Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Accessing [6,6,5,6] tetracyclic indeno-quinolines via a photomediated cascade reaction of electron-rich 1,7-enynes†
Pooja
Yadav
,
A. Anagha
Varma
and
Purushothaman
Gopinath
 *
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER) Tirupati, Tirupati 517619, India. E-mail: gopi@iisertirupati.ac.in
First published on 8th September 2025
Abstract
Synthesis of various sulfonyl- and trifluoromethyl-decorated tetracyclic indeno-quinolines from 1,7-enynes via a cascade radical addition–cyclization–aromatization sequence is presented. The reaction was found to show broad substrate scope with good functional group tolerance on the sulfonyl and o-alkynyl aniline derivatives. The protocol was successfully extended for late-stage modification of various sulfonyl-containing drugs and other radical precursors to obtain the corresponding indeno-quinolines in good yields. Control experiments corroborated the proposed photomediated radical cascade mechanism.
Photoredox-mediated radical cascade reactions have shown tremendous potential in rapidly and effectively accessing various hetero- and carbo-cycles.1–6 In this regard, 1,n-enynes have emerged as versatile synthons for radical cascade addition–cyclization reactions to access many interesting molecular scaffolds.7–14 Although 1,n-enynes have been well studied, electron-deficient alkene12–15 partners have most commonly been used as the starting materials to control the chemo- and regioselectivity of their radical addition step. Conversely, employing electron-rich alkenes can give access to new and interesting scaffolds.
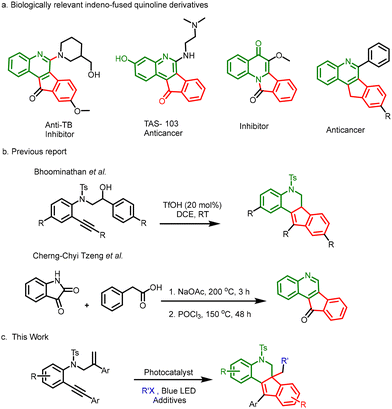
Indeno-fused quinolines play a crucial role in the drug and agrochemical industry (Scheme 1a).16 In general, synthesis of indeno-quinoline derivatives involves a tedious multiple-step process, due to the presence of a complex tetracyclic core (Scheme 1b).17,18 This multi-step process greatly limits the introduction of other functional groups or substituents onto the indeno-quinoline core, as they appear to not withstand the reaction conditions. In this regard, Bhoominathan et al.19 achieved the synthesis of indeno-quinolines at room temperature via a Lewis acid (LA)-catalysed reaction in a single step—but again, the method does not provide any prospect to introduce additional functional groups or substituents. Herein, we have developed a protocol involving the use of photoredox catalysis to access various functionalized indeno-quinolines from electron-rich 1,7-enynes via a cascade addition–cyclization-aromatization sequence (Scheme 1c).
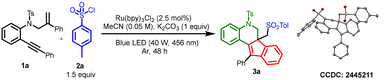
We started the screening with a 2-(phenylethynyl)-N-(2-phenylallyl)-aniline derivative, 1a, as a model substrate and tosyl chloride (TsCl) as the sulfonyl source. Using Ru(bpy)3Cl2 as the photocatalyst with K2CO3 as the base in acetonitrile as solvent under blue LED irradiation (wavelength of 456 nm, 40 W) for 48 h, we obtained an 83% yield of the desired tetracyclic indeno-quinoline derivative (entry 1, Table 1). Other solvents including methanol and EtOH did not furnish the desired product in good yields (entries 2–8, Table 1). Similarly, other bases and additives including Na3PO4, Na2HPO4, NaH2PO4, and NaHCO3 also gave lower yields of the desired product (entries 10 and 11, Table 1). When the reaction was carried out with organic bases such as triethylamine and pyridine, the yield of the desired product was reduced further. When the photocatalyst was changed to Eosin Y or Irppy3, we obtained 64% and 30% yields, respectively (entries 16 and 17, Table 1).
| Entry | Deviation from standard condition | Yield |
|---|---|---|
| 1 | None | 83% |
| 2 | MeOH, EtOH, Me-THF, toluene, DMF, EtOAc, i-PrOH | <35% |
| 3 | DCM | 50% |
| 4 | THF | 45% |
| 5 | Acetone | 40% |
| 6 | t-BuOH | 47% |
| 7 | CHCl3 | 67% |
| 8 | 1,4-Dioxane | 61% |
| 9 | 12 W | 30% |
| 10 | 16 W | 45% |
| 11 | 24 W | 65% |
| 12 | Na3PO4, Na2HPO4 | <70% |
| 13 | NaH2PO4, NaHCO3, KH2PO4, Na2CO3 | <50% |
| 14 | NEt3, pyridine | <50% |
| 15 | Without base | 45% |
| 16 | Irppy3 | 30% |
| 17 | Eosin Y | 65% |
| 18 | Ru(bpy)3Cl2 (1 mol%) | 65% |
| 19 | Ru(bpy)3Cl2 (5 mol%) | 45% |
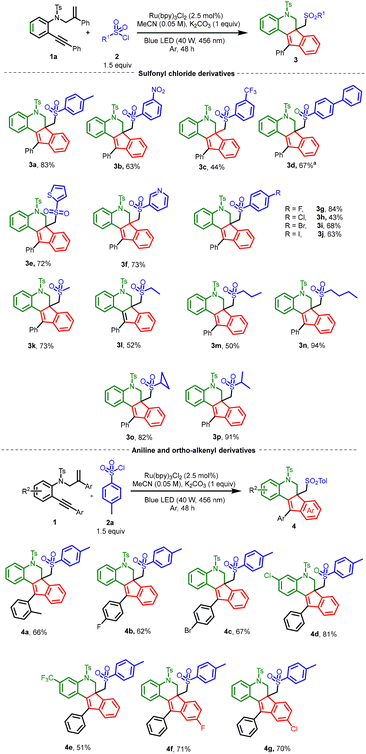
With the optimized conditions in hand, we next investigated the scope of sulfonyl chloride derivatives using ortho-alkynyl aniline derivative 1a as the standard (Scheme 2). Halogens such as F, Cl, Br, and I withstood the reaction conditions and afforded good yields of the expected products (3g–3j). 3-Nitro sulfonyl chloride gave (3b) a 63% yield. In addition, biphenyl sulfonyl chloride gave a 67% yield of the desired product (3d), but required 72 h for the reaction, due to its lower reactivity. Other sulfonyl chloride derivatives containing heterocyclic rings such as pyridine sulfonyl chloride and thiophene sulfonyl chloride were also promising substrates and afforded the resultant products in good yields (3e and 3f). Interestingly, alkyl sulfonyl chlorides gave moderate to good yields of the expected products (3m–3r). Next, we investigated the scope of o-alkynyl aniline derivatives with p-tosyl chloride 2a as the standard (Scheme 2). Having an ortho methyl substituent on the alkynyl phenyl ring afforded a 66% yield of product (4a). Similarly, halogen substituents on the o-alkynyl aniline derivatives also worked well and afforded the desired tetracyclic indeno-quinolines in moderate to good yields (4b–4g).
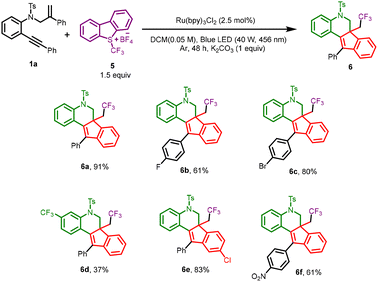
Next, we extended our method to access triflouromethylated indeno-quinolines, as fluoro substituents have been shown to enhance certain properties, such as metabolic stability, and lipophilicity, of pharmaceuticals and agrochemicals. (Scheme 3). Accordingly, substrate 1a and Umemoto reagent, 5 (1.5 equiv.) with Rubpy3Cl2 as photocatalyst and K2CO3 (1 equiv.) as base in DCM upon irradiation with blue LED for 48 h afforded a 91% yield of the desired product 6a. With the optimized conditions in hand, various o-alkynyl derivatives were investigated. Halogen substituents on the alkynyl and styrene ring worked well and gave good yields of the expected tetracyclic cores (6b, 6c, and 6e). Having an electron-withdrawing group, like a trifluoromethyl group or a nitro substituent, afforded moderate yields of the respective desired products (6d and 6f), due to their lower reactivity.
In order to demonstrate the synthetic versatility of the method, various sulfonyl-containing drug derivatives and bio-active molecules such as sulphonamide and valdecoxib were used for the transformation, and the corresponding indeno-quinoline-tethered drug derivatives were obtained in moderate to good yields. Similarly, the reaction was also performed on a gram scale, and the resultant product was obtained in 71% yield, showing the potential for large-scale applications. Next, the reaction was performed with diethyl (bromodifluoromethyl) phosphonate using Irppy3 as the photocatalyst; interestingly, the desired indeno-quinoline phosphonate ester, 8a, was obtained in 73% yield (Scheme 4).
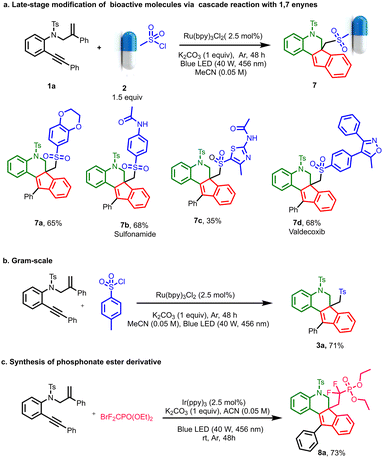 | ||
| Scheme 4 (a) Late stage modification of bioactive molecules via cascade reaction with 1,7-enynes. (b) Gram-scale reaction. (c) Synthesis of phosphonate ester derivative. | ||
In order to understand the mechanism, we performed several control experiments. When the reaction was performed in the presence of oxygen, no product was formed, which indicated the requirement of an inert atmosphere (Scheme 5a). Reactions in the presence of the radical quencher TEMPO did not yield any expected product 3a (Scheme 5b). HRMS analysis of the reaction mixture showed tosyl-TEMPO adduct, which indicated a radical pathway. In the absence of photocatalyst, only a 12% yield of the desired product was obtained. Similarly, there was no product formation in the absence of light, which suggested the need for both photocatalyst and light for this transformation (Scheme 5c and d).
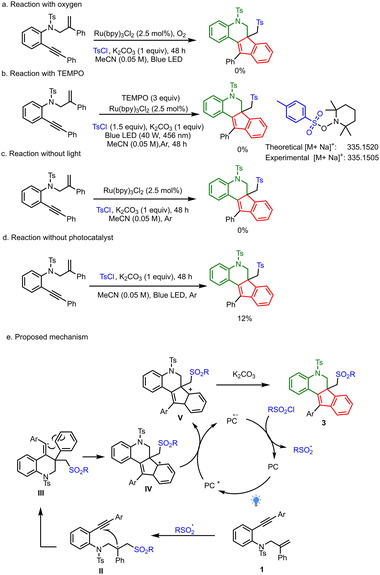 | ||
| Scheme 5 (a) Reaction with oxygen. (b) Radical quenching studies with TEMPO. (c) Reaction without light. (d) Reaction without photocatalyst. (e) Proposed mechanism. | ||
Based on these mechanistic studies and literature reports, we propose the following mechanism. At first, the photogenerated sulfonyl radical chemoselectively adds to the alkene of 1,7 enyne to give radical intermediate II; this intermediate, upon intramolecular cyclization, generates radical intermediate III. Radical intermediate III then undergoes intramolecular cyclization to give radical intermediate IV. At this point, the excited-state photocatalyst (PC*) undergoes SET with intermediate IVvia a reductive quenching cycle to generate cationic intermediate V and reduced photocatalyst, PC˙−. Intermediate V quickly undergoes aromatization in the presence of base to give the desired product 3a, and finally, PC˙− is then oxidized by sulfonyl chloride to regenerate ground-state photocatalyst (PC) and sulfonyl radical, which then reinitiates the cascade reaction (Scheme 5e).
In conclusion, we showcased the synthesis of various sulfonylated and triflouromethylated indeno-quinoline derivatives via a photoredox-catalyzed radical cascade reaction. The reaction was found to work well with a variety of substituents on the sulfonyl and ortho-alkynyl aniline derivatives. Various sulfonyl-containing drug derivatives were demonstrated as the sulfonyl source, and the corresponding sulfonyl-tethered indeno-quinoline cores were obtained in good yields. This method can offer a wide potential for the synthesis of other substituted indeno-quinoline derivatives, by changing the radical precursors. Control experiments were performed to understand the mechanism of the reaction.
We are grateful to SERB (CRG/2022/008440) for financial support. P. Y. thanks PMRF and A. A. V. thanks CSIR for financial support.
Conflicts of interest
There is no conflicts to declare.Data availability
The data underlying this study are available in the published article and its SI. All experimental procedures, characterization data, 1H, 13C and 19F NMR spectras for all new compounds. See DOI: https://doi.org/10.1039/d5cc04023j.CCDC 2445211 contains the supplementary crystallographic data for this paper.20
Notes and references
- S. S. Babu, A. A. Varma and P. Gopinath, Chem. Commun., 2022, 58, 1990–1993 RSC.
- L. Yuan, S. M. Jiang, Z. Z. Li, Y. Zhu, J. Yu, L. Li, M. Z. Li, S. Tang and R. R. Sheng, Org. Biomol. Chem., 2018, 16, 2406–2410 RSC.
- S. S. Babu and P. Gopinath, J. Org. Chem., 2022, 87, 9414–9418 CrossRef CAS PubMed.
- M. Wang, Y. Wang, C. Zhang and L. Zhang, Org. Lett., 2024, 26(50), 10714–10718 CrossRef CAS.
- A. P. Shaji, N. Sudarshan, B. König and P. Gopinath, J. Org. Chem., 2025, 90, 5550–5563 CrossRef CAS PubMed.
- J. Liao, X. Yang, L. Ouyang, Y. Lai, J. Huang and R. Luo, Org. Chem. Front., 2021, 8, 1345–1363 RSC.
- X. S. Zhang, Y. P. Han and Y. M. Liang, Adv. Syn. Catal., 2024, 366, 324–356 CrossRef CAS.
- M. H. Huang, Y. L. Zhu, W. J. Hao, A. F. Wang, D. C. Wang, F. Liu, P. Wei, S. J. Tu and B. Jiang, Adv. Synth. Catal., 2017, 359, 2229–2234 CrossRef CAS.
- J. Xuan and A. Studer, Chem. Soc. Rev., 2017, 46, 4329–4346 RSC.
- V. Michelet, P. Y. Toullec and J. P. Genêt, Angew. Chem., 2008, 4268–4315 CrossRef CAS.
- P. Yadav, P. Sinha and P. Gopinath, J. Org. Chem., 2025, 90(3), 1333–1343 CrossRef CAS PubMed.
- Z. Cai, S. Trienes, K. Liu, L. Ackermann and Y. Zhang, Org. Chem. Front., 2023, 10, 5735–5745 RSC.
- K. Zhuang, Y. Cui, X. Yuan, L. Qin, Q. Sun, X. Duan, L. Chen, X. Zhang, J. Qiu and K. Guo, ACS Sustainable Chem. Eng., 2020, 8, 11729–11736 CrossRef CAS.
- S. Xiang, Q. Ni, Q. Liu, S. Zhou, H. Wang, Y. Zhou and Y. Liu, J. Org. Chem., 2023, 88(18), 13248–13261 CrossRef CAS PubMed.
- L. Zhang, M. Liu, J. Zhao and S. Li, J. Org. Chem., 2025, 90, 7468–7475 CrossRef CAS PubMed.
- C. C. Bernal, A. R. R. Bohórquez, J. A. Henao and M. A. Macías, J. Mol. Struct., 2021, 1233, 130095 CrossRef CAS.
- C. H. Tseng, Y. L. Chen, P. J. Lu, C. N. Yang and C. C. Tzeng, Bioorg. Med. Chem., 2008, 16, 3153–3162 CrossRef CAS PubMed.
- A. Suresh Kshirsagar and R. S. Liu, Adv. Synth. Catal., 2025, 367, e202400968 CrossRef CAS.
- S. S. K. Boominathan and J. J. Wang, Adv. Synth. Catal., 2017, 359, 1844–1848 CrossRef CAS.
- CCDC 2445211: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2n2fsz.
Footnote |
| † Dedicated to Prof. S. Chandrasekaran on his 80th birthday. |
| This journal is © The Royal Society of Chemistry 2025 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction time 72 h.
Reaction time 72 h.