Understanding the mechanism behind improved cycling stability of FeF3 cathode in lithium bis(fluorosulfonyl)amide-concentrated electrolytes†
Yuta
Maeyoshi
 *a,
Noboru
Taguchi
a,
Kazuki
Yoshii
*a,
Noboru
Taguchi
a,
Kazuki
Yoshii
 a,
Masahiro
Shikano
a,
Masahiro
Shikano
 a and
Hikari
Sakaebe
a and
Hikari
Sakaebe
 ab
ab
aResearch Institute of Electrochemical Energy, Department of Energy and Environment, National Institute of Advanced Industrial Science and Technology (AIST), 1-8-31 Midorigaoka, Ikeda, Osaka 563-8577, Japan. E-mail: y.maeyoshi@aist.go.jp
bInstitute for Materials Chemistry and Engineering, Kyushu University, 6-1 Kasuga koen, Kasuga-Shi, Fukuoka 816-8580, Japan
First published on 8th May 2025
Abstract
We investigate the mechanism behind the improved discharge/charge cycling stability of FeF3 cathode in lithium bis(fluorosulfonyl)amide (LiFSA)-concentrated electrolytes. Macroscopic and microscopic analyses reveal that the concentrated electrolyte effectively suppresses the formation of inactive Fe and LiF in the cycled FeF3 at the charged state, thus improving the cycling stability.
Increasing demand for electrification toward a sustainable society has emphasized the need to develop rechargeable batteries with high energy density. Compared with conventional intercalation-type cathodes, conversion-type transition metal fluoride cathodes are promising candidates for high-energy-density batteries because of their higher specific capacity due to the ability to store multiple Li+ per metal centre via multi-electron reactions.1 Among them, iron fluorides have gained much attention as next-generation cathode materials for lithium batteries due to their high specific capacity (571 mA h g−1 for FeF2; 712 mA h g−1 for FeF3) and abundance of constituent elements.1–3 However, the practical use is hindered by the poor cycling stability. The degradation of iron fluoride cathodes in conventional electrolytes, typically 1 mol dm−3 (M) LiPF6 in organic carbonate solvents, has been attributed to aggregation of LiF and Fe, dissolution of active materials, decomposition of electrolytes, and growth of cathode electrolyte interphases (CEI).3–7
To overcome these issues, electrolyte design is one of the most effective strategies.3 Particularly, lithium bis(fluorosulfonyl)amide (LiFSA)-based electrolytes have been reported to improve the cycling stability of iron fluoride cathodes.8–14 Several studies have shown that FSA-derived CEI is formed on the cathode surface in the electrolytes.8–14 The FSA-derived CEI has been considered to prevent the dissolution of Fe from the cathodes and the continuous decomposition of electrolytes, thus leading to the improvement in cycling stability.8,10,11,13 However, the CEI chemistry and the suppression of Fe dissolution are insufficient to explain the improved cycling stability of iron fluoride cathodes in the electrolytes, and the precise mechanism remains controversial. Elucidating the mechanism is crucial for the electrolyte design and the electrode/electrolyte interfacial engineering for iron fluoride cathodes because the cycling stability must be further improved for practical use.
In this work, we investigate the mechanism behind the improved cycling stability of FeF3 cathode in LiFSA-based electrolytes. The liquid electrolytes with different LiFSA concentrations are prepared, and the discharge/charge characteristics of FeF3 cathodes in these electrolytes are evaluated. By macroscopic and microscopic analyses, we reveal that the LiFSA-concentrated electrolyte, which prevents the Fe dissolution from FeF3 and forms the FSA-derived CEI, suppresses the formation of inactive Fe and LiF in the cycled FeF3 at the charged state, thus improving the cycling stability.
Ethylene carbonate (EC) and propylene carbonate (PC) were used as the solvents for LiFSA-based electrolytes because of their good compatibility with FeF3 cathode.5 These solvents also have higher oxidation stability than ethers such as 1,2-dimethoxyethane (DME), enabling accurate evaluation of FeF3 at the relatively high charge cutoff voltage of 4.5 V in this study. We prepared conventional 1.2 M, intermediate 3.2 M, and near saturated 5.3 M LiFSA/EC:PC electrolytes, which correspond to LiFSA:EC:PC molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and 1
1.5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.65
0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.65, respectively. Their physicochemical properties are shown in Fig. S1 and Table S1 (ESI†). The 5.3 M electrolyte has higher viscosity and lower ionic conductivity than the 1.2 M electrolyte, which are common challenges for the practical use of salt-concentrated electrolytes.15,16 The solution structures were studied by Raman spectroscopy (Fig. S2, ESI†). As reported in previous literature, increasing the LiFSA concentration increased the number of the EC and PC molecules coordinated to Li+ and enhanced the ion-pairing of FSA−–Li+ to form contact ion pairs (CIPs) and aggregates (AGGs-I, one FSA− coordinating two Li+; AGGs-II, one FSA− coordinating three Li+).17–19 In the 5.3 M electrolyte, almost all solvent molecules were coordinated to Li+ and the anion formed CIPs and AGGs, which was quite different from the 1.2 M electrolyte with many uncoordinated (free) solvent molecules and anions.
0.65, respectively. Their physicochemical properties are shown in Fig. S1 and Table S1 (ESI†). The 5.3 M electrolyte has higher viscosity and lower ionic conductivity than the 1.2 M electrolyte, which are common challenges for the practical use of salt-concentrated electrolytes.15,16 The solution structures were studied by Raman spectroscopy (Fig. S2, ESI†). As reported in previous literature, increasing the LiFSA concentration increased the number of the EC and PC molecules coordinated to Li+ and enhanced the ion-pairing of FSA−–Li+ to form contact ion pairs (CIPs) and aggregates (AGGs-I, one FSA− coordinating two Li+; AGGs-II, one FSA− coordinating three Li+).17–19 In the 5.3 M electrolyte, almost all solvent molecules were coordinated to Li+ and the anion formed CIPs and AGGs, which was quite different from the 1.2 M electrolyte with many uncoordinated (free) solvent molecules and anions.
The discharge/charge characteristics of FeF3 cathodes in the LiFSA/EC:PC electrolytes were evaluated using Li/FeF3 cells (Fig. 1 and Fig. S3, ESI†). We used FeF3 electrodes with relatively high active material loading (3.1 mg cm−2) and areal capacity (2.0 mA h cm−2), which is more practical than the previously reported cathodes.8,11–13 The mechanism study using more practical FeF3 cathodes should be beneficial for developing commercially competitive technologies.3 The performance of the Li/FeF3 cells mainly depends on the FeF3 cathodes because of the low Li utilization (below 3%), high Coulombic efficiency (above 95%) and low overpotential of Li plating/stripping reactions in the LiFSA/EC:PC electrolytes.16,19 Fig. S3a (ESI†) shows the initial galvanostatic discharge/charge curves of the Li/FeF3 cells using the LiFSA/EC:PC electrolytes at 0.1C rate in a voltage range of 1.0–4.5 V. All cells exhibited typical discharge/charge features of FeF3 regardless of the electrolytes.20 The discharge/charge reactions of FeF3 proceed according to the following equation: FeF3 + 3Li+ + 3e− ⇄ Fe + 3LiF.1,2 The large discharge capacities and low Coulombic efficiencies were observed in the initial few cycles (Fig. 1 and Fig. S3, ESI†), which should be mainly due to the decomposition of electrolytes and the formation of CEI on the cathode.11 Higher LiFSA concentration in the electrolytes increased the initial discharge capacity and decreased the initial Coulombic efficiency (Fig. 1 and Fig. S3, ESI†). This would be related to the enhanced reduction potential of FSA− in the concentrated electrolytes, which induces the FSA− reduction and the passivating CEI formation.8 Increasing the salt concentration greatly suppressed the increase in the overpotential between discharge/charge reactions during cycling (Fig. 1a, b and Fig. S3b, ESI†). As a result, higher salt concentration of the electrolytes reduced the capacity degradation (Fig. 1a–c and Fig. S3b, c, ESI†), which is consistent with the previous report using the concentrated LiFSA/DME electrolyte.8 The 5.3 M electrolyte achieved the best cycling stability with 52.5% capacity retention after 50 cycles than the 1.2 M (14.3% capacity retention after 50 cycles) and 3.2 M (37.0% capacity retention after 50 cycles) electrolytes as shown in Fig. 1c and Fig. S3c (ESI†).
To clarify the mechanism behind the improved cycling performance of FeF3 in the LiFSA-concentrated electrolyte, we focused on the 1.2 M and 5.3 M electrolyte systems. It has been reported that the dissolution of transition metals from transition metal-based cathodes including iron fluorides closely relates to the degradation of the active materials.6,8,19,21 Thus, we investigated the dissolution of Fe from the FeF3 electrodes after discharge/charge cycling. The mass of Fe deposited on the Li foil after cycling in the Li/FeF3 cells was measured by inductively coupled optical atomic emission spectroscopy (ICP-OES). To avoid Fe contamination and improve the quantitative accuracy of the ICP-OES, the Li/FeF3 pouch cells were assembled using Al laminated films and large area FeF3 electrodes (4 cm2). As shown in Fig. 2a, the mass of Fe deposited on the Li foil after 5 cycles was lower in the 5.3 M electrolyte (19.6 μg) compared with the 1.2 M electrolyte (49.4 μg), indicating that the LiFSA-concentrated electrolyte suppressed the Fe dissolution from FeF3, as previously reported.8 The suppressed dissolution of Fe should be attributed to the solution structure of the electrolyte with almost no free solvent molecules and anions (Fig. S2, ESI†)18,22 as well as the formation of FSA-derived CEI on FeF3 (described later).8,10,11,13 The mass of Fe deposited on the cycled Li foil in the 5.3 M electrolyte was increased to 42.7 μg after 20 cycles. These results mean that even though almost all solvent molecules and anions were coordinated to Li+ in the bulk electrolyte, Fe dissolution cannot be completely inhibited during the electrode reactions.
We calculated Fe dissolution from the cycled FeF3 electrodes by dividing the mass of Fe deposited on the cycled Li foil by the mass of Fe in the pristine FeF3 electrodes (Fig. 2b). The Fe dissolution after 20 cycles was 0.84% and 0.69% for the 1.2 M and 5.3 M electrolytes, respectively. On the other hand, the observed capacity losses of the Li/FeF3 cells after 20 cycles were 50.8% and 26.8% for the 1.2 M and 5.3 M electrolytes (Fig. 1c), which were much higher than the calculated Fe dissolution. These results indicate that the observed capacity loss is mainly due to other degradation mechanisms rather than the Fe dissolution. In electrolytes that easily dissolve Fe from FeF3, dissolution/redeposition of Fe from/on the cathode may occur, inducing the particle growth of Fe by Ostwald ripening of Fe particles.10 Additionally, the Fe dissolution should form electron-insulating LiF on the surface of FeF3 particles.6 The formation of Fe and LiF in FeF3 must degrade the cathode and reduce the reversibility of discharge/charge reactions.
To verify the above hypothesis on the Fe dissolution and FeF3 degradation, we analysed the FeF3 electrodes before and after discharge/charge cycling from macroscopic and microscopic viewpoints. Note that all the analyses of the FeF3 electrodes were performed at the charged (delithiated) state. When the charge reaction of Fe and LiF fully proceeds, only FeF3 is theoretically formed in the electrode. First, the magnetic behaviour of the FeF3 electrodes was investigated. Interestingly, the FeF3 electrode after 20 discharge/charge cycles in the 1.2 M electrolyte stuck to a ferrite magnet (Fig. 2c), implying that considerable amounts of Fe particles remained in the cycled FeF3 electrode even at the charged state. In contrast, the pristine FeF3 electrode and the electrode cycled in the 5.3 M electrolyte were not stuck to the magnet (Fig. 2c). The backscattered electron images for the cross-section of the FeF3 electrodes are shown in Fig. 2d. Many bright spots were observed in the FeF3 electrode cycled in the 1.2 M electrolyte, evidencing the formation of unreacted Fe particles in the electrode. To the best of our knowledge, this is the first observation of such large inactive Fe particles (10–100 nanometre-order) in the cycled FeF3 electrodes at the charged state, in contrast to the formation of several nanometre-order Fe particles in the degraded iron fluoride cathodes in the conventional 1 M LiPF6/organic carbonates electrolytes.4,23 The inactive Fe particles are directly related to the reduced reversibility of FeF3 discharge/charge reaction and capacity fading in the 1.2 M electrolyte (Fig. 1a and c). The FeF3 electrode cycled in the 5.3 M electrolyte showed no bright spots and appeared almost identical to the pristine electrode (Fig. 2d). These results reveal that the LiFSA-concentrated electrolyte, which prevented the Fe dissolution from FeF3, effectively suppressed the formation of inactive Fe particles in the cycled FeF3 electrode at the charged state, thus improving the cycling stability. The suppressed growth of Fe particles is likely attributed to the prevented Ostwald ripening of Fe particles via dissolution/redeposition of Fe from/on the cathode as well as the protection of FeF3 by superior FSA-derived CEI (shown later) in the 5.3 M electrolyte.8,10,11
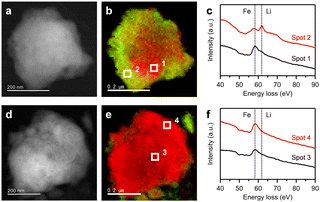
Additionally, we analysed the cycled FeF3 particles by a scanning transmission electron microscope (STEM) equipped with electron energy-loss spectroscopy (EELS). Fig. 3a, b, d and e display the annular dark-field (ADF)-STEM images and the overlapped concentration maps of Li K-edge (green) and Fe M-edge (red) signals for the FeF3 particles after 20 discharge/charge cycles in the electrolytes. For the FeF3 particle cycled in the 1.2 M electrolyte, Li K-edge signal was strongly detected near the surface of the particle, along with Fe M-edge signal in the entire particle (Fig. 3b). In contrast, almost only Fe M-edge signal was detected from the entire particle cycled in the 5.3 M electrolyte (Fig. 3e). Fig. 3c and f show the EELS spectra of Li K-edge and Fe M-edge region taken from square spots on the particles in Fig. 3b and e. As shown in Fig. 3c, a sharp peak attributed to LiF was observed near the surface of the FeF3 particle cycled in the 1.2 M electrolyte (spot 2). A weak LiF peak was also seen in the ELLS spectrum taken from the inside of the particle (spot 1). These results demonstrate that unreacted LiF remained near the surface and inside of the cycled FeF3 particle at the charged state. As shown in a report on the degradation analysis of FeF3 in conventional electrolytes, the LiF formation will be due in part to the Fe dissolution from FeF3.6 Furthermore, a Fe M-edge peak in spot 2 shifted to slightly lower energy loss compared with that in spot 1, suggesting the presence of reduced state Fe along with LiF in the particle. This result correlates well with the formation of inactive Fe in the FeF3 electrode cycled in the 1.2 M electrolyte (Fig. 2c and d). In stark contrast, no LiF and reduced state Fe peaks were observed in the EELS spectra taken from inside (spot 3) and near the surface (spot 4) of the FeF3 particle cycled in the 5.3 M electrolyte (Fig. 3f), indicating that the LiFSA-concentrated electrolyte suppresses the formation of unreacted LiF and Fe in the cycled particle at the charged state. The X-ray diffraction patterns of the pristine and cycled FeF3 electrodes also support the suppression of inactive LiF formation by the 5.3 M electrolyte (Fig. S4, ESI†). Therefore, the superior cycling stability of FeF3 in the LiFSA-concentrated electrolyte is attributed to the suppression of inactive Fe and LiF formation in the cycled FeF3 at the charged state by the limited Fe dissolution. However, even in the 5.3 M electrolyte, Fe gradually dissolved into the electrolyte from FeF3 (Fig. 2a and b), which forms some LiF in the cycled FeF3 (Fig. 3e and Fig. S4, ESI†), leading to the gradual capacity loss of the Li/FeF3 cell (Fig. 1b and c).
The surface chemistry of the cycled FeF3 electrodes was studied by X-ray photoelectron spectroscopy (XPS). Fig. 4 and Fig. S5 (ESI†) display XPS spectra of the FeF3 before and after five discharge/charge cycles in the electrolytes. In the F 1s spectra, the intensity of Li–F peak was higher for the FeF3 electrode cycled in the 5.3 M electrolyte.4,8 The S 2p and N 1s spectra show that more sulfur-containing compounds and N–SOx species were formed on the FeF3 electrode cycled in the 5.3 M electrolyte.8,11,15 These results indicate that the formation of CEI composed of more decomposition products of FSA− on the surface of the FeF3 electrode cycled in the electrolyte. The STEM-EDS analyses also support the FSA-derived CEI formation on the cycled FeF3 particle (Fig. S6–S8, ESI†). The FSA-derived CEI has been considered to prevent the dissolution of Fe from FeF3 and the decomposition of electrolytes.8,11 We believe that the FSA-derived CEI probably prevents the Fe dissolution, protects the FeF3 surface, and suppresses the inactive Fe and LiF formation in the cycled FeF3 in the 5.3 M electrolyte. Further studies are required to elucidate the precise mechanism and function of CEI.
 | ||
| Fig. 4 F 1s and S 2p XPS spectra of FeF3 electrodes before and after five discharge/charge cycles in the electrolytes. | ||
In summary, we revealed that the improved cycling stability of the FeF3 cathode in the LiFSA-concentrated electrolyte is directly attributed to the suppressed formation of inactive Fe and LiF in the cycled FeF3 at the charged state. The LiFSA-concentrated electrolyte with almost no free solvent molecules and anions inhibits the dissolution of Fe from FeF3 and forms the FSA-derived CEI on the active material, preventing the Fe agglomeration and electron-insulating LiF generation in the cycled FeF3. This study advances the understanding of the mechanism behind improved cycling stability of FeF3 in the LiFSA-concentrated electrolytes, promoting the design of electrolytes and the development of electrode/electrolyte interfacial engineering technologies for high-energy-density lithium batteries with not only iron fluoride cathodes but also other conversion-type cathode materials. LiFSA-based solid electrolytes are expected to further inhibit the degradation such as Fe dissolution and improve the stability of iron fluoride cathodes.21
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan under the “Research and Development Initiative for Scientific Innovation of New Generation Batteries 2 (RISING2) (JPNP16001)”.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- F. Wu and G. Yushin, Energy Environ. Sci., 2017, 10, 435–459 RSC.
- G. G. Amatucci and N. Pereira, J. Fluorine Chem., 2007, 128, 243–262 CrossRef CAS.
- L. F. Olbrich, A. W. Xiao and M. Pasta, Curr. Opin. Electrochem., 2021, 30, 100779 CrossRef CAS.
- M. Sina, R. Thorpe, S. Rangan, N. Pereira, R. A. Bartynski, G. G. Amatucci and F. Cosandey, J. Phys. Chem. C, 2015, 119, 9762–9773 CrossRef CAS.
- H. Senoh, K. Matsui, M. Shikano, T. Okumura, H. Kiuchi, K. Shimoda, K. Yamanaka, T. Ohta, T. Fukunaga, H. Sakaebe and E. Matsubara, ACS Appl. Mater. Interfaces, 2019, 11, 30959–30967 CrossRef CAS PubMed.
- K. Yoshii, N. Taguchi, T. Miyazaki, M. Shikano and H. Sakaebe, Chem. Commun., 2020, 56, 4878–4881 RSC.
- K. Shimoda, M. Shikano, M. Murakami and H. Sakaebe, J. Power Sources, 2020, 477, 228772 CrossRef CAS.
- W. Gu, O. Borodin, B. Zdyrko, H.-T. Lin, H. Kim, N. Nitta, J. Huang, A. Magasinski, Z. Milicev, G. Berdichevsky and G. Yushin, Adv. Funct. Mater., 2016, 26, 1507–1516 CrossRef CAS.
- W. Fu, E. Zhao, Z. Sun, X. Ren, A. Magasinski and G. Yushin, Adv. Funct. Mater., 2018, 28, 1801711 CrossRef.
- Q. Huang, K. Turcheniuk, X. Ren, A. Magasinski, D. Gordon, N. Bensalah and G. Yushin, Adv. Energy Mater., 2019, 9, 1803323 CrossRef.
- A. W. Xiao, H. J. Lee, I. Capone, A. Robertson, T.-U. Wi, J. Fawdon, S. Wheeler, H.-W. Lee, N. Grobert and M. Pasta, Nat. Mater., 2020, 19, 644–654 CrossRef CAS PubMed.
- B. R. Wygant, L. C. Merrill, K. L. Harrison, A. A. Talin, D. S. Ashby and T. N. Lambert, Adv. Sci., 2022, 9, 2105803 CrossRef CAS PubMed.
- C. Choi, H. Yoon, S. Kang, D. I. Kim, J. Hong, M. Shin, D.-J. Yoo and M. Kim, Adv. Sci., 2024, 11, 2410114 CrossRef CAS PubMed.
- L. F. Olbrich, A. W. Xiao, M. Schart, J. Ihli, G. Matthews, M. Sanghadasa and M. Pasta, Cell Rep. Phys. Sci., 2024, 5, 101787 CrossRef CAS.
- Y. Maeyoshi, D. Ding, M. Kubota, H. Ueda, K. Abe, K. Kanamura and H. Abe, ACS Appl. Mater. Interfaces, 2019, 11, 25833–25843 CrossRef CAS PubMed.
- Y. Maeyoshi, K. Yoshii and H. Sakaebe, Electrochemistry, 2022, 90, 047001 CrossRef CAS.
- J. L. Allen, O. Borodin, D. M. Seo and W. A. Henderson, J. Power Sources, 2014, 267, 821–830 CrossRef CAS.
- J. Wang, Y. Yamada, K. Sodeyama, C. H. Chiang, Y. Tateyama and A. Yamada, Nat. Commun., 2016, 7, 12032 CrossRef CAS PubMed.
- Y. Maeyoshi, K. Yoshii, M. Shikano and H. Sakaebe, ACS Appl. Energy Mater., 2021, 4, 13627–13635 CrossRef CAS.
- X. Hua, A. S. Eggeman, E. Castillo-Martinez, R. Robert, H. S. Geddes, Z. Lu, C. J. Pickard, W. Meng, K. M. Wiaderek, N. Pereira, G. G. Amatucci, P. A. Midgley, K. W. Chapman, U. Steiner, A. L. Goodwin and C. P. Grey, Nat. Mater., 2021, 20, 841–850 CrossRef CAS PubMed.
- Y. Maeyoshi, K. Yoshii, H. Sano, H. Sakaebe, R. Tamate, T. Kaneko and K. Sodeyama, ACS Appl. Polym. Mater., 2025, 7, 1629–1638 CrossRef CAS.
- Y. Yamada, H. Chiang, K. Sodeyama and J. Wang, ChemElectroChem, 2015, 2, 1687–1694 CrossRef CAS.
- T. Takami, K. Matsui, H. Senoh, N. Taguchi, M. Shikano, H. Sakaebe and T. Fukunaga, J. Alloys Compd., 2018, 769, 539–544 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01817j |
| This journal is © The Royal Society of Chemistry 2025 |