 Open Access Article
Open Access ArticleInnovative and sustainable approaches to NIR-active coatings for next-generation medical devices
G. S.
Lekshmi
*a,
Karthika
Prasad
 b,
Katia
Alexander
b and
Vignesh
Kumaravel
b,
Katia
Alexander
b and
Vignesh
Kumaravel
 *a
*a
aInternational Centre for Research on Innovative Biobased Materials (ICRI-BioM)—International Research Agenda, Lodz University of Technology, Lodz 90-537, Poland. E-mail: vignesh.kumaravel@p.lodz.pl; lekshmi.gopakumari-satheesh-chandran@p.lodz.pl
bSchool of Engineering, College of Systems and Society, The Australian National University, Canberra, ACT 2601, Australia
First published on 13th June 2025
Abstract
Designed to reflect or absorb near-infrared (NIR) light, smart NIR coatings have emerged as a transformative and sustainable solution in healthcare and biomedical fields. As longer wavelength allow for reduced scattering and absorption, NIR light exhibits superior penetration through biological tissues when compared to visible light, making NIR-based technologies extremely useful for both therapeutics and diagnostics. NIR coatings can be utilized for non-invasive imaging to monitor and control the performance of implantable devices, including drug release, biofilm disintegration and infection prevention, providing several advantages over the traditional drug administration, sterilization or antibiotic strategies. In this review, we explore key advantages of using NIR coatings in medical devices, highlighting the impact of their use on device efficiency, operational lifespan and performance, and their role in reducing the environmental impact of medical devices. Using recent examples, we identify pathways by which the use of NIR coatings can continue to drive the improvements in the key performance characteristics of medical devices while supporting the principles of circular economy, highlighting critical challenges and opportunities for this family of technologies.
1. Introduction
The circular economy (CE) model is a transformative approach that aims at maximizing resource productivity, reducing waste, and promoting sustainable innovation. In contrast to the linear economy that follows a “take-make-dispose” model, the circular economy is oriented towards keeping materials in use, designing out waste, and regenerative natural systems.1 In the healthcare sector, where the single-use mindset is driven by concerns over infection control and toxicity, and results in the generation of significant volumes of potentially hazardous healthcare waste that persists in the environment, the adoption of CE principles is essential for sustainable and responsible innovation.2 By shifting towards highly efficient smart and biodegradable materials, the healthcare industry can reduce its environmental footprint without compromising on safety, efficiency, and performance.One of the emerging solutions that is strongly aligned with the principles of circular economy is the development of near-infrared (NIR)-active coatings for future medical devices.3 Their unique properties make NIR-active coatings ideally suited for non-invasive sensing, photothermal therapy, and controlled-rate drug delivery, allowing them to meet increasingly complex demands of modern healthcare. However, traditional NIR-active materials are based on synthetic polymers and high-energy processing, raising questions about resource exhaustion and waste to the environment associated with their manufacturing and disposal. A circular economy model attempts to replace traditional materials with biocompatible, recyclable, and sustainable alternatives without compromising on medical devices being not only high-performing but also eco-friendly.4,5
Harmful chemicals such as phthalates, bisphenol A (BPA) and epoxy resins that are commonly used in medical devices, posing a significant risk to both patient wellbeing and the environment.5 The impact of leaching of such chemicals from medical devices like implants, catheters, and dental scaffolds have been reported to be more significant in vulnerable populations like children and dialysis patients.5 Increased awareness of the health and environmental risks associated with certain materials has driven the demand for safer, and more sustainable materials. Therefore, efforts to harmonize new materials and processes with sustainability goals have risen. Multifunctional coating is a promising approach which can improve the functionality of healthcare products and reduce waste generation. By integrating multiple advanced properties such as biocompatibility, antimicrobial activity, and wear resistance onto a device's surface in a single step, these coatings offer a multi-faceted solution to a variety of medical issues. The capacity of these coatings to impart substrates with customized properties makes them useful in various medical devices6 particularly in surgical instruments where coatings have the potential to enhance performance and lifespan.7
Of the different techniques, the application of NIR-active coatings is advantageous to the medical community in assisting in sustainability objectives. These materials not only contribute to extending the life of medical devices, but also play a significant role in enabling energy-efficient procedures and reduce dependency on disposables, marking a promising step towards a more sustainable healthcare. The greatest advantage, though, might come from their capacity to lessen the need for revision surgeries and the risk of hospital-acquired infections. These advancements directly address the major challenges in healthcare such as long hospital stays and the requirement of revision surgical procedures. While single-use disposable materials are relatively less expensive, the real costs come from avoidable complications. Since contaminated medical waste must first undergo decontamination, volume reduction, and specialized disposal before it can be dumped in a landfill, its management poses both financial and environmental challenges. In this regard, using long-lasting smart coatings presents an appealing way to improve patient outcomes while supporting environmental and financial sustainability objectives.
Nanoparticles, dyes, and polymers have been used in designing NIR-active coatings with appropriate electronic structures, allowing them to absorb light and effectively convert it into thermal energy through a photothermal effect.8,9 Compared to visible (380–700 nm) and NIR-I light (700–100 nm), NIR-II light (1000–1500 nm) has a better tissue penetration capacity, reaching depths of 3–6 mm,10,11 offering advantages of deeper tissue penetration and higher spatiotemporal resolution necessary for more detailed imaging of biological structures, e.g. solid tumours, and processes, and their targeted treatment. In clinical diagnostics and therapy, precise visualisation of deeper tissues is essential for early detection, real-time monitoring, and focused disease treatment. This is particularly important in applications like image-guided surgery, photothermal therapy, and drug delivery. The capacity to obtain detailed information from deeper layers minimizes the need for invasive procedures, enhances treatment planning, and improves overall outcomes.
NIR-active coatings can also be integrated into medical imaging techniques to enhance the visibility of surgical equipment, which can help improve the precision with which surgical interventions are performed. Increased penetration and reduced interference of NIR light with biological tissues allow surgical instruments to stand out clearly, enabling real-time monitoring and augmented reality integration for accurate navigation. This enhances surgical accuracy, reduces chances of accidental tissue damage, and increases overall safety of the procedure and patient outcomes. Therefore, NIR-active materials can be effectively utilized to coat a range of surgical instruments, including fiducials, catheters, surgical sutures, and gauzes,6 not only to show how adaptable these coatings are to different substrates, but also to allow for thermal activation, real-time intraoperative imaging, or antimicrobial activity.
Given their potential to deliver significant advances in the medical and healthcare technologies, in the recent years, the design and development of NIR-active coatings, and strategies for their integration in traditional and modern medical process and devices has been a subject of notable research efforts, producing a wide range of advanced materials and architectures. However, the potential impact of the use of NIR-active coatings on the environment footprint of healthcare has not been fully explored. This paper aims to bridge this gap by focusing on the key benefits the NIR-active coatings deliver across medicine and healthcare and linking them to the key challenges faced by modern and future healthcare with respect to meeting its sustainability objectives. To achieve this aim, the recent history and the current state-of-the-art of NIR-active coatings and their use in medical care are explored. It is not the intention of this article to provide a comprehensive overview of all the excellent research papers on various NIR-active material designs and uses that are available in the literature, as these have already been reviewed extensively by many. Therefore, the significance focuses on how key functionalities afforded by the use of NIR-active coatings can enhance the performance of medical devices and procedures in the context of the impact of these improvements on the device lifecycle, from manufacturing to disposal, timely and accurate diagnostics, treatment success and patient outcomes. Indeed, while introducing sustainable practices into e.g. material development and waste out design can deliver immediate direct benefits in reducing the environmental impact of the healthcare sector, and is often a focus for the material science community, the indirect benefits of improved patient outcomes (inclusive of accurate diagnostics, continuous active monitoring and preventative care, precise treatment and reduced medical errors, and patient safety post-treatment that can all be improved by the integration of NIR-active coatings) can play an even greater role by alleviating the burden on all aspects of healthcare, from energy consumption to transport. After that, the opportunities and challenges in the integration of NIR-coatings into existing and future diagnostic and therapeutic strategies are highlighted, in the context of regulatory constraints.
2. NIR-active coatings and the scope of their use in modern healthcare
2.1 A brief history of NIR-active coatings
Fig. 1 represents a road map of NIR-active materials that can be used for medical devices to enhance performance and sustainability, with the field developing rapidly, driven in part by the innovations in the design and synthesis of advanced materials. A biocompatible organic nanoparticle (2,3-bis(4-(phenyl(4-(1,2,2-triphenyl vinyl) phenylamino) phenyl) fumaronitrile (TPETPAFN)) for NIR-active medical imaging was developed in 2014.12 In 2015, research on functionalized gold nanoparticles (Au NPs) was reported, focusing on their potential to improve imaging quality and combat catheter-related infections (CRIs).13 The development of NIR-active semiconducting polymer nanoparticles in 2016 marked another milestone in improving imaging and therapeutic methods.14 The advancement of NIR-active superhydrophobic coatings for medical devices was a significant accomplishment in 2017 to modify the interactions between protein and cell-surface.15 This is important for applications that need to minimise unwanted cell adhesion or integrate tissue in a controlled manner. Additionally, one of the main issues with implant-associated infections effectively tackled by these coatings, as their intrinsic non-wettability significantly lowers bacterial attachment and biofilm formation. Advanced multimodal imaging nanoparticles were developed in 2018,16 facilitating combined visualization of molecules and detection of diseases. A novel NIR-active photocatalyst was developed in 2019 for antimicrobial applications in medical devices,17 where the Au/D-TiO2 structure provided for enhanced light absorption and effective a NIR light-triggered photocatalytic payload release of antimicrobial drugs, such as ampicillin sodium, the bacteria inactivating activity of which was further enhanced by the generation of reactive oxygen species. In 2020, the employment of NIR-II fluorescence imaging technology for surgical procedures was a significant milestone,18 delivering improved surgical precision by providing greater contrast between normal and abnormal tissues during surgical procedures.19 In 2022, eco-friendly and biocompatible hybrid films were fabricated with waterborne polyurethane coated with polydopamine (WPU-PDA).20 Subsequently, wearable health monitoring gadgets that use NIR coating were created to augment the functionality and utility of NIR as a pivotal technology for digital health.21 Over the past decade, a major technology breakthroughs in non-invasive medical devices have significantly improved the efficiency and accuracy of data collection in wearable health monitors, setting the stage for more precise, real-time health monitoring and tailored treatment in the years to come.Stretchable intrinsic photodiodes have been proven essential in advanced biomedicine and imaging technologies. A device that remains functional even when deformed, utilizing the spatially modularizable-assembled elastic (SAME) photoactive layer allows for spatial modular assembly of the elastic body. Two primary applications of NIR technology include (1) secure imaging with cryptography, which is enhanced by NIR light, enabling visibility through an opaque mask to the underlying pattern, and (2) real-time photoplethysmography (PPG) signal detection, demonstrating the device's capability to identify heartbeats and other signals under the maximum strains of up to 100%.22 These practical applications demonstrate that stretchable NIR-active coatings can be utilized for flexible optoelectronics and non-invasive, wearable medical diagnostics, particularly where optimal conformal contact with human skin is required. This research on NIR-active photodiode involves the utilization of a high-performance polymer donor, poly[(2,6-(4,8-bis(5-(2-ethylhexyl-3-fluoro)thiophene-2-yl)benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1',3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione)] (PM6), alongside a non-fullerene organic semiconductor, Y6, which effectively absorbs NIR light up to 950 nm. To achieve inherent stretchability and enhance optoelectronic performance without compromise, the active layer is combined with styrene–ethylene–butylene–styrene (SEBS) elastomer, facilitating conformal and lasting application on dynamic surfaces like human skin.22
2.2 Types of NIR-active materials
NIR-active dyes, graphene-based materials, Au NPs, carbon dots, other fluorescent materials, indocyanine green (ICG), polydopamine and black phosphorus (BP) have been commonly used to improve real-time monitoring, cancer treatment, and antimicrobial applications. Their properties are often optimized for a specific application. For instance, for implantable devices where the light is to penetrate biological tissues, the absorption and emission windows need to be optimized to prevent excessively strong absorption and scattering of light by said tissues, and consequently loss of imaging quality and illumination intensity, as well as potential tissue damage. In some particles, e.g. silver sulfide quantum dots, this can be achieved by changing the size of the particle, in others the wavelength of the surface plasmon resonance can be shifted towards NIR by changing the particle shape, with the hot spots preferentially created at the sharp tips of e.g. anisotropic gold particles having up to 106 times enhanced light intensity, which is likely to significantly affect the catalytic and thermal properties of these particles. Particle size may also play an important role in the emission quantum yield, as is the case in lanthanide-doped nanomaterials. In addition to fine-tuning the absorption and emission windows, surface engineering is also frequently employed to control the interactions between the nanoparticles and their environment to e.g. prevent agglomeration or stabilize dispersion (when used alone or during coating synthesis), control protein- and cell-surface interactions to prevent non-specific fouling, target a specific cell type, render the particle more biocompatible, or facilitate the attachment of bioactive agents for spatiotemporally-controlled drug delivery. The internal structure of the particle can also be engineered to enable drug loading and transport, improve particle stability, or tune its NIR light response. It is worth noting that the fabrication of NIR-active particles with a highly controlled architecture and surface functionalization, and additional functionalities, often requires the use of more complex and resource intensive, multi-step processes, high purity input materials and more controlled processing environments, which present a challenge for reducing the environmental footprint of such materials. For this reason, the development of single-step, low-energy one-pot fabrication processes that can use more readily available minimally-processed inputs remains a major priority for the materials research community.Integrating these NIR-active materials as coatings into medical devices is a logical extension of their application in healthcare to improve clinical effectiveness, reduce complications, and extend the lifespan of medical devices. Table 1 represents a comprehensive overview of the most advanced NIR-active coatings used for medical devices, highlighting the current directions for the field. It is worth noting that this summary focuses explicitly on the examples that represent the significant progress and outline the scope of their practical uses in this field, rather than being a comprehensive overview of all the available state-of-the-art. The use of NIR-active coatings on medical devices presents many advantages, such as accurate diagnostics and tailored targeted delivery of therapeutic agents. However, as in the case of the design of NIR-active particles with an increased degree of complexity and function, the environmental impact of the fabrication and integration of these particles into the coatings without compromising their function should be considered, and methods that minimize the use of resource-intensive steps actively pursued.
| Materials | Functional properties | Application | Ref. |
|---|---|---|---|
| Au NPs | Improved cell adhesion, aiding bone-implant integration | Implants | 23, 34 and 35 |
| Enhanced photothermal effects and bacterial eradication | Cancer treatment | ||
| Facilitated drug release | Stents and coronary angioplasty devices | ||
| Biocompatible and hydrophilic | Biosensors | ||
| Drug delivery | |||
| Graphene based materials | High photothermal conversion efficiency | Disinfection biointerfaces | 24 |
| Significant penetration depth and reduced off-target heating | Drug delivery systems | ||
| Carbon dots | Tunable fluorescence for targeted imaging | Implants | 26 and 28 |
| Easily functionalized for drug binding | Bioimaging | ||
| Inhibited biofilm formation | |||
| Sustainable synthesis process, energy-efficient and biocompatible | |||
| NIR-fluorescent materials | Deeper tissue penetration with minimal scattering | NIR active tattoos | 29 |
| Tumor identification and labeling | Surgical needles | 36 | |
| Fluoresce under UV and NIR light | Theranostic agents | 37 and 38 | |
| Improved in vivo imaging and support antibody–drug conjugates activated by NIR light | Implants | ||
| Indocyanine green (ICG) | FDA-approved NIR dye with excellent photothermal conversion | Cancer phototherapy | 39 |
| Real-time imaging and targeted delivery | Lymph node tracking | ||
| Short circulation time can be tuned with carriers | Wound monitoring | ||
| Polydopamine | NIR absorption | Orthopedic and dental implants | 30 |
| PTT and PDT effects with good surface anchoring properties and photostability | Wound healing | ||
| Black phosphorus (BP) | High NIR absorption, photothermal, and photodynamic properties | Bone regeneration | 32 |
| Biodegradable in physiological environments | Antibacterial coatings | ||
| Antibacterial and osteoinductive | |||
| Lanthanide-doped NIR-active materials | Dual-modality sensing | Smart orthopedic implants | 32 |
| High stability | |||
| High mechanical strength |
Photothermal treatment (PTT) with Au NPs is one of the most intensively researched ways of benefiting from the NIR-active behavior of these materials. Due to their distinctive optical properties across a wide spectrum from UV-vis to NIR, plasmonic nanoparticles and nanostructures based on Au NPs have numerous potential uses not only in medicine, but also in other fields, with the medical field benefiting from the advances in other sectors concerned with the development of novel particle designs and methods for their synthesis and modification, with positive impact on the speed of optimization due to growing understanding of the property-function behavior and the fundamental mechanisms which underpin NIR activity. In terms of their use in medical devices, an excellent example is the use of Au NPs to produce a thin plasmonic gold film on bare metal implantable stents, where the addition of the photothermal layer effectively reduces clots within the stent lumen, as demonstrated in multiple in vitro studies, including those simulating blood flow conditions.23 In another example, the NIR light can be used to locally increase the temperature at the surface of a bone implant, with the mild heat shock conditions (39–41 °C) effectively enhancing the production of proteins such as alkaline phosphatase (ALP) and heat shock protein (HSP), consequently facilitating bone mineralization.24
Graphene-based materials also exhibit significant potential for application in light-based therapies, attributed to their high photothermal conversion efficiency and exceptional broad-band optical absorption capabilities that stem from the closely spaced energy levels of loosely held π electrons. This facilitates heat production as the light-excited electrons return to their ground states.25 The absorption of NIR irradiation by graphene-based materials enhances their applicability in biomedical fields, as the longer wavelengths of NIR light and its minimal interactions with biological constituents (within the therapeutic window of 700–950 nm, where tissue absorption and scattering are diminished) facilitate significant penetration depth and reduced off-target heating. When integrated on the surface of devices as a bio-interface, NIR-activated graphene-based platforms can be used to e.g. mitigate bacterial attachment and biofilm formation, and in doing so may evolve into a more cost-effective disinfection strategy for surfaces and systems in the medical field. However, it should be noted that the environmental impact of the fabrication of high-quality graphene with a high degree of control of their functionalization and defects at a sufficiently large scale remains a challenge and a subject of intense research efforts.
Another important type of NIR-active materials, namely the NIR absorbing carbon dots (CDs), utilize polaron engineering to enable NIR-II in vivo imaging and photothermal therapy.26 Here, surface modification of CDs is often used to optimize their NIR-II emission in a variety of aqueous media, facilitating angiographic imaging and the identification of inflammatory sites.27 NIR-II-emitting CDs are capable of activation by an 808 nm laser, and these carbon dots demonstrated exceptional characteristics, including strong luminescence in the 900–1200 nm region, a quantum yield (QY) of 0.4%, and nontoxicity, indicating their potential as effective agents for NIR-II bioimaging in vivo.28
NIR-fluorescence materials have been developed as promising labeling agents for the sensitive detection and imaging of biological targets. In the near-infrared spectrum, biological samples exhibit minimal background fluorescence, resulting in a high signal-to-noise ratio (Fig. 2).29 A new endoscopic tattooing technique utilizes an NIR fluorescent marker instead of conventional blue dye was reported. The NIR fluorescent marker AFS81x makes it possible to mark colonic locations for at least 10 days without obscuring the view of the surgical planes or surrounding anatomical features. This method is an excellent example of how to incorporate NIR-fluorescent materials for real-time intraoperative guidance into clinical workflows. Building on this idea, coatings containing comparable NIR-active agents could be incorporated into implantable devices, sutures, or surgical instruments to allow for continuous visualisation, position tracking, or even photo-activated therapeutic effects both during and after surgery.
 | ||
| Fig. 2 Identification of locoregional lymphatics (A) and lymph nodes (B) on day one following endoscopic submucosal injection of NIR fluorescent dye AFS81x. Reproduced with permission from ref. 29. Copyright (2023), Springer Nature, open access article distributed under the terms of the Creative Commons CC BY license. | ||
Polydopamine (PDA) based coating containing such NIR-active materials as cyanine can facilitate the integration of efficient photothermal antibacterial properties with improved osseointegration. Polydopamine effectively converts NIR light to heat due to its conjugated structure, hence imparting photothermal properties to the coated material.19 For example, a PDA-based coating created by anchoring TiO2 NSs-Cy7 composites onto the titanium surface has been shown to effectively mitigate bacterial attachment and biofilm formation, while at the same time promoting osseointegration of the implant into the bone matrix. Post-operative infection and aseptic implant loosening due to limited osseointegration are key factors of implant failure, and their mitigation comes at a considerable cost to patient quality of life, and the healthcare, as implant removal and replacement is often required. TiO2 nanostructures facilitate a uniform dispersion of Cy7 within the PDA coating, enabling effective and uniform PTT treatment. The exceptional dual-photothermal conversion properties of PDA and Cy7 enable the formulated photo thermal coating to deliver localized heating that is sufficient to induce substantial leakage of bacterial cytoplasm, resulting in remarkable PTT antibacterial efficacy. Additionally, photothermal coatings can proficiently eradicate biofilms formed by E. coli, S. aureus, S. mutans, and P. gingivalis,30 providing a more effective alternative to systemic antibiotics that often have limited potency against bacteria in their biofilm form due to the protective nature of the latter.
Black phosphorus (BP) exhibits a significant absorption coefficient in the visible and near-infrared spectral ranges, rendering it a suitable candidate for optoelectronic applications. The absorption coefficient is approximately 105 cm−1, significantly exceeding that of other two-dimensional materials, including graphene and transition metal dichalcogenides.31 BP demonstrates significant light–matter interaction owing to its substantial oscillator strength and excitonic effects. Excitons are electron–hole pairs bound by the Coulomb interaction, significantly influencing the optical characteristics of BP. The exciton binding energy of BP is around 0.4–0.5 eV, significantly exceeding that of other two-dimensional materials, including graphene and MoS2.32 BP also demonstrates robust photoluminescence (PL) in the visible and NIR spectral ranges. The photoluminescence emission results from exciton recombination and can be adjusted by varying the material's thickness.32
Neodymium (Nd) and ytterbium (Yb) co-activated Sr3Sn2O7 phosphors (Sr3Sn2O7:Nd3+,Yb3) have been actively investigated for their ability to deliver upconversion luminescence (UCL) in response to temperature and mechanoluminescence (ML) when subjected to mechanical stimuli, such as mechanical force, bending, and twisting, and consequently their potential in monitoring the outcomes of the e.g. knee replacement surgeries, as localized increases in temperature can be indicative of infection and inflammation of the peri-implant milieu in the absence of local fever. It is also worth noting that delayed postoperative implant infections, where the infectious agent is not necessarily introduced during surgery but instead introduced via e.g. blood transfer from another infected site (e.g. during dental work) months or years after the surgery, is another common cause of implant failure that can be potentially mitigated using NIR-active coatings. These coatings are made of two sensing systems, force and temperature, which are monitored noninvasively and stimulated with NIR. This enables real-time assessment of the success of knee replacements, and orthopedic and load-bearing medical implants more broadly.33
3. Significance of NIR-active coatings in healthcare and medical devices
There has been a significant interest in smart coatings in recent years due to their remarkable properties, such as self-healing, self-cleaning, anti-fouling, anti-corrosion, and superhydrophobic functionalities.40 For NIR-active coatings, the possibility of integrating multiple functionalities into a single coating is an area of research that is actively pursued. For example, NIR-responsive coatings with a self-repair property have been developed to extend the functional life-time of the coating, and in doing so increase the operating life-span of medical devices and delay their replacement.41 Here, the multilayered nanocomposites of SHPP (SIM@HMSs@PDA/PEG/PPy) combine drug delivery with photothermal responsiveness.41Fig. 3 shows the efficiency and photothermal behavior of SHPP coatings under NIR laser irradiation at 808 nm. Fig. 3(a) shows a rise in temperature from 27.1 °C to 79.0 °C within 4 min of NIR light exposure, indicating NIR light absorption and efficient conversion of energy to heat. Importantly, the material exhibited excellent thermal cycling stability over numerous ON/OFF irradiation cycles, which is crucial for maintaining a consistent therapeutic output over time (Fig. 3(b)). Fig. 3(c) demonstrates the tunable heating effect at varying laser intensities, providing precise thermal control based on the clinical need, which is an important factor in precision medicine where the aim is to tailor the treatment to maximise the therapeutic efficacy against the target cells or tissues while at the same time minimizing side-effects, such as thermal damage of adjacent healthy tissues. These properties underscore the transformative potential of NIR-active coatings in developing the next-generation medical devices that perform structural functions along with smart therapeutic functions, such as localized infection control, wound healing, and targeted drug delivery with minimal patient discomfort and high biocompatibility. Fig. 3(d)–(i) provide illustrations of the coating system's self-healing behaviour. The material can self-repair integrity, as evidenced by the recovery of surface cracks and scratches following thermal activation in SEM images (Fig. 3(d) and (e)) and optical micrographs (Fig. 3(f) and (g)). This is explained by the material's ability to flow and fill in damaged areas due to the heat-responsive mobility of embedded components like wax or polymer domains. The suggested self-healing mechanism, in which heat causes material redistribution and crack closure across the damaged interface, is further illustrated in the schematics (Fig. 3(h) and (i)). Such self-healing properties are especially useful for preserving coating performance in dynamic or damage-prone biomedical environments, particularly when triggered by localised photothermal effects (as illustrated in Fig. 3(a)–(c)). | ||
| Fig. 3 (a)–(c) SHPP nanocontainers and associated systems’ photothermal and self-healing capabilities. SHPP's photothermal behavior under 808 nm NIR irradiation, demonstrating efficient and consistent heating at various power densities. Reproduced with permission from ref. 41 Copyrights (2023), Elsevier. (d)–(g) SEM and optical micrographs showing surface recovery and crack closure before and after thermal treatment.40 (h) and (i) A schematic illustration of the coating's thermally induced self-healing mechanism.40 Reproduced with permission from ref. 41 Copyrights (2024), John Wiley and Sons, open access article distributed under the terms of the Creative Commons CC-BY-NC license. | ||
In addition to imparting additional functionalities on the surfaces of permanent devices, e.g. titanium implants, NIR-active coatings hold significant potential in tissue engineering, contributing to improved therapeutic outcomes as well as allowing researchers to continuously observe the important scaffold behavior, e.g. material degradation dynamics, under in vivo conditions without the need for explanation. This is important for scaffold optimization, as well as for timely detection of scaffold failure due to e.g. premature mechanical failure that could compromise function restoration and tissue regeneration outcomes. For example, when a biodegradable citrate-based scaffolds (BPLPMGd) containing NIR-fluorescent lanthanide complexes were implanted into the body, NIR imaging was successfully used to track scaffold integrity, degradation and performance over time. As shown in Fig. 4(A)–(C), upon irradiation, the scaffolds emitted a powerful NIR signal right immediately after implantation, followed by a slow decline in emission intensity that coincided with the scaffold material deterioration. Such minimally invasive highly spatially- and temporally-resolved real-time imaging of scaffold position, integrity, and interaction with the biological milieu have the potential to not only increase patient control and treatment accuracy, but also open the door to the development of sophisticated intelligent implants that are able to self-report their biological and structural status. Such advances will alter the way that diagnostic tools and existing materials are studied and used in clinical settings.42
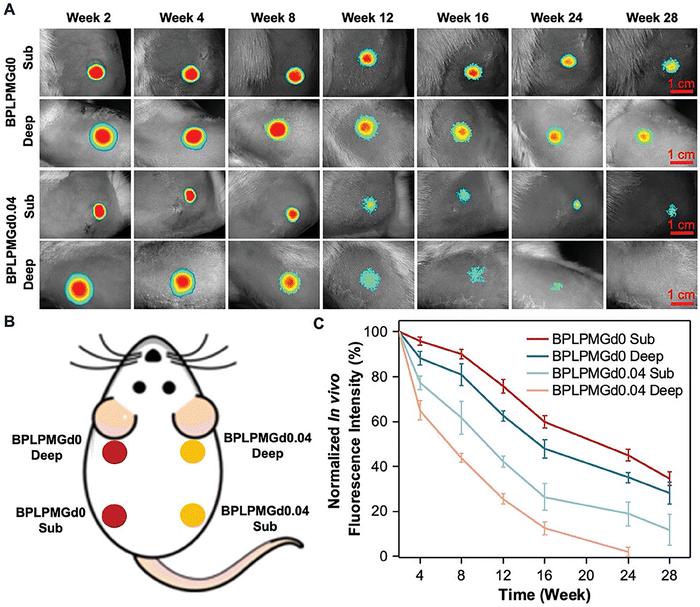 | ||
| Fig. 4 (A) In vivo fluorescence pictures of BPLPMGd0 and BPLPMGd0.04 scaffolds implanted at various time points are compared. (B) A map showing where the scaffolds are implanted. One side of the SD rat received subcutaneous and intramuscular implantation of BPLPMGd0 scaffolds (designated as BPLPMGd0 Deep and BPLPMGd0 Sub, respectively), while the other side received subcutaneous and intramuscular implantation of BPLPMGd0.04 scaffolds (designated as BPLPMGd0.04 Deep and BPLPMGd0.04 Sub, respectively). (C) BPLPMGd scaffolds’ in vivo fluorescence patterns alter as they degrade. Reproduced with permission from ref. 42. Copyright (2025), John Wiley and Sons, Open access article distributed under the terms of the Creative Commons CC-BY-NC license. | ||
As summarized in Fig. 5, by prolonging the life and enhancing the performance of devices across the range of medical devices and healthcare applications presented in Fig. 1 the use of these coatings can help the healthcare sector meet several United Nation's sustainable development goals (SDGs), including those related to health, industry, and sustainability, by improving the efficiency and durability of medical devices, promoting better health care outcomes and delivering reliable health care services (SDG 3). As previously noted, the main sustainability benefits of using NIR-active coatings are related to improving patient outcomes (though continued potentially non-invasive monitoring essential for prevention and early intervention, timely and accurate imaging and diagnostics, and more precise surgical interventions and targeted therapeutic delivery with a greater degree of spatiotemporal control), reducing the use of potentially hazardous chemicals (by providing a more effective alternative to the current range of devices and treatments), and increasing the durability, longevity and safety of medical devices (thus minimizing waste and positively contributing to the aforementioned two strategies).
In this context, the following sub-sections will explore several other examples of how the use of NIR-active coatings can help reduce the environmental impact of healthcare, while also addressing some of the common concerns regarding their use in the selected applications.
3.1 Next-generation imaging and diagnostics
As previously noted, the outcome of surgical procedures in e.g. the treatment of cancer is closely linked to the ability of the surgeon to remove all of the diseased tissues while minimizing the excision of healthy tissues, as incomplete removal may result in cancer returning or metastasizing, whereas excessive removal of healthy tissues can delay healing and reduce function, and NIR-based technologies have been used to accurately image diseased tissues. Similarly, the ability to monitor the position of the surgical instruments at all times prevents unintentional damage to tissues and organs during all types of surgery. Imagine-guided surgery holds much promise for the delivery of precision surgical outcomes and improvement of patient safety, however its practical realization requires the development of coatings with very high brightness and photostability. With this goal in mind, researchers have used lipophilic dyes from the cyanine 7.5 family and poly(methyl methacrylate) (PMMA) to develop a near-infrared coating of equipment (NICE), which demonstrated significantly enhanced brightness and photostability when compared to conventional coatings based on ICG. Fig. 6 shows the performance of these coatings during surgery. The use of cyanine 7.5 (Cy) dyes containing hydrophobic alkyl chains (C18), which are a rigidified polymethine backbone, resulted in better photostability, whereas bulk tetraphenylborate (TPB) counterions prevented dye aggregation and subsequent leakage from the coating. These dyes were embedded in a biocompatible PMMA matrix, resulting in coatings that were 15 to 20 times brighter than traditional ICG-based dyes. In addition to exhibiting high NIR emissions, the coatings were shown to be resistant to biological degradation. The biocompatibility test of these coatings with HeLa cells showed normal adhesion, morphology, and over 90% viability, with no signs of cytotoxicity to the tested cell line. The concept has been validated in porcine and human cadaver models. This example demonstrates the potential of NIR-active coatings to assist surgeons in accurately detecting and tracking the devices in real-time, leading to improved precision, visualization, and the ability to perform minimally invasive treatments, leading to expedited healing periods, less patient distress, and reduced levels of radiation exposure.3 Additionally, NIR-active coatings have the ability to provide real-time feedback on the device location and movements, allowing surgeons to refine their technique and achieve optimal placement of implantable devices. | ||
| Fig. 6 In vivo imaging in porcine models. (a)–(c) The ureter (indicated by two arrows) is identified during the image-guided surgery using an inserted catheter highlighted with a Cy-C18 TPB-based coating material. (d) and (e) The gauze coated with Cy-C18 TPB-based coating material soaked in blood during a surgical procedure was identified using a NIR laparoscopic system. (f) Four corners of the pathologic lesion inside the stomach were endoscopically marked by Cy-C18 TPB-coated polyglactin surgical suture pieces and visualized transmurally (from the exterior gastric surface) using the NIR laparoscopic system. While light and NIR imaging were performed at the same time. Reproduced with permission from ref. 3 Copyright (2020), Elsevier. | ||
3.2 Reducing reliance on harsh chemicals
The benefit from using NIR-active coatings in the context of reducing the reliance of healthcare sector on the potentially-harmful agents is two-fold. For one, the use of non-toxic NIR-active coatings can potentially either limit or eliminate the use of chemicals or contrast agents during diagnostic procedures and surgery, due to their ability to emit light when subjected to NIR irradiation. The use of conventional contrast agents and chemicals used in medical imaging and treatment often gives rise to side effects, from allergic reactions to kidney injury, or even cancer. Hence, their replacement with technologies that rely on NIR-active materials is likely to enhance patient safety,43 and minimize the environmental footprint related to the manufacture, packaging, transportation, and disposal of such chemicals, which aligns with the increasing focus on sustainable practices in the healthcare sector. In addition, by introducing the NIR-active coatings onto surgical, implantable, and monitoring devices, the expenses incurred from purchasing, storage, and disposal of contrast agents can also be reduced, providing a more cost-effective alternative for the hospitals. Therefore, using NIR-coatings on medical devices will transform the field of medical imaging, both from the point of view of patient safety and ecological sustainability. Secondly, by integrating functions such as minimally-invasive monitoring and disinfection, directly onto the implantable devices, the number of procedures, and therefore single-use materials and devices that are commonly associated with hospital visits and stays can be reduced.3.3 Biocompatibility
As noted earlier, in medicine, many of the uses of NIR-active materials rely on both their biocompatibility and ability to deliver the intended function when in contact with biological media, which holds true for both short- and long-term applications. This is because undesirable chemical reactions between the NIR-active materials and e.g. thiols may not only result in cell damage and death, but also compromise their optical properties, resulting in fluorescence quenching or signal loss,44 which creates significant challenges for high-quality imaging. To circumvent these issues, several NIR-active dyes have been specifically designed to resist interaction with thiols. One such example is a CS-thiol fluorescent probe that has been created to precisely and accurately detect biothiols, such as cysteine and glutathione, in living organisms.44 This probe has a significant disparity between the excitation and emission wavelengths, also known as a Stokes shift. This property overcomes the limitation of tiny Stokes shifts encountered in earlier NIR probes and enables effective imaging in biological applications. Additionally, CS-thiol is mainly non-toxic and has been successfully employed to image thiols in vitro and in vivo in animal models.44 It should be noted that although CS-thiol and similar agents have been engineered for biological imaging, a detailed examination of its chemical composition, production process, and degradability must be conducted to comprehensively assess their environmental footprint.Strategies that improve the biocompatibility of NIR-active materials are also being actively investigated, including by leveraging their NIR activity. For example, in a recent study, Ti3C2 was used to create a bioactive nanocomposite coating containing polydopamine and poly(vinylidene fluoride trifluoroethylene), referred to as PDA/Ti3C2/P(VDF-TrFE), where NIR-triggered photothermal effect was used to enhance implant osseointegration.45 Mild thermal stimulation from NIR irradiation was shown to improve osteogenic differentiation, promote cell growth, and upregulate the key osteogenic markers while exhibiting antibacterial ability against Staphylococcus aureus and Escherichia coli, with the results shown in Fig. 7. The coating also induced macrophage polarization, which makes it highly promising for bone tissue repair and regeneration in the clinical use of implants. Through rigorous checks on non-toxicity, manufacturers can make NIR-active coatings effective and harmless for a broad range of medical uses, contributing to improved patient outcomes and enhanced acceptance into clinical use.
 | ||
| Fig. 7 Photographs of the bacterial colonies of S. aureus (a) and E. coli (b) after 24 h of incubation with different coating samples under NIR (+) or NIR (−). Bacterial live/dead staining images (c) and (d) and bacteria reduction rate (e) and (f) of S. aureus and E. coli incubated with different samples under NIR (+) or NIR (−). Reproduced with permission from ref. 45. Copyright (2024) Elsevier. | ||
3.4 Photostability
Photostability is crucial for NIR-active medical coatings to ensure consistent performance over their intended operation lifetime, however this can be particularly challenging when several functions are integrated within a single platform. The photostability of the coating can be significantly improved by carefully selecting and integrating multiple elements within a single coating. For example, a robust and stable phototherapeutic system was developed using a combination of polydopamine (PDA), black phosphorus nanosheets, and zinc oxide (ZnO) nanowires on titanium (Ti) substrates.46 Under the NIR light irradiation, this system demonstrated long-term durability and high efficiency in mitigating bacterial attachment and biofilm development by using synergistic dissipation and killing. Even though black phosphorus nanosheets and ZnO are not inherently photostable, integrating them with PDA increases their photostability significantly, especially on Ti plates since Ti plates are photostable and chemically inert. The antibacterial capability of the system was enhanced over time due to the prolonged release of Zn2+ ions from ZnO, further extending the effective lifetime of the functional coating.Inversely, the elements in the composite can be selected so that the behavior of one promotes the degradation of the other under a predetermined set of conditions, with one application being the controlled degradation of Mg-based implants where the degradation of the NIR-protective coating controls the rate of degradation in the substrate. One example of such a coating is a composite coating containing hybrid polycaprolactone/ICG (H-PCL/ICG).47 When coated onto AZ31 magnesium alloys, in the absence of NIR light, the coating afforded good protection to the substrate, with ICG enhancing the compactness of the PCL matrix, and hence its degradation-retarding ability. When exposed to NIR light with a wavelength of 808 nm, however, the ICG absorbed heat, with the resultant localized heating triggering the glass transition in H-PCL, increasing chain mobility and allowing for greater penetration of the electrolyte to the underlying Mg alloy substrate, and the more rapid degradation of the latter. In vitro biological experiments indicated that the synthesized coating exhibited good cytocompatibility, the inherent photobleaching and degradation of ICG performance was observed over multiple NIR light exposure cycles. The latter issue may be addressed by replacing ICG with more photostable dyes with good NIR absorption, such as lipophilic cyanine-7.5 dyes, especially for coatings where NIR activity for extended periods of time is required.3 Despite this limitation, the study showcases yet another strategy for effectively controlling the behavior of implantable devices in vivo, in this instance by actively controlling their degradation, which is difficult to achieve with more conventional biodegradable coatings.
3.5 Degradation
For effective and safe functioning of biomedical devices, NIR-active coatings must degrade in a predictable and controlled manner, to avoid the coating degrading prematurely or remaining longer than necessary, affecting the performance of the device or causing biocompatibility issues. Controlled degradation mechanisms allow for timely release of therapeutic drugs, minimize toxic byproducts, and allow for natural excretion from the body. Importantly, as already touched upon in the example in the previous section, the photothermal activity of NIR-active dyes such as ICG can be used to initiate and control the temporal dynamics of the degradation of the underlying substrate. Polypyrrole (PPy) is another photothermal material that can be integrated with PCL into a coating designed to degrade under specific light irradiation,46 and in doing so trigger the degradation of biodegradable Mg alloys. When exposed to NIR at 808 nm, the photothermally-active PPy produces localized heating, reaching the low melting point temperature of PCL within 6 min. The rapid increase in temperature enables the coating to react automatically to scratches or defects and restore its corrosion-resistant function. The controlled degradation property of the Mg alloy is achieved based on synergistic effects between PCL's low-melting-point plastic deformation and PPy's photothermal effect. This cutting-edge coating technology can also find potential application in biomedical devices with controlled degradation and self-healing capabilities.46 This method also allows for the development of highly sophisticated NIR-active coatings that integrate functionality, safety, biocompatibility, and degradability.In another study, a nano-enhanced thermogenic stent (NETS) was developed, featuring a thin layer of Au nanorods to facilitate photothermal ablation for dissolving blood clots under NIR irradiation to prevent thrombolysis.23 The coating converted NIR light into localized heat for producing photothermal therapeutic effects, consistently reaching the temperature of around 60 °C. The stability of coating was evaluated by perfusing a buffer over the coated stents at an arterial shear rate of 1500 s−1 for 6 to 8 h each day over a period of 30 days, as shown in Fig. 8. The results indicated that the temperature generated upon irradiation with the NIR laser remained consistent for 30 days, with recorded temperatures of 60, 61, 58, and 59 °C on day 0, 10, 20, and 30 of operation, respectively.23 NETS could effectively induce photothermal clot ablation in vivo and in vitro in thrombus models, with up to 73% lysis confirmed through biochemical and imaging assays. This coating has the potential to be used in biomedical devices requiring controlled degradation while also facilitating the development of sophisticated NIR-active coatings that integrate functionality, safety, and biocompatibility.
 | ||
| Fig. 8 IR thermal images of a stent coated with an NIR-active material subjected to a hydrodynamic shear at 1500 s−1 to mimic the arterial shear, and operated for 30 days. Within each panel, the cursor represents the spot temperature, and the vertical pseudo-color bar signifies temperature intensity from high (yellow) to low (dark blue). Reproduced from ref. 23 (ESI). Copyright (2024), Royal Society of Chemistry, open access article distributed under the terms of the Creative Commons CC-BY 3.0 license. | ||
3.6 Immunological response
It is essential to screen NIR dyes for their ability to cause an immune response. NIR coatings should thus be screened for their ability to cause phototoxicity, cytotoxicity, and genotoxicity, since these characteristics can affect their safety and effectiveness when used in biomedical applications.48 At the same, controlled induction of the immune system can be leveraged to enhance the therapeutic outcomes. For example, local photothermal and photodynamic therapy can induce apoptosis in cancer cells by generating local heat and reactive oxygen species (ROS), respectively. High temperatures can trigger immune responses by inducing the transcription of heat shock proteins and enhancing the binding of lymphocytes to tissues.48 The release of cancer-specific antigens and signaling molecules from damaged cancer cells can in turn activate the immune response, acting as a form of immunotherapy, and augmenting anticancer efficacy of the treatment. The application of melanin-like polydopamine-coated nanoparticles, which combine immune modulation and NIR-induced PTT, is an effective example. These nanoparticles produced photo-induced heat to destroy tumour tissue when exposed to laser radiation, upregulating HSP70 and improving antigen presentation to dendritic cells, which ultimately resulted in systemic antitumor immunity.49 Black phosphorus nanosheets functionalised for NIR-PDT were used in another study, which showed the potential of NIR-responsive materials as cancer immunotherapy enhancers by ablating tumours and causing the release of tumor-associated antigens (TAAs) and cytotoxic T cell activation.50 In this study, anti-CD47 antibody (aCD47) immunotherapy was used in conjunction with black phosphorous nanosheets as photothermal agents. The BP nanosheets produced enough localised heat to ablate tumour tissues when exposed to NIR laser radiation. Tumor-associated antigens (TAAs) were released, and cytotoxic T cells were activated as a result of this photothermal effect in conjunction with aCD47-mediated immune checkpoint blockade. Both innate and adaptive immune responses were improved by the synergistic approach, indicating the potential of NIR-responsive materials as enhancers of cancer immunotherapy.4. Circular economy
Fig. 9 illustrates the key aspects of the adoption of NIR-active coatings in healthcare in the context of addressing the principles of circular economy and sustainability. An important fundamental principle in circular economy is longevity, because it helps to reduce waste and resource consumption in a long term.51 As environmental impacts associated with the premature failure of devices intended to operate over a long term and disposal of single-use medical devices are a growing concern in the era of technology-driven healthcare, both from the perspective of resource consumption associated with manufacturing and waste management of devices themselves and of extended hospitalization and revision surgeries, increasing the operational life span of medical instruments using polymer-based NIR-active coatings on metallic surfaces is a potentially effective strategy to address both. The of such coatings can reduce corrosion, abrasion and frequency of replacements by increasing the self-healing properties of the coatings.52 Since biodegradable polymers like PLA and PCL naturally break down, including NIR-active compounds within the polymer matrix can improve performance of both permanent and biodegradable implants while lowering waste generation. By using minimally toxic carbonaceous materials like CQDs as part of the NIR-active coatings based on polymeric networks, the environmental impact can be minimized further by using sustainable sources such as food, agricultural or biomass wastes and non-toxic chemicals for their fabrication.53 By incorporating polymeric materials in the coatings with reversible bonds, NIR-active coatings could be impacted with self-healing properties,54 extending the useful life of both the coatings and the underlying substrate and hence reducing waste generation and resource consumption associated with their fabrication, replacement and disposal. Over time, this strategy can help mitigate the release of waste into the environment.The process of making, applying and curing of NIR-active coatings have the potential to be more energy efficient, reducing the overall carbon footprint related to medical device manufacturing. NIR-active coatings can be cured and activated without the use of high temperatures. This makes such coatings helpful in reducing energy consumption, lowering emission and can be used as an ecofriendly alternative to conventional strategies.55 When applied to surgical devices, these coatings can actively resist bacterial attachment and biofilm development due to their ability to self-sterilize using NIR light, allowing for their safe reuse rather than disposal. Thereby it can contribute to the circular economic practices in healthcare.
Medical devices are ranked high on the target list in countries where remanufacturing is a well-developed industry. As it stands now, North America and Germany are the main leaders in the field of remanufacturing medical devices. General electric (GE), Stryker, Vanguard AG, Meditek ReNew, and the Association of Medical Device Preprocessors are involved in such practices. Stryker, an American company, does medical remanufacturing services by following a six-point procedure to ensure safety, quality and compliance.56 The six-point procedure includes collecting medical waste, sorting and identifying it, disassembly and cleaning, inspection and testing, reassembly and performance verification, and sterilizing and packing. Medical centers are increasingly turning to recycling plans that address the disposal of items such as plastics, metals, and electronic scrap (e-waste) from medical devices.57 This enables subsequent use of valuable materials and diminishes the quantity of waste sent to landfills. However, the recycling of medical waste is energy- and resource-intensive, and its efficiency is largely hindered by the problems connected with sorting and decontamination. It also relies on the collective initiative and efforts from both the healthcare system and recycling enterprises in order for the medical waste to be recycled. The use of NIR-active coatings to extend the lifetime and reuse of medical devices can complement the implementation of sustainable reuse initiatives, by reducing the number of devices that require remanufacturing while maintaining their safety and efficacy.
One more typical challenge in the implementation of principles of circular economy in healthcare is to do with the management of drugs, with current initiatives focused on finding less toxic alternatives, and setting up take-back programs aimed at preventing the incorrect disposal of unused or expired medication to mitigate environmental pollution.58 By developing multi-functional NIR-active coatings capable of targeted drug delivery or non-drug-based therapeutics, it may be possible to reduce the environmental impacts resulting from the production, use and disposal of conventional medication, thereby improving sustainability of the healthcare sector.
5. Regulatory and safety considerations
Ensuring the safety of patients and medical staff is key for the successful use of NIR-active coatings in biomedical applications. It is therefore essential that regulatory and safety measures, as well as industry standards, are met at all points in their lifecycle.5.1 Regulatory compliance
Biocompatibility measurements should be made using ISO standards to ensure the NIR-active coatings are safe. It shows the fundamental and vital facts, such as the clinical effectiveness of medical devices. This consists of cytotoxicity, genotoxicity, and systemic toxicity, which are implemented in the quality management system. The FDA classifies devices for medical purposes into category 1, 2, or 3, and for each class, thorough toxicological tests are administered by the manufacturers during their production. The example of the producer's compliance with the guidelines of ISO and FDA shows their concern for the patient's safety, preventing adverse reactions, and the assurance of product safety. With the help of these regulations, companies can get their product approved more rapidly and more efficiently into the market.5.2 Toxicological assessment
Even though NIR-active medical coatings are promising, they require thorough toxicological analysis because of their dynamic interaction with heat, light, and ROS generation. If not, they can damage tissues, cause systemic toxicity, or cause regulatory failure. A comprehensive toxicological assessment of an NIR-active coating is mandatory, which includes the evaluation of potential leaching of any chemicals from the coatings to the environment or surrounding living tissues.59 Furthermore, the possibility and impact of the accumulation of degradation products over prolonged use on the human health and the environment should be comprehensively evaluated. Even though the end-of-life circularity of medical coatings through chemical recycling is a significant advancement towards sustainable design, assessing the fate of any potential degradation products that may enter the environment during the remanufacturing process should also be considered, as these products too can accumulate and impact the ecosystems. Therefore, evaluating their long-term stability and possible toxicity is important to ensure that the closed-loop recyclability of the material does not inadvertently introduce new risks. This dual focus on circularity and in-build biodegradation pathways aligns with the principles of sustainable and safe-by-design materials for healthcare applications.60 Since the constituent materials that make up NIR-active coatings may retain their light-activated enhanced chemical and biological reactivity after their intended use, their potential toxicity with respect ROS generation, cancer induction, and effect on reproductive health need to be investigated not only in the context of their intended use but also when they enter the ecosystem, considering differences in the physical and chemical properties of environment, as well as different stages of degradation. It is important to note that despite significant progress in the design and out understanding of in vitro systems, they often fail to fully replicate the complexity of in vivo or ecological systems. Therefore, it is essential that in vitro studies are complemented by appropriate in vivo studies and post-approval FDA monitoring for human applications, and comprehensive environmental assessment for materials in accordance with pathways in which they may enter the environment. By performing these toxicology tests, the risks associated with the use of NIR-active coatings to human and environment can be identified and reduced.5.3 Clinical evaluation
Before implementing the NIR-active coatings in the clinical testing, it is important to conduct clinical trials to ensure their safety and effectiveness. These trials can help identify the potential dangers associated with the NIR coatings and help acquire crucial data on their performance as well. Even though human studies are important for assessing clinical effectiveness, preclinical animal studies can offer better insights into the biological aspects in a more controlled manner.61 However, more real-time clinical studies are required to confirm the long-term biocompatibility and safety of the NIR-active coatings. Most current research is performed at the laboratory level and often focuses on understanding the theoretical benefits of their use, which hinders the translation and broader adoption of NIR-active coatings in healthcare settings.The study involving benzyl violet 4B (BV-4B), a fluorescent dye added to FDA-approved surgical sutures, is a noteworthy illustration of clinical research in this field.62 BV-4B has been used to monitor the in vivo degradation of sutures and provides real-time, non-invasive visualisation of sutures under both NIR-I and NIR-II imaging (Fig. 10). The study's clinical significance rests in its successful demonstration of safe, dependable imaging in a human-relevant setting, which is a crucial step towards regulatory approval and wider clinical adoption, even though the dye performed mainly as anticipated. The practical value of incorporating NIR-active components into well-established medical materials is further supported by the potential benefits of real-time visualisation of suture degradation, including improved patient outcomes, decreased need for exploratory procedures, and improved post-operative monitoring.
 | ||
| Fig. 10 NIR-II fluorescence imaging of surgical sutures in patients intraoperatively: (a) schematic illustration of NIR-II and NIR-I imaging of BV-4B-coated surgical sutures in a surgical wound using a multispectral system (excitation: 792 nm; emission: 1000 nm for NIR-II, 850 nm for NIR-I). (b) NIR-II fluorescence image of surgical sutures (exposure: 1000 ms). (c) NIR-I image of the same field as (b) (exposure: 1000 ms). (d) Schematic illustration of imaging sutures in a blood-covered surgical wound using the same multispectral parameters as (a). (e) NIR-II fluorescence image of blood-covered sutures (exposure: 1000 ms). (f) NIR-I image of the same field as (e) (exposure: 1000 ms). Reproduced with permission from ref. 62. Copyright (2023) John Wiley and Sons, open access article distributed under the terms of the Creative Commons CC-BY-NC license. | ||
It is also important to conduct continual post-market surveillance after the product is introduced into the markets to identify any potential hazards present, whether it adheres to the current regulatory standards or any suspicious uncommon effects that may not have been presented in the initial trials.63 Implementing this two-step process guarantees that NIR-active coatings are carefully examined to ensure their safety and efficacy. This safeguards patients and upholds the public's confidence in medical innovations.
6. Challenges and opportunities
The main challenge of NIR fluorophore dyes is their lower QY. Generally, visible light fluorophores exhibit higher QY compared to that of NIR.64 Improving the fluorescence efficiency of NIR dyes is essential for enhancing signal-to-noise ratios in imaging applications. Improving the fluorescence efficiency of NIR dyes is vital to strengthening signal-to-noise ratios in imaging applications. Compared to ICG, NIR-II molecules with higher QY, such as protein complexes with a sulfonated NIR-II organic dye, outperform ICG in lymph node imaging in mice.65 Limited QY in tissue imaging is also a big challenge in advanced imaging techniques.Developing NIR-active materials, particularly those with advanced properties, can be expensive. Therefore, developing cost-effective and scalable techniques in this field is quite challenging.66 Substantial chemical changes are necessary to obtain specific characteristics, like targeting tissues or cells with NIR dyes, which can be highly demanding because of their structural complexity, photostability, and limited scalability of some of the synthesis methods that are currently used. This problem can be minimized by carefully optimizing reaction conditions, developing screening libraries, and using modular approaches to synthesis.67 Therefore, more diverse and cost-effective NIR-active materials should be designed to improve therapeutic efficiency.
In the NIR-active photocatalytic microbial disinfection process, band gap engineering is a good strategy for conserving energy and reducing cost. Employing the plasmon effect and sensitization methods could be a significant strategy for cost reduction. The process is still in an emerging stage due to the lack of research activities in this field. Using a combination of metallic and non-metallic materials, such as integrating plasmonic metals (Au, Ag) with semiconducting oxides (TiO2, ZnO) or chalcogenides (MoS2, WS2), can be a good strategy for achieving better light absorption and also deliver higher efficiency due to an improved charge separation process.68
NIR-active coatings allow for high-resolution imaging of deep tissues, which is advantageous for visualizing internal organs and facilitating the development of novel non-invasive diagnostic methods.69 NIR-II-triggered semiconducting polymer brushes with a thermosensitive CO donor can enhance PTT and mitigate tumor thermotolerance through promoting mitochondrial dysfunction and suppressing the expression of heat shock proteins, enabling effective cancer treatment.69
These coatings can significantly change diagnostics and therapeutics, enabling the development of more sustainable healthcare. Atomically precise metal clusters with specific features could be used as NIR-II fluorescence probes for in vivo imaging with a higher QY, and their emission wavelength is changeable.70 These clusters exhibit favorable properties such as water solubility and high stability, making them suitable for imaging applications in brain, kidney, gastrointestinal, and tumor metastasis monitoring. Recent advances have also demonstrated the biosafety of Au nanoclusters at ultrahigh concentrations, further supporting their potential as molecular probes for biomedical imaging.70
A series of better-performing conformationally restricted coumarin-hemocyanins (RCHCs) has been synthesized, with RSHS showing not only greater QY but also quite a large Stokes shift. The RCHC platform can undergo further modifications to produce a carboxy-functionalized derivative (RCHC1-COOH) capable of distinguishing between cancer and normal cells by selectively aggregating lipid rafts found in cancer cells.70
Advanced computational models can be used to predict the band alignments in NIR-active materials to provide insights for improving their performance. Density function theory (DFT) could predict the energy band gaps by calculating band structure, using Green's function and screened Coulomb interaction (GW) approximation for accuracy, and deriving effective masses for the conduction and valence bands.71 Hence, computational simulations can be used to predict charge absorption, separation, and transfer and realize the electron excitation and recombination mechanisms, minimizing energy loss.
7. Summary and outlook
In summary, the domain of NIR-responsive coatings has advanced rapidly in recent years, emerging as one of the extensively researched sectors of medical biotechnology and materials science due to its potential significance in improving biocompatibility, energy efficiency, photostability, degradability, and immunological response in current therapies, and mitigating healthcare-associated environmental impacts. In the area of implants, where e.g. traditional orthopaedic implants fall short in a number of areas, including osseointegration, aseptic loosening, and post-operative problems such biofilm formation and microbial infections, stimuli-responsive coatings such as the NIR-active coatings can deliver multiple functions to address this gap. Considering the significant burden of infections linked to medical devices and limited efficacy of some of the conventional coatings, NIR-active coatings can significantly restrict microbial colonization while stimulating tissue healing and integration, and delivering bioactive agents through highly spatiotemporally controlled drug release. For biodegradable implants, they can be used to control implant degradation kinetics, and monitor implant degradation and inflammation. In surgery, NIR-active coatings can improve the safety and precision of imaging-guided surgery.However, despite an increasing number of reports on novel NIR-active materials and their potential applications, the potential impact of their adoption on the environment, and on the environmental footprint of the healthcare sector more broadly has not been explored. This study aimed to bridge that gap by considering not only the critical attributes that NIR-active coatings need to exhibit to improve their effectiveness and expand their applicability, but also the environmental considerations for their use. This study examines the potential impact of the integration of NIR-active coatings in on the sustainability of healthcare, from their ability to enhance energy efficiency and significantly decrease energy requirements in the medical sector to how their disposal may align with the principles of circular economy through resource optimization and waste minimization. As the healthcare continues to evolve and transition to prevention, early diagnostics and patient-centered precision therapeutics, NIR-active coatings may offer a better way to develop smart devices for real-time monitoring and diagnostics, and minimally invasive, responsive and effective therapies that minimize systemic drug use and improve patient safety and treatment outcomes. These technologies may result in revolutionary advances in healthcare, which can be utilized for diagnostics and therapeutics and can also help ensure the overall sustainability of healthcare products.
Although the literature includes a wide range of promising examples of NIR-active coatings, the majority of these materials remain in the experimental phase. Only a small number of materials have undergone clinical trials, with even fewer used for clinical procedures. The coating's stability may vary depending on the applications, leading to inadequate research on stability analysis. To build a consistent and generally recognized validation procedure, there is a necessity for enhanced education and awareness, facilitating its acceptance in clinical settings. Integrating multiple fields, such as artificial intelligence, computational chemistry, materials science, biomedical engineering, microbiology, clinical medicine, and regulatory science can significantly enhances the rate of development and clinical adoption of NIR-active coatings.
GSL and VK would like to thank the International Research Agendas PLUS programme of the Foundation for Polish Science, co-financed by the European Union under the European Regional Development Fund (MAB PLUS/2019/11).
Data availability
No primary research results, software or codes have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- A. Alcayaga and E. G. Hansen, R&D Manage., 2025, 55, 508–530 Search PubMed.
- E. Ebrahimzadehsarvestani, E. M. Sarvestani, H. Safarzadeh, M. Enayati and F. Di Maria, J. Solid Waste Technol. Manage., 2025, 51, 14–24 CrossRef PubMed.
- A. H. Ashoka, S.-H. Kong, B. Seeliger, B. Andreiuk, R. V. Soares, M. Barberio, M. Diana and A. S. Klymchenko, Biomaterials, 2020, 261, 120306 CrossRef CAS PubMed.
- A. Molero, M. Calabrò, M. Vignes, B. Gouget and D. Gruson, Ann. Lab. Med., 2021, 41, 139–144 CrossRef PubMed.
- G. R. Warner and J. A. Flaws, Toxicol. Sci, 2018, 166, 246–249 CrossRef CAS PubMed.
- X. Chen, J. Zhou, Y. Qian and L. Zhao, Mater. Today Bio, 2023, 19, 100586 CrossRef CAS PubMed.
- H. Yang, Y. Yang, Y. Li, J. Hope and W. Choo, ACS Omega, 2023, 8, 26650–26662 CrossRef CAS PubMed.
- C. Xu and K. Pu, Chem. Soc. Rev., 2021, 50, 1111–1137 RSC.
- K. Prasad, J. Weerasinghe, O. Bazaka, E. P. Ivanova, I. Levchenko and K. Bazaka, Funct. Nanocompos. Hydrogels, 2023, 395–427 Search PubMed.
- L. Lin, H. He, R. Xue, Y. Zhang, Z. Wang, S. Nie and J. Ye, Med-X, 2023, 1, 9 CrossRef CAS.
- X. Ge, Q. Fu, L. Bai, B. Chen, R. Wang, S. Gao and J. Song, New J. Chem., 2019, 43, 8835–8851 RSC.
- J. Geng, Z. Zhu, W. Qin, L. Ma, Y. Hu, G. G. Gurzadyan, B. Z. Tang and B. Liu, Nanoscale, 2014, 6, 939–945 RSC.
- O. Khantamat, C.-H. Li, F. Yu, A. C. Jamison, W.-C. Shih, C. Cai and T. R. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 3981–3993 CrossRef CAS PubMed.
- H. Zhu, Y. Fang, X. Zhen, N. Wei, Y. Gao, K. Q. Luo, C. Xu, H. Duan, D. Ding and P. Chen, Chem. Sci., 2016, 7, 5118–5125 RSC.
- S. A. Mahadik, F. Pedraza, S. S. Mahadik, B. P. Relekar and S. S. Thorat, J. Sol-Gel Sci. Technol., 2017, 81, 791–796 CrossRef CAS.
- N.-N. Zhang, C.-Y. Lu, M.-J. Chen, X.-L. Xu, G.-F. Shu, Y.-Z. Du and J.-S. Ji, J. Nanobiotechnol., 2021, 19, 132 CrossRef PubMed.
- J. Xu, N. Liu, D. Wu, Z. Gao, Y.-Y. Song and P. Schmuki, ACS Nano, 2019, 14, 337–346 CrossRef PubMed.
- M. Chen, S. Feng, Y. Yang, Y. Li, J. Zhang, S. Chen and J. Chen, Nano Res., 2020, 13, 3123–3129 CrossRef.
- B. Alkan-Taş, E. Berksun, C. E. Taş, S. Ünal and H. Ünal, Prog. Org. Coat., 2022, 164, 106669 CrossRef.
- D. C. Santra, S. Mondal, B. Prusti and M. Higuchi, ACS Appl. Opt. Mater., 2024, 2, 1117–1127 CrossRef CAS.
- V. T. N. Linh, S. Han, E. Koh, S. Kim, H. S. Jung and J. Koo, Biomaterials, 2024, 122865 Search PubMed.
- M. Qin, Y. Bian, C. Wang, J. Sun, W. Shi, K. Liu, Y. Zheng, F. Zhang, G. Liu and M. Shao, Adv. Funct. Mater., 2024, 34, 2403770 CrossRef CAS.
- N. Singh, P. P. Kulkarni, P. Tripathi, V. Agarwal and D. Dash, Nanoscale Adv., 2024, 6, 1497–1506 RSC.
- Q. Feng, X. Zhou and C. He, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2024, 16, e1925 CAS.
- Y. Zou, Y. Zhang, Q. Yu and H. Chen, Biomater. Sci., 2021, 9, 10–22 RSC.
- T. Zhang, B. Wang, Q. Cheng, Q. Wang, Q. Zhou, L. Li, S. Qu, H. Sun, C. Deng and Z. Tang, Sci. Adv., 2024, 10, eadn7896 CrossRef CAS PubMed.
- T. Han, Y. Wang, S. Ma, M. Li, N. Zhu, S. Tao, J. Xu, B. Sun, Y. Jia and Y. Zhang, Adv. Sci., 2022, 9, 2203474 CrossRef CAS PubMed.
- M. Behi, L. Gholami, S. Naficy, S. Palomba and F. Dehghani, Nanoscale Adv., 2022, 4, 353–376 RSC.
- M. Thomaschewski, M. Lipp, C. Engelke, J. Harder, I. Labod, T. Keck and K. Mittmann, Surg. Endosc., 2023, 37, 9690–9697 CrossRef PubMed.
- G. Yang, R. Deng, Y. Chang and H. Li, Int. J. Biol. Macromol., 2024, 281, 136481 CrossRef CAS PubMed.
- C. Tan, X. Cao, X.-J. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han and G.-H. Nam, Chem. Rev., 2017, 117, 6225–6331 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, M. Zhang, J. Xie, Z. Zhang, Z. Liu, J. Li, Y. Liang and H. Dai, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- W. Li, S. Wang, M. Jin, L. Wang, J. Nan, C. Wang, P. Xiong, Q. M. Hu, L. Liu and J. Ren, Small, 2024, 20, 2310180 CrossRef CAS PubMed.
- G. Kaur, V. Kaur, N. Kaur, C. Kaur, K. Sood, A. Shanavas and T. Sen, ChemPhysChem, 2023, 24, e202200809 CrossRef CAS PubMed.
- X. Han, G. Boix, M. Balcerzak, O. H. Moriones, M. Cano-Sarabia, P. Cortés, N. Bastús, V. Puntes, M. Llagostera and I. Imaz, Adv. Funct. Mater., 2022, 32, 2112902 CrossRef CAS.
- E. P. Ward, J. Yang, J. C. Delong, T.-W. Sung, J. Wang, C. Barback, N. Mendez, S. Horgan, W. Trogler and A. C. Kummel, Surgery, 2018, 163, 883–888 CrossRef PubMed.
- A. P. Gorka, R. R. Nani and M. J. Schnermann, Acc. Chem. Res., 2018, 51, 3226–3235 CrossRef CAS PubMed.
- X. Ni, Y. Gao, X. Zhang, Y. Lei, G. Sun and B. You, Chem. Eng. J., 2021, 406, 126725 CrossRef CAS.
- D. Hu, M. Zha, H. Zheng, D. Gao and Z. Sheng, Research, 2025, 8, 0583 CrossRef CAS PubMed.
- Q. X. Ma, L. Xu, Y. Fan, L. Wang, J.-N. Xu, J. Zhao and X.-B. Chen, Small, 2024, 20, 2406912 CrossRef CAS PubMed.
- Y. Zhao, P. He, J. Yao, M. Li, B. Wang, L. Han, Z. Huang, C. Guo, J. Bai, F. Xue, Y. Cong, W. Cai, P. K. Chu and C. Chu, Biomaterials, 2023, 301, 122237 CrossRef CAS PubMed.
- D. Shan, D. Wang, Y. Ma, Z. Liang, D. J. Ravnic, N. Zhang and J. Yang, Adv. Funct. Mater., 2025, 35, 2414400 CrossRef CAS.
- S. Yang, N. Li, H. Xiao, W. Gui-long, F. Liu, Q. Pan, L. Tang, X. Tan and Q. Yang, Theranostics, 2022, 12, 7853–7883 CrossRef CAS PubMed.
- K. Liu, H. Shang, X. Kong and W. Lin, J. Mater. Chem. B, 2017, 5, 3836–3841 RSC.
- S. Xia, D. Liu, K. Jiang, M. Cao, Z. Lou, R. Cheng, J. Yi, A. Yin, Y. Jiang, K. Cheng, W. Weng, B. Shi and B. Tang, Mater. Today Bio, 2024, 27, 101156 CrossRef CAS PubMed.
- J. Fang, Y. Wan, Y. Sun, X. Sun, M. Qi, S. Cheng, C. Li, Y. Zhou, L. Xu, B. Dong and L. Wang, Chem. Eng. J., 2022, 435, 134935 CrossRef CAS.
- Z.-X. Han, X.-M. Liu, L. Tan, Z.-Y. Li, Y.-F. Zheng, K. W.-K. Yeung, Z.-D. Cui, Y.-Q. Liang, S.-L. Zhu and S.-L. Wu, Rare Met., 2021, 40, 2538–2551 CrossRef CAS.
- L. Huang, J. Li, W. Yuan, X. Liu, Z. Li, Y. Zheng, Y. Liang, S. Zhu, Z. Cui, X. Yang, K. W. K. Yeung and S. Wu, Corros. Sci., 2020, 163, 108257 CrossRef CAS.
- N. Xu, A. Hu, X. Pu, J. Li, X. Wang, J. Wang, Z. Huang, X. Liao and G. Yin, ACS Appl. Mater. Interfaces, 2022, 14, 15894–15910 CrossRef CAS PubMed.
- Z. Xie, M. Peng, R. Lu, X. Meng, W. Liang, Z. Li, M. Qiu, B. Zhang, G. Nie and N. Xie, Light: Sci. Appl., 2020, 9, 161 CrossRef CAS PubMed.
- J. Korhonen, A. Honkasalo and J. Seppälä, Ecol. Econ., 2018, 143, 37–46 CrossRef.
- N. Kutner, K. R. Kunduru, L. Rizik and S. Farah, Adv. Funct. Mater., 2021, 31, 2104105 CrossRef.
- N. Azam, M. Najabat Ali and T. Javaid Khan, Front. Mater., 2021, 8, 758928 Search PubMed.
- X. Liu, J. Wu, Z. Tang, J. Wu, Z. Huang, X. Yin, J. Du, X. Lin, W. Lin and G. Yi, ACS Appl. Mater. Interfaces, 2022, 14, 45678–45691 Search PubMed.
- A. L. Soares, S. C. Buttigieg, B. Bak, S. McFadden, C. Hughes, P. McClure, J. G. Couto and I. Bravo, Int. J. Health Policy Manage., 2023, 12, 6947 CrossRef PubMed.
- K. Oturu, W. Ijomah, A. Orr, L. Verpeaux, B. Broadfoot, S. Clark and R. Devine, Health Technol., 2022, 12, 273–283 CrossRef PubMed.
- C.-K. Yang, H.-W. Ma and M.-H. Yuan, Sustainable Environ. Res., 2023, 33, 29 CrossRef CAS.
- J. Han, Environ. Chem. Lett., 2022, 20, 2989–3003 CrossRef CAS PubMed.
- P. Laux, C. Riebeling, A. M. Booth, J. D. Brain, J. Brunner, C. Cerrillo, O. Creutzenberg, I. Estrela-Lopis, T. Gebel, G. Johanson, H. Jungnickel, H. Kock, J. Tentschert, A. Tlili, A. Schäffer, A. J. A. M. Sips, R. A. Yokel and A. Luch, NanoImpact, 2017, 6, 69–80 CrossRef PubMed.
- Z. E. Ozcelik, B. Alkan Tas, N. Ozcelik, B. S. Chee, E. L. Garcia, M. Mojicevic, M. Brennan Fournet and C. E. Tas, ACS Sustainable Chem. Eng., 2024, 12, 17936–17951 CrossRef CAS.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, M. Zhang, J. Xie, Z. Zhang, Z. Liu, J. Li, Y. Liang and H. Dai, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- D. Li, H. Shi, Q. Qi, B. Chang, Y. Jiang, K. Qian, X. Guan, P. Kang, N. Ma, Y. Zhang, Z. Zhang, X. Shi, C. Qu, Y. Wu, W. Chen, H. Chen, B. Li, L. Chen, Z. Li, S. Ma, L. Xu, Y. Zhang, J. Tian, Z. Hu, W. Jia and Z. Cheng, Adv. Sci., 2023, 10, 2303491 CrossRef PubMed.
- J. Pane, R. D. C. Francisca, K. M. C. Verhamme, M. Orozco, H. Viroux, I. Rebollo and M. Sturkenboom, Pharmacoepidemiol. Drug Saf., 2019, 28, 1155–1165 CrossRef PubMed.
- X. Chen, J. Li, S. Roy, Z. Ullah, J. Gu, H. Huang, C. Yu, X. Wang, H. Wang, Y. Zhang and B. Guo, Adv. Healthcare Mater., 2024, 13, 2304506 CrossRef CAS PubMed.
- A. L. Antaris, H. Chen, S. Diao, Z. Ma, Z. Zhang, S. Zhu, J. Wang, A. X. Lozano, Q. Fan, L. Chew, M. Zhu, K. Cheng, X. Hong, H. Dai and Z. Cheng, Nat. Commun., 2017, 8, 15269 CrossRef CAS PubMed.
- C. T. Jackson, S. Jeong, G. F. Dorlhiac and M. P. Landry, iScience, 2021, 24, 102156 CrossRef CAS PubMed.
- C. Maller, F. Schedel and M. Köhn, J. Org. Chem., 2024, 89, 3844–3856 CrossRef CAS PubMed.
- T. Kong, A. Liao, Y. Xu, X. Qiao, H. Zhang, L. Zhang and C. Zhang, RSC Adv., 2024, 14, 17041–17050 RSC.
- C. Zhu, M. Yu, J. Lv, F. Sun, A. Qin, Z. Chen, X. Hu, Z. Yang and Z. Fang, J. Nanobiotechnol., 2024, 22, 708 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X.-D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- D. F. Macias-Pinilla, C. Echeverría-Arrondo, A. F. Gualdrón Reyes, S. Agouram, V. Muñoz-Sanjosé, J. Planelles, I. Mora-Seró and J. I. Climente, Chem. Mater., 2021, 33, 420–429 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |



