Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cross-linking teichoic acids by click chemistry prevents bacterial cell growth†
Morgane
Baudoin
a,
Anne
Chouquet
b,
Célia
Boyat
b,
Cédric
Laguri
 b,
André
Zapun
b,
André
Zapun
 b,
Basile
Pérès
a,
Cecile
Morlot
b,
Basile
Pérès
a,
Cecile
Morlot
 b,
Yung-Sing
Wong
b,
Yung-Sing
Wong
 *a and
Claire
Durmort
*a and
Claire
Durmort
 *b
*b
aUniv. Grenoble Alpes, CNRS, DPM, 38000 Grenoble, France. E-mail: yung-sing.wong@univ-grenoble-alpes.fr
bUniv. Grenoble Alpes, CNRS, CEA, IBS, 38000 Grenoble, France. E-mail: claire.durmort@ibs.fr
First published on 30th May 2025
Abstract
This work identifies a novel antibacterial mechanism that targets the cell wall of the human pathogen Streptococcus pneumoniae. Unlike conventional cell-wall targeting antibiotics, which inhibit the natural cross-linking of peptidoglycan, we introduce artificial cross-links in the other main component of the Gram-positive cell wall, the teichoic acids, and show that it leads to impaired cell growth.
Faced with the growing problem of antibiotic resistance, the discovery of new antibacterial targets remains a high priority.1 The bacterial cell wall is a prime target that has already led to valuable antibiotics such as penicillin and vancomycin.2,3 Its main component, peptidoglycan (PG, Fig. 1), is a biopolymer composed of repeating units of β-1,4-linked N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) (green in Fig. 1). MurNAc residues harbor peptide stems (purple in Fig. 1) that can cross-link to other PG strands, forming a mesh-like structure. During bacterial growth and division, PG synthesis and remodeling ensure cell morphogenesis, and resistance to cytoplasmic turgor pressure.
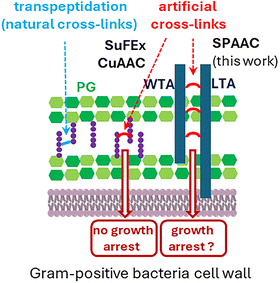 | ||
| Fig. 1 Structure of the cell wall of Gram-positive bacteria and illustration of the artificial cross-linking mechanisms described in previous publications with peptidoglycan (PG)4–6 and in this work with teichoic acids (TAs). | ||
PG biosynthesis is targeted by several classes of antibiotics, leading to growth arrest and breaches in the PG mesh, ultimately causing cell lysis. In search of new antibacterial mechanisms, a key question is whether introducing unnatural cross-links into the cell wall can impair bacterial growth. Schultz et al. first achieved synthetic PG cross-linking in Escherichia coli4 and Bacillus subtilis5 by metabolically incorporating D-alanine analogues bearing a latent electrophilic sulfonyl fluoride group. Following SuFEx click chemistry,6 these analogues could subsequently cross-link with a neighboring nucleophile (proximity-induced ligation). No significant effect on bacterial growth was observed, except in B. subtilis ΔdacA, a strain lacking the D-Ala-D-Ala carboxypeptidase PBP5. In this mutant, enhanced persistence of the D-alanine analogue led to high levels of unnatural cross-linking, resulting in slower growth and cell shape defects (elongated and curly cells). Later, Siegrist et al. incorporated D-alanine derivatives bearing azido and alkyne groups into PG, with cross-linking subsequently induced by CuAAC.7 Again, no impact on growth was noticed, and contrary to the desired effect, a protection against broad-spectrum β-lactams was reported.
The Gram-positive bacterial cell wall contains another biopolymer, teichoic acids (TAs, deep blue in Fig. 1), made of repeating phosphate, saccharide, N-acetylgalactosamine and alditol groups. There are two types: wall teichoic acids (WTAs), covalently bound to PG, and lipoteichoic acids (LTAs), anchored in the cytoplasmic membrane. TAs are involved in numerous cellular processes that include cell elongation and division, and maintenance of the Gram-positive periplasmic space, which helps resist turgor pressure.8,9 In this context, we investigated whether TA cross-linking could impair bacterial growth. We exploited the fact that S. pneumoniae TAs are decorated with phosphocholines, which can be selectively replaced by clickable choline analogues.10–12 Here, we evaluated the incorporation efficiency of various choline analogues into S. pneumoniae TAs, and explored the cross-linking of one analogue with an additional agent to artificially interconnect TAs and potentially hinder bacterial growth.
We first aimed to determine if choline metabolization into the TA can tolerate structural and functional variations of the clickable motifs. The SPAAC reaction was chosen because it involves the small azido group. The different types of “choline-like” azido compounds (Fig. 2) were inspired by the work of Tomasz, who reported that in the absence of choline in the growth medium, S. pneumoniae is able to incorporate ethanol amine instead, demonstrating that the presence of a tetra-alkyl ammonium function is not mandatory.13,14 Interestingly, Badger also showed that S. pneumoniae can grow in the presence of tri-alkylated ethanol amine (tertiary amines), but they did not demonstrate that tri-alkylated ethanol amine is actually incorporated into the TA.15 In addition to the conventional azido compound 2,11 its di-ethylated analogue 3 and the corresponding tertiary amine 4 were made.
Two analogues with a longer alkyl chain containing an azido group, the ammonium analogue 5 and the tertiary amine 6, were also synthesized, as well as the quaternary ammonium analogue 7 and tertiary amine 8 bearing two ethanol groups. Finally, a bulky group, inspired by the fluorogenic azido-coumarin16 used by Siegrist et al.,7 was grafted onto the choline unit to generate compound 9.
These molecules were evaluated based on three criteria: their impact on bacterial growth, their labelling efficiency during a 10 minute pulse-labelling period (Fig. S1, see ESI†), and their competitiveness compared to the incorporation of native choline (Fig. 2). Incorporation of the modified choline into TA was carried out at the same time as fluorescent labelling with DBCO-AF488 in C-medium, as reported previously.12 Fluorescence intensities were measured by flow cytometry, and the mean value obtained with compound 2 was defined as the reference. The incorporation of choline 2 into TA was also shown by NMR analysis of the LTA extract (Fig. 3 and Fig. S3, see ESI†). For pulse labelling, the quaternary ammonium compounds 2, 3 and 5 were globally effective, while the di-ethanol analogue 7 was inefficient. For tertiary amine analogues 4 and 6, fluorescence microscopy showed for the first time their incorporation into TA, albeit with less efficiency for compound 6 (62 ± 26%, p < 0.05), which harbors the longest alkyl chain. The azido-coumarin choline compound 9 was used with DIBO 10 to trigger a fluorogenic labelling,7,16 but no fluorescent signal was observed, indicating that the choline derivative was not incorporated by the bacteria, probably due to its bulkiness. Analysis of the bacterial growth rate showed that, compared to native choline, compound 2 is the least harmful of all the choline derivatives (Fig. S2, see ESI†). Taken together, these observations show that the metabolic incorporation of modified choline and bacterial cell growth tolerate moderate hindrance and modification. Incorporation into TA seems more permissive to choline modifications than cell growth, as illustrated by the data obtained with the quaternary ammonium compounds 3 and 5, and the tertiary amine compounds 4 and 6, which allow TA labelling but impair cell growth (Fig. 2).
 | ||
| Fig. 3 Portion of the 1D 1H spectrum of LTAs labelled with azido-choline 2, shown in Fig. S3 (see ESI†). The peaks corresponding to the methyl groups of choline and azido-choline were integrated, revealing a 60% content of azido-choline in the LTA sample. | ||
Following TA labelling with compound 2, the next step was to cross-link these polymers using the external agent 11, which has two DBCO groups on a polyethylene glycol chain (Fig. 4 and Fig. S4, see ESI†). To compare the effect of TA cross-linking with that of artificial PG cross-linking, we used azido-D-alanine-D-alanine (azido-DADA) 12,17–19 which places an azido group at position 4 of the pentapeptide stem in the PG. The concentrations were set at 250 μM and 2 mM for optimal incorporation of analogues 2 and 12, respectively. Bacteria were first treated for 10 min (pulse 1, Fig. S4, ESI†) with either native choline 1, azido-choline 2, azido-DADA 12, or both compounds 2 and 12, in chemically defined C-medium.20 After centrifugation to remove excess compounds, cells were resuspended in brain-heart infusion (BHI) medium with or without linker 11 (100 μM), and incubated for 10 min (pulse 2). Cultures were then diluted 30-fold in BHI and grown overnight in 24-well plates. When TAs were labelled with azido-choline 2, a 10 min incubation with 100 μM linker 11 significantly inhibited growth (57 ± 9% relative to the control without linker 11). To allow the SPAAC reaction to continue after the dilution into the growth medium, linker 11 was further added as a supplement at 10 μM, reducing the growth rate ratio to 25 ± 12%, relative to the control condition. At 10 μM, linker 11 showed no toxicity of its own. In the presence of 50 μM linker 11, the relative growth rate was reduced to 7 ± 2%. However, this was possibly due to a slight intrinsic toxicity of 11 at high concentration, as found in the control condition with choline 1 and 50 μM linker 11 (64 ± 6%). In contrast, the use of azido-DADA 12 and linker 11 did not significantly impact growth, suggesting that synthetic PG cross-links do not strongly impair cell growth, as previously reported for E. coli and B. subtilis.4,5 The reduced growth seen with azido-DADA 12 and 50 μM linker 11 (56 ± 1% relative to the control condition) was quite similar to that of choline 1 with 50 μM linker 11 (64 ± 6%), supporting a toxic effect of linker 11 at this concentration. It is noteworthy to observe that the joint addition of azido-choline 2 and azido-DADA 12 reproduced the same growth defect as the single addition of azido-choline 2. Therefore, there is no additional effect due to possible cross-linking between TA and PG. In a bacterium lacking choline-decorated TA like B. subtilis, incubation with azido-choline 2 and linker 11 had no impact on growth (Fig. S5, see ESI†). Similarly, E. coli, which lacks TA in its cell wall, was unaffected by the compounds.
To assess the impact of cross-linking on cell wall synthesis, S. pneumoniae cells treated with azido-choline 2 and linker 11 were incubated with a DBCO-linked fluorophore for 10 min (chase period, Fig. 5 and Fig. S6, see ESI†). Remaining free azido functions on TA were conjugated with the DBCO-fluorophore by SPAAC reaction. The distance between fluorescent bands reflects wall synthesis at the midcell following TA cross-linking. Demograph analysis (Fig. 5) revealed that linker 11 inhibited wall expansion, with a significant decrease of the mean distance between labelled bands (0.77 nm to 0.45 nm in the absence and presence of linker 11, respectively; p < 0.01). With longer chase periods (20, 40 and 60 min, Fig. 6), minimal change in the fluorescent banding patterns was observed, with most cells retaining midcell labelling. In contrast, cells not treated with linker 11 showed normal division, progressively diluting the initial fluorescence signal over time.
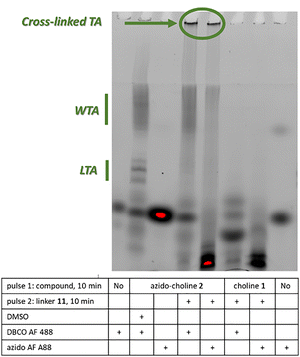
To confirm TA cross-linking, we applied our recently developed gel electrophoresis and fluorescence imaging method (Fig. 7 and Fig. S7, see ESI†).9 Cells labelled with azido-choline 2, with or without linker 11, were treated with PG hydrolases to digest the cell wall. Remaining free azido or DBCO groups were then reacted with complementary fluorophore-linked probes. Samples were analyzed by tris-tricine SDS-PAGE, and labelled species were visualized under UV. LTAs appeared as a ladder due to their lipidic nature and SDS interaction, whereas WTAs migrated as an upper smear bound to PG fragments. In the presence of linker 11, the LTAs shifted upward and became unresolved, with additional high-molecular-weight species retained in the wells. The appearance of these large, low electrophoretic mobility species indicates successful TA cross-linking via SPAAC between azido-choline 2 and linker 11.
In conclusion, we have demonstrated that, unlike artificial PG cross-linking, TA cross-linking can inhibit the growth of S. pneumoniae. This growth inhibition may be explained by a much higher grafting yield of azido functions into TAs compared to PG, or by the fact that TAs are not cross-linked by nature (unlike PG). Whereas conventional cell-wall targeting antibiotics inhibit the natural cross-linking of the bacterial wall, our mechanism operates in the opposite way, by artificially inducing cross-linking where none normally occurs. This entirely new mode of action constitutes a novel and promising therapeutic target, particularly relevant in the preoccupying context of antimicrobial resistance.
We acknowledge the financial support for this work from Agence Nationale de la Recherche (ANR-19-CE07-0035 and ANR-23-CE11-0029). We thank R.-L. Revel-Goyet, O. Glushonkov and J.-P. Kleman (IBS, Grenoble) for support and access to the M4D cell imaging platform. This work used the platforms of the Grenoble Instruct-ERIC center (ISBG; UAR 3518 CNRS-CEA-UGA-EMBL) within the Grenoble Partnership for Structural Biology (PSB), supported by FRISBI (ANR-10-INBS-0005-02) and Labex ARCANE and Labex GRAL, financed within the University Grenoble Alpes graduate school (Ecoles Universitaires de Recherche) CBH-EUR-GS (ANR-17-EURE-0003). IBS acknowledges integration into the Interdisciplinary Research Institute of Grenoble (IRIG, CEA). The authors acknowledge support from Institut de Chimie Moléculaire de Grenoble (ICMG, UAR 2607) for analysis facilities.
Data availability
Data presented in this work are available within the article and/or its ESI,† and upon request to the authors.Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Chen, S. Kumar and H. Wu, Arch. Microbiol., 2023, 205, 356 CrossRef PubMed.
- M. F. Chellat, L. Raguž and R. Riedl, Angew. Chem., Int. Ed., 2016, 55, 6600 CrossRef PubMed.
- H. Cho, J. Microbiol., 2023, 61, 359 CrossRef PubMed.
- D. A. Dik, N. Zhang, J. S. Chen, B. Webb and P. G. Schultz, J. Am. Chem. Soc., 2020, 142, 10910 CrossRef PubMed.
- D. A. Dik, N. Zhang, E. J. Sturgell, B. B. Sanchez, J. S. Chen, B. Webb, K. G. Vanderpool and P. G. Schultz, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2100137118 CrossRef PubMed.
- J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9430 CrossRef PubMed.
- S. L. Rivera, A. Espaillat, A. K. Aditham, P. Shieh, C. Muriel-Mundo, J. Kim, F. Cava and M. S. Siegrist, Cell Chem. Biol., 2021, 28, 213 CrossRef CAS PubMed.
- H. P. Erickson, Front. Microbiol., 2021, 12, 664704 CrossRef PubMed.
- M. Nguyen, E. Bauda, C. Boyat, C. Laguri, C. Freton, A. Chouquet, B. Gallet, M. Baudoin, Y.-S. Wong, C. Grangeasse, C. Moriscot, C. Durmort, A. Zapun and C. Morlot, eLife, 2025, 14, RP105132 CrossRef PubMed.
- A. Tomasz, Science, 1967, 157, 694 CrossRef CAS PubMed.
- A.-M. Di Guilmi, J. Bonnet, S. Peiβert, C. Durmort, B. Gallet, T. Vernet, N. Gisch and Y.-S. Wong, Chem. Commun., 2017, 53, 10572 RSC.
- J. Bonnet, Y.-S. Wong, T. Vernet, A.-M. Di Guilmi, A. Zapun and C. Durmort, ACS Chem. Biol., 2018, 13, 2010 CrossRef PubMed.
- A. Tomasz, Proc. Natl. Acad. Sci. U. S. A., 1968,(59), 86–93 CrossRef PubMed.
- T. Briese and R. Hakenbeck, Eur. J. Biochem., 1985, 146, 417 CrossRef PubMed.
- E. Badger, J. Biol. Chem., 1944, 153, 183 CrossRef.
- K. Sivakumar, F. Xie, B. M. Cash, S. Long, H. N. Barnhill and Q. Wang, Org. Lett., 2004, 6, 4603 CrossRef PubMed.
- G. W. Liechti, E. Kuru, E. Hall, A. Kalinda, Y. V. Brun, M. VanNieuwenhze and A. T. Maurelli, Nature, 2014, 506, 507 CrossRef PubMed.
- J. Trouve, A. Zapun, C. Arthaud, C. Durmort, A.-M. Di Guilmi, B. Söderström, A. Pelletier, C. Grangeasse, D. Bourgeois, Y.-S. Wong and C. Morlot, Curr. Biol., 2021, 31, 2844 CrossRef PubMed.
- E. Kuru, A. Radkov, X. Meng, A. Egan, L. Alvarez, A. Dowson, G. Booher, E. Breukink, D. I. Roper, F. Cava, W. Vollmer, Y. Brun and M. VanNieuwenhze, ACS Chem. Biol., 2019, 14, 2745 CrossRef PubMed.
- S. Lacks and R. D. Hotchkiss, Biochim. Biophys. Acta, 1960, 39, 508 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc02577j |
| This journal is © The Royal Society of Chemistry 2025 |