Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A Mn(III)-catalysed domino process for C-3 substituted dihydrocoumarins from 2-hydroxybenzyl alcohols and 4-hydroxy-2H-chromen-2-ones†
Gokul S.
Londhe
,
Shankhajit
Mondal
and
Boopathy
Gnanaprakasam
 *
*
Department of Chemistry, Indian Institute of Science Education and Research Dr Homi Bhabha Road, Pashan, Pune-411008, India. E-mail: gnanaprakasam@iiserpune.ac.in
First published on 23rd June 2025
Abstract
A Mn-catalysed efficient domino process for the synthesis of new C-3 substituted dihydrocoumarins from 2-hydroxybenzyl alcohols and 4-hydroxy-2H-chromen-2-ones under one-pot conditions is described. This reaction proceeds via a series of reactions in one-pot, such as o-quinone methide formation, Michael addition, intramolecular transesterification, and skeletal rearrangement to access dihydrocoumarins.
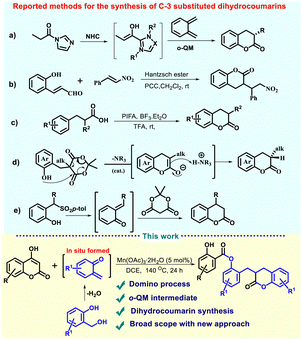
Dihydrocoumarin serves as an important natural compound, known to be an exclusive class of lactone and characterized by its distinctive sweet, vanillin-like olfactory profile.1 Therefore, it has been used as a key compound in many fragrance and flavor industries. Several natural products and drug molecules possessing these structural motifs exhibit promising biological activities, such as anti-inflammatory, antibiotic, anticancer, etc.2,3 Owing to its significance in the scientific community, many synthetic methods have been documented in the literature for the synthesis of dihydrocoumarins by using various reagents and catalysts.4 Notably, the synthesis of C-3 substituted dihydrocoumarins was also demonstrated in the literature due to their promising biological significance. For instance, NHC-catalysed synthesis of dihydrocoumarin has been presented by Scheidt using ortho quinone methides (Scheme 1a).5 Then, Liu and coworkers reported the synthesis of C3 alkylated dihydrocoumarins from conjugated aldehydes and nitro compounds using Hantzsch ester as a catalyst (Scheme 1b).6 The PIFA-based oxidative cyclization of 3-arylpropionic acids to 3,4-dihydrocoumarins has also been demonstrated to investigate the reaction mechanism (Scheme 1c).7 Recently, an organocatalytic asymmetric version of the synthesis of dihydrocoumarins was developed by Sylvan and coworkers via transesterification using pre-functionalized Meldrum's acid derivatives (Scheme 1d).8 Previously, this approach was studied for the synthesis of 4-substituted dihydrocoumarin using specially designed sulfonate derivatives as a source of ortho-quinone methide with Meldrum's acid through Michael addition and transesterification (Scheme 1e).9 In recent studies, the ortho quinone methides (o-QMs) have been extensively used for the synthesis of substituted dihydrocoumarins.10,11 Although the synthesis of substituted dihydrocoumarins is well documented, very few studies are present in the literature, specifically for the synthesis of C-3 substituted dihydrocoumarins.5–8 These methodologies are constrained by a few aspects, such as the use of expensive catalysts and pre-functionalized substrates, a multistep approach, hazardous reagents, and the requirement for harsh reaction conditions with low yields. Consequently, to address such drawbacks, and increase the significance of C-3 substituted dihydrocoumarins, the development of a direct and step-economical process to access structurally diverse and elegant compounds by exploiting readily accessible substrates is always intended in synthetic organic chemistry.
The domino process refers to a sequence of multiple bond-forming reactions that occur under the same reaction conditions, without isolating intermediates or adding new reagents throughout the whole process.12 This process plays a pivotal role in modern synthetic chemistry due to its selectivity, sustainability, and efficiency. To the best of our knowledge, there is no existing report on an Mn(III) catalyzed domino process for the synthesis of C3-substituted dihydrocumarins utilizing 4-hydroxy coumarins and 2-hydroxy benzyl alcohols. Herein, we report an earth abundant and inexpensive Mn(OAc)3 catalyzed synthetic protocol to synthesize C3-substituted new dihydrocumarins using a domino process through ortho-quinone methide (o-QM) as a reactive intermediate.
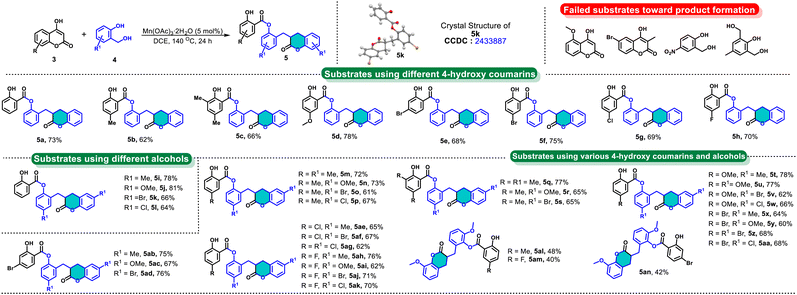
Our investigation was commenced by taking 4-hydroxy-2H-chromen-2-one 3a and 2-(hydroxymethyl)phenol 4a as model substrates to optimize the reaction conditions for the synthesis of 2-((2-oxochroman-3-yl)methyl)phenyl 2-hydroxybenzoate 5a. A control experiment in the absence of a catalyst using toluene as a solvent at 140 °C revealed that the catalyst is necessary to conduct the reaction towards the desired product 5a (Table S1, entry 1, ESI†). Subsequently, we examined various metal complexes as a catalyst, such as Ru, Fe, In, Ni, Cu, Mn, etc., indicating that Mn complexes are more efficient (see Table S1, ESI†). As a result, Mn(OAc)3 rendered 55% yield (Table S1, entry 12, ESI†) and other complexes conferred 23% to 51% yields. From these studies, we observed that the metal complexes bearing –OAc ligand are more efficient for this transformation. The more feasibility of these catalysts might be due to the formation of the acetate ion in the process, which can abstract the proton from phenol to form acetic acid, which can drive the intramolecular esterification (refer to the mechanism). To confirm this, a reaction was performed with 5 mol% and stoichiometric amounts of acetic acid to afford 16% and 40% yields of the product 5a, respectively (Table S1, entries 26 and 27, ESI†). When we examined the Brønsted acid catalysts such as Amberlyst-15 and p-toluene sulfonic acid (p-TSA), no product formation was observed. All these results reveal that Mn(OAc)3 is an efficient catalyst for the synthesis of product 5a. Increasing the catalyst loading up to 10 mol% and extending the reaction time to 48 h resulted in no improvement in the yields (Table 1, entries 1 and 2). In order to improve the product yield, a reaction was performed with different solvent media such as 1,4 dioxane, 2-methyl THF, ethyl acetate (EtOAc), acetonitrile (ACN), dichloroethane (DCE), dimethyl sulfoxide (DMSO), and N,N-dimethyl formamide (DMF). Among all the solvents tested, dichloroethane (DCE) was found to be the most suitable solvent, providing a 74% yield of 5a after 36 h (Table 1, entry 7). DMSO was an inefficient solvent for this transformation (Table 1, entry 9). However, other solvents have produced poor to moderate yields (Table 1, entries 3 to 6 and 10). Other reaction conditions were tested and did not improve the yield of product 5a (Table 1, entries 11 to 14). From the optimisation studies, 5 mol% Mn(OAc)3 catalyst and 24 h reaction time were found to be the best reaction conditions for the synthesis of product 5a.
| S. no. | Mn(OAc)3·2H2O (mol%) | Solvent | % Yield (5a) |
|---|---|---|---|
| Reaction conditions: Mn(OAc)3·2H2O (mol%), compound 3a (0.3 mmol), compound 4a (0.6 mmol), and solvent (3 mL) were stirred in a sealed tube in a preheated oil bath at 140 °C for 36 h.a 48 h.b 4a (0.75 mmol).c 24 h.d 120 °C, ND = not detected. All mentioned yields are isolated yields. | |||
| 1. | Mn(OAc)3·2H2O (10) | Toluene | 54 |
| 2.a | Mn(OAc)3·2H2O (5) | Toluene | 55 |
| 3. | Mn(OAc)3·2H2O (5) | Dioxane | 20 |
| 4. | Mn(OAc)3·2H2O (5) | 2-Me-THF | 25 |
| 5. | Mn(OAc)3·2H2O (5) | EtOAc | 20 |
| 6. | Mn(OAc)3·2H2O (5) | ACN | 54 |
| 7. | Mn(OAc)3·2H2O (5) | DCE | 74 |
| 8. | Mn(OAc)3·2H2O (10) | DCE | 75 |
| 9. | Mn(OAc)3·2H2O (5) | DMSO | ND |
| 10. | Mn(OAc)3·2H2O (5) | DMF | 12 |
| 11.a | Mn(OAc)3·2H2O (10) | DCE | 75 |
| 12.b | Mn(OAc)3·2H2O (5) | DCE | 71 |
| 13.c | Mn(OAc) 3 ·2H2O (5) | DCE | 73 |
| 14.d | Mn(OAc)3·2H2O (5) | DCE | 58 |
Next, the scope of this reaction was elaborated with various coumarins and benzylic alcohols under the optimal conditions (Scheme 2). 4-Hydroxy-2H-chromen-2-ones with diverse substituents, such as 5-Me, 2,4-di-Me, 5-OMe, 4-Br, 5-Br, 5-Cl, and 5-F, were reacted with 2-(hydroxymethyl)phenol to achieve the respective 2-((2-oxochroman-3-yl)methyl)phenyl 2-hydroxybenzoates 5b to 5h in good yields (62% to 78%). Subsequently, several 2-(hydroxymethyl)phenols were subjected to reaction with different 4-hydroxy-2H-chromen-2-ones to deliver the associated 2-((2-oxochroman-3-yl)methyl)phenyl 2-hydroxybenzoates 5i to 5l in good to excellent yields (64% to 81%). Afterward, the compounds 5m to 5p were synthesized in good yields (61% to 72%) by reacting them with 4-hydroxy-6-methyl-2H-chromen-2-one under the standard reaction conditions.
These alcohols reacted smoothly with 4-hydroxy-6,8-dimethyl-2H-chromen-2-one to afford the compounds 5q to 5s in 65% to 77% yields and with 4-hydroxy-6-methoxy-2H-chromen-2-one to generate compounds 5t to 5w in good yields (62% to 78%). The reactivity of bromo-substituted 4-hydroxy-6-methoxy-2H-chromen-2-ones was also examined with various alcohols to construct the anticipated products 5x–5ad in 60% to 76% yields. The 5-Me, 5-Br, and 5-Cl-containing benzylic alcohols productively reacted with 6-chloro-4-hydroxy-2H-chromen-2-one and 6-fluoro-4-hydroxy-2H-chromen-2-one to afford 5ae–5ag and 5ah–5ak (62% to 76% yields), respectively. The reaction of 2-(hydroxymethyl)-6-methoxyphenol was performed with different 4-hydroxy-2H-chromen-2-ones to deliver products 5al–5an in moderate yields. In contrast, a few coumarins, namely 4-hydroxy-5-methoxy-2H-chromen-2-one and 6-bromo-4-hydroxy-3-methyl-2H-chromen-2-one, as well as alcohols such as 2-(hydroxymethyl)-4-nitrophenol and (2-hydroxy-5-methyl-1,3-phenylene)dimethanol were unable to deliver the desired products. The failure of these substrates to yield the desired products could be attributed to steric hindrance and electronic factors that interfere with the reaction. To demonstrate gram-scale synthesis, the reaction of 3a (10.0 mmol) and 4a (20.0 mmol) was performed on a large scale in a sealed tube under standard reaction conditions to afford product 5a (2.47 g, 66% yield).
Next, to understand the reaction pathway, a series of experiments using radical quenchers such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), ∝-methyl styrene and butylated hydroxytoluene (BHT) were performed. This set of experiments indicated that the reaction may not involve the generation of radicals throughout the process and follows the ionic pathway (Scheme 3, entry 1). When the model reaction was tested at low reaction temperature (100 °C), the formation of 4-hydroxy-3-(2-hydroxybenzyl)-2H-chromen-2-one 8a as a major product was observed with 42% yield and 5a with 26% yield (Scheme 3, entry 2). The formation of 8a was further confirmed by reacting with 2-(hydroxymethyl)phenol 4a under the standard reaction conditions, providing 5a with a 73% yield (Scheme 3, entry 3).
Based on these experiments, two possible mechanistic pathways (path A and path B) have been depicted for product formation, as disclosed in Scheme 4. Path A follows the Michael addition reaction, while Path B follows the [4+2] cycloaddition reaction. To gain insights into the reaction pathways, further control experiments have been performed. Replacement of the –OH group from 4-hydroxy-2H-chrome-2-one with methyl/methoxy/phenyl resulted in no product formation when reacted with 2-(hydroxymethyl)phenol 4a (Scheme 3, entry 4). This experiment shows that a free –OH is necessary to form the product, and the reaction may not follow the cycloaddition reaction pathway. Furthermore, to confirm the formation of the o-QM intermediate, the reaction of 4-hydroxy-2H-chromen-2-one 3a and (2-methoxyphenyl)methanol 4ab, was performed to obtain 3-(2-hydroxyphenyl)-3-oxopropanoate 11 (Scheme 3, entry 5). In a similar fashion, when fully protected alcohol, 1-methoxy-2-(methoxymethyl)benzene 4ac, was reacted with 4-hydroxy-2H-chromen-2-one 3a, no reaction was observed under the standard conditions (Scheme 3, entry 6). These reactions clearly indicate that the free –OH is required to generate an o-QM intermediate, which is formed in our model reaction and confirmed by HRMS.
From the control experiments and literature findings,8,10,11 we proposed the transformation route for the current protocol (Scheme 5). The process initiates with the Mn(III)-catalyzed dehydration of 2-(hydroxymethyl)phenol 4a to generate an o-QM intermediate [A]. The acetate ion generated from Mn(OAc)3 abstracts a proton from 4-hydroxy-2H-chromen-2-one 3a and reacts with intermediate [A]via Michael addition through the C-3 carbon to deliver mono-alkylated product 8a and bis-alkylated product [B]. This species, upon skeletal rearrangement in the presence of an Mn(III) catalyst, forms a compound [C]. In the last step, the desired product 5a was formed by intramolecular transesterification of compound [C] promoted by an Mn(III)-catalyst under the standard reaction conditions.
In conclusion, we developed a Mn(OAc)3-catalysed domino process for the synthesis of diverse C-3 substituted dihydrocoumarins using several coumarins and hydroxybenzyl alcohols under one-pot conditions. This process involves a series of reactions, such as o-QM formation, Michael addition, and intramolecular transesterification, followed by skeletal rearrangement. This protocol enables the synthesis of a library of C-3 substituted dihydrocoumarins (40 compounds) with moderate to good yields. The proposed mechanistic pathway has been supported by the detection of the reactive intermediate (o-QM) and several control experiments.
This research was supported by the SERB (Grant No. CRG/2023/002452), India. G. S. L. thanks UGC. S. M. thanks IISER-Pune for the fellowship. B. G. thanks SERB and IISER-Pune for the research support.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†References
- (a) S. Serra, A. Castagna and M. Valentino, Catalysts, 2019, 9, 665 CrossRef CAS; (b) T. B. Adams, D. B. Greer, J. Doull, I. C. Munro, P. Newberne, P. S. Portoghese, R. L. Smith, B. M. Wagner, C. S. Weil, L. A. Woods and R. A. Ford, Food Chem. Toxicol., 1998, 36, 249 CrossRef CAS PubMed.
- (a) N. Baral, D. R. Mishra, N. P. Mishra, S. Mohapatra, B. P. Raiguru, P. Panda, S. Nayak, M. Nayak and P. S. Kumar, J. Heterocycl. Chem., 2020, 57, 575–589 CrossRef CAS; (b) J. H. G. Lago, C. S. Ramos, D. C. C. Casanova, A. D. A. Morandim, D. C. B. Bergamo, A. J. Cavalheiro, V. D. S. Bolzani, M. Furlan, E. F. Guimaraes, M. C. M. Young and M. J. Kato, J. Nat. Prod., 2004, 67, 1783–1788 CrossRef CAS PubMed.
- (a) D. P. Kamat, S. G. Tilve, V. P. Kamat and J. K. Kirtany, Org. Prep. Proced. Int., 2015, 47, 1–79 CrossRef CAS; (b) G. C. L. Ee, S. H. Mah, S. S. Teh, M. Rahmani, R. Go and Y. H. Taufiq-Yap, Molecules, 2011, 16, 9721–9727 CrossRef CAS PubMed; (c) Z. Leitis, Chem. Heterocycl. Compd., 2016, 52, 527–529 CrossRef CAS.
- (a) G. T. Li, Z. K. Li, Q. Gu and S. L. You, Org. Lett., 2017, 19, 1318–1321 CrossRef CAS PubMed; (b) F. Han, S. Xun, L. Jia, Y. Zhang, L. Zou and X. Hu, Org. Lett., 2019, 21, 5907–5911 CrossRef CAS PubMed; (c) S. Shee, S. Barik, A. Ghosh and A. T. Biju, Org. Lett., 2021, 23, 8039–8044 CrossRef CAS PubMed; (d) J. Lai, C. Yang, R. Csuk, B. Song and S. Li, Org. Lett., 2022, 24, 1329–1334 CrossRef CAS PubMed; (e) Z. Chen, H. Zhang, G. Lin, W. Yao, J. Xing and X. Dou, J. Org. Chem., 2025, 90, 6611–6616 CrossRef CAS PubMed; (f) Z. P. Zhang, K. X. Xie, C. Yang, M. Li and X. Li, J. Org. Chem., 2018, 83, 364–373 CrossRef CAS PubMed; (g) P. Sharma, S. Singh and C. K. Hazra, J. Org. Chem., 2023, 88, 16104–16115 CrossRef CAS PubMed; (h) S. Y. Zhang, M. Lv, S. J. Yin, N. K. Li, J. Q. Zhang and X. W. Wang, Adv. Synth. Catal., 2016, 358, 143–153 CrossRef CAS.
- A. Lee and K. A. Scheidt, Chem. Commun., 2015, 51, 3407–3410 RSC.
- Y. H. Chen, X. L. Sun, H. S. Guan and Y. K. Liu, J. Org. Chem., 2017, 82, 4774–4783 CrossRef CAS PubMed.
- H. Zeng, Z. Ye, A. Chai, Y. Jiang, Y. Zou, F. Wu, Z. Li and L. Zhou, J. Org. Chem., 2024, 89, 5287–5297 CrossRef CAS PubMed.
- T. Martzel, J. Annibaletto, V. Levacher, J. F. Brière and S. Oudeyer, Adv. Synth. Catal., 2019, 361, 995–1000 CrossRef CAS.
- L. Caruana, M. Mondatori, V. Corti, S. Morales, A. Mazzanti, M. Fochi and L. Bernardi, Chem. – Eur. J., 2015, 21, 6037–6041 CrossRef CAS PubMed.
- (a) Z. J. W. M. L. Gao, G. F. Jiang and Y. G. Zhou, Chem. Commun., 2017, 53, 3531 RSC; (b) J. H. Jin, X. Li, X. Luo, J. S. Fossey and W. P. Deng, J. Org. Chem., 2017, 82, 5424–5432 CrossRef CAS PubMed; (c) Y. Wang, J. Pan, J. Dong, C. Yu, T. Li, X. S. Wang, S. Shen and C. Yao, J. Org. Chem., 2017, 82, 1790–1795 CrossRef CAS PubMed; (d) R. Ukis and C. Schneider, J. Org. Chem., 2019, 84, 7175–7188 CrossRef CAS PubMed; (e) M. Spanka and C. Schneider, Org. Lett., 2018, 20, 4769–4772 CrossRef CAS PubMed; (f) X. Chen, R. Song, Y. Liu, C. Y. Ooi, Z. Jin, T. Zhu, H. Wang, L. Hao and Y. R. Chi, Org. Lett., 2017, 19, 5892–5895 CrossRef CAS PubMed.
- (a) M. S. Singh, A. Nagaraju, N. Anand and S. Chowdhury, RSC Adv., 2014, 4, 55924 RSC; (b) S. Saha, S. K. Alamsetti and C. Schneider, Chem. Commun., 2015, 51, 1461–1464 RSC; (c) S. Saha and C. Schneider, Chem. – Eur. J., 2015, 21, 2348–2352 CrossRef CAS PubMed; (d) M. Xiang, C. Y. Li, X. J. Song, Y. Zou, Z. C. Huang, X. Li, F. Tian and L. X. Wang, Chem. Commun., 2020, 56, 14825–14828 RSC; (e) A. A. Jaworski and K. A. Scheidt, J. Org. Chem., 2016, 81, 10145–10153 CrossRef CAS PubMed; (f) S. Saha and C. Schneider, Org. Lett., 2015, 17, 648–651 CrossRef CAS PubMed; (g) M. Spanka and C. Schneider, Org. Lett., 2018, 20, 4769–4772 CrossRef CAS PubMed.
- (a) B. Delayre, Q. Wang and J. Zhu, ACS Cent. Sci., 2021, 7, 559–569 CrossRef CAS PubMed; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed; (c) Y. Sharma, G. P. Pawar and V. D. Chaudhari, J. Org. Chem., 2023, 88, 701–710 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2433887. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc02950c |
| This journal is © The Royal Society of Chemistry 2025 |