 Open Access Article
Open Access ArticleHigh throughput screening of high entropy spinel electrolytes for multivalent batteries†
Mahesh J.
Dheerasinghe‡
a,
Yi
Gan‡
b,
Lin
Wang
a,
Yufang
He
 a,
Zhengda
He
a,
Gui-Liang
Xu
a,
Zhengda
He
a,
Gui-Liang
Xu
 c,
Yang
Zhao
c,
Yang
Zhao
 *b and
Bin
Ouyang
*b and
Bin
Ouyang
 *a
*a
aDepartment of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, USA. E-mail: bouyang@fsu.edu
bDepartment of Mechanical and Materials Engineering, The University of Western Ontario, Canada
cChemical Sciences and Engineering Division, Argonne National Laboratory, Lemont, IL, USA
First published on 19th June 2025
Abstract
High-entropy (HE) design emerged as a promising path for discovering multivalent superionic conductors. This work provides a computational exploration of the synthesizability of HE spinel-based electrolytes among the typical chemical space. Design principles have been established, while experimental synthesis has supported the stability rules predicted by computational data.
With the rapid advancement of electric vehicles, portable electronics, and mobile robotics, the demand for rechargeable batteries with higher energy density and improved safety continues to grow.1 Electrolytes are key to enhancing long-term cycling stability by effectively suppressing the formation of lithium dendrites-a major safety concern.2 To further improve energy storage capabilities, multivalent-ion batteries have gained attention as promising alternatives due to their potential for higher volumetric energy density. Unlike monovalent ions such as Li+/Na+/K+, multivalent ions (e.g., Mg2+, Al3+) carry multiple charges per ion, enabling greater energy storage potential.3–5 Moreover, multivalent metals exhibit reduced tendency to form dendrites compared to lithium, contributing to enhanced safety and stability in multivalent systems.6
Despite substantial developments in multivalent electrode7 materials, the relative deficiency of robust solid-state electrolytes presents a significant challenge to developing safer energy storage technologies. Materials with spinel structures are widely recognized for their potential in various rechargeable batteries applications.8–12 The general chemical formula AM2X4 allows divalent cations to occupy the A site, which adopts tetrahedral coordination, while charge balance is maintained by cations at the octahedral M sites. The anion site, represented by X, can be occupied by oxygen, sulfur or halogens. In this work, we will focus primarily on oxide-based spinel due to their exceptional phase stability and electrochemical stability. Despite the advantages, spinel-based multivalent systems suffer from sluggish ion migration within the lattice.13 This challenge might be mitigated through HE strategy, which boost ionic conductivity via compositional disorder14 that creates overlapping site energies which promote the formation of ion-percolating networks. Moreover, the synthetic accessibility of such materials has the potential to be enhanced by entropy stabilization mechanism.15
In this work, we present a systematic high-throughput screening of potential spinel oxide-based HE electrolytes, with a focus on addressing the synthesis challenges of these materials. We computationally investigated the phase stability of AM2O4 HE spinel oxides, with Mg, Ca and Zn being the mobile ions at A site, while the M sites are contributed by 4 equal-molar species out of 14 cations that can reach charge balance. Phase diagrams have been analyzed across 381 compounds to reveal the thermodynamic stability principles for compositional design. Finally, experimental synthesis has been performed to support the viability of our theoretical predictions.
This study focused on spinel electrolytes based on Mg2+, Zn2+, and Ca2+, where these three divalent cations occupy the tetrahedral sites within the spinel structures. Accordingly, all spinels were categorized as Mg-, Zn-, and Ca-based, respectively. The spinel structure with a single metal at the M site is used as a template to generate HE versions by substituting the octahedral sites (M) with four different metal cations, selected in equimolar concentrations from a pool of 14 redox inactive ions: Mg2+, Zn2+, Ca2+, Al3+, Ga3+, In3+, Sc3+, Zr4+, Hf4+, Ti4+, Sn4+, Nb5+, Ta5+, and Sb5+, while ensuring overall charge neutrality. As a result, 127 distinct HE compositions have been generated for each divalent metal in A site.
Subsequently, we performed a high-throughput phase diagram16 calculations among the identified 381 HE spinels using Density function theory (DFT) to assess their stability. (see ESI† for computational methods) DFT simulations were employed to all three spinel categories, utilizing the ground states from materials project database to construct phase diagrams.16,17 One representative candidate, chosen as Zn2(AlCaGaSn)O8, is illustrated in Fig. 1 to clarify this process. In this material, Zn2+ stays in tetrahedral site, while four other metals will occupy octahedral sites. From phase diagram calculations, there are four competing phases-ZnAl2O4, ZnGa2O4, ZnO, and CaSc2O4 for the targeted composition, while the energy above the convex hull (Ehull) made from these four competing phases can serve as quantitative metric for evaluating the synthesizability. Ehull, defined as the decomposition energy of a target material into the ground state (i.e., the convex hull) in the corresponding phase diagram, has been shown in various prior studies to possess remarkable capability in quantifying synthesizability,5,18–21 and is therefore adopted in this work. A total of 26, 35, and 24 competing phases were identified for Mg-, Zn-, and Ca-based spinels, respectively. Fig. 2 presents the frequency distribution of these competing phases. As depicted, the most encountered competing phases for Mg-, Zn-, and Ca-based systems are MgO, ZnO, and CaIn2O4, respectively (see ESI† for competing phases).
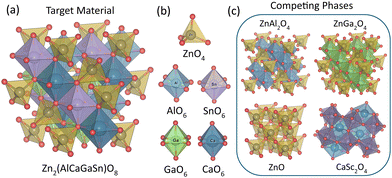 | ||
| Fig. 1 (a) One example of HE Zn-spinel; (b) tetrahedral site and octahedral sites of metals, (c) competing phases of the selected material. | ||
As HE materials become increasingly stable at elevated temperatures due to configurational entropy contributions, the temperature at which the target phase becomes thermodynamically favored is defined as the entropy stabilization temperature (Tstabilized), calculated as: Tstabilized = Ehull/Sconfig (refer to the ESI† for the Tstabilized values).15,21 Based on this analysis, 53 target phases exhibit entropy stabilization temperatures below 1473 K, which is set us a proxy for the upper temperature limit for classical solid-state reaction. The application of this filtering criterion yields 19, 26, and 8 stable phases from the Mg-, Zn-, and Ca-based spinels, respectively. This also suggests that spinel structures incorporating Zn2+ at the tetrahedral site demonstrate the greatest synthetic stability under ambient conditions, whereas Ca-based spinels appear to be least stable within the same temperature range.
To provide a better picture of the thermodynamic stability trends, heatmaps and boxplots derived from the calculated Ehull values are presented in Fig. 3(a–c) displays heatmaps representing the average Ehull values among all compositions that have the given pairs of metals. These plots reveal trends in cation compatibility, where darker regions indicate more stable cation pairings (lower Ehull), and lighter regions correspond to less stable combinations (higher Ehull). Such patterns provide insights into favorable elemental substitutions that help enhance the thermodynamic stability in HE spinel systems.20–24 Meanwhile, Fig. 3(d–f) display box plots illustrating the distribution of Ehull values for individual cations occupying octahedral-sites in Mg-, Zn-, and Ca-based spinels. Cations with lower median Ehull values are associated with higher stability, while the width of the distribution reflects their sensitivity to the surrounding chemical environment. Narrow distributions indicate consistent stability across compositions, whereas broader ones suggest greater variability. It can be inferred from Fig. 3 that among the Mg- and Zn-based spinels, the most stabilizing elements in the octahedral sublattice include Mg2+, Sn4+, and Sb5+ whereas Ca2+ and Nb5+ appear to be the least favorable. In Ca-based spinels, however, the stability trend slightly diverges. Although Ca2+ contributes to instability when present in the octahedral sites of Mg- and Zn-based spinels, it exhibits moderate stability within its own species of Ca-based spinels. For these systems, Al3+ and Nb5+ turn out to be the least stable elements, whereas Sb5+, Sn4+, and In3+ displayed strong stabilizing behavior.
To further understand the fundamental chemical handles for tuning the synthesizability of the HE spinels, we attempted several elemental descriptors, e.g., electronegativity, ionic radii and oxidation number, and have identified that average electronegativity (![[small chi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d8.gif) ) and the standard deviation of ionic radius (σrion) of octahedral-site elements correlate best with phase stability. In Mg- and Zn-based spinels,
) and the standard deviation of ionic radius (σrion) of octahedral-site elements correlate best with phase stability. In Mg- and Zn-based spinels, ![[small chi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d8.gif) shows a moderate negative linear correlation with Ehull, with Pearson correlation coefficients (r) of −0.552 and −0.619, respectively (Fig. 4a and b). However, this trend is absent in Ca-based spinels, where the correlation is negligible, (e.g., r = 0.108, as shown in Fig. 4c). Meanwhile, the σrion exhibits a strong positive linear relationship with Ehull for Mg- and Zn-based spinels, with r values of 0.813 and 0.793, respectively (Fig. 4e and f). Once again, Ca-based spinels deviate from this behavior, showing minimal correlation (r = 0.020; Fig. 4f). The strength of the inverse relationship between σrion and thermodynamic stability also follows the trend Ca ≪ Zn < Mg, which inversely correlates with the ionic radii of the tetrahedral site cations (Ca2+: 1.00 Å, Zn2+: 0.60 Å, Mg2+: 0.57 Å).
shows a moderate negative linear correlation with Ehull, with Pearson correlation coefficients (r) of −0.552 and −0.619, respectively (Fig. 4a and b). However, this trend is absent in Ca-based spinels, where the correlation is negligible, (e.g., r = 0.108, as shown in Fig. 4c). Meanwhile, the σrion exhibits a strong positive linear relationship with Ehull for Mg- and Zn-based spinels, with r values of 0.813 and 0.793, respectively (Fig. 4e and f). Once again, Ca-based spinels deviate from this behavior, showing minimal correlation (r = 0.020; Fig. 4f). The strength of the inverse relationship between σrion and thermodynamic stability also follows the trend Ca ≪ Zn < Mg, which inversely correlates with the ionic radii of the tetrahedral site cations (Ca2+: 1.00 Å, Zn2+: 0.60 Å, Mg2+: 0.57 Å).
Even though stability of Ca-spinels is less correlated with ![[small chi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d8.gif) and σrion, there are much better correlations with standard deviation of electronegativity (σχ) and average ionic radius (
and σrion, there are much better correlations with standard deviation of electronegativity (σχ) and average ionic radius (![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif) ion) instead. Particularly, the Pearson correlation with σχ and
ion) instead. Particularly, the Pearson correlation with σχ and ![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif) ion are −0.437 and −0.634 respectively (Fig. 4d and h). These deviations in the Ca-based spinels may be attributed to the significantly larger ionic radius and lower electronegativities of Ca2+, which likely disrupt structural compatibility within the spinel lattice. As a result, the major factor for stability will be whether the metal ions have closer (larger) ionic radii compared to Ca2+ and possess sufficiently varied electronegativities to be compatible with the Ca-spinel framework. (See ESI† for the relationship between X and rion of octahedral site elements.)
ion are −0.437 and −0.634 respectively (Fig. 4d and h). These deviations in the Ca-based spinels may be attributed to the significantly larger ionic radius and lower electronegativities of Ca2+, which likely disrupt structural compatibility within the spinel lattice. As a result, the major factor for stability will be whether the metal ions have closer (larger) ionic radii compared to Ca2+ and possess sufficiently varied electronegativities to be compatible with the Ca-spinel framework. (See ESI† for the relationship between X and rion of octahedral site elements.)
To further understand the chemical bonding origin of stability, crystal orbital Hamilton population (−COHP) analysis was performed on the Mg-, Zn-, and Ca-based spinel systems, each featuring identical M-site (octahedral) compositions.25 As shown by Fig. 5, the analysis focuses on interactions at the tetrahedral sites across these systems. As can be observed from Fig. 5(a), (c) and (e), Mg–O bond shows mostly bonding states, indicated by only green (positive) –COHP values. As a contrast, the Zn–O bond shows moderate antibonding character, while the Ca–O bond exhibits predominantly antibonding states, e.g., red (negative) –COHP values. Such distinct chemical bonding features highlight the instability of Ca-based spinels mainly arises from the unstable Ca–O bond. Fig. 5(b), (d) and (f) can further support this point, as the M–O bond are quite similar across all three systems. The observations from chemical bond analysis are also consistent with the statistic observation that Ca tends to destabilize in tetrahedron environment in compounds like spinel compared to Mg and Zn based compounds.26
To experimentally validate the computational predictions, six selected spinel structures, two each from the Mg-, Zn-, and Ca-based spinel groups are picked. The corresponding powder X-ray diffraction (XRD) patterns are presented in Fig. 6, with ZnFe2O4 (ICSD: PDF# 01-070-6393) used as a reference.27 Notably, the samples Mg2(ZnMgSbIn)O8, Mg2(ZnMgSbGa)O8, Zn2(MgZnSbIn)O8, and Zn2(MgZnSbGa)O8 exhibit diffraction patterns consistent with the spinel phase, closely matching the reference material. In contrast, both Ca-based spinel samples are not synthesized successfully, as being indicated by the extra diffraction peaks in Fig. 6. The calculated Ehull values are provided together with each of the diffraction patterns. The synthesis outcome is consistent with the relative values of Ehull, while the two Mg-spinels and two Zn-spinels selected show much lower Ehull values, turn out to be phase pure. As a contrast, the two Ca-spinels, even though with lowest Ehull and third lowest Ehull values, still cannot be made phase pure.
 | ||
| Fig. 6 Powder XRD patterns of synthesized HE spinel samples. Compositions are indicated in each panel of XRD patten. | ||
To sum up, this study presents a high-throughput computational analysis, complemented by experimental validation of Mg-, Zn-, and Ca-based HE spinels as candidates for multivalent-ion electrolytes. Thermodynamic stability in HE spinels was found to correlate with the ![[small chi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d8.gif) and the σrion of octahedral site elements for Mg- and Zn-based spinels. However, Ca-based spinels show a divergent trend and unique correlation with σχ and
and the σrion of octahedral site elements for Mg- and Zn-based spinels. However, Ca-based spinels show a divergent trend and unique correlation with σχ and ![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif) ion. Based on the elemental study, the next challenge is to determine the optimal stoichiometry of the favorable metals, including Mg/Zn/Ca that maximizes ionic conductivity.22,28
ion. Based on the elemental study, the next challenge is to determine the optimal stoichiometry of the favorable metals, including Mg/Zn/Ca that maximizes ionic conductivity.22,28
This work was supported by startup funding from Florida State University. Computational resources were provided by the Advance Cyberinfrastructure Coordination Ecosystem: Services and Support (ACCESS) and the National Energy Research Scientific Computing Center (RCC) at Florida State University. Additional computation and data processing were supported by supercomputing recourses from the Department of Energy's Office of Energy Efficiency and Renewable Energy at the National Renewable Energy Laboratory. Research at Argonne National Laboratory was funded by the US Department of Energy (DOE), Vehicle Technologies Office. Support from S. Thompson and T. Duong of the US DOE's Office of Vehicle Technologies Program is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†References
- D. Zhang, Y. You, F. Wu, X. Cao, T.-Y. Lü, Y. Sun, Z.-Z. Zhu and S. Wu, Exploring the Relationship between Composition and Li-Ion Conductivity in the Amorphous Li–La–Zr–O System, ACS Mater. Lett., 2024, 6(5), 1849–1855 CrossRef CAS.
- Q. Liu, Z. Geng, C. Han, Y. Fu, S. Li, Y.-B. He, F. Kang and B. Li, Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries, J. Power Sources, 2018, 389, 120–134 CrossRef CAS.
- Q. Y. Zhao, G. Y. Yin, Y. F. Liu, R. R. Tang, X. W. Wu and X. X. Zeng, Recent advances in material chemistry for zinc enabled redox flow batteries, Carbon Neutralization, 2023, 2(1), 90–114 CrossRef CAS.
- M. Al-Abbasi, Y. Zhao, H. He, H. Liu, H. Xia, T. Zhu, K. Wang, Z. Xu, H. Wang and W. Zhang, Challenges and protective strategies on zinc anode toward practical aqueous zinc-ion batteries, Carbon Neutralization, 2024, 3(1), 108–141 CrossRef CAS.
- L. Wang, J. Wang and B. Ouyang, Computational Investigation of MAX as Intercalation Host for Rechargeable Aluminum-Ion Battery, Adv. Energy Mater., 2023, 13(46), 2302584 CrossRef CAS.
- Y. Tian, G. Zeng, A. Rutt, T. Shi, H. Kim, J. Wang, J. Koettgen, Y. Sun, B. Ouyang and T. Chen, Promises and challenges of next-generation “beyond Li-ion” batteries for electric vehicles and grid decarbonization, Chem. Rev., 2020, 121(3), 1623–1669 CrossRef PubMed.
- Z. Rong, R. Malik, P. Canepa, G. Sai Gautam, M. Liu, A. Jain, K. Persson and G. Ceder, Materials design rules for multivalent ion mobility in intercalation structures, Chem. Mater., 2015, 27(17), 6016–6021 CrossRef CAS.
- Z. Cai, B. Ouyang, H.-M. Hau, T. Chen, R. Giovine, K. P. Koirala, L. Li, H. Ji, Y. Ha and Y. Sun, In situ formed partially disordered phases as earth-abundant Mn-rich cathode materials, Nat. Energy, 2024, 9(1), 27–36 CrossRef CAS.
- M. Liu, A. Jain, Z. Rong, X. Qu, P. Canepa, R. Malik, G. Ceder and K. A. Persson, Evaluation of sulfur spinel compounds for multivalent battery cathode applications, Energy Environ. Sci., 2016, 9(10), 3201–3209 RSC.
- Z. Cai, H. Ji, Y. Ha, J. Liu, D.-H. Kwon, Y. Zhang, A. Urban, E. E. Foley, R. Giovine and H. Kim, Realizing continuous cation order-to-disorder tuning in a class of high-energy spinel-type Li-ion cathodes, Matter, 2021, 4(12), 3897–3916 CrossRef CAS.
- P. Canepa, S.-H. Bo, G. Sai Gautam, B. Key, W. D. Richards, T. Shi, Y. Tian, Y. Wang, J. Li and G. Ceder, High magnesium mobility in ternary spinel chalcogenides, Nat. Commun., 2017, 8(1), 1759 CrossRef PubMed.
- L. Yin, B. J. Kwon, Y. Choi, C. J. Bartel, M. Yang, C. Liao, B. Key, G. Ceder and S. H. Lapidus, Operando X-ray diffraction studies of the Mg-ion migration mechanisms in spinel cathodes for rechargeable Mg-ion batteries, J. Am. Chem. Soc., 2021, 143(28), 10649–10658 CrossRef CAS PubMed.
- P. Canepa, G. Sai Gautam, D. C. Hannah, R. Malik, M. Liu, K. G. Gallagher, K. A. Persson and G. Ceder, Odyssey of multivalent cathode materials: open questions and future challenges, Chem. Rev., 2017, 117(5), 4287–4341 CrossRef CAS PubMed.
- Y. Zeng, B. Ouyang, J. Liu, Y.-W. Byeon, Z. Cai, L. J. Miara, Y. Wang and G. Ceder, High-entropy mechanism to boost ionic conductivity, Science, 2022, 378(6626), 1320–1324 CrossRef CAS PubMed.
- B. Ouyang and Y. Zeng, The rise of high-entropy battery materials, Nat. Commun., 2024, 15(1), 973 CrossRef CAS PubMed.
- T.-L. Pham, L. Wang and B. Ouyang, Design principles for anode stable solid-state electrolytes, J. Mater. Chem. A, 2024, 12(31), 19979–19987 RSC.
- S. P. Ong, W. D. Richards, A. Jain, G. Hautier, M. Kocher, S. Cholia, D. Gunter, V. L. Chevrier, K. A. Persson and G. Ceder, Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis, Comput. Mater. Sci., 2013, 68, 314–319 CrossRef CAS.
- S. P. Ong, L. Wang, B. Kang and G. Ceder, Li− Fe− P− O2 phase diagram from first principles calculations, Chem. Mater., 2008, 20(5), 1798–1807 CrossRef CAS.
- W. Sun, S. T. Dacek, S. P. Ong, G. Hautier, A. Jain, W. D. Richards, A. C. Gamst, K. A. Persson and G. Ceder, The thermodynamic scale of inorganic crystalline metastability, Sci. Adv., 2016, 2(11), e1600225 CrossRef PubMed.
- B. Ouyang, J. Wang, T. He, C. J. Bartel, H. Huo, Y. Wang, V. Lacivita, H. Kim and G. Ceder, Synthetic accessibility and stability rules of NASICONs, Nat. Commun., 2021, 12(1), 5752 CrossRef CAS PubMed.
- L. Wang, N. Sunariwal, Y. He, D.-H. Kim, D.-H. Yeon, Y. Zeng, J. Cabana and B. Ouyang, Elemental Stability Rules for High Entropy Disordered Rocksalt Type Li-Ion Battery Positive Electrodes, Adv. Energy Mater., 2025, 2404982 CrossRef CAS.
- Y. Chen, Z. Lun, X. Zhao, K. P. Koirala, L. Li, Y. Sun, C. A. O’Keefe, X. Yang, Z. Cai and C. Wang, Unlocking Li superionic conductivity in face-centred cubic oxides via face-sharing configurations, Nat. Mater., 2024, 23(4), 535–542 CrossRef CAS PubMed.
- Z. Lun, B. Ouyang, D.-H. Kwon, Y. Ha, E. E. Foley, T.-Y. Huang, Z. Cai, H. Kim, M. Balasubramanian and Y. Sun, Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries, Nat. Mater., 2021, 20(2), 214–221 CrossRef CAS PubMed.
- L. Wang, Z. He and B. Ouyang, Data driven design of compositionally complex energy materials, Comput. Mater. Sci., 2023, 230, 112513 CrossRef CAS.
- P. C. Müller, C. Ertural, J. Hempelmann and R. Dronskowski, Crystal orbital bond index: Covalent bond orders in solids, J. Phys. Chem. C, 2021, 125(14), 7959–7970 CrossRef.
- D. Waroquiers, X. Gonze, G.-M. Rignanese, C. Welker-Nieuwoudt, F. Rosowski, M. Gobel, S. Schenk, P. Degelmann, R. André and R. Glaum, Statistical analysis of coordination environments in oxides, Chem. Mater., 2017, 29(19), 8346–8360 CrossRef CAS.
- M. Bini, M. Ambrosetti and D. Spada, ZnFe2O4, a green and high-capacity anode material for lithium-ion batteries: A review, Appl. Sci., 2021, 11(24), 11713 CrossRef CAS.
- H. Ji, J. Wu, Z. Cai, J. Liu, D.-H. Kwon, H. Kim, A. Urban, J. K. Papp, E. Foley and Y. Tian, Ultrahigh power and energy density in partially ordered lithium-ion cathode materials, Nat. Energy, 2020, 5(3), 213–221 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc02095f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |




