Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Alkyl-chain-grafted 13X zeolite for steady CO2 capture from humid flue gas
Beining Caoab,
Qian Jia *a,
Guoqiang Lia,
Haitao Cuia,
Feng Lia,
Bo Peng*c and
Lei Li*a
*a,
Guoqiang Lia,
Haitao Cuia,
Feng Lia,
Bo Peng*c and
Lei Li*a
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, 030001, P. R. China. E-mail: lilei@sxicc.ac.cn
bUniversity of the Chinese Academy of Sciences, Beijing, 100049, P. R. China
cSINOPEC Research Institute of Petroleum Processing Co., Ltd., Beijing 100083, P. R. China
First published on 22nd September 2025
Abstract
13X zeolite shows potential for CO2 capture, but its performance is diminished by the steam present in real-world flue gas. To overcome this limitation, a facile and efficient approach is to graft straight-chain alkyl groups to the 13X structure. Adsorbents with anchored alkyl groups exhibit both high CO2 uptake and excellent resistance to humidity.
Anthropogenic CO2 emissions pose a significant threat to global climate stability.1 To address this issue, the following technical routes have been proposed: (1) direct air capture (DAC),2 which extracts CO2 directly from the atmosphere (420 ppm CO2); and (2) point-source carbon capture,3 primarily applied in industrial sectors with high-concentration CO2 emissions. Nowadays, industrial sectors, such as power plants and petrochemical industries, contribute to over 25% of global carbon emissions.4 Therefore, it is essential to develop high-performance adsorbents for CO2 removal from flue gas. However, efficient CO2 capture remains challenging, mainly due to the negative impact of the water vapor in flue gas (∼10 vol%) on CO2 adsorption.5 Physical adsorption generally describes the adherence of an adsorbate to the surface of an adsorbent through weak interactions, such as van der Waals forces and electrostatic forces, without involving the making or breaking of chemical bonds, and typically occurs in a reversible fashion. Physical adsorbents typically exhibit stronger affinity for H2O, due to its dipole moment, compared to CO2, which relies on weaker quadrupolar interactions.6 Thus, water vapor competitively adsorbs with CO2, reducing overall CO2 uptake. For example, at 90% relative humidity (3.4 vol%), the CO2 capture capacity of 13X zeolite is reduced by 18.5% after multiple vacuum swing adsorption cycles.7 Similarly, the static CO2 adsorption capacity of Na-LSX zeolite was reduced by 50% following exposure to moisture pretreatment conditions.8
To mitigate the impact of humidity, pure silica materials9 and carbon materials with a hydrophobic nature10,11 were previously used as CO2 adsorbents because of their low affinity for H2O molecules. However, this improved resistance to humidity was normally achieved by sacrificing CO2/N2 selectivity. Zeolites, 3-dimensional crystalline structures constructed from SiO44− tetrahedra bridged by O atoms, offer an alternative. Although substituting Si atoms with elements like Al generates an overall negatively charged framework that attracts neutralizing cations (e.g., H+, Na+, Ca2+, and La3+) and enhances hydrophilicity, zeolites remain highly promising for CO2 capture, as these incorporated cations can promote strong interactions with CO2 and lead to high CO2/N2 selectivity.11,12
To prohibit water vapor access, several feasible strategies have been proposed to modify zeolite surfaces: (1) polymerizing polyethylene terephthalate (PET) films on the zeolite surface,13 (2) combining ZIF materials with zeolites via a glass transition,14 (3) utilizing azide reagents as binders for composites,15 etc. However, these methods are complicated and may not be suitable for large-scale applications.
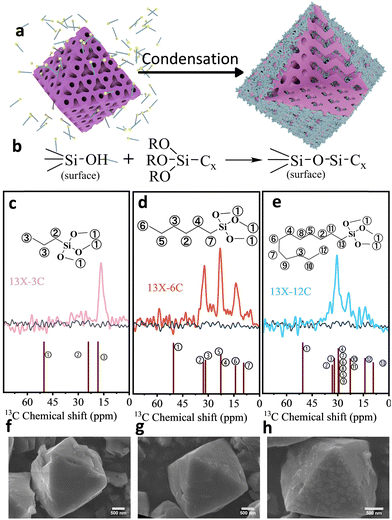
In this work, an efficient approach was introduced by grafting different alkyl molecules onto 13X zeolite via methanol condensation between hydroxyl groups of zeolite and methoxy groups of silanes (Fig. 1a and b).16 Alkyl chains with different carbon numbers were tested, i.e. trimethoxy(propyl)silane (3C), hexyltrimethoxysilane (6C), and dodecyltrimethoxysilane (12C). It is important to note that the kinetic diameter of hexyltrimethoxysilane (∼1.1 nm) fits the largest pore opening of 13X zeolite. The influence of alkyl-modified zeolites on the CO2 adsorption performance has been investigated under humid flue gas conditions. The modified pore structure endows the material with remarkable CO2 adsorption capacity, water resistance and excellent cycling stability, suggesting potential applications for post-combustion CO2 capture.
According to the sol–gel poisoned Eden model, the three methoxy groups from the grafting agent can undergo two competing reactions: (1) condensation with surface hydroxy groups of zeolite or (2) self-condensation to form a low-fractal dimension16 and loosely structured carbon layer. The presence of hydroxyl groups in the zeolite can be confirmed by the broad Fourier transform infrared spectroscopy absorption peaks within the range of 3500–3100 cm−1 and 1H NMR peaks at 1.2 ppm (silanol groups) and 3.6 ppm (bridging oxygen hydroxyl groups) (Fig. S5 and S13). Compared with the 13C NMR spectra of the pure grafting agents (Fig. 1c–e), the disappearance of the methoxy signal (50 ppm; ①) from modified zeolite indicated condensation between the methoxy and surface hydroxyl groups. In addition, the attenuated signals at 9.3 ppm (⑦ and  ) for 13X-6C and 13X-12C after grafting suggest a significant change in the local chemical environment for carbons adjacent to silicon atoms. To see the effect of the grafted groups, the crystalline structures of modified zeolites were compared with their parent materials (Fig. S2), and this shows that the introduction of alkyl molecules has no effect on the crystallinity, but the diffraction peaks shift slightly towards higher angles, which has a certain impact on the periodicity of the material.
) for 13X-6C and 13X-12C after grafting suggest a significant change in the local chemical environment for carbons adjacent to silicon atoms. To see the effect of the grafted groups, the crystalline structures of modified zeolites were compared with their parent materials (Fig. S2), and this shows that the introduction of alkyl molecules has no effect on the crystallinity, but the diffraction peaks shift slightly towards higher angles, which has a certain impact on the periodicity of the material.
SEM confirmed that the grafting of alkyl molecules can form a carbon layer on the zeolite surface (Fig. 1f–h). In addition, prominent interparticle adhesion of carbon layers can be detected on 13X-12C, which is a consequence of the dispersion of longer chain molecules combined with silane condensation among methoxy groups (Fig. S3h). N2 adsorption isotherms (at 77 K) exhibited a slight reduction in pore volume for modified zeolites, possibly due to the relatively small mass contribution of the alkyl groups (Fig. S6). The NLDFT model was applied to analyze the pore size distribution within the structure.17 Notably, both 13X-3C and 13X-6C displayed additional apertures of around 1 nm and 30 nm (Fig. 2). The presence of these apertures can be tentatively attributed to the entanglement of alkyl groups fixed on the zeolite surface. Depending on the locations of alkyl groups, the small aperture (13X-3C: 0.6 nm) was probably created within the zeolite pores, while the large aperture (30 nm) was formed by grafted alkyl molecules on the outer surface. In contrast, 13X-dodecyl only exhibited the large aperture (30–40 nm). This can be explained by the reduced pore aperture with long-chain alkyl groups, which cause severe pore blockages and hinder nitrogen diffusion in the low-pressure range. Thus, its micropore characteristics are masked by mesoporous pores.
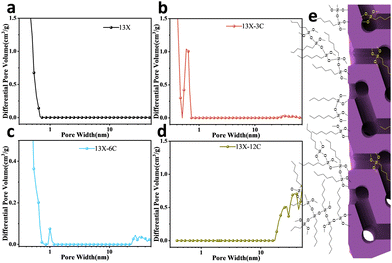 | ||
| Fig. 2 The aperture distributions of (a) 13X, (b) 13X-3C, (c) 13X-6C, and (d) 13X-12C, and (e) a schematic diagram of the structure of modified 13X. | ||
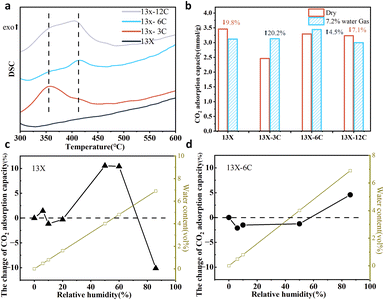
The relevant contact angle measurements also verified the modification of the material surface with alkyl groups (Fig. S4). Upon increasing the length of alkyl chains, the resistance of the carbon layer to water molecules was more pronounced, as indicated by the higher contact angle for the zeolites 13X-hexyl (158.2°) and 13X-dodecyl (145.5°). The thermal decomposition behaviour of the modified alkyl groups was characterized using DSC (Fig. 3a). The peaks at 360 °C and 416 °C were assigned to the loss of alkyl groups. Due to the Brønsted acidity of zeolite18 and the confinement effect on alkyl groups,19 the thermal decomposition temperature of the modified alkyl groups in the zeolite phase is lower than that of the alkyl groups themselves. Therefore, the peak at 360 °C originated from the modified alkyl groups in the zeolite phase,19 while the peak at 416 °C was related to the outer carbon layer structure on the zeolite surface originating from the self-condensation of the grafted silanes. For small-sized groups, such as propyl, the exothermic peak (360 °C) indicates that the modified alkyl groups are largely present in the zeolite phase, while 13X-6C and 13X-12C maintain a relatively high exothermic peak at 416 °C, indicating the presence of abundant silane-based carbon layer structures on the zeolite surface.
The performance of zeolite adsorbents was assessed by measuring CO2 adsorption in a fixed-bed reactor (Fig. S1). Fig. 3b shows the CO2 uptake from dynamic column breakthrough experiments at 41 °C using standard feed gas simulating real-world flue gas (7.2% H2O, 13.92% CO2 and 78.88% N2 vs 15% CO2 and 85% N2). Under dry conditions, the CO2 uptake of 13X-3C was reduced compared to its counterparts. This can be attributed to the higher content of alkyl groups in the zeolite pores. As such, CO2 transport will be affected, resulting in lower uptake. In contrast, the bulkier hexyl and dodecyl groups were partially or even largely fixed on the outer surface, resulting in a slight change in CO2 uptake. However, under humid conditions, the adsorbent with dodecyl groups displayed a significant drop in CO2 uptake, similar to 13X. In contrast, the CO2 uptake of 13X-3C and 13X-6C increased, suggesting the enhancing effect of water vapor on CO2 uptake in the presence of such alkyl groups in the pore structure. This could be related to the improved water resistance of zeolites, by which the adsorbed amount of water vapor was reduced. In general, the smaller propyl and hexyl groups have a more pronounced effect on the water resistance of zeolite adsorbents compared to dodecyl groups, but propyl molecules can easily access the pore structure, hindering CO2 diffusion. In this context, 13X-6C was optimum for application in flue gas treatment due to its stable CO2 adsorption performance under both dry and humid conditions. Therefore, only 13X-6C is discussed in terms of further investigations.
The influence of water vapor on CO2 adsorption was studied. Fig. 3c and d compares the CO2 adsorption capacities of zeolite adsorbents at varying relative humidity levels. Different scenarios are shown for the original 13X and modified 13X-6C adsorbents. At lower humidity (<20%), the presence of water had a negligible effect on the CO2 uptake by both materials. However, at a higher water content, an increase in CO2 uptake was detected for 13X, which could be related to the formation of carbonates according to previous studies,20,21 while the CO2 adsorption of 13X-6C remained nearly constant. When the relative humidity exceeded 80%, the CO2 uptake from 13X experienced a dramatic drop, whereas 13X-6C showcased an appreciable increase in CO2 uptake. A possible explanation is that water molecules easily cluster into the pore structure of unmodified 13X, reducing the available space. However, the inclusion of alkyl groups hindered the transport of water molecules and prohibited the formation of water clusters.22,23 Water vapor adsorption was measured (Fig. S12), and this indicated that grafting alkyl groups can reduce the saturated adsorption capacity of H2O. In addition, the heat of H2O adsorption from 13X and 13X-6C was estimated using two-cycle DSC measurements by integrating the desolvation peak (Fig. S10). Compared with 13X, the heat of water vapor adsorption for 13X-6C shows a slight decrease, but its desorption temperature does not change significantly, indicating that the alkyl groups do not alter the adsorption characteristics of the zeolite towards water.
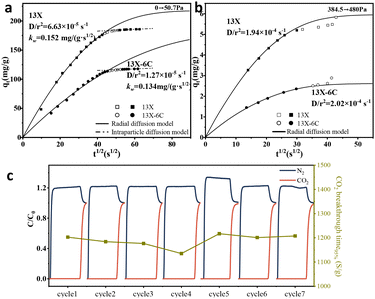
To further explore the influence of alkyl groups on the water vapor adsorption dynamics, the mutual diffusion and self-diffusion of H2O molecules were studied. For mutual diffusion, the radial diffusion model24 is used to determine the initial-stage diffusion coefficient (D) of H2O molecules, and the intraparticle diffusion model25 is employed to measure the adsorption rate constant of H2O molecules (kw) in the final stage of adsorption. At low water vapor pressure (50.7 Pa, Fig. 4a), D values for 13X-6C are significantly smaller than those for pristine 13X, indicating that hexyl grafting effectively inhibits the diffusion of H2O molecules in the initial stage (Fig. 4a); and in the final stages, the smaller differences in the kw values of the two suggest a reduced variation in the adsorption rate. At low vapor pressure, two models are required to represent the diffusion patterns in different time periods, indicating a change in the diffusion mechanism: the dominant diffusion mode seems to shift from molecular diffusion at low vapor loading to intracrystalline diffusion at higher vapor loading (Fig. 4a).26 However, the alkyl-group inhibition effect becomes less pronounced at elevated vapor loadings (approximately 82.3%, Fig. 4b), where the diffusion coefficients for both initial and final stages converge. This convergence indicates that alkyl modification primarily influences H2O molecule diffusion under low-loading conditions, while its impact diminishes appreciably when approaching saturation.
For the self-diffusion of molecules, the adsorption and equilibrium diffusion of water vapor on 13X and 13X-6C were simulated using grand canonical Monte Carlo (GCMC) and molecular dynamics (MD) methods.27 The randomly selected diffusion paths of water molecules in the MD simulations are shown in Fig. S14. The probability of H2O molecules passing through the zeolite cavity after alkyl modification decreased. Moreover, the self-diffusion coefficient of water molecules decreased for alkyl-modified zeolites (Table S1). Finally, the cycling stability of modified 13X-6C was evaluated (Fig. 4c). During the measurements, the same batch of 13X-6C was tested 7 times using CO2/N2/H2O mixed gas (13.89/78.88/7.2 v/v/v). Its adsorption capacity remained at around 3.46 mmol g−1, and the performance did not decline.
In conclusion, hydrophobic alkyl molecules can anchor either within pores or on the surface of zeolites, depending on their molecular size. This differential anchoring manner creates micropores (for smaller molecules) or mesopores (for larger ones). The resulting alkyl-modified zeolite shows improved water vapor resistance, which is favourable for post-combustion CO2 capture. Breakthrough experiments under simulated flue gas conditions indicate that the CO2 adsorption capacity of 13X zeolite shows the phenomenon of first increasing and then decreasing with an increase in humidity. Moreover, zeolite with appropriate alkyl modification has lower sensitivity to humidity changes. Furthermore, through a study of water diffusion, it was found that alkyl inhibition of water diffusion occurs when the water loading is low, which is a key point. This provides a new perspective relating to the bed layer diffusion kinetics of the actual adsorption process.
The project was supported by the National Natural Science Foundation of China (U23A20100), Key R&D Program of Shanxi Province (202202090301013, 202102090301008), ICC CAS SCJC-DT-2023-03, and the independent research project of the State Key Laboratory of Coal Conversion (2024BWZ006)
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article are included in the SI. See DOI: https://doi.org/10.1039/d5cc03810c.Notes and references
- S. Solomon, G.-K. Plattner, R. Knutti and P. Friedlingstein, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 1704–1709 CrossRef PubMed.
- A. Goeppert, M. Czaun, G. K. Surya Prakash and G. A. Olah, Energy Environ. Sci., 2012, 5, 7833–7853 RSC.
- A. G. Olabi, T. Wilberforce, K. Elsaid, E. T. Sayed, H. M. Maghrabie and M. A. Abdelkareem, J. Cleaner Prod., 2022, 362, 132300 CrossRef.
- B. Metz, O. Davidson, H. De Coninck, M. Loos and L. Meyer, IPCC special report on carbon dioxide capture and storage, Cambridge University Press, Cambridge, 2005 Search PubMed.
- R. Zevenhoven and P. Kilpinen, Control of pollutants in flue gases and fuel gases, Helsinki University of Technology, Espoo, Finland, 2001 Search PubMed.
- N. Hedin, L. Chen and A. Laaksonen, Nanoscale, 2010, 2, 1819–1841 RSC.
- G. Li, P. Xiao, P. Webley, J. Zhang, R. Singh and M. Marshall, Adsorption, 2008, 14, 415–422 CrossRef.
- F. Brandani and D. M. Ruthven, Ind. Eng. Chem. Res., 2004, 43, 8339–8344 CrossRef.
- L. Ronchi, H. Nouali, T. J. Daou, J. Patarin and A. Ryzhikov, New J. Chem., 2017, 41, 15087–15093 RSC.
- D. Xu, P. Xiao, J. Zhang, G. Li, G. Xiao, P. A. Webley and Y. Zhai, Chem. Eng. J., 2013, 230, 64–72 CrossRef.
- S. De, A. M. Balu, J. C. van der Waal and R. Luque, ChemCatChem, 2015, 7, 1608–1629 CrossRef.
- D. Bonenfant, M. Kharoune, P. Niquette, M. Mimeault and R. Hausler, Sci. Technol. Adv. Mater., 2008, 9, 013007 CrossRef.
- A. Zhou, C. Yang, M. Xue, B. Xue, J. Zheng, X. Li, F. Nie, X. Zhao and J. Mi, Sep. Purif. Technol., 2025, 355, 129689 CrossRef.
- S. Chi, Y. Ye, X. Zhao, J. Liu, J. Jin, L. Du and J. Mi, Sep. Purif. Technol., 2023, 307, 122738 CrossRef.
- D.-i Kwon, J.-C. Kim, H. Lee, W. Lee and C. Jo, Chem. Eng. J., 2022, 427, 131461 CrossRef.
- C. J. Brinker and G. W. Scherer, Sol-gel science: the physics and chemistry of sol-gel processing, Academic press, 2013 Search PubMed.
- C. Lastoskie, K. E. Gubbins and N. Quirke, J. Phys. Chem., 1993, 97, 4786–4796 CrossRef.
- P. del Campo, C. Martínez and A. Corma, Chem. Soc. Rev., 2021, 50, 8511–8595 RSC.
- W. Wang, J. Jiao, Y. Jiang, S. S. Ray and M. Hunger, Chem. Phys. Chem., 2005, 6, 1467–1469 CrossRef PubMed.
- R. V. Siriwardane, M.-S. Shen and E. P. Fisher, Energy Fuels, 2003, 17, 571–576 CrossRef.
- H. Veldhuizen, S. A. Butt, A. van Leuken, B. van der Linden, W. Rook, S. van der Zwaag and M. A. van der Veen, ACS Appl. Mater. Interfaces, 2023, 15, 29186–29194 CrossRef PubMed.
- B. S. Bal'zhinimaev, E. A. Paukshtis, A. V. Toktarev, E. V. Kovalyov, M. A. Yaranova, A. E. Smirnov and S. Stompel, Microporous Mesoporous Mater., 2019, 277, 70–77 CrossRef.
- J. Hunger, I. A. Beta, H. Böhlig, C. Ling, H. Jobic and B. Hunger, J. Phys. Chem. B, 2006, 110, 342–353 CrossRef PubMed.
- J. Crank, The mathematics of diffusion, Oxford University Press, 1979 Search PubMed.
- F.-C. Wu, R.-L. Tseng and R.-S. Juang, Chem. Eng. J., 2009, 153, 1–8 CrossRef.
- D. M. Ruthven, Principles of adsorption and adsorption processes, John Wiley & Sons, 1984 Search PubMed.
- D. Dubbeldam, S. Calero, D. E. Ellis and R. Q. Snurr, Mol. Simul., 2016, 42, 81–101 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |