Open Access Article
Open Access ArticleDivergent late stage functionalisation of luminescent europium(III) complexes for targeting and imaging applications
Tsz-Lam
Cheung
 ,
Carlson
Alexander
,
Carlson
Alexander
 ,
Huishan
Li
,
Huishan
Li
 and
David
Parker
and
David
Parker
 *
*
Department of Chemistry, Hong Kong Baptist University, Kowloon Tong, 999077, Hong Kong SAR, China. E-mail: davidparker@hkbu.edu.hk
First published on 2nd October 2025
Abstract
An efficient approach to six functionalised europium(III) complexes based on a common alkyne intermediate is exemplified by syntheses with morpholino and triphenylphosphonium moieties exhibiting a dominant lysosomal localisation profile, two dye conjugates showing intramolecular energy transfer, and two competitive antagonists, including vismodegib that binds to a G-protein coupled receptor in the Hedgehog signalling pathway and masupirdine, designed to target the serotonin GPCR.
Luminescent Eu(III) complexes have found a wide range of applications in sensing and bio-imaging, notably in the creation of time-resolved luminescence bioassays and the development of environmentally responsive probes.1–4 Key aspects of probe design include the need for the desired complexes to be kinetically stable and amenable to excitation in the range from 337 to 375 nm in an efficient sensitisation process. No less important is that the synthetic pathway should be versatile and allow the introduction of functionality in the last step, e.g. for the preparation of conjugates with targeting or directing groups.
Successful strategies have included the introduction of a 4-bromo-pyridine group into the ligand framework of the Eu complex, so that a large number of transformations can be undertaken by activation of the C–Br bond under Pd catalysis, as used by Charbonnière and Starck,5,6 in a related manner to early work with luminescent Ir systems by Williams.6 An alternative approach has been to introduce a 4-nitropyridine nucleofuge into the complex, wherein coordination of the Eu ion to the pyridine nitrogen atom activates ipso-nucleophilic attack under ambient conditions in water, allowing the displacement of the nitro group by various soft nucleophiles.6,7 Here, we use an alkyne group in the final ligand synthesis step, as it is readily transformed to another functionality by copper catalysed click reactions on the derived metal complex.8 As a further design consideration, we sought to create a nonadentate ligand that would lead to a charge neutral complex (with no quenching water coordinated to Eu), and based the strategy on the ubiquitous cyclen (12-N-4) macrocycle, with AB2C functionality around the four ring nitrogen atoms. To avert the problem of forming constitutional isomers, the ‘B’ substituents were chosen to be trans-related carboxymethyl groups.
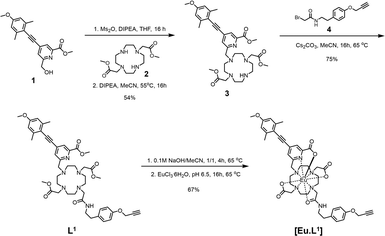
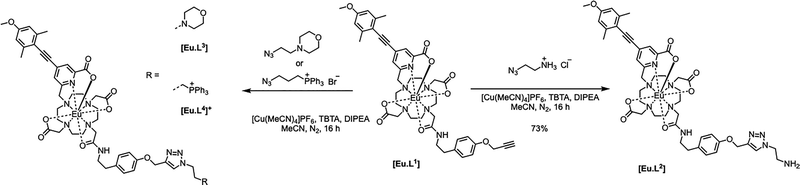
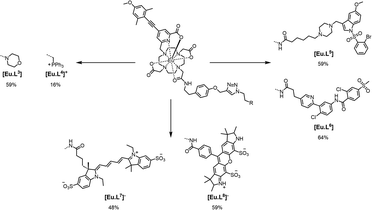
With this background in mind, we prepared a small family of Eu(III) ligands, using an established aryl-alkynyl-pyridylcarboxylate sensitiser, 1,1,9 starting from trans-1,7-bis(carboxymethyl)-1,4,7,10-tetraazacyclododecane, 2 (Scheme 1 and Scheme S1). Controlled alkylation of 2 with the mesylate ester of 1 in warm MeCN afforded the trisubstituted amine, 3. Subsequent alkylation with the α-bromo-amide, 4, gave L1 bearing a remote alkyne group that could be used in Cu(II) catalysed click reactions on its Eu complex, [Eu.L1] (Scheme 2 and Schemes S2, S3), to form the modified complex directly, e.g. the morpholine derivative, [Eu.L3] and the phosphonium salt, [Eu.L4]+. Alternatively, reaction of [Eu.L1] with 2-azidoethylamine yielded the amine [Eu.L2], which was converted to the amides, [Eu.L5–8] (Scheme 3), using standard peptide coupling methods. The morpholine and phosphonium groups were selected to test the commonly accepted strategy that such simple groups can be used to direct intracellular localisation. In the former case, the rationale is observational and empirical,10 and in the latter case it has been hypothesised that lipophilic and cationic phosphonium salts favour intracellular probe localisation to mitochondria, simply because of attraction to the negative surface potential of the mitochondrial outer membrane.11
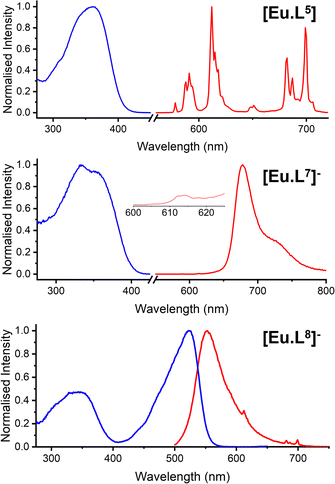
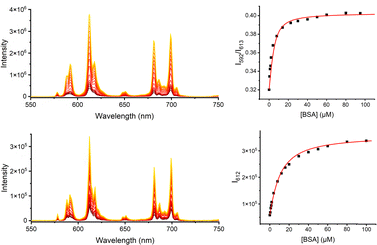
Two other targeting vectors were introduced, ([Eu.L5] and [Eu.L6]), based on competitive antagonists for important G-protein coupled receptors (GPCRs). Masupirdine (SUVN 502; Scheme S4)12 is a potent, selective 5-HT6 (serotonin) receptor antagonist (Ki = 2 nM for the human 5-HT6 GPCR), with pro-cognitive properties in mammalian models of cognition. It is being evaluated for the treatment of agitation in Alzheimer's disease. The piperazine ring, with a pKa value of 8.4, is protonated at physiological pH. In [Eu.L6], the Eu complex is coupled to vismodegib,13 a compound that inhibits the Hedgehog signalling pathway (IC50 = 3 nM) and is used clinically to treat patients with basal-cell carcinoma and medulloblastoma. It binds selectively to the GPCR, smoothened (SMO), a transmembrane receptor considered to be essential for embryonic development and tissue homeostasis.14,15 Finally, the Eu complex was linked to two emissive visible dyes. The cyanine dye, Dy647-CO2H, was coupled to [Eu.L2] to afford [Eu.L7]−, in which very efficient intramolecular FRET was expected to occur from the Eu donor to the red-emitting dye acceptor.16 Indeed, following excitation at 375 nm into the sensitising chromophore, intense dye emission was observed at 670 nm, in which two components were observed (Fig. S31): one due to direct dye fluorescence with a lifetime of 1.3 ns; the other, with a lifetime of 0.18 ms, echoed the lifetime of the Eu donor. Only very weak Eu emission was discernible at 612 nm (Fig. 1 centre, Table 1). In contrast, with the unmatched donor–acceptor pair in [Eu.L8]− (sometimes termed an ‘off resonance’ or ‘non-overlapping’ FRET pair),17 excitation at 375 nm (a wavelength used in confocal microscopy, at which both the dye and the sensitising group absorb) led to the observation of both dye fluorescence at 550 nm and Eu luminescence, in which emission associated with the 5D0 to 7F4 transition manifold around 700 nm was most apparent, clear of the tail of the broad dye emission band (Fig. 1 lower).
| λ max [nm] | ε [M−1 cm−1] | ϕ H2O [%] | B [M−1 cm−1] | τ H2O(τD2O)b [ms] | q | |
|---|---|---|---|---|---|---|
| a EuPh3dpqC(CF3SO3)3 was used as the standard, with excitation at 348 nm (293 K). b The experimental errors for quantum yield and lifetime measurements are ±10%. c In each of these systems, the lifetime and intensity of Eu and dye emission are temperature dependent, owing to both thermally activated back energy transfer from the Eu 5D1 and 5D0 states to the intermediate ICT triplet of the alkynyl chromophore and changes in medium viscosity; further details will be reported later.9b | ||||||
| [Eu.L3] | 347 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.3 | 160 | 0.60 (0.78) | 0.1 |
| [Eu.L4]+ | 348 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.9 | 140 | 0.57 (0.73) | 0.1 |
| [Eu.L5] | 360 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.6 | 140 | 0.57 (0.73) | 0.1 |
| [Eu.L6] | 366 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
3.8 | 1100 | 0.81 (1.06) | 0 |
| [Eu.L7]− | 349 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
n.a. | n.a. | 0.18 (0.23) | n.a. |
| 650 | 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
|||||
| [Eu.L8]− | 346 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
n.a. | n.a. | 0.45 (0.58) | n.a. |
| 524 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
|||||
Binding constants for the association of each complex with bovine serum albumin (BSA) were determined by spectral titrations (Fig. 2), with the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K values estimated to be 5.7, 5.7, 6.1, 5.1, and 4.9 for [Eu.L3–6] and [Eu.L8], respectively, assuming a dominant 1
K values estimated to be 5.7, 5.7, 6.1, 5.1, and 4.9 for [Eu.L3–6] and [Eu.L8], respectively, assuming a dominant 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry in accordance with predominant binding of the hydrophobic aromatic moiety to one high affinity site. With [Eu.L7]− no significant variation in emission intensity was observed up to the addition of 0.1 mM protein. However, a large change in Eu emission intensity was observed in every other case, with intensity increases of 6 to 10 times that observed in buffer at pH 7.4 and, with [Eu.L8]−, the increase in Eu emission at 699 nm following BSA binding was much greater than the dye emission increase at 550 nm (Fig. 2). Significant variations of emission spectral form were not seen, nor did the temperature dependence of Eu emission (293 to 318 K) change much in the protein-bound state compared to the free complex. Such behaviour is suggestive of the occurrence of aggregation of the individual Eu complexes via inclusion of the pendant chromophore to the serum albumin binding site,18 even at the micromolar concentrations used. In the protein bound form, the enhanced emission may simply be associated with de-aggregation of the complex and a reduced sensitivity to vibrational relaxation.
1 stoichiometry in accordance with predominant binding of the hydrophobic aromatic moiety to one high affinity site. With [Eu.L7]− no significant variation in emission intensity was observed up to the addition of 0.1 mM protein. However, a large change in Eu emission intensity was observed in every other case, with intensity increases of 6 to 10 times that observed in buffer at pH 7.4 and, with [Eu.L8]−, the increase in Eu emission at 699 nm following BSA binding was much greater than the dye emission increase at 550 nm (Fig. 2). Significant variations of emission spectral form were not seen, nor did the temperature dependence of Eu emission (293 to 318 K) change much in the protein-bound state compared to the free complex. Such behaviour is suggestive of the occurrence of aggregation of the individual Eu complexes via inclusion of the pendant chromophore to the serum albumin binding site,18 even at the micromolar concentrations used. In the protein bound form, the enhanced emission may simply be associated with de-aggregation of the complex and a reduced sensitivity to vibrational relaxation.
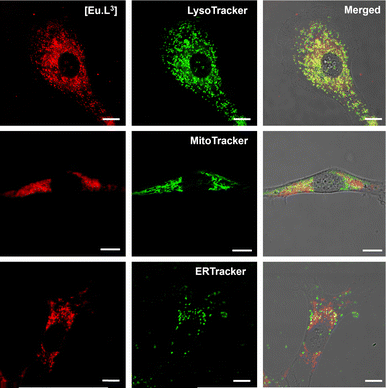
The cellular localisation profiles of each complex were examined in preliminary studies with NIH-3T3 or A-549 cells, after a 4 to 8 h incubation time. With [Eu.L3] (over 90% protonated at pH 7.4) and [Eu.L4]+, MTT assays (24 h, Fig. S32) showed only moderate toxicity (IC50 values of 50(3) μM and >60 μM, respectively), with a predominant lysosomal uptake observed, and no significant selective uptake in the mitochondria or endoplasmic reticulum (Fig. 3). Each of the dye conjugates exhibited very spotty confocal microscope images, suggestive of the presence of a precipitated or aggregated complex, while in preliminary cell uptake studies, the vismodegib complex, [Eu.L8] led to cell death in both cell lines examined. Further imaging studies are underway, in which the Eu complex will be modified to create overall negative charge in this moiety, in order to suppress non-specific binding1c and the tendency to aggregate.
A versatile strategy has allowed the expeditious synthesis of a set of six emissive europium(III) probes of interest for sensing and cell imaging applications.7 By gaining control over the selective functionalisation of the cyclen ring, an efficient pathway to AB2C substituted 12-N-4 macrocyclic ligands has been devised, in which the side arm containing a monosubstituted alkyne is introduced in the final ligand synthesis step, in contrast to related approaches.8 The complex, [Eu.L1] serves as a common intermediate for the preparation of six emissive complexes. The introduction of a directing or targeting moiety or conjugation with a matched FRET or nFRET fluorescent acceptor is made in the last synthetic transformation of the Eu complex.
Mouse embryo NIH3T3 (ATCC®CRL-1658™) fibroblasts were purchased from ATCC (Manassas, VA). Human lung cancer A549 cells were obtained from the Cell Culture Bank of the Chinese Academy of Sciences’ Type Culture Collection Committee (Shanghai).
The manuscript was written by DP and TLC; TLC carried out the ligand and complex synthesis and characterisation work and the measurements of binding and rate constants at steady state; CA performed the synthesis of the masupirdine intermediate in [Eu.L5], and HL performed the cell imaging and toxicity studies.
D. P. thanks the Hong Kong Jockey Club Charities Trust for equipment support and the Hong Kong Government and Hong Kong Baptist University for support under the Global STEM Professorship scheme.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental methods, synthetic schemes (S1–S6), procedures and characterisation, MTT assay data and lifetime measurements. See DOI: https://doi.org/10.1039/d5cc04784f.Notes and references
- (a) D. Parker, J. D. Fradgley and K.-L. Wong, Chem. Soc. Rev., 2021, 50, 8193–8213 CAS; (b) S. E. Bodman and S. J. Butler, Chem. Sci., 2021, 12, 2716–2734 RSC; (c) M. Delbianco, V. Sadovnikova, E. Bourrier, G. Mathis, L. Lamarque, J. M. Zwier and D. Parker, Angew. Chem., Int. Ed., 2014, 53, 10718–10722 CrossRef CAS PubMed; (d) S. J. Butler, M. Delbianco, L. Lamarque, B. K. McMahon, E. R. Neil, R. Pal, D. Parker, J. W. Walton and J. M. Zwier, Dalton Trans., 2015, 44, 4791–4803 RSC; (e) M. Soulie, F. Latzko, E. Bourrier, V. Placide, S. J. Butler, R. Pal, J. W. Walton, P. L. Baldeck, B. Le Guennic, C. Andraud, J. M. Zwier, L. Lamarque, O. Maury and D. Parker, Chem. – Eur. J., 2014, 20, 8636–8646 CrossRef CAS PubMed.
- (a) C. Alexander, Z. Guo, P. B. Glover, S. Faulkner and Z. Pikramenou, Chem. Rev., 2025, 125, 2269–2370 CrossRef CAS PubMed; (b) Z. Wang, Y. Huang, B. Song, Y. Shi and J. Yuan, Inorg. Chem., 2025, 64, 8685–8693 CrossRef PubMed; (c) S. E. Bodman, P. Stachelek, U. Rehman, F. Plasser, R. Pal and S. J. Butler, Chem. Sci., 2025, 16, 5602–5612 RSC; (d) P. Harvey, A. Nonat, C. Platas-Iglesias, L. S. Natrajan and L. Charbonnière, Angew. Chem., Int. Ed., 2018, 31, 9921–9924 CrossRef PubMed.
- A. Emami-Nemini, T. Roux, M. Leblay, E. Bourrier, L. Lamarque, E. Trinquet and M. J. Lohse, Nat. Protoc., 2013, 8, 1307–1320 CrossRef PubMed.
- D. E. Barry, J. A. Kitchen, K. Pandurangan, A. J. Saviasachi, R. D. Peacock and T. Gunnlaugsson, Inorg. Chem., 2020, 59, 2646–2650 CrossRef CAS PubMed.
- P. Kadjane, M. Starck, F. Carmarel, D. Hill, N. Hildebrandt, R. Ziessel and L. Charbonnière, Inorg. Chem., 2009, 48, 4601–4603 CrossRef CAS PubMed.
- (a) S. J. Butler, M. Delbianco, N. H. Evans, A. T. Frawley, R. Pal, D. Parker, R. S. Puckrin and D. S. Yufit, Dalton Trans., 2014, 43, 5721–5730 RSC; (b) V. L. Whittle and J. A. G. Williams, Dalton Trans., 2009, 3929–3940 RSC.
- M. Starck, J. D. Fradgley, D. F. De Rosa, A. S. Batsanov, M. Papa, M. J. Taylor, J. E. Lovett, J. C. Lutter, M. J. Allen and D. Parker, Chem. – Eur. J., 2021, 27, 17921–17927 CrossRef CAS PubMed.
- B. Chartier, N. Hamon, D. Akl, A. Sickinger, L. Corne, G. Micoucin, A. N. Banyasz, O. Maury, S. Erbek, V. Martel-Frachet, M. Beyler, O. Senèque and R. Tripier, Chem. – Eur. J., 2025, 31, e202502044 CrossRef PubMed , and references therein to earlier examples using an alkyne intermediate in the overall ligand synthesis.
- (a) D. F. De Rosa, M. Starck, R. Pal and D. Parker, Chem. – Eur. J., 2024, 30, e2023322 CrossRef PubMed; (b) T. L. Cheung, Z. Ju, W. Zhang, D. Parker and R. Deng, ACS Appl. Mater. Interfaces, 2024, 16, 43933–43941 CrossRef CAS PubMed.
- (a) H. Ma, B. Song, Y. Wang, C. Liu, X. Wang and J. Yuan, Dyes Pigm., 2017, 140, 407–416 CrossRef CAS; (b) D. K. Mai, I. W. Badan, J. M. Lim, T. P. Voles, C. Kim, J. Yang, J. Lee and H.-J. Kim, Dyes Pigm., 2023, 208, 110856 CrossRef.
- J. Zielanka, J. Joseph, A. Sikosa, M. Hardy, O. Quari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef PubMed.
- R. Nirogi, R. Abraham, J. Ravula, S. Jetta, V. Benade, A. K. Shinde, M. A. Rasheed, R. K. Badange, V. K. Goyal, V. R. C. Palacharia, R. Subramanian and P. Jayarajan, Alzheimer's Dementia, 2023, 19, e074068 CrossRef.
- A. Sekulic, M. R. Migden, A. E. Oro, L. Dirix, K. D. Lewis, J. D. Hainsworth, J. A. Solomon and A. Hauschild, New Engl. J. Med., 2012, 366, 2171–2179 CrossRef CAS PubMed.
- A. M. Arensdorf, S. Marada and S. K. Ogden, Trends Pharmacol. Sci., 2015, 37, 62–72 CrossRef PubMed.
- E. F. X. Byrne, R. Sircar, P. S. Miller, G. Hedger, G. Luchetti, S. Nachtergaele, M. D. Tully, L. Mydock-McGrane, D. F. Covey, R. P. Rambo, M. S. P. Sansom, S. Newstead, R. Rohatgi and C. Siebold, Nature, 2016, 535, 517–522 CAS.
- D. Parker, J. D. Fradgley, M. Delbianco, M. Starck, J. W. Walton and J. M. Zwier, Faraday Discuss., 2022, 234, 159–174 CAS.
- J. Vuojola, I. Hypannen, M. Nummela, J. Kankare and T. Soukka, J. Phys. Chem. B, 2011, 115, 13685–13694 CAS.
- H. Li, S. L.-W. Ng, D. J. Black, W. Han, R. Pal and D. Parker, Chem. Sci., 2025, 16, 14544–14552 CAS.
| This journal is © The Royal Society of Chemistry 2025 |