An unsymmetrically sandwiched bis(O3S2-macrocycle) lead(II) complex via an endo/exo-coordination mode†
Seulgi
Kim
 *,
Kyunghye
Ju
*,
Kyunghye
Ju
 ,
Taehun
Kim
,
Taehun
Kim
 and
In-Hyeok
Park
and
In-Hyeok
Park
 *
*
Graduate School of Analytical Science and Technology, Chungnam National University, Daejeon 34134, South Korea. E-mail: sgkim7495@gmail.com; ipark@cnu.ac.kr
First published on 3rd December 2024
Abstract
An unsymmetrically sandwiched complex was isolated from the reaction of O3S2-macrocycle with lead(II) perchlorate. The lead(II) centre is bound to one macrocycle in an endocyclic (face) mode and the other in an exocyclic (edge) mode, resulting in a di-capped trigonal prismatic geometry. The edge-to-face sandwich complex might be associated with the hemispherical lead(II) coordination.
Metal–ligand interactions play a crucial role in the design and functional control of metal complexes, metal–organic frameworks (MOFs), and various macromolecular compounds.1–11 The precise regulation of such interactions can impart unique chemical properties and reactivities, making crown ethers and their derivatives notable for their ability to selectively bind metal ions.12,13 Crown ethers have attracted much attention due to the preferential endocyclic (metal-in-cavity) complexation with alkali and alkali earth metal ions in a good fit mode.14 Sometimes, crown ether-type macrocycles form unusual stoichiometric complexes including sandwich types with a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (metal-to-ligand) ratio, club sandwiches (2
2 (metal-to-ligand) ratio, club sandwiches (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and higher-ordered ones.15–23 In general, sandwich-type complexes are formed when the cavity is relatively small to fully accommodate the metal ion.
3) and higher-ordered ones.15–23 In general, sandwich-type complexes are formed when the cavity is relatively small to fully accommodate the metal ion.
On the other hand, various modifications of crown ethers including donor atoms allow new reactivities and functionalities.24–26 Thiacrowns (or thiamacrocycles), for example, form not only endocyclic complexes but also exocyclic (a metal ion binds outside of the cavity) coordination products due to the preferential exo-orientation of sulfur donors.27–32 Interestingly, the exo-coordination between sulfur donors (soft base) and thiaphilic metal ions (soft acid)33 so often provides an opportunity to construct diverse types of metallosupramolecules from monomers and cyclic oligomers to polymers as reviewed by the Lee group.34–36 In addition, some controllable endo- and exo-coordination modes37,38 and their applications in preparing CunIn (n = 2 or 4) clusters,39 photochemical sensors40–43 and chiral inversion44 have been reported.
Considering the contribution of the exo-coordination, the sandwich-type macrocyclic complexes could be extended to three types (A–C in Scheme 1, see Fig. S1†) depending on the combination of the endo- (face) and exo-coordination (edge). Undoubtedly, type A (face-to-face) is the most common because large alkali metal ions tend to form this symmetrical sandwich.15–23 Types B and C involve the edge mode, which appears in the exo-coordinated soft metal complexes of thiamacrocycles.34–38 In particular, type C (edge-to-face) complexes are unsymmetrical because one macrocycle has most of the donors coordinated in the same plane, while one part of the donors in the other macrocycle participates in the coordination environment. Thus, isolating type C complexes is challenging, especially in a one-pot reaction.
 | ||
| Scheme 1 Sandwich-type macrocyclic complexes with different coordination modes: (a) type A (face-to-face), (b) type B (edge-to-edge) and (c) type C (edge-to-face). | ||
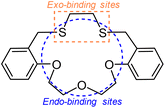
More recently, we have reported a 17-membered O3S2-macrocycle (L in Scheme 2) as one of the ditopic model ligands together with its interdonor (sulfur-to-sulfur) distance-dependent cis-palladium(II) complex and infinite one-dimensional copper(II) complex.45,46 Due to three consecutive oxygen donors, L has a hard base nature for endo-binding with hard or borderline metal ions (Scheme 2). Meanwhile, two sulfur donors could act as soft bases for the exo-binding sites with soft metal ions. In the present work, we employed the ligand L to gain a further understanding of non-soft metal complexations.
We have extended such sandwich-type complexes of macrocycles with hard and soft metal ions to borderline metal ions including lead(II) because their complexation behaviours are important in coordination chemistry, materials science and biological areas.47,48 Lead(II) may bind to both oxygen and sulfur donors in L with variable coordination numbers and unusual coordination geometries. Practically, plenty of hemidirected lead(II) complexes involving both Pb–O and Pb–S bonds have been reported so far.49–55 In this work, we have isolated an unsymmetrically sandwiched lead(II) complex of L with an edge-to-face mode (type C in Scheme 1) as the first non-soft metal species in this category. The details of our investigations are presented below.
The O3S2-macrocycle L was prepared as described previously.37 One-pot reaction of L in dichloromethane with Pb(ClO4)2·3H2O in acetonitrile afforded a colourless crystalline product 1 after slow evaporation at room temperature (yield 32%). X-ray analysis revealed that 1 crystallises in the monoclinic space group P21/n (Table S1†). Product 1 features a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (metal-to-ligand) complex of type [Pb(L)2(ClO4)]ClO4·0.5CH2Cl2·0.5CH3CN that involves one coordinated anion in the complex part (Fig. 1a). The asymmetric unit contains one formula unit.
2 (metal-to-ligand) complex of type [Pb(L)2(ClO4)]ClO4·0.5CH2Cl2·0.5CH3CN that involves one coordinated anion in the complex part (Fig. 1a). The asymmetric unit contains one formula unit.
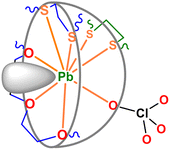
In 1, as shown in Fig. 1a, the complex part [Pb(L)2(ClO4)]+ comprises an unsymmetrical sandwich structure in which both endo-dentate (LA) and exo-dentate (LB) macrocycles are bonded to the lead(II) centre in an edge-to-face mode (type C in Scheme 1c). The lead(II) centre is eight-coordinated with one macrocycle (LA) bound to one side of the metal ion via the O3S2 donors (Pb1–S1 2.902(13), Pb1–S2 2.990(12), Pb1–O1 2.854(3), Pb1–O2 2.806(3), Pb1–O3 2.951(1) Å). Interestingly, the coordination sphere is completed by two exo-dentate sulfur donors (Pb1–S3 2.902(11), Pb1–S4 3.100(12) Å) from the other macrocycle LB in an edge mode and one perchlorate oxygen atom (Pb1–O8 2.781(9) Å).
The eight-coordinated geometry of 1 can be best described as a distorted di-capped trigonal prismatic geometry (Fig. 1b). The trigonal faces of the prism are defined by S1–S2–S3 and O1–O2–O8. One sulfur atom (S4) and one oxygen atom (O3) cap the corresponding rectangular faces (S1–S3–O8–O1 and S2–S3–O8–O2). Thus, the capping bond lengths of Pb1–S4 (3.100(12) Å) and Pb1–O3 (2.951(1) Å) are slightly longer than those of other Pb1–S (2.902(11)–2.990(12) Å) and Pb1–O (2.781(9)–2.854(3) Å) bonds, respectively. Similar to the Pb–S bonds (2.902 (11)–3.100(12) Å), the Pb–Oether bond lengths (2.806(3)–2.951(1) Å) fall in the longer part of the literature range for such bonds (2.5–2.9 Å).56 The elongated bond lengths seem to be caused by the steric hindrance between two macrocycles. Also, these are the typical signs of a hemidirected complex discussed in the latter part.49–55
Notably, the lead(II) centre is located 1.00 Å above the average plane of the O3S2 donors in LA with a perching manner (Fig. 1c). In general, the perching position in the sandwich-type macrocyclic complexes is mainly due to the larger size of metal ions compared to the macrocyclic cavity. In 1, however, the perching position of the lead(II) centre is mainly caused by the exo-coordination of the macrocycle LB that lifts the lead(II) centre. Unlike the face-to-face mode, which shields the metal centre effectively from anions and solvent molecules, the edge-to-face mode in 1 allows some open metal space between the two macrocycles (Fig. 1c and d). Due to this reason, one ClO4− ion participates to the coordination environment (Pb1–O8 2.781(9) Å), with another ClO4− ion remaining uncoordinated (Pb1⋯O14 3.243(6) Å) (Fig. 1a). The IR spectrum for 1 shows an intense ClO4− peak at 1045 cm−1 (Fig. S6†).
As mentioned, lead(II) often shows a hemidirected coordination sphere.49–55,57 Indeed, the eight-coordinated O4S4 coordination environment in 1 occupies only one hemisphere keeping the other hemisphere unoccupied except for the lone pair electron (Fig. 2). In our previous work, the assembly reactions of lead(II) and zinc(II) with a mixture of the rod-like 1,4-bis(4-pyridyl)piperazine and V-shaped 4,4′-sulfonyldibenzoic acid generate a polyrotaxane and a polycatenane, respectively.54 In this case, interestingly, the polyrotaxane formation is attributed to the hemisphere configuration of the lead(II) centre. The Penner-Hahn and Godwin groups reported the hemisphere coordination of lead(II) with thiol-rich peptides, which do not induce protein folding unlike the zinc(II)-analogue, as the origin of the lead poisoning.58 Considering the coordination numbers, elongated bond lengths, anion coordination and steric configuration, the formation of unsymmetrical sandwich-type complex 1 might be responsible for the hemispherical coordination of the lead(II) centre. To the best of our knowledge, this is the first example of unsymmetrically sandwiched macrocyclic complexes with non-soft metal ions. When the analogue product with the soft metal ion shown in Fig. S1c† is involved, this is the second example in the Type C category (Scheme 1c).
For the understanding of the complexation behaviours of L with lead(II) perchlorate in solution, we attempted NMR and ESI-mass experiments. In the 1H NMR titration, the addition of lead(II) (0–1.7 equiv.) to L induced all of the proton peaks to shift downfield, reflecting a stable complexation with an exchange rate being fast on the NMR time scale (Fig. 3a and S3†). The titration curves show that the magnitudes of the chemical shifts follow the order H4 > H3 ≫ H2 > H1 > Har (Fig. 3b). The larger shifts for H4 and H3 than those for H2 and H1 indicate the more favourable binding of lead(II) to sulfur donors than oxygens. Above 1.0 equiv., no significant chemical shifts were observed probably due to the predominant formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 product in this region.
1 product in this region.
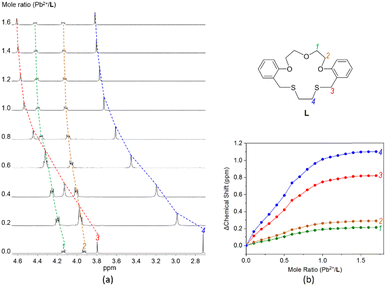 | ||
Fig. 3 (a) 1H NMR titration of L (1.0 × 10−3 M) with lead(II) perchlorate in CDCl3/CD3CN (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (b) titration curves for each proton in L. 1) and (b) titration curves for each proton in L. | ||
The stability constant of the complexation was obtained by HyperNMR software (Fig. S4†).59 This complexation could not be described using a mixed model of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios (metal-to-ligand). Instead, a good fit of the data with the 1
2 ratios (metal-to-ligand). Instead, a good fit of the data with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model yields the log
1 model yields the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K value of 5.9 ± (0.5), indicating the formation of a typical endocyclic mononuclear lead(II) complex, unlike the crystal structure. It is not surprising that the crystal structure differs from that in solution because of the solvation of the lead(II) with acetonitrile, a dipolar aprotic solvent.
K value of 5.9 ± (0.5), indicating the formation of a typical endocyclic mononuclear lead(II) complex, unlike the crystal structure. It is not surprising that the crystal structure differs from that in solution because of the solvation of the lead(II) with acetonitrile, a dipolar aprotic solvent.
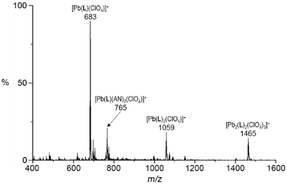
Alternatively, ESI-mass spectroscopy is quite sensitive in monitoring labile or less stable supramolecules assembled in solution. The ESI-mass spectrum of L with 1.0 equiv. of lead(II) perchlorate was dominated by peaks for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (metal-to-ligand) complexes such as [Pb(L)(ClO4)]+ (m/z 683) and [Pb(L)(AN)2(ClO4)]+ (m/z 765, AN = CH3CN) (Fig. 4). In the same spectrum, the formation of both 1
1 (metal-to-ligand) complexes such as [Pb(L)(ClO4)]+ (m/z 683) and [Pb(L)(AN)2(ClO4)]+ (m/z 765, AN = CH3CN) (Fig. 4). In the same spectrum, the formation of both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes is also confirmed by the peaks for [Pb(L)2(ClO4)]+ (m/z 1059) and [Pb2(L)2(ClO4)3]+ (m/z 1465), respectively. This result indicates that the 1
2 complexes is also confirmed by the peaks for [Pb(L)2(ClO4)]+ (m/z 1059) and [Pb2(L)2(ClO4)3]+ (m/z 1465), respectively. This result indicates that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric species is less stable than in the solid state probably due to the influence of the solvation.
2 stoichiometric species is less stable than in the solid state probably due to the influence of the solvation.
In summary, an unsymmetrically sandwiched 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (metal-to-ligand) complex with an edge-to-face mode via exo/endo-coordination was isolated from the reaction of a ditopic O3S2-macrocycle L with lead(II) perchlorate. In its crystal structure, the lead(II) centre in a hemisphere configuration adopts an eight-coordinated di-capped trigonal prismatic geometry with the elongated Pb–O and Pb–S bond distances matched to satisfy the steric and electronic conditions required. In solution, the mononuclear bis(macrocycle) product was traced by ESI-mass spectroscopy but such evidence was not observed in the 1H NMR titration probably due to the solvation effect. The knowledge of such a sandwich-type complex is expected to provide insight into the lead(II) coordination found in biosystems and materials.
2 (metal-to-ligand) complex with an edge-to-face mode via exo/endo-coordination was isolated from the reaction of a ditopic O3S2-macrocycle L with lead(II) perchlorate. In its crystal structure, the lead(II) centre in a hemisphere configuration adopts an eight-coordinated di-capped trigonal prismatic geometry with the elongated Pb–O and Pb–S bond distances matched to satisfy the steric and electronic conditions required. In solution, the mononuclear bis(macrocycle) product was traced by ESI-mass spectroscopy but such evidence was not observed in the 1H NMR titration probably due to the solvation effect. The knowledge of such a sandwich-type complex is expected to provide insight into the lead(II) coordination found in biosystems and materials.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NRF of Korea (2021R1C1C1006765, 2022R1A4A1022252 and RS-2023-00245420), COMPA (2024-24020010-11r, R&D Equipment Engineer Education Program), and the research fund of Chungnam National University.References
- G. Chakraborty, I.-H. Park, R. Medishetty and J. J. Vittal, Chem. Rev., 2021, 121, 3751–3891 CrossRef CAS PubMed.
- M. Kim, J. Yi, S.-H. Park and S. S. Park, Adv. Mater., 2023, 35, 2203791 CrossRef CAS PubMed.
- J. Park, A. Adhikary and H. R. Moon, Coord. Chem. Rev., 2023, 497, 215402 CrossRef CAS.
- C. Bae, M. Gu, Y. Jeon, D. Kim and J. Kim, Bull. Korean Chem. Soc., 2023, 44, 112–124 CrossRef CAS.
- B. B. Rath, D. Kottilil, W. Ji and J. J. Vittal, ACS Appl. Mater. Interfaces, 2023, 15, 26939–26945 CrossRef CAS PubMed.
- Z. Zheng, A. H. Alawadhi, S. Chheda, S. E. Neumann, N. Rampal, S. Liu, H. L. Nguyen, Y.-H. Lin, Z. Rong, J. I. Siepmann, L. Gagliardi, A. Anandkumar, C. Borgs, J. T. Chayes and O. M. Yaghi, J. Am. Chem. Soc., 2023, 145, 28284–28295 CrossRef CAS PubMed.
- J. Miao, W. Graham, J. Liu, E. C. Hill, L.-L. Ma, S. Ullah, H.-L. Xia, F.-A. Guo, T. Thonhauser, D. M. Proserpio, J. Li and H. Wang, J. Am. Chem. Soc., 2024, 146, 84–88 CrossRef CAS PubMed.
- I.-H. Choi, J.-M. Gu, H.-C. Kim, Y. Kim and S. Huh, Bull. Korean Chem. Soc., 2023, 44, 780–787 CrossRef CAS.
- A. H. Alawadhi, S. Chheda, G. D. Stroscio, Z. Rong, D. Kurandina, H. L. Nguyen, N. Rampal, Z. Zheng, L. Gagliardi and O. M. Yaghi, J. Am. Chem. Soc., 2024, 146, 2160–2166 CrossRef CAS PubMed.
- D. Kim, H. Yoo, K. Kim, D. Kim, K. T. Kim, C. Kim, J. Y. Kim, H. R. Moon and M. Kim, Chem. Commun., 2022, 58, 5948–5951 RSC.
- J. Kim, C. Na, Y. Son, M. Prabu and M. Yoon, Bull. Korean Chem. Soc., 2023, 44, 507–515 CrossRef CAS.
- I. Hwang, S.-Y. Huang, S. Smith, V. Lynch, R. Custelcean, B. A. Moyer, N. Kumar, V. S. Bryantsev and J. L. Sessler, J. Am. Chem. Soc., 2023, 145, 14387–14394 CrossRef CAS PubMed.
- C.-H. Wang, Y.-C. Lin, S. Bhunia, Y. Feng, P. Kundu, C. L. Stern, P.-L. Chen, J. F. Stoddart and M. Horie, J. Am. Chem. Soc., 2023, 145, 21378–21386 CrossRef CAS PubMed.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 2495–2496 CrossRef CAS.
- R. Rencsok, K. A. Jackson, T. A. Kaplan, J. F. Harrison and M. R. Pederson, Chem. Phys. Lett., 1996, 262, 207–212 CrossRef CAS.
- R. W. Saalfrank, N. Löw, S. Kareth, V. Seitz, F. Hampel, D. Stalke and M. Teichert, Angew. Chem., Int. Ed., 1998, 37, 172–175 CrossRef CAS.
- E. N. Ushakov, S. P. Gromov, O. A. Fedorova, Y. V. Pershina, M. V. Alfimov, F. Barigelletti, L. Flamigni and V. Balzani, J. Phys. Chem. A, 1999, 103, 11188–11193 CrossRef CAS.
- J. Kim, M. Shamsipur, S. Z. Huang, R. H. Huang and J. L. Dye, J. Phys. Chem. A, 1999, 103, 5615–5620 CrossRef CAS.
- J. W. Steed, Coord. Chem. Rev., 2001, 215, 171–221 CrossRef CAS.
- S. P. Gromov, A. I. Vedernikov, N. A. Lobova, L. G. Kuz'mina, S. S. Basok, Y. A. Strelenko, M. V. Alfimov and J. A. K. Howard, New J. Chem., 2011, 35, 724–737 RSC.
- A. Bey, O. Dreyer and V. Abetz, Phys. Chem. Chem. Phys., 2017, 19, 15924–15932 RSC.
- H.-R. Yu, J.-Q. Hu, X.-H. Lu, X.-J. Ju, Z. Liu, R. Xie, W. Wang and L.-Y. Chu, J. Phys. Chem. B, 2015, 119, 1696–1705 CrossRef CAS PubMed.
- I. Oral and V. Abetz, Soft Matter, 2022, 18, 934–937 RSC.
- R. M. Izatt, J. S. Bradshaw, S. A. Nielsen, J. D. Lamb, J. J. Christensen and D. Sen, Chem. Rev., 1985, 85, 271–339 CrossRef CAS.
- L. F. Lindoy, The Chemistry of Macrocyclic Ligand Complexes, Cambridge University Press, Cambridge, 1989 Search PubMed.
- G. W. Gokel, Crown Ethers and Cryptands, The Royal Society of Chemistry, 1991 Search PubMed.
- R. E. Wolf, Jr., J. R. Hartman, J. M. E. Storey, B. M. Foxman and S. R. Cooper, J. Am. Chem. Soc., 1987, 109, 4328–4335 CrossRef.
- G. H. Robinson and S. A. Sangokoya, J. Am. Chem. Soc., 1988, 110, 1494–1497 CrossRef CAS.
- J. Buter, R. M. Kellogg and F. van Bolhuis, J. Chem. Soc., Chem. Commun., 1991, 910–912, 10.1039/C39910000910.
- A. J. Blake, G. Reid and M. Schröder, J. Chem. Soc., Chem. Commun., 1992, 1074–1076, 10.1039/C39920001074.
- Y. Jin, I. Yoon, J. Seo, J.-E. Lee, S.-T. Moon, J. Kim, S. W. Han, K.-M. Park, L. F. Lindoy and S. S. Lee, Dalton Trans., 2005, 788–796, 10.1039/B415794J.
- M. Heller, Z. Anorg. Allg. Chem., 2006, 632, 441–444 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- S. Park, S. Y. Lee, K.-M. Park and S. S. Lee, Acc. Chem. Res., 2012, 45, 391–403 CrossRef CAS PubMed.
- E. Lee, S. Y. Lee, L. F. Lindoy and S. S. Lee, Coord. Chem. Rev., 2013, 257, 3125–3138 CrossRef CAS.
- S. Kim, L. F. Lindoy and S. S. Lee, Coord. Chem. Rev., 2014, 280, 176–202 CrossRef CAS.
- H. J. Kim and S. S. Lee, Inorg. Chem., 2008, 47, 10807–10809 CrossRef CAS PubMed.
- E. Lee and S. S. Lee, Inorg. Chem., 2011, 50, 5803–5807 CrossRef CAS PubMed.
- S. Kim, A. D. Siewe, E. Lee, H. Ju, I.-H. Park, K.-M. Park, M. Ikeda, Y. Habata and S. S. Lee, Inorg. Chem., 2016, 55, 2018–2022 CrossRef CAS PubMed.
- S. J. Lee, J. E. Lee, J. Seo, I. Y. Jeong, S. S. Lee and J. H. Jung, Adv. Funct. Mater., 2007, 17, 3441–3446 CrossRef CAS.
- C. S. Park, J. Y. Lee, E.-J. Kang, J.-E. Lee and S. S. Lee, Tetrahedron Lett., 2009, 50, 671–675 CrossRef CAS.
- H. Lee and S. S. Lee, Org. Lett., 2009, 11, 1393–1396 CrossRef CAS PubMed.
- E. Lee, H. Ju, I.-H. Park, S. Park, M. Ikeda, Y. Habata and S. S. Lee, Analyst, 2020, 145, 1667–1676 RSC.
- E. Lee, H. Ju, I.-H. Park, J. H. Jung, M. Ikeda, S. Kuwahara, Y. Habata and S. S. Lee, J. Am. Chem. Soc., 2018, 140, 9669–9677 CrossRef CAS PubMed.
- S. Kim, H. Ryu, J. K. Clegg, L. F. Lindoy and S. S. Lee, Inorg. Chem., 2020, 59, 15807–15812 CrossRef CAS PubMed.
- S. Kim, I.-H. Park, H.-B. Choi, H. Ju, E. Lee, T. S. Herng, J. Ding, J. H. Jung and S. S. Lee, Dalton Trans., 2020, 49, 1365–1369 RSC.
- R. L. Davidovich, V. Stavila, D. V. Marinin, E. I. Voit and K. H. Whitmire, Coord. Chem. Rev., 2009, 253, 1316–1352 CrossRef CAS.
- R. L. Davidovich, V. Stavila and K. H. Whitmire, Coord. Chem. Rev., 2010, 254, 2193–2226 CrossRef CAS.
- R. Luckay, I. Cukrowski, J. Mashishi, J. H. Reibenspies, A. H. Bond, R. D. Rogers and R. D. Hancock, J. Chem. Soc., Dalton Trans., 1997, 901–908, 10.1039/A605068I.
- L. Shimoni-Livny, J. P. Glusker and C. W. Bock, Inorg. Chem., 1998, 37, 1853–1867 CrossRef CAS.
- M. L. Golden, J. H. Reibenspies and M. Y. Darensbourg, Inorg. Chem., 2004, 43, 5798–5800 CrossRef CAS PubMed.
- I. Persson, K. Lyczko, D. Lundberg, L. Eriksson and A. Płaczek, Inorg. Chem., 2011, 50, 1058–1072 CrossRef CAS PubMed.
- X. X. Wu, B. Ding, J. H. Li, P. Yang, Y. Wang and G. X. Du, Inorg. Chem. Commun., 2013, 33, 170–174 CrossRef CAS.
- H. Ju, E. Lee, S. Kim, I.-H. Park, J.-H. Lee and S. S. Lee, CrystEngComm, 2016, 18, 2621–2625 RSC.
- S. Mirdya, S. Roy, S. Chatterjee, A. Bauzá, A. Frontera and S. Chattopadhyay, Cryst. Growth Des., 2019, 19, 5869–5881 CrossRef CAS.
- Y.-M. Yang, C. Feng, Y.-H. Jiang, D.-H. Du, H. Zhao, G.-N. Zhang and Y.-C. Wang, Transition Met. Chem., 2023, 48, 55–61 CrossRef CAS.
- G. K. Kole, A. M. P. Peedikakkal, B. M. F. Toh and J. J. Vittal, Chem. – Eur. J., 2013, 19, 3962–3968 CrossRef CAS PubMed.
- J. S. Magyar, T.-C. Weng, C. M. Stern, D. F. Dye, B. W. Rous, J. C. Payne, B. M. Bridgewater, A. Mijovilovich, G. Parkin, J. M. Zaleski, J. E. Penner-Hahn and H. A. Godwin, J. Am. Chem. Soc., 2005, 127, 9495–9505 CrossRef CAS PubMed.
- https://www.hyperquad.co.uk/hypnmr.htm .
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, characterization, NMR titration, and crystal structure data. CCDC 2389314 (1). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ce01045k |
| This journal is © The Royal Society of Chemistry 2025 |