect of densification. The three parameters, viz., compressibility, tabletability, and compactability are interrelated as shown in Fig. 7A. For convenience, the three plots can be merged and represented in a three-dimensional format which is shown in Fig. 7B.101
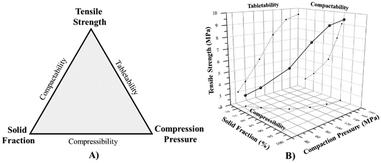 | ||
| Fig. 7 A) Tablet compaction triangle indicating the compressibility, tabletability, and compactability profiles, B) three-dimensional compression profile. | ||
Crystal habit modification can significantly improve the compression and compaction profiles of the API.102,103 Tabular and platy crystals have high compressibility due to their layered structure and packing density (example, celecoxib and dabigatran etexilate).104,105 However, it is not always the case that other crystal habits show poor compaction behavior. For instance, polyhedral and platy paracetamol and ibuprofen crystals showed excellent tabletability.106,107 Various other examples involving glimepiride,108 dapagliflozin propanediol monohydrate,57 erythromycin A dihydrate,70etc., have shown a positive impact of crystal habit modification on the compaction properties of the crystals. It is crucial to understand the mechanical properties of the API to enhance its compaction.109 The powder compaction properties can be analyzed through Heckel or Kawakita plots, and the reliable method to be employed is determined based on various factors like nature of material, pressure applied and so on.110
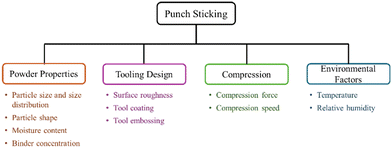
Impact of crystal habit on punch sticking
Adhesion of the powder material to the tooling surfaces is one of the primary issues faced during tablet punching, and the phenomenon is called punch sticking. Even though extensive research is being done to find effective solutions, to date no single root cause of sticking has been identified. Various factors like crystal habit,111,112 punch-particle interactions, API-excipient interactions, geometry of the punch,113 particle size, electrostatic properties of the API114,115 are under scrutiny and are depicted in Fig. 8.The physicochemical characteristic of a drug largely varies due to the anisotropic surface chemistry of crystalline substances. Since the interactions between API, punch, and excipients depend on the API's surface properties, crystal habit modification is proposed to considerably impact punch sticking. For instance, the crystal habit of ibuprofen was discovered to be the primary factor in the drug's propensity for sticking, and the platy crystals had exhibited higher level of sticking than needles/lath-shaped crystals.116 The molecular aspects of crystalline compounds can be considered to explain the punch-sticking tendency. In case of mefenamic acid, the exposure of polar functional groups from the crystal facets increases the interaction of the particles with the punch surface and hence increases sticking.112 The abundancy in hydrogen bond donors and acceptors and other surface phenomena in ibuprofen form I that enhance particle–particle interactions can also be attributed to sticking.117 Plenty of work has been done on punch sticking, emphasizing that punch sticking is less dependent on particle size and more on crystal habit modification, among other factors, because of the changes in the interparticle bonding areas and surface energy.118 Studies indicate that using lubricants can significantly reduce the tendency to stick for pharmaceutical powders. The addition of magnesium stearate, the most commonly used lubricant, to tablet formulations modifies surface properties like roughness, and the sticking tendency decreases significantly with an increase in lubricant concentration.119 Lubricants reduce friction between the punch face and powder particles. This is called boundary lubrication, and is commonly employed in the pharmaceutical industry.120
Impact of crystal habit on dissolution rate
Dissolution is a process in which the solid substances (particles/crystals) are converted into a molecular state in a solution form.121 The dissolution rate of a compound depends upon various factors such as its solid state (crystalline or amorphous), polymorphic form, particle size, API-solvent interactions, etc.122 In addition to the mentioned factors, crystal habit can also play a vital role in determining the dissolution rate of APIs.Research in this area has made it evident that crystal habit modification can change the dissolution profile of an API. For instance, numerous studies have shown that crystal habit modification of poorly soluble drugs such as tolfenamic acid, terbinafine hydrochloride, etc. can result in an improved dissolution rate.123,124 Crystals with the highest surface area per volume, for instance, needles and plates, tend to have high dissolution rates.125–127 However, other factors, along with crystal habit, might be responsible for changes in the dissolution profile, and we cannot solely attribute it to the crystal habit of the API.128 When the dissolution rate of a drug is enhanced, its bioavailability can be increased, since a greater fraction of a drug can be absorbed.129 The surface anisotropy in crystals contributes to the increased dissolution rate after habit modification. The surface anisotropy in crystals is a result of differences in the atomic arrangements, bonding orientations, and surface energies on each crystal face. As a result, the type of functional groups exposed on each facet of the crystal is modified during the crystal habit experiments.130 Through meticulous use of crystal growth inhibitors, the growth rate of facets expressing hydrophobic functional groups can be limited to increase the surface area of facets possessing hydrophilic functional groups, potentially improving the interaction of facets with water molecules during dissolution. This results in an increase in the polar energies of the compound, thereby improving its dissolution rate.131,132
Other research works that have employed crystal habit modification to improve flowability, compaction, and dissolution rate have been summarized in Table 4. The readers are directed towards the individual research works reported in the literature to obtain thorough understanding of the mechanisms of pharmaceutical property improvement after habit modification.
| API | Crystal habit | Property improved |
|---|---|---|
| Metformin HCl | Equidimensional133 | Flowability |
| Hollow, spherical134 | ||
| Mesalazine | Planks135 | |
| Celecoxib | Lath-shaped136 | Flowability, compaction, dissolution |
| Acicular15 | ||
| Plates15 | ||
| Carbamazepine | Block-shaped137 | |
| Paracetamol | Prismatic11 | Compaction |
| BMS-561390 (investigational anti-HIV drug) | Irregular138 | |
| Paracetamol | Irregular tabular139 | |
| Carbamazepine | Needle-like140 | Dissolution, compaction |
| Phenytoin | Long thin plates141 | Dissolution rate |
| Aspirin | Plates/prisms142 | |
| Meloxicam | Prismatic143 | |
| Tolbutamide | Circular, platy128 | |
| Simvastatin | Rods144 | |
| Piroxicam | Cubical145 | |
| Carbamazepine | Needle146 | |
| Ticagrelor | Plates147 | |
| Dipyridamole | Needle-like148 | Bioavailability |
| Trimethoprim | Rods149 | Stability |
Impact of crystal habit on stability
Stability of pharmaceutical products refers to the ability of a drug product to retain its physical, chemical, microbiological, and therapeutic properties until consumption under specific storage conditions. Considering the example of a suspension formulation of trimethoprim (TRM), which is expected to show a degree of physical stability with respect to its sedimentation volume. However, it was found that the habit of TRM influenced the sedimentation rate, with the order sedimentation volume of trimethoprim suspension being thin rods > thick rods > cubes > polyhedra.150 Hygroscopicity can also cause serious stability issues due to absorption of moisture from environment which may lead to either drug degradation or cause handling and processability issues. Platy and needle-like crystal exhibit enhanced hygroscopicity due to higher surface area to volume ratio. For instance, Prestidge and Tsatouhas studied the wettability of morphine sulphate crystallized from different solvent systems. It was found that the morphine sulphate samples showed different crystal habits in different solvents and as a result, its wettability profile also changed due to difference in the elemental composition of the different faces of morphine sulphate.151 Also, the degradation of crystals is known to be dependent on the crystal habit. For instance, the crystals of poly(hexamethylene adipate) were known to undergo enzymatic degradation mainly from the (110) face.152Characterization studies after crystal habit modification experiments
Morphological characterization
Solid state characterization
Solids are made of repetitive molecules known as unit cells which pack/arrange in a specific manner, resulting in a particular solid form. The specific intramolecular and intermolecular forces guide this packing/arrangement; changes in these forces also result in changes in the packing behavior, which results in changes in the solid-state form (polymorphs or amorphous form). Though exhibiting the same chemical composition, the polymorphs of compounds show remarkable changes in their physicochemical properties.155 Hence, during crystallization experiments, it is necessary to confirm the polymorphic form of a compound. The polymorphic confirmation of compounds can be made by estimating the unit cell parameters of compounds before and after the habit modification experiments. Any changes in the unit cell parameters is an indicative of polymorphic transformation which is undesired during habit modification experiments. Studies such as powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), Fourier-transform infra-red spectroscopy (FTIR) can be carried out to confirm the polymorphic identity of APIs.156Sometimes, a phenomenon called as “Preferred orientation” is seen during PXRD studies, where certain facets of a crystal tend to align parallel to the sample surface which then shows enhanced diffraction intensities from these facets. In such cases, certain peaks appear with high intensity and certain peaks may not appear in the diffractogram leading to erroneous interpretation of the PXRD results. Such effects are mostly seen in acicular and platy crystals and can be avoided by using a rotating sample holder or spinning capillary method solves the problem of preferred orientation.158,159
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 w/w), followed by preparation of a thin transparent compact using a hydraulic press. The compact can then be placed in the sample holder to record FTIR spectra. Alternatively, if attenuated total reflectance (ATR) mode is available, the powder sample can directly be placed on the ATR crystal to record IR spectra (between 400–4000 cm−1).57
100 w/w), followed by preparation of a thin transparent compact using a hydraulic press. The compact can then be placed in the sample holder to record FTIR spectra. Alternatively, if attenuated total reflectance (ATR) mode is available, the powder sample can directly be placed on the ATR crystal to record IR spectra (between 400–4000 cm−1).57
Powder rheology
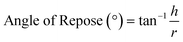 | (1) |
The United States Pharmacopoeia (USP) has defined the flow behavior of powders based on their AOR values, as shown in Table 5.
| Flow property | Angle of repose (°) | Carr's index (%) | Hausner ratio |
|---|---|---|---|
| Excellent | 25–30 | ≤10 | 1.00–1.11 |
| Good | 31–35 | 11–15 | 1.12–1.18 |
| Fair | 36–40 | 16–20 | 1.19–1.25 |
| Passable | 41–45 | 21–25 | 1.26–1.34 |
| Poor | 46–55 | 26–31 | 1.35–1.45 |
| Very poor | 56–65 | 32–37 | 1.46–1.59 |
| Very, very poor | >66 | >38 | >1.60 |
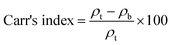 | (2) |
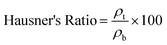 | (3) |
The bulk density of a sample can be determined by pouring a weighted quantity of the powder sample in a 100 mL measuring cylinder and noting down the volume (called bulk volume). Tapped density can be determined by subjecting the powder sample to around 1250 tapping using suitable apparatus (such as tapped density testers). The volume after the tapping is noted down as tapped volume. Bulk and tapped densities can be calculated using the following formulae (eqn (4) and (5)).
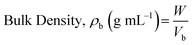 | (4) |
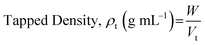 | (5) |
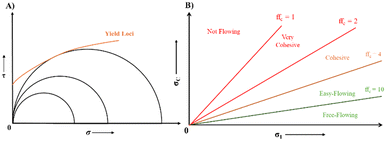 | ||
| Fig. 9 A) Measurement of yield strength of sample through Mohr stress circles using a σ–τ diagram, B) flow function and lines of constant flowability. | ||
ffc < 1 not flowing.
1 < ffc < 2 very cohesive.
2 < ffc < 4 cohesive.
4 < ffc < 10 easy flowing.
10 < ffc free-flowing.
Surface changes
In this method, a minimum of three probe liquids must be used for contact angle studies and to determine the above-mentioned parameters. Different polarity liquids (for instance, water as a polar liquid, diiodomethane (DIM) as a non-polar liquid, and ethylene glycol as a semi-polar liquid) are commonly used in the study. The total surface energy, dispersive and polar energies, and acidic and basic components are calculated using eqn (6).
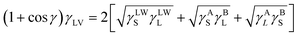 | (6) |
X-ray photoelectron spectroscopy (XPS)
XPS is a surface-sensitive analytical technique that is based on the photoelectric effect. In XPS, the surface of the material is bombarded with X-rays, which emit electrons. This method is highly surface sensitive (∼10 nm), and the chemical state information of the sample can be studied. Through this method, all elements except hydrogen and helium can be detected. Habit modification of APIs changes its surface chemistry (anisotropy), and thus XPS is a suitable technique to study the surface changes occurring in the sample. Changes in the hydrophilicity of the samples can be supported through XPS studies. For instance, after habit modification, the surface composition of hydrophilic moieties such as –OH, –NH increases or decreases. This change can be studied through XPS, wherein the % elemental composition of oxygen and nitrogen is increased in the modified samples. Thus, XPS serves as an important analytical tool to characterize the surface chemistry of samples.168Dissolution rate/solubility analysis
When conventional IDR studies are not possible (in cases where API quantity available is too low), powder dissolution studies can be carried out which utilize very less amount of API sample.171 In contrast to IDR studies, powder dissolution studies use powder sample without compression in a USP Type-IV “Flow through cell” apparatus. It is possible to monitor the dissolution rate of individual crystal through this method in combination with shadowgraph imaging technique. Herein, a high-resolution camera is utilized to capture video/images and tag and track individual crystals/particles in the flow through cell during dissolution studies. Further, the video analysis can be carried out using software such as Matlab to track the surface area of dissolving crystal/particle.172,173 The results obtained by powder dissolution studies can also be correlated with that of pellet IDR studies.171
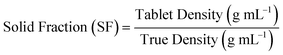
Compaction behaviour
In CTC profiling, tablet porosity and solid fraction can be used interchangeably, and the solid fraction is calculated using eqn (7).
 | (7) |
For flat round tablets:
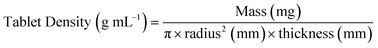 | (8) |
For oblong convex shape tablets:
 | (9) |
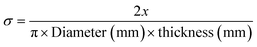 | (10) |
The experimental procedure involves the preparation of compacts/tablets of fixed weight and dimensions at different compression pressures using a hydraulic press or tablet compression machine. The tablet hardness and dimensions are then measured for each tablet, and CTC plots are prepared.
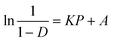 | (11) |
In a Heckel plot the natural logarithm of the reciprocal of tablet porosity is plotted against the applied compaction pressure.176 Such plots can be used to determine and compare the compaction properties of the API. Heckel plots are linear for intermediate pressure ranges, and there are significant deviations when the pressure is either too high or too low. In the context of Heckel plots, API powders are classified into three types based on their compaction behavior: type A (undergoes plastic deformation and has a linear Heckel plot), type B (undergoes fracture at lower pressures resulting in initial curvature followed by a linear region in the plot), and type C (undergoes particle fragmentation with little plastic deformation). Larger values of ‘K’ indicate increased hardness of tablets. Likewise, K′ decreases when the concentration of lubricants in the tablet increases. Thus, Heckel plots can also aid in choosing the appropriate binding agents.109
Regulatory perspective on control over crystal habit
Crystal habit modification can lead to changes in the processability (powder flowability, compressibility, filterability), stability (hygroscopicity, degradation rate, etc.) and performance (solubility/dissolution rate, bioavailability, etc.). It is absolute necessity for the manufacturers to maintain these quality attributes in the drugs to avoid regulatory repercussion. The United States Food and Drug Administration (USFDA) in its guidance documents mentions the need for the analysis of the particle size distribution and crystal habit of drugs wherever such factors are found to affect its performance.177 From the discussion presented in this tutorial, it is evident that to control the habit and size distribution of drugs, the crystallization process must be well understood, and adequate control measures must be in place. Additionally, the regulatory agencies recommend to use quantitative statistical assessment tools such as design of experiment (DoE) to study the effect of process parameters on the process robustness and the product (drug) quality. For thorough understanding of the DoE topic, the readers are advised to refer standard texts by Montgomery,178 Snee and Hoerl,179etc.Summary
As the pharmaceutical industry continues to strive for innovation, and regulating crystal habit remains a critical aspect in medication development and production, the factors that influence crystal habit modification are being investigated in detail. Modifying the crystal habit to improve physicochemical properties of an API can be considered as a cost-effective and simple alternative to other techniques such as the preparation of cocrystals, milling, amorphous solid dispersion formulation, etc. With appropriate process controls, crystal habit modification can yield excellent results in terms of improved processability (e.g. filterability, flowability, compaction) and dissolution performance of an API. In summary, the present tutorial details a procedure for carrying out crystal habit modification experiments and highlights the possible effects of different critical process parameters on crystal habit modification experiments. Various methods of crystallization (evaporative, cooling and anti-solvent crystallization) are also explained in brief, as is the effect of additives on crystal habit during crystallization. Further characterization techniques (morphological, physicochemical, rheological, and surface characterization), which can be employed to characterize the modified API, are discussed.Data availability
No primary research results and no new data were generated or analysed as a part of this review.Author contributions
Niranj H. Ram: conceptualization, literature survey, writing – original draft; Madhukiran R. Dhondale: conceptualization, content structuring, visualization, writing – original draft; Maan Singh: writing – review and editing; Brahmeshwar Mishra: supervision, Ashish Kumar Agrawal: writing – review and editing; Anne Marie Healy: writing – review and editing; Dinesh Kumar: conceptualization, visualization, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Madhukiran R. Dhondale acknowledges the funding from PMRF, India under the grant number 1102018.References
- A. Tiwary, Drug Dev. Ind. Pharm., 2001, 27, 699–709 CrossRef CAS PubMed.
- J. Prywer, Prog. Cryst. Growth Charact. Mater., 2005, 50, 1–38 CrossRef CAS.
- R. Ho, D. A. Wilson and J. Y. Heng, Cryst. Growth Des., 2009, 9, 4907–4911 CrossRef CAS.
- S. J. Satyawati, in Modern Aspects of Bulk Crystal and Thin Film Preparation, ed. K. Nikolai and B. Elena, IntechOpen, Rijeka, 2012, ch. 18, DOI:10.5772/28451.
- M. Okayasu, S. Kikkawa, H. Hikawa and I. Azumaya, CrystEngComm, 2021, 23, 7760–7770 RSC.
- F. M. Mahdi, A. P. Shier, I. S. Fragkopoulos, J. Carr, P. Gajjar and F. L. Muller, Pharm. Res., 2020, 37, 231 CrossRef CAS PubMed.
- C. Y. Ma and X. Z. Wang, J. Process Control, 2012, 22, 72–81 CrossRef CAS.
- M. R. Dhondale, A. G. Nambiar, M. Singh, A. R. Mali, A. K. Agrawal, N. R. Shastri, P. Kumar and D. Kumar, J. Pharm. Sci., 2023, 112, 2010–2028 CrossRef CAS PubMed.
- Y. Kawashima, M. Imai, H. Takeuchi, H. Yamamoto, K. Kamiya and T. Hino, Powder Technol., 2003, 130, 283–289 CrossRef CAS.
- O. Gyulai and Z. Aigner, Powder Technol., 2018, 336, 144–149 CrossRef CAS.
- H. A. Garekani, J. L. Ford, M. H. Rubinstein and A. R. Rajabi-Siahboomi, Int. J. Pharm., 1999, 187, 77–89 CrossRef CAS PubMed.
- Y. Wang, H. Zhang, L. Cai, F. Xue, H. Chen, J. Gong and S. Du, Ultrason. Sonochem., 2023, 97, 106475 CrossRef CAS PubMed.
- A. Maharana, A. Das, J. Kumar and D. Sarkar, Comput. Chem. Eng., 2024, 184, 108651 CrossRef CAS.
- T. Munk, S. Baldursdottir, S. Hietala, T. Rades, S. Kapp, M. Nuopponen, K. Kalliomaki, H. Tenhu and J. Rantanen, Mol. Pharmaceutics, 2012, 9, 1932–1941 CrossRef CAS PubMed.
- S. R. Modi, A. K. R. Dantuluri, V. Puri, Y. B. Pawar, P. Nandekar, A. T. Sangamwar, S. R. Perumalla, C. C. Sun and A. K. Bansal, Cryst. Growth Des., 2013, 13, 2824–2832 CrossRef CAS.
- M. K. Bommaka, M. K. Chaitanya Mannava, S. K. Rai, K. Suresh and A. K. Nangia, Cryst. Growth Des., 2021, 21, 5573–5585 CrossRef CAS.
- D. Liu and M. Z. Yates, Langmuir, 2006, 22, 5566–5569 CrossRef CAS PubMed.
- Y. Masui, Y. Kitaura, T. Kobayashi, Y. Goto, S. Ando, A. Okuyama and H. Takahashi, Org. Process Res. Dev., 2003, 7, 334–338 CrossRef CAS.
- T. Wu, L. Valencia, T. Pfohl, B. Heck, G. Reiter, P. J. Lutz and R. Mülhaupt, Macromolecules, 2019, 52, 4839–4846 CrossRef CAS.
- I. Wünsch, J. H. Finke, E. John, M. Juhnke and A. Kwade, Int. J. Pharm., 2022, 626, 122117 CrossRef PubMed.
- G. Perini, C. Avendano, W. Hicks, A. R. Parsons and T. Vetter, Chem. Eng. Sci., 2021, 230, 116151 CrossRef CAS.
- H. Kalman, Powder Technol., 2021, 393, 582–596 CrossRef CAS.
- D. Geldart, E. Abdullah, A. Hassanpour, L. Nwoke and I. Wouters, China Particuol., 2006, 4, 104–107 CrossRef.
- M. Maghsoodi, Adv. Pharm. Bull., 2015, 5, 13 CAS.
- C. Noiriel, M. Oursin, G. Saldi and D. Haberthür, ACS Earth Space Chem., 2018, 3, 100–108 CrossRef.
- Y. Tsume, D. M. Mudie, P. Langguth, G. E. Amidon and G. L. Amidon, Eur. J. Pharm. Sci., 2014, 57, 152–163 CrossRef CAS PubMed.
- S. Mirza, I. Miroshnyk, J. Heinämäki, O. Antikainen, J. Rantanen, P. Vuorela, H. Vuorela and J. Yliruusi, AAPS PharmSciTech, 2009, 10, 113–119 CrossRef CAS PubMed.
- S. Clercq, A. Mouahid, G. Pèpe and E. Badens, Cryst. Growth Des., 2020, 20, 6863–6876 CrossRef CAS.
- H. Gu, R. Li, Y. Sun, S. Li, W. Dong and J. Gong, J. Cryst. Growth, 2013, 373, 146–150 CrossRef CAS.
- D. Kumar and N. R. Shastri, Cryst. Growth Des., 2014, 14, 326–338 CrossRef CAS.
- K. Chadwick, J. Chen, E. E. Santiso and B. L. Trout, in Handbook of Industrial Crystallization, ed. A. S. Myerson, D. Erdemir and A. Y. Lee, Cambridge University Press, Cambridge, 3rd edn, 2019, pp. 136–171, DOI:10.1017/9781139026949.005.
- T. Tari, P. Szabó-Révész and Z. Aigner, Crystals, 2019, 9, 295 CrossRef CAS.
- A. Bártová, R. Gabriel, B. B. Prudilová, E. Otyepková, L. Malina and M. Otyepka, Powder Technol., 2022, 402, 117334 CrossRef.
- R.-Q. Song and H. Cölfen, CrystEngComm, 2011, 13, 1249–1276 RSC.
- K. N. Olafson, R. Li, B. G. Alamani and J. D. Rimer, Chem. Mater., 2016, 28, 8453–8465 CrossRef CAS.
- A. V. Tulcidas, Ph.D. thesis, Universidade Do Porto, 2019 Search PubMed.
- Z. Gao, S. Rohani, J. Gong and J. Wang, Engineering, 2017, 3, 343–353 CrossRef.
- M. Shahid, G. Sanxaridou, S. Ottoboni, L. Lue and C. Price, Org. Process Res. Dev., 2021, 25, 969–981 CrossRef CAS PubMed.
- T. Lee, H. Y. Lin and H. L. Lee, Org. Process Res. Dev., 2013, 17, 1168–1178 CrossRef CAS.
- S. Pu and K. Hadinoto, Chem. Eng. Res. Des., 2024, 201, 45–66 CrossRef CAS.
- I. Nikolakakis, K. Kachrimanis and S. Malamataris, Int. J. Pharm., 2000, 201, 79–88 CrossRef CAS PubMed.
- E. M. Soper, R. Y. Penchev, S. M. Todd, F. Eckert and M. Meunier, J. Cryst. Growth, 2022, 591, 126712 CrossRef CAS.
- A. T. Karunanithi, C. Acquah, L. E. K. Achenie, S. Sithambaram and S. L. Suib, Comput. Chem. Eng., 2009, 33, 1014–1021 CrossRef CAS.
- A. T. Karunanithi, L. E. Achenie and R. Gani, Chem. Eng. Sci., 2006, 61, 1247–1260 CrossRef CAS.
- L. E. Hatcher, W. Li, P. Payne, B. Benyahia, C. D. Rielly and C. C. Wilson, Cryst. Growth Des., 2020, 20, 5854–5862 CrossRef CAS.
- T. D. Turner, L. E. Hatcher, C. C. Wilson and K. J. Roberts, J. Pharm. Sci., 2019, 108, 1779–1787 CrossRef CAS PubMed.
- Z. Liang, M. Zhang, F. Wu, J.-F. Chen, C. Xue and H. Zhao, Comput. Chem. Eng., 2017, 99, 296–303 CrossRef CAS.
- P. Neugebauer, J. Cardona, M. O. Besenhard, A. Peter, H. Gruber-Woelfler, C. Tachtatzis, A. Cleary, I. Andonovic, J. Sefcik and J. G. Khinast, Cryst. Growth Des., 2018, 18, 4403–4415 CrossRef CAS PubMed.
- C.-H. Chang, C.-M. Hsieh and C.-S. Su, Cryst. Res. Technol., 2021, 56, 2000182 CrossRef CAS.
- C. J. Callahan and X.-W. Ni, CrystEngComm, 2014, 16, 690–697 RSC.
- Z. Yu, R. Tan and P. Chow, J. Cryst. Growth, 2005, 279, 477–488 CrossRef CAS.
- L. Jia, D. Wu, P. Cui, L. Zhou and Q. Yin, Particuology, 2023, 82, 146–156 CrossRef CAS.
- E. Kougoulos, C. Chadwick and M. Ticehurst, Powder Technol., 2011, 210, 308–314 CrossRef CAS.
- R. Chow, R. Blindt, R. Chivers and M. Povey, Ultrasonics, 2003, 41, 595–604 CrossRef CAS PubMed.
- J. R. G. Sander, B. W. Zeiger and K. S. Suslick, Ultrason. Sonochem., 2014, 21, 1908–1915 CrossRef CAS PubMed.
- R. Prasad and S. V. Dalvi, Chem. Eng. Sci., 2020, 226, 115911 CrossRef CAS.
- M. Singh, M. R. Dhondale, A. K. Agrawal, S. Panda and D. Kumar, Chem. Eng. Res. Des., 2024, 207, 308–319 CrossRef CAS.
- G. Ruecroft, D. Hipkiss, T. Ly, N. Maxted and P. W. Cains, Org. Process Res. Dev., 2005, 9, 923–932 CrossRef CAS.
- A. G. Shtukenberg, M. D. Ward and B. Kahr, J. Cryst. Growth, 2022, 597, 126839 CrossRef CAS.
- S. Xu, D. Cao, Y. Liu and Y. Wang, Cryst. Growth Des., 2022, 22, 2001–2022 CrossRef CAS.
- N. Rodríguez-hornedo and D. Murphy, J. Pharm. Sci., 1999, 88, 651–660 CrossRef PubMed.
- A. L. Sarode, P. Wang, S. Obara and D. R. Worthen, Eur. J. Pharm. Biopharm., 2014, 86, 351–360 CrossRef CAS PubMed.
- Y. Wang, F. Xue, S. Yu, Y. Cheng, M. Yin, S. Du and J. Gong, J. Mol. Liq., 2021, 343, 116967 CrossRef CAS.
- D. Kumar, R. Thipparaboina, S. R. Modi, A. K. Bansal and N. R. Shastri, CrystEngComm, 2015, 17, 1615–1624 RSC.
- M. A. Bellucci, A. Marx, B. Wang, L. Fang, Y. Zhou, C. Greenwell, Z. Li, A. Becker, G. Sun and J. G. Brandenburg, Small Methods, 2023, 7, 2201692 CrossRef CAS PubMed.
- W. Kaialy, H. Larhrib, B. Chikwanha, S. Shojaee and A. Nokhodchi, Int. J. Pharm., 2014, 464, 53–64 CrossRef CAS PubMed.
- L. Y. Pfund, C. P. Price, J. J. Frick and A. J. Matzger, J. Am. Chem. Soc., 2015, 137, 871–875 CrossRef CAS PubMed.
- M. N. Femi-Oyewo and M. Spring, Int. J. Pharm., 1994, 112, 17–28 CrossRef CAS.
- S. K. Poornachary, V. D. Chia, Y. Yani, G. Han, P. S. Chow and R. B. Tan, Cryst. Growth Des., 2017, 17, 4844–4854 CrossRef CAS.
- S. Mirza, I. Miroshnyk, J. Heinämäki, J. Rantanen, O. Antikainen, P. Vuorela, H. Vuorela and J. Yliruusi, Cryst. Growth Des., 2008, 8, 3526–3531 CrossRef CAS.
- K. Smiti and C. Garima, Yakugaku Zasshi, 2008, 128, 281–289 CrossRef PubMed.
- F. Tian, S. Baldursdottir and J. Rantanen, Mol. Pharmaceutics, 2009, 6, 202–210 CrossRef CAS PubMed.
- Y. Wang, H. Zhang, L. Cai, F. Xue, H. Chen, J. Gong and S. Du, Ultrason. Sonochem., 2023, 106475 CrossRef CAS PubMed.
- A. R. Klapwijk, E. Simone, Z. K. Nagy and C. C. Wilson, Cryst. Growth Des., 2016, 16, 4349–4359 CrossRef CAS.
- W. Zhu, F. S. Romanski, X. Meng, S. Mitra and M. S. Tomassone, Eur. J. Pharm. Sci., 2011, 42, 452–461 CrossRef CAS PubMed.
- A. Kuldipkumar, Y. T. Tan, M. Goldstein, Y. Nagasaki, G. G. Zhang and G. S. Kwon, Cryst. Growth Des., 2005, 5, 1781–1785 CrossRef CAS.
- J. Jennings, M. F. Butler, M. McLeod, E. Csányi, A. J. Ryan and O. O. Mykhaylyk, Cryst. Growth Des., 2018, 18, 7094–7105 CrossRef CAS.
- S. Zhang, L. Zhou, W. Yang, C. Xie, Z. Wang, B. Hou, H. Hao, L. Zhou, Y. Bao and Q. Yin, Cryst. Growth Des., 2020, 20, 1609–1617 CrossRef CAS.
- X. Cheng, X. Huang, Y. Hao, B. Wang, C. Sun, J. Shu and H. Hao, Ind. Eng. Chem. Res., 2022, 61, 7193–7203 CrossRef CAS.
- D. Prasad, H. Chauhan and E. Atef, Mol. Pharmaceutics, 2016, 13, 756–765 CrossRef CAS PubMed.
- F. Liu, L. Wang, W. Li, M. Li, J. Gong, Y. Wang and D. Han, Cryst. Growth Des., 2021, 21, 3818–3830 CrossRef CAS.
- E. Simone, M. V. Cenzato and Z. K. Nagy, J. Cryst. Growth, 2016, 446, 50–59 CrossRef CAS.
- S. Xie, S. K. Poornachary, P. S. Chow and R. B. Tan, Cryst. Growth Des., 2010, 10, 3363–3371 CrossRef CAS.
- V. Chikhalia, R. T. Forbes, R. A. Storey and M. Ticehurst, Eur. J. Pharm. Sci., 2006, 27, 19–26 CrossRef CAS PubMed.
- K. Patchigolla and D. Wilkinson, Ind. Eng. Chem. Res., 2008, 47, 804–812 CrossRef CAS.
- M. Sultana and K. F. Jensen, Cryst. Growth Des., 2012, 12, 6260–6266 CrossRef CAS.
- S. Saifoori, W.-P. Goh, M. Ali and M. Ghadiri, Powder Technol., 2020, 361, 651–662 CrossRef CAS.
- B. Li, H. Zhang, K. Saranteas and M. A. Henson, Sep. Purif. Technol., 2021, 278, 119462 CrossRef.
- D. Acevedo, R. Peña, Y. Yang, A. Barton, P. Firth and Z. K. Nagy, Chem. Eng. Process.: Process Intesif., 2016, 108, 212–219 CrossRef CAS.
- K. S. Gouthami, D. Kumar, R. Thipparaboina, R. B. Chavan and N. R. Shastri, Int. J. Pharm., 2015, 491, 26–34 CrossRef PubMed.
- F. Podczeck and Y. Mia, Int. J. Pharm., 1996, 144, 187–194 CrossRef CAS.
- M. Manish, J. Harshal and P. Anant, Eur. J. Pharm. Sci., 2005, 25, 41–48 CrossRef CAS PubMed.
- D. Wilson, M. Bunker, D. Milne, A. Jawor-Baczynska, A. Powell, J. Blyth and D. Streather, Powder Technol., 2018, 339, 641–650 CrossRef CAS.
- M. R. Dhondale, M. Singh, A. K. Agrawal and D. Kumar, Powder Technol., 2025, 457, 120885 CrossRef CAS.
- K. Kedia and S. Wairkar, Powder Technol., 2019, 344, 665–672 CrossRef CAS.
- K. K. Moravkar, D. S. Shah, A. G. Magar, B. A. Bhairav, S. D. Korde, K. M. Ranch and S. S. Chalikwar, J. Drug Delivery Sci. Technol., 2022, 71, 103265 CrossRef CAS.
- A. Nokhodchi, A. Homayouni, R. Araya, W. Kaialy, W. Obeidat and K. Asare-Addo, RSC Adv., 2015, 5, 46119–46131 RSC.
- V. R. Nalluri and M. Kuentz, Eur. J. Pharm. Biopharm., 2010, 74, 388–396 CrossRef CAS PubMed.
- N. Pudasaini, P. P. Upadhyay, C. R. Parker, S. U. Hagen, A. D. Bond and J. Rantanen, Org. Process Res. Dev., 2017, 21, 571–577 CrossRef CAS.
- O. A. Odeku, Pharm. Rev., 2007, 5, 1–20 Search PubMed.
- United States Pharmacopoeia (USP), 〈1062〉 Tablet Compression Characterization, USP, 2022, pp. 1–15, DOI:10.31003/USPNF_M99395_02_01.
- L. Keshavarz, M. Pishnamazi, U. R. Khandavilli, S. Shirazian, M. N. Collins, G. M. Walker and P. J. Frawley, Arab. J. Chem., 2021, 14, 103089 CrossRef CAS.
- A. Nokhodchi, N. Bolourtchian and R. Dinarvand, Int. J. Pharm., 2003, 250, 85–97 CrossRef CAS PubMed.
- S. R. Modi, K. S. Khomane and A. K. Bansal, Int. J. Pharm., 2014, 460, 189–195 CrossRef CAS PubMed.
- S. R. Datir and M. H. Bele, J. Cryst. Growth, 2023, 606, 127047 CrossRef CAS.
- N. Rasenack and B. W. Müller, Int. J. Pharm., 2002, 244, 45–57 CrossRef CAS PubMed.
- P. Di Martino, M. Beccerica, E. Joiris, G. F. Palmieri, A. Gayot and S. Martelli, J. Cryst. Growth, 2002, 243, 345–355 CrossRef CAS.
- S. R. Datir, D. Kumar and M. H. Bele, J. Cryst. Growth, 2022, 592, 126711 CrossRef CAS.
- S. Jain, Pharm. Sci. Technol. Today, 1999, 2, 20–31 CrossRef CAS PubMed.
- S. Pundir and A. Badola, Int. J. Pharm. Life Sci., 2013, 2, 141–157 CrossRef.
- H. Chen, S. Paul, H. Xu, K. Wang, M. K. Mahanthappa and C. C. Sun, Mol. Pharmaceutics, 2020, 17, 1387–1396 CrossRef CAS PubMed.
- V. Waknis, E. Chu, R. Schlam, A. Sidorenko, S. Badawy, S. Yin and A. S. Narang, Pharm. Res., 2014, 31, 160–172 CrossRef CAS PubMed.
- M. Roberts, J. L. Ford, G. S. MacLeod, J. T. Fell, G. W. Smith, P. H. Rowe and A. M. Dyas, J. Pharm. Pharmacol., 2004, 56, 947–950 CrossRef CAS PubMed.
- L. Samiei, K. Kelly, L. Taylor, B. Forbes, E. Collins and M. Rowland, Powder Technol., 2017, 305, 509–517 CrossRef CAS.
- M. U. Ghori, E. Šupuk and B. R. Conway, Eur. J. Pharm. Sci., 2014, 65, 1–8 CrossRef CAS PubMed.
- D. Hooper, F. Clarke, R. Docherty, J. Mitchell and M. Snowden, Int. J. Pharm., 2017, 531, 266–275 CrossRef CAS PubMed.
- A. A. Moldovan and A. G. P. Maloney, Cryst. Growth Des., 2024, 24, 4160–4169 CrossRef CAS PubMed.
- S. Paul, L. J. Taylor, B. Murphy, J. F. Krzyzaniak, N. Dawson, M. P. Mullarney, P. Meenan and C. C. Sun, Mol. Pharmaceutics, 2020, 17, 1148–1158 CrossRef CAS PubMed.
- C. A. Gunawardana, A. Kong, D. Wanapun, D. O. Blackwood, C. T. Powell, J. F. Krzyzaniak, M. C. Thomas, J. E. Kresevic and C. C. Sun, Int. J. Pharm., 2023, 123016 CrossRef CAS PubMed.
- J. Li and Y. Wu, Lubricants, 2014, 2(1), 21–43 CrossRef.
- H. S. Purohit, G. G. Zhang and Y. Gao, J. Pharm. Sci., 2023, 112, 290–303 CrossRef CAS PubMed.
- C. U. Phan, J. Shen, K. Yu, J. Mao and G. Tang, Molecules, 2021, 26, 3469 CrossRef CAS PubMed.
- H.-H. Chen, C.-S. Su, J.-J. Liu and M.-T. Sheu, J. Supercrit. Fluids, 2015, 101, 17–23 CrossRef CAS.
- G. Kuminek, G. S. Rauber, M. K. Riekes, C. E. M. de Campos, G. A. Monti, A. J. Bortoluzzi, S. L. Cuffini and S. G. Cardoso, J. Pharm. Biomed. Anal., 2013, 78, 105–111 CrossRef PubMed.
- D. Kumar, R. Thipparaboina, S. R. Modi, A. K. Bansal and N. R. Shastri, J. Cryst. Growth, 2015, 422, 44–51 CrossRef CAS.
- Y. Gao, B. Glennon, V. K. Kamaraju, G. Hou and P. Donnellan, Org. Process Res. Dev., 2018, 22, 328–336 CrossRef CAS.
- P. Bukovec, P. Benkič, M. Smrkolj and F. Vrečzer, Pharmazie, 2016, 71, 263–268 CAS.
- R. A. Keraliya, T. G. Soni, V. T. Thakkar and T. R. Gandhi, Dissolution Technol., 2010, 17, 16–21 CrossRef CAS.
- D. Kumar, R. Thipparaboina and N. R. Shastri, Org. Process Res. Dev., 2015, 19, 1912–1917 CrossRef CAS.
- R. Adhiyaman and S. K. Basu, Int. J. Pharm., 2006, 321, 27–34 CrossRef CAS PubMed.
- S. R. Datir, D. Kumar, P. Kumar, S. Jain, A. K. Bansal, B. Nallamothu, S. D. Thakore and M. H. Bele, Appl. Sci., 2021, 11, 5604 CrossRef CAS.
- S. R. Modi, A. K. R. Dantuluri, S. R. Perumalla, C. C. Sun and A. K. Bansal, Cryst. Growth Des., 2014, 14, 5283–5292 CrossRef CAS.
- M. K. Raval, J. M. Patel, R. K. Parikh and N. R. Sheth, Part. Sci. Technol., 2014, 32, 431–444 CrossRef CAS.
- J. Hansen and P. Kleinebudde, Eur. J. Pharm. Biopharm., 2021, 159, 170–176 CrossRef CAS PubMed.
- P. P. Upadhyay, N. Pudasaini, M. K. Mishra, U. Ramamurty and J. Rantanen, Chem. Eng. Res. Des., 2018, 136, 447–455 CrossRef CAS.
- S. Banga, G. Chawla, D. Varandani, B. Mehta and A. K. Bansal, J. Pharm. Pharmacol., 2007, 59, 29–39 CrossRef CAS PubMed.
- V. Verma, R. V. Peddapatla, C. M. Crowley, A. M. Crean, P. Davern, S. Hudson and B. K. Hodnett, Cryst. Growth Des., 2018, 18, 338–350 CrossRef CAS.
- D. S. Bindra and S. Desikan, Pharm. Dev. Technol., 2015, 20, 129–138 CrossRef CAS PubMed.
- C. Thompson, M. C. Davies, C. J. Roberts, S. J. Tendler and M. J. Wilkinson, Int. J. Pharm., 2004, 280, 137–150 CrossRef CAS PubMed.
- C. N. Bolour, A. Nokhoudchi and R. Dinarvand, Daru, J. Pharm. Sci., 2001, 9, 12–22 Search PubMed.
- A. H. Chow, J. D. Gordon, A. Szeitz and J. W. Young, Int. J. Pharm., 1995, 126, 11–19 CrossRef CAS.
- A. Watanabe, Y. Yamaoka and K. Takada, Chem. Pharm. Bull., 1982, 30, 2958–2963 CrossRef CAS.
- N. Bolourchian, M. Nili, S. M. Foroutan, A. Mahboubi and A. Nokhodchi, J. Drug Delivery Sci. Technol., 2020, 55, 101485 CrossRef CAS.
- P. Bukovec, A. Meden, M. Smrkolj and F. Vrečer, Acta Chim. Slov., 2015, 62, 958–966 CrossRef CAS PubMed.
- L. Lyn, H. Sze, A. Rajendran, G. Adinarayana, K. Dua and S. Garg, Acta Pharm., 2011, 61, 391 CAS.
- Y. Javadzadeh, A. Mohammadi, N. S. Khoei and A. Nokhodchi, Acta Pharm., 2009, 59, 187–197 CAS.
- Y. Ren, J. Shen, K. Yu, C. U. Phan, G. Chen, J. Liu, X. Hu and J. Feng, Crystals, 2019, 9, 556 CrossRef CAS.
- R. Adhiyaman and S. K. Basu, Int. J. Pharm., 2006, 321, 27–34 CrossRef CAS PubMed.
- A. K. Tiwary and G. M. Panpalia, Pharm. Res., 1999, 16, 261–265 CrossRef CAS PubMed.
- A. K. Tiwary and G. M. Panpalia, Pharm. Res., 1999, 16, 261–265 CrossRef CAS PubMed.
- C. A. Prestidge and G. Tsatouhas, Int. J. Pharm., 2000, 198, 201–212 CrossRef CAS PubMed.
- S. Gestí, A. Almontassir, M. T. Casas and J. Puiggalí, Biomacromolecules, 2006, 7, 799–808 CrossRef PubMed.
- C. G. Jones, in Forensic Microscopy for Skeletal Tissues: Methods and Protocols, ed. L. S. Bell, Humana Press, Totowa, NJ, 2012, pp. 1–20, DOI:10.1007/978-1-61779-977-8_1.
- M. S. R. N. Kiran, S. Varughese, C. M. Reddy, U. Ramamurty and G. R. Desiraju, Cryst. Growth Des., 2010, 10, 4650–4655 CrossRef CAS.
- Y. Zhou, J. Wang, Y. Xiao, T. Wang and X. Huang, Curr. Pharm. Des., 2018, 24, 2375–2382 CrossRef CAS PubMed.
- N. Salunke, R. Thipparaboina, R. B. Chavan, A. Lodagekar, S. Mittapalli, A. Nangia and N. R. Shastri, J. Pharm. Biomed. Anal., 2018, 149, 185–192 CrossRef CAS PubMed.
- A. A. Bunaciu, E. G. Udriştioiu and H. Y. Aboul-Enein, Crit. Rev. Anal. Chem., 2015, 45, 289–299 CrossRef CAS PubMed.
- S. Halder and S. Ray, Int. J. Ceram. Eng. Sci., 2022, 4, 309–314 CrossRef CAS.
- C. F. Holder and R. E. Schaak, ACS Nano, 2019, 13, 7359–7365 CrossRef CAS PubMed.
- D. Faroongsarng, AAPS PharmSciTech, 2016, 17, 572–577 CrossRef CAS PubMed.
- N. Saadatkhah, A. Carillo Garcia, S. Ackermann, P. Leclerc, M. Latifi, S. Samih, G. S. Patience and J. Chaouki, Can. J. Chem. Eng., 2020, 98, 34–43 CrossRef CAS.
- H. M. Beakawi Al-Hashemi and O. S. Baghabra Al-Amoudi, Powder Technol., 2018, 330, 397–417 CrossRef CAS.
- R. B. Shah, M. A. Tawakkul and M. A. Khan, AAPS PharmSciTech, 2008, 9, 250–258 CrossRef CAS PubMed.
- D. Schulze, in Powders and Bulk Solids: Behavior, Characterization, Storage and Flow, ed. D. Schulze, Springer International Publishing, Cham, 2021, pp. 57–100, DOI:10.1007/978-3-030-76720-4_3.
- D. Schulze, Annu. Trans. – Nord. Rheol. Soc., 2013, 21, 99–106 Search PubMed.
- A. R. Balkenende, H. J. A. P. van de Boogaard, M. Scholten and N. P. Willard, Langmuir, 1998, 14, 5907–5912 CrossRef CAS.
- F. M. Etzler, Rev. Adhes. Adhes., 2013, 1, 3–45 CrossRef CAS.
- F. A. Stevie and C. L. Donley, J. Vac. Sci. Technol., A, 2020, 38, 063204 CrossRef CAS.
- K. Sing, Colloids Surf., A, 2001, 187–188, 3–9 CrossRef CAS.
- United States Pharmacopoeia (USP), 〈1087〉 Intrinsic Dissolution—Dissolution Testing Procedures for Rotating Disk and Stationary Disk, USP, 2020, pp. 1–7, DOI:10.31003/USPNF_M99806_02_01.
- K. Tsinman, A. Avdeef, O. Tsinman and D. Voloboy, Pharm. Res., 2009, 26, 2093–2100 CrossRef CAS PubMed.
- D. R. Serrano, T. Persoons, D. M. D'Arcy, C. Galiana, M. A. Dea-Ayuela and A. M. Healy, Eur. J. Pharm. Sci., 2016, 89, 125–136 CrossRef CAS PubMed.
- D. M. D'Arcy and T. Persoons, J. Pharm. Sci., 2011, 100, 1102–1115 CrossRef PubMed.
- S. Patel, A. M. Kaushal and A. K. Bansal, Crit. Rev. Ther. Drug Carrier Syst., 2006, 23, 1–66 CrossRef CAS PubMed.
- H. Triboandas, K. Pitt, M. Bezerra, D. Ach-Hubert and W. Schlindwein, Pharmaceutics, 2022, 14, 2398 CrossRef CAS PubMed.
- G. Vreeman, C. Wang, C. M. Reddy and C. C. Sun, Cryst. Growth Des., 2021, 21, 6655–6659 CrossRef CAS.
- USFDA, Drug Substance Chemistry, Manufacturing, and Controls Information, Guidance for Industry, 2010, pp. 1–59 Search PubMed.
- D. C. Montgomery, Design and analysis of experiments, John Wiley & Sons, 2017 Search PubMed.
- R. Snee and R. Hoerl, Strategies for formulations development: a step-by-step guide using JMP, SAS Institute, 2016 Search PubMed.
Footnote |
| † Joint first authors. |
| This journal is © The Royal Society of Chemistry 2025 |