Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surfactant-free co-solvent exfoliation strategy for semiconducting quasi-1D Nb2Pd3Se8 nanowires†
Kyung Hwan
Choi‡
b,
Yeongjin
Kim‡
a,
Jinsu
Kang
a,
Kyung
In Kim
a,
Jeong Su
Park
a,
Hyung-Suk
Oh
 ade,
Hak Ki
Yu
ade,
Hak Ki
Yu
 *c and
Jae-Young
Choi
*c and
Jae-Young
Choi
 *abe
*abe
aSchool of Advanced Materials Science & Engineering, Sungkyunkwan University, Suwon, 16419, Korea. E-mail: jy.choi@skku.edu
bSKKU Advanced Institute of Nanotechnology (SAINT), Sungkyunkwan University, Suwon, 16419, Korea
cDept. of Materials Science and Engineering & Dept. of Energy Systems Research, Ajou University, Suwon, 16499, Korea. E-mail: hakkiyu@ajou.ac.kr
dClean Energy Research Center, Korea Institute of Science and Technology, Seoul, 02792, Korea
eKIST-SKKU Carbon-Neutral Center, Sungkyunkwan University, Suwon, 16419, Korea
First published on 21st January 2025
Abstract
Nb2Pd3Se8, a quasi-one-dimensional (1D) van der Waals material, has recently gained attention owing to its intriguing structural, electrical and optoelectrical properties. In this work, we studied co-solvent exfoliation of Nb2Pd3Se8 in a mixture of water and ethanol or isopropanol. By adjusting the compositions of the co-solvent, the dispersion behavior of Nb2Pd3Se8 was interpreted in terms of surface tension and dielectric constant. This approach is effective for substituting the previously reported optimal solvent of NMP, which is toxic with a high boiling point of 204 °C. The Nb2Pd3Se8 nanowire prepared through co-solvent exfoliation presented a high electronic property with an electron mobility of 10.3 cm2 V−1 s−1.
1. Introduction
One-dimensional (1D) van der Waals (vdW) materials have recently attracted attention because of their unique structural, physical, electrical and magnetic properties.1–4 In particular, the ultimate downscaled structure, known as a 1D single-chain atomic crystal, holds promise for the fabrication of highly integrated electronic devices owing to its minimized dimensionality and lack of dangling bonds on surfaces.5–7 Extensive research has been conducted on these materials to address the stability at the ultimate atomic scale and explore associated downscaling processes.8–11Recently, we successfully prepared a novel quasi-1D vdW Nb2Pd3Se8 material as a promising semiconducting building block for nano-electronic fields.12–14 Its dissociative nature allowed for the separation of several nanometers thick Nb2Pd3Se8 nanowires via mechanical exfoliation technique. The properties of mechanically exfoliated Nb2Pd3Se8 nanowires have been intensively studied for application in field-effect transistors (FETs) and photodetectors.12,15 Furthermore, research on liquid phase exfoliation of Nb2Pd3Se8 has been studied to expand the preparation methods.16 By testing various exfoliation solvents, it was confirmed that N-methyl-2-pyrrolidone (NMP) is the optimal solvent for exfoliation and stabilization of Nb2Pd3Se8 nanowires owing to its well-matched surface tension and polarity. However, NMP is considered highly toxic, and its high boiling point of 204 °C limits its broader applications.17
In this work, we utilized a co-solvent exfoliation strategy using a mixture of water and ethanol (EtOH) or isopropanol (IPA) to identify alternative exfoliation solvents without using a dispersant (Scheme 1). We tested a total of nine solvent compositions for each co-solvent system to compare the dispersibility of Nb2Pd3Se8.
 | ||
| Scheme 1 Co-solvent exfoliation strategy of Nb2Pd3Se8 nanowires for substituting the conventional, highly toxic, high-boiling point NMP solvent. | ||
2. Experimental section
2.1 Synthesis of Nb2Pd3Se8
Nb2Pd3Se8 was prepared using Nb powder (99.99%, Alfa Aesar), Pd powder (≥99.9, Alfa Aesar) and Se powder (99+%, Alfa Aesar). A stoichiometric mixture of Nb, Pd and Se was pelletized and sealed in a 10 cm-long evacuated quartz tube. The sealed quartz tube was placed inside a box furnace and heated at 600 °C for 120 h before cooling at 4 °C h−1. The resulting material was a dark grey sintered powder.2.2 Liquid phase exfoliation
Initially, 10 mg of Nb2Pd3Se8 powder was immersed in 10 mL of each solvent. The initial large powder particles were crushed via ultrasonication using a probe sonicator (VC 505, Sonics & Materials, Inc.). The probe sonicator was operated for 5 min at a 2 s on/2 s off interval. Subsequently, bath sonication (B2005S-68 K, 68 kHz, 200 W, KODO Technical) was conducted for 3 h. After ultrasonication, centrifugation at 6000 rpm for 10 min was performed to remove insufficiently dispersed chains.2.3 Characterization
X-ray diffraction (XRD, D8 Advance, Bruker) was performed using Cu-Kα radiation (λ = 0.154 nm) at a scan rate of 5° min−1. Morphological and compositional analyses were conducted using field-emission scanning electron microscopy (FE-SEM, Hitachi, S-4300SE). Topographic analysis was performed in a tapping mode using a Park system with Si cantilevers (∼300 kHz resonant frequency) coated with Al (Tap300 Al, Budget Sensors Inc.) with a scan rate of ≈0.4 Hz. UV-visible absorbance was determined using a UV-vis spectrophotometer (Agilent Technologies Inc., Agilent 89090A).2.4 Device fabrication
Nb2Pd3Se8 nanowires exfoliated in 50% IPA were chosen for device fabrication. Nb2Pd3Se8 dispersion was spin-coated on a highly doped SiO2 (100 nm)/Si substrate. The device was patterned using the standard photolithography method, and the Cr/Au (5/50 nm) electrodes were deposited using an e-beam evaporator under high vacuum conditions.3. Results and discussion
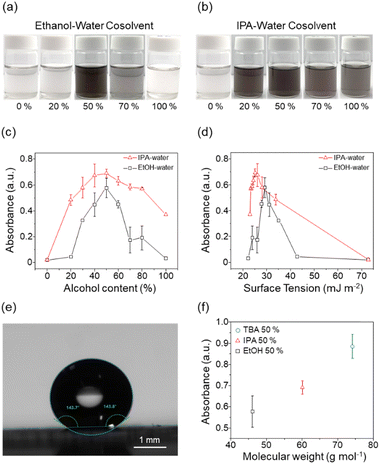
Nb2Pd3Se8 consists of the smallest unit ribbon structures forming in a zigzag shape (Fig. 1a). As studied in a previous report, the inter-ribbon forces exhibit a low binding energy as weak as that exhibited by vdW forces, allowing miniaturization through the exfoliation process.12 Bulk Nb2Pd3Se8 powder was synthesized through the solid-state reaction of elements at 600 °C, as described in previous studies.16 As shown in the SEM image, the bulk crystals are of several micrometers in size and consist of separable wire-like structures (Fig. 1b). The obtained powder was further characterized using X-ray diffraction and Raman spectroscopy, resulting in the well-matched diffraction pattern and vibration modes, respectively (Fig. 1c and d).Co-solvent exfoliation of Nb2Pd3Se8 was performed using nine different mixing volume ratios with varying alcohol content (Table 1). Nb2Pd3Se8 powder was immersed in each solvent with an initial concentration of 1 mg mL−1. Next, the starting solutions were ultrasonicated and centrifuged at 6000 rpm to obtain the resulting dispersions. Fig. 2a and b show images of the representative final Nb2Pd3Se8 dispersions processed in EtOH–water and IPA–water. When using water (0%) and ethanol (100%) as single solvents, successful exfoliation of Nb2Pd3Se8 was not observed. However, in the case of co-solvent, dark-colored dispersions were produced, indicating effective exfoliation. In the case of IPA–water, sufficient exfoliation was observed over a wider range of volume ratios compared to EtOH–water. The images for all solvent compositions are summarized in Fig. S1.† To compare the concentration of Nb2Pd3Se8 nanowires in each solvent, UV-visible absorbance was performed for all test solvents (Fig. S2 and S3†). The concentrations of the dispersions were evaluated based on the measured absorbance at a wavelength of 400 nm (Fig. 2c). Overall, the IPA–water system revealed higher dispersibility than EtOH–water. To investigate the dispersion behavior of Nb2Pd3Se8, the absorbance results were plotted as a function of the surface tension of solvents used (Fig. 2d). First, as described earlier, there was no exfoliation in water (72.75 mJ m−2). Then, as EtOH or IPA was introduced into the solvent system, a peak in the exfoliation yield started to appear. Notably, an upturn in the absorbance curve was observed for EtOH–water at 30% (35.0 mJ m−2), whereas IPA–water showed high exfoliation yield even at 20% (33.87 mJ m−2), indicating a higher amount of EtOH is required to reach a critical point compared to IPA. This difference would be explained by the variation in steric repulsion between EtOH and IPA due to their molecular structures.18 During exfoliation, the hydrophobic –CH3 groups in EtOH and IPA face the hydrophobic surface of Nb2Pd3Se8 (Fig. 2e), while their hydrophilic –OH groups interact with surrounding water. Thus, IPA, with more –CH3 groups and a higher molecular weight, could exert a greater driving force for exfoliation and stabilization compared to EtOH.18 To further evaluate the effect of molecular weight of the co-solvent, tert-butyl alcohol (TBA) was tested as a co-solvent, which has higher molecular weight with the extra hydrophobic –CH3 groups compared to EtOH and IPA (Fig. S4†). Exfoliation of Nb2Pd3Se8 in a 50% TBA–water showed a very high exfoliation efficiency with a linear trend in the measured absorbance with respect to the molecular weights of alcohols in the co-solvent (Fig. 2f and S5†). Overall, Nb2Pd3Se8 exhibited high dispersibility within a surface tension range of 23.4–35.0 mJ m−2 and a dielectric constant range of 30.3–63.4.
| IPA% | Surface tension (mJ m−2) | Dielectric constant | EtOH% | Surface tension (mJ m−2) | Dielectric constant |
|---|---|---|---|---|---|
| 0 | 72.75 | 80.10 | 0 | 72.75 | 80.1 |
| 20 | 33.87 | 67.66 | 20 | 42.9 | 68.94 |
| 30 | 28.08 | 61.44 | 30 | 35.0 | 63.36 |
| 40 | 26.16 | 55.22 | 40 | 31.1 | 57.78 |
| 50 | 25.13 | 49.00 | 50 | 29.2 | 52.20 |
| 60 | 24.43 | 42.78 | 60 | 27.9 | 46.62 |
| 70 | 23.78 | 36.56 | 70 | 26.0 | 41.04 |
| 80 | 23.39 | 30.34 | 80 | 24.0 | 35.46 |
| 100 | 23.00 | 17.90 | 100 | 21.82 | 24.3 |
We compared the efficiency of the co-solvent approach with previous LPE results in NMP. The concentrations of the dispersions obtained through the same exfoliation process was confirmed using UV-vis absorbance measurements (Fig. 3a). It was observed that both IPA–water and EtOH–water can achieve dispersion levels comparable to NMP in terms of concentration. Subsequently, three dispersions were vacuum filtered onto anodized aluminum oxide membranes to examine the morphology of the exfoliated Nb2Pd3Se8 nanowires. The widths of Nb2Pd3Se8 nanowires in each solvent were measured, and results were organized into a histogram (Fig. 3b). Similar to the absorbance trend, 50% IPA showed the smallest average width of 21.0 ± 5.4 nm, while 50% EtOH produced relatively thicker nanowires of 30.4 ± 7.9 nm. This can be attributed to the larger steric repulsion effect provided by greater molecular weight of IPA, promoting more sufficient exfoliation and preventing restack.18Fig. 3c shows the SEM images of the Nb2Pd3Se8 nanowires exfoliated in the representative co-solvent compositions. The composition with the highest measured absorbance exhibited the smallest nanowire width (Fig. S6†). To evaluate the dispersion stability based on co-solvent compositions, the absorbance intensities of four Nb2Pd3Se8 dispersions, processed in 50% IPA, 70% IPA, 50% EtOH and 80% EtOH, were monitored over a seven-day period (Fig. S7†). Solvents with higher exfoliation efficiency and absorbance tended to exhibit a smaller reduction in the measured absorbance after seven days. Dispersion stability appears to be significantly influenced by the size of Nb2Pd3Se8 nanowires, which depends on exfoliation efficiency and enhances the solvation effect of solvent molecules.
Finally, FET devices using Nb2Pd3Se8 nanowires were fabricated to evaluate the effectiveness of co-solvent exfoliation. Nb2Pd3Se8/50% IPA dispersion was spin-coated onto a 100 nm SiO2/Si substrate. Next, Cr/Au (5/50 nm) electrodes were deposited using standard photolithography and e-beam evaporation (Fig. 4a). We measured the transfer curve of the 13.3 nm-thick Nb2Pd3Se8 FET, displaying n-type transport behavior as confirmed through previous studies (Fig. 4b). Its electron mobility was calculated using the following equation:
| μ = (L/WCoxVDS)(dIDS/dVG), |
4. Conclusions
In conclusion, the co-solvent exfoliation of quasi-1D Nb2Pd3Se8 nanowires was successfully demonstrated using IPA–water and EtOH–water. By testing various mixing volume ratios of the co-solvent, we identified the surface tension range in which efficient exfoliation takes place. The optimal composition of each system achieved high exfoliation efficiency, which was comparable to that of the traditional single solvent exfoliation in NMP. We believe that this approach could expand the range of applications owing to its low toxicity and low range of boiling points.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
K. H. Choi and Y. Kim equally contributed to this work. K. H. Choi and Y. Kim were responsible for conceptualization, investigation, methodology, and writing – original draft. J. Kang, K. I. Kim, J. S. Park, and H.-S. Oh supported the analysis and investigation. H. K. Yu and J.-Y. Choi supervised the whole project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-00208311). In addition, this work was supported by the KIST Institutional Program (Project No. 2E31854-22-066) from the Korea Institute of Science and Technology.References
- J. Teeter, N. Y. Kim, T. Debnath, N. Sesing, T. Geremew, D. Wright, M. Chi, A. Z. Stieg, J. Miao and R. K. Lake, Adv. Mater., 2024, 2409898 CrossRef CAS PubMed.
- D. L. M. Cordova, Y. Zhou, G. M. Milligan, L. Cheng, T. Kerr, J. Ziller, R. Wu and M. Q. Arguilla, Adv. Mater., 2024, 2312597 CrossRef CAS PubMed.
- J. Klein, B. Pingault, M. Florian, M.-C. Heißenbüttel, A. Steinhoff, Z. Song, K. Torres, F. Dirnberger, J. B. Curtis and M. Weile, ACS Nano, 2023, 17, 5316–5328 CrossRef CAS PubMed.
- Y. Lee, Y. W. Choi, K. Lee, C. Song, P. Ercius, M. L. Cohen, K. Kim and A. Zettl, Adv. Mater., 2023, 35, 2307942 CrossRef CAS PubMed.
- Y. Meng, W. Wang and J. C. Ho, ACS Nano, 2022, 16, 13314–13322 CrossRef CAS PubMed.
- X. Liu, J. Liu, L. Y. Antipina, J. Hu, C. Yue, A. M. Sanchez, P. B. Sorokin, Z. Mao and J. Wei, Nano Lett., 2016, 16, 6188–6195 CrossRef CAS PubMed.
- X. Tan, Q. Li and D. Ren, Phys. Chem. Chem. Phys., 2023, 25, 2056–2062 RSC.
- J.-K. Qin, P.-Y. Liao, M. Si, S. Gao, G. Qiu, J. Jian, Q. Wang, S.-Q. Zhang, S. Huang and A. Charnas, Nat. Electron., 2020, 3, 141–147 CrossRef CAS.
- J. Kim, J. Lee, J. M. Lee, A. Facchetti, T. J. Marks and S. K. Park, Small Methods, 2024, 8, 2300246 CrossRef CAS PubMed.
- Y. Ma, H. Yi, H. Liang, W. Wang, Z. Zheng, J. Yao and G. Yang, Mater. Futures, 2024, 3, 012301 CrossRef CAS.
- L. Du, Y. Zhao, L. Wu, X. Hu, L. Yao, Y. Wang, X. Bai, Y. Dai, J. Qiao and M. G. Uddin, Nat. Commun., 2021, 12, 4822 CrossRef CAS PubMed.
- B. J. Jeong, K. H. Choi, J. Jeon, S. O. Yoon, Y. K. Chung, D. Sung, S. Chae, B. J. Kim, S. Oh and S. H. Lee, Adv. Funct. Mater., 2022, 32, 2108104 CrossRef CAS.
- B. J. Jeong, B. Lee, K. H. Choi, D. Sung, S. Ghods, J. Lee, J. Jeon, S. Cho, S. H. Lee and B. J. Kim, Nano Lett., 2023, 23, 6269–6275 CrossRef CAS PubMed.
- S. H. Lee, B. J. Jeong, K. H. Choi, J. Jeon, B. Lee, S. Cho, D. Kim, G. T. Gudena, D. D. Megersa and S. H. Kim, CrystEngComm, 2024, 26, 4541–4550 RSC.
- Q. Qin, W. Gao, H. Zhang, J. Chen, Y. Yan, K. Zhu, M. Long, G. Li, S. Yin and Y. Du, J. Mater. Chem. A, 2023, 11, 11517–11525 RSC.
- K. H. Choi, J. Jeon, B. J. Jeong, S. Chae, S. Oh, C. Woo, T. Y. Kim, J. Ahn, J. H. Lee and H. K. Yu, Adv. Mater. Interfaces, 2022, 9, 2200620 CrossRef CAS.
- J. Shen, J. Wu, M. Wang, P. Dong, J. Xu, X. Li, X. Zhang, J. Yuan, X. Wang and M. Ye, Small, 2016, 12, 2741–2749 CrossRef CAS PubMed.
- U. Halim, C. R. Zheng, Y. Chen, Z. Lin, S. Jiang, R. Cheng, Y. Huang and X. Duan, Nat. Commun., 2013, 4, 2213 CrossRef PubMed.
- Y. Sui and J. Appenzeller, Nano Lett., 2009, 9, 2973–2977 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ce01136h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |