 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembly under continuous flow conditions
Liqun
Guo
,
Qiang
Zhu
and
Anna G.
Slater
 *
*
Department of Chemistry and Materials Innovation Factory, University of Liverpool, Liverpool L69 7ZD, UK. E-mail: Liqun.Guo2@liverpool.ac.uk
First published on 30th May 2025
Abstract
Self-assembly plays a crucial role in the formation of hierarchical supramolecular structures and functional materials. The use of flow chemistry for self-assembly processes has garnered significant attention due to its enhanced ability to control the formation of complex molecular and nanoscale architectures. Compared to conventional batch processes, flow-based systems offer improved regulation of key reaction parameters, such as mixing dynamics, temperature gradients, and residence time, facilitating continuous and scalable synthesis. This review examines recent advances in self-assembly under flow conditions, highlighting advantages in four domains: scalable production, opportunities to control selectivity, improved product crystallinity, and precise regulation of size and morphology in particle formation. Selected case studies from the literature, including our work in this field, will be discussed to demonstrate that flow-based technology is valuable for the fabrication of supramolecular structures and represents a promising approach for advanced materials science.
1. Introduction
Self-assembled structures form from individual building blocks that collectively organise over time into the most thermodynamically favoured configuration, driven by non-covalent interactions such as hydrogen bonding, electrostatic interactions, π–π stacking, metal–ligand coordination, and van der Waals forces.1,2 By leveraging these interactions, small molecules can assemble into larger supramolecular architectures whose combined properties and behaviours differ significantly from that of their individual precursor building blocks.3,4Governed by the principles of reversibility and ‘error-correction’, self-assembly presents a powerful strategy to achieve long-range order and/or hierarchical structures that are extremely difficult to obtain via stepwise synthesis.5–7 As such, the principles of error-correction and assembly driven by overall thermodynamic stability have also been used to form multicomponent molecular species using reversible covalent bonds,8 and, through crystallisation processes, porous crystalline materials.9
The range of such supramolecular systems is vast, including materials such as metal–organic frameworks (MOFs),10–13 covalent organic frameworks (COFs)14–16 and coordination polymers (CPs);17–20 porous organic cages (POCs),21,22 metal organic cages (MOCs),23–25 molecular knots,26–28 macrocycles,29,30 and hydrogels.31,32 Collectively, these materials exhibit significant potential for various applications, including host–guest chemistry for molecular storage, separation, and recognition,33–35 drug delivery,34,36–38 catalysis,39 sensing,37,38 and beyond. However, to achieve this potential, the reliable, sustainable, and scalable production of such structures is required: a remaining challenge for the field.40,41
Traditionally, self-assembly processes are carried out in batch reactors, which present several limitations including poor reproducibility, inefficient mixing, and challenges in scalable production due to inconsistent reaction conditions.42 Furthermore, self-assembly often requires high-dilution conditions to avoid formation of kinetic by-products (e.g., polymeric vs. discrete species), or relies on templating approaches, meaning that substantial solvent and waste would be generated upon scale-up of supramolecular syntheses for industrial applications.43,44
To address these challenges, flow reactors have emerged as a powerful alternative to batch due to their unique capabilities: precisely controlled reaction parameters, enhanced mass and heat transfer, and continuous operation.45,46 The well-defined reaction environment in flow systems can afford a better control over nucleation and growth processes in self-assembly, which can result in improved uniformity, higher yields, enhanced reproducibility, and improved crystallinity in solid-state materials.47–52 The ability to modulate parameters such as flow rate and temperature allows the formation of tuneable size and shape controlled self-assemblies, enabling the development of process-structure–function relationships.53–55 Controlling selectivity via defined flow pathways and parameters rather than using high-dilution approaches also opens opportunities to increase concentration and throughput, minimising solvent use and waste.56
Compared to conventional batch processes, continuous flow systems offer precise control over key reaction parameters, enhancing reaction efficiency, selectivity, sustainability, and reproducibility, as summarised in recent reviews.45,46,51,57–59 Two advantages that are particularly relevant to the examples in this review are (a) reproducible mixing and (b) improved temperature control.
Efficient mixing is essential for homogeneous reaction environments and consistent product quality. In batch systems, mixing can be impacted by slow diffusion and/or variable shear forces, leading to concentration gradients and reduced selectivity—especially during scale-up. Flow chemistry can help to overcome these limitations by allowing improved control over shear rate through adjustments in flow rate, residence time, and channel geometry.46
Temperature is a key determinant of both the kinetics and selectivity of chemical reactions. The high surface area-to-volume ratios of flow reactors provide rapid and uniform heat transfer, enabling tight control over thermal conditions.45,50 In combination with the use of back-pressure regulators which allow solvents to be heated to elevated temperatures compared with reactors under ambient pressure, this capability allows researchers to access reaction regimes that are difficult or unsafe to achieve in batch processes.
In this review, we summarise recent progress in the synthesis of self-assembled structures using flow reactors, highlighting the advantages of flow chemistry in four areas: (a) routes to scalable production, (b) opportunities to control selectivity of kinetic vs. thermodynamic products, (c) enhanced crystallinity, and (d) improved size/morphology control compared to batch processes (Fig. 1). As such, we aim to highlight opportunities for supramolecular chemists to benefit from the technique, as well as future directions for technological development in this interdisciplinary area.
 | ||
| Fig. 1 Schematic representation of the advantages and enhancements offered by flow-based technology in the formation of self-assembled supramolecules. | ||
2. Advantages of flow compared to batch
2.1 Routes to scalable production
Scalable production remains a major challenge for supramolecular materials, primarily due to the lack of uniform reaction conditions in batch reactors. Most supramolecular compounds are synthesized on a limited scale (mg to g) and typically require prolonged reaction times (days), resulting in low production rates. Translation of a mg g−1-scale synthesis to kg and beyond typically requires re-optimisation even for well-understood batchwise reactions, as demonstrated in pharmaceutical process chemistry;60 this is exacerbated for processes involving weak, non-covalent interactions or reversible covalent bonds due to increased sensitivity to reaction conditions. It has been demonstrated that a move to flow can significantly reduce re-optimisation requirements on scale-up of active pharmaceutical ingredients,61 due to easier replication of desired mass-transport and heat-transfer requirements across length scales,62 although this is not universal and depends on the reaction and reactor in use.63,64 Such advantages are beginning to be used for supramolecular chemistry: the use of flow-based devices to improve throughput, and hence scalability, while reducing reagent and solvent consumption has been exemplified in the synthesis of MOFs,65–76 COFs,77–80 POCs,81–84 and other supramolecular assemblies, such as polymers85–87 and hydrogels.55,88–90 Although many of these studies remain on the g scale, improving throughput from mg h−1 to g h−1 is important to demonstrate scalability in principle, allowing assessment of the requirements of a pilot-scale process to begin without excessive use of resources.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159 kg m−3 day−1 (5.6 kg h−1), demonstrating the potential for commercial-scale MOF production (Fig. 2A).74 Compared to batch processes, scaling up in a flow reactor provides a safer and more sustainable approach, particularly for MOF synthesis, which often requires prolonged high temperatures in sealed environments followed by cooling. Additionally, flow systems minimize exposure to hazardous solvents like N,N′-dimethylformamide, a toxic mutagen with environmental risks.92 To date, multiple studies have reported the successful scale-up of MOFs in flow, including MIL-53 (Al),92 UIO-66,66,71,76 HKUST-1,67,70,76,93 ZIF-8,68,73 MOF-575 and zirconium MOFs.65,69
159 kg m−3 day−1 (5.6 kg h−1), demonstrating the potential for commercial-scale MOF production (Fig. 2A).74 Compared to batch processes, scaling up in a flow reactor provides a safer and more sustainable approach, particularly for MOF synthesis, which often requires prolonged high temperatures in sealed environments followed by cooling. Additionally, flow systems minimize exposure to hazardous solvents like N,N′-dimethylformamide, a toxic mutagen with environmental risks.92 To date, multiple studies have reported the successful scale-up of MOFs in flow, including MIL-53 (Al),92 UIO-66,66,71,76 HKUST-1,67,70,76,93 ZIF-8,68,73 MOF-575 and zirconium MOFs.65,69
 | ||
| Fig. 3 Schematic representation of the formation of hexamer and SOD in a flow reactor. Adapted with permission from ref. 95 (copyright 2024 Elsevier). | ||
The above breakthroughs highlight the potential and diversity of flow-based methodologies for the scalable synthesis of supramolecular materials, paving the way for their expanded applications. However, multiple possible products can arise from self-assembly, and it is also advantageous to be able to identify, scale up, and test the properties of minor products, such as kinetically trapped species, which may have superior properties to the most thermodynamically stable structure. Here, flow can offer routes to alter selectivity and access novel structures that may not be scalable or even obtainable in batch.
2.2 Kinetically controlled self-assembly in flow
The synthesis of supramolecular assemblies with well-defined structures typically requires the careful design of building blocks and precise regulation of their behaviour during the assembly process. In conventional batch reactions, supramolecular assembly often requires low concentrations and prolonged reaction times, with product formation predominantly governed by thermodynamic control. This reliance on thermodynamic equilibrium presents a significant challenge in obtaining kinetically controlled assemblies.43,100 A direct approach to modulating the assembly process is to alter the microenvironment surrounding the self-assembling molecules, an advantage readily accessible in flowing solutions within microchannels.101 Flow chemistry enables precise control over key parameters such as temperature, reactant ratios, and residence time along with inline analytical techniques, which can detect short-lived intermediates and provide real-time reaction feedback. Furthermore, efficient heat transfer and pressurised reactors open opportunities to achieve rapid and extreme temperature swings, alongside telescoping with inline quenching to stabilise and isolate kinetically trapped products. As such, the selectivity of kinetic vs. thermodynamic assembly products can be controlled with improved accuracy.102 | ||
| Fig. 4 Schematic representation of the formation of varied ratios of stack-dimer under microfluidic conditions. Adapted with permission from ref. 103 (copyright 2013 Royal Society of Chemistry). | ||
In the same year, Kozawa and colleagues reported the formation of kinetically trapped guanosine 5′-monophosphate (GMP) fibres through stepwise activation of hydrogen bonding and π–π stacking within a microfluidic channel (Fig. 5).104 This metastable configuration was sustained exclusively under continuous-flow conditions. Introducing a linker into these trapped nanofibers facilitated the formation of micron-sized fibrous aggregates, whereas conventional batch mixing of GMP and the linker failed to induce nanofiber or microstructure assembly.
 | ||
| Fig. 5 Schematic representation of the formation of 1D self-assembly of GMP and subsequent hierarchical organization of GMP nanofibers under laminar microfluidic conditions. Adapted with permission from ref. 104 (copyright 2013 Wiley-VCH). | ||
In 2015, Sakurai and co-workers used a microfluidic device to form a kinetic microfilm product on the micrometre scale through synchronized protonation of zinc(II)-5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (Fig. 6(A)).105 Microfluidic chemistry also demonstrates significant potential as a powerful tool for exploring pathway complexity in supramolecular polymerization. In 2015, Nogami and co-workers reported the flow synthesis of an oligo(p-phenylenevinylene) (OPV)-based amphiphilic self-assembled fibre. The metastable aggregates undergo a time-dependent morphological transition from flexible fibres to bundled structures, fan-shaped sheets, and discrete dots after collection in vials (Fig. 6(B)).106 Self-assembly was initiated by the controlled diffusion of water as an anti-solvent within a cross-point microfluidic setup. The laminar mixing conditions facilitated the formation of an energetically unfavourable self-assembled state under kinetic control, demonstrating an approach that would be difficult to achieve through conventional batch synthesis. The application of microfluidics in the formation of metastable fibres was further demonstrated by Numata and co-workers in a subsequent study. In this work, a solution of Camide-C1 as the assembling monomer and a solution of Namide-C1 as molecular wedges were introduced into a microfluidic device (Fig. 6(C)).107 The degree of wedge separation was precisely controlled by adjusting the concentration, and the subsequent degradation of the wedged fibres resulted in the formation of one-dimensional metastable nanofibers with capped ends. Later in 2019, Kitamura and colleagues reported the accelerated polymerization of perylene bisimides within a microfluidic device using a sheath flow strategy (Fig. 6(D)).101 In this approach, monomers dissolved in DCM were introduced into the central stream, while hexane was used as the surrounding sheath fluid. This controlled microfluidic environment facilitated aggregation even at lower monomer concentrations—an outcome not observed in conventional batch reactions.
 | ||
| Fig. 6 Schematic representation of the formation of (A) porphyrin-based self-assembly; (B) OPV-based amphiphilic self-assembled fibres, (C) Namide-C1-capped Camide-C1 nanofibres and (D) PBI-based polymers under sheath flow based microfluidic conditions. Adapted with permission from ref. 105 (copyright 2015 Chemical Society of Japan), ref. 106 (copyright 2015 Royal Society of Chemistry) ref. 107 (copyright 2015 American Chemical Society) and 101 (copyright 2017 Wiley-VCH). | ||
 | ||
| Fig. 7 Schematic representation of the formation of kinetically trapped P9L18 and zinc(II) pentameric circular helicate under continuous flow conditions. Adapted with permission from ref. 84 (copyright 2023 Wiley-VCH). | ||
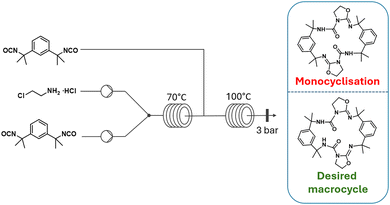 | ||
| Fig. 8 Schematic representation of the two-step formation of clamp-like macrocycle under continuous flow conditions. Adapted with permission from ref. 108 (copyright 2021 American Chemical Society). | ||
Opportunities for precise temperature control can be similarly advantageous for the formation of solid particles or crystalline materials. The formation of self-assembled aggregates is conceptually similar to crystal nucleation and growth, as both processes involve the spontaneous organization of molecules or particles into ordered structures driven by non-covalent interactions. In both cases, nucleation serves as the initial step, where a critical number of units come together to form a stable nucleus that can further grow. Once nucleation occurs, the system follows a pathway dictated by intermolecular forces, concentration gradients, and environmental conditions, leading to the expansion of the aggregate or crystal. Here, flow can offer advantages of improved crystallinity and control over particle size and morphology.
2.3 Improved crystallinity
Crystallinity plays a crucial role in defining the mechanical, optical, and electronic properties of self-assembled materials. Amorphous structures can exhibit distinct physicochemical properties compared to their crystalline counterparts, such as differences in solubility.54,109 However, traditional batch synthesis often leads to inconsistencies in crystallisation due to uncontrolled concentration fluctuations, temperature gradients, and limited mass transfer, which can introduce defects in crystal structures. In contrast, flow reactors enable continuous processing under precisely controlled conditions, including regulated supersaturation levels, uniform temperature distribution, and homogeneous reactant dispersion within the flow system. These factors facilitate well-defined nucleation events and can significantly enhance crystallinity.110,111 Moreover, flow reactors minimize polydispersity, which can result in morphological variations, by fine-tuning parameters such as flow rate, residence time, and shear forces. This level of control promotes ordered molecular packing and yields superior crystalline quality,43 although care must be taken to avoid fouling and blockages. | ||
| Fig. 9 Schematic representation of the synthesis of MF-COF-1 under sheath flow based microfluidic conditions. Adapted with permission from ref. 80 (copyright 2016 Royal Society of Chemistry). | ||
 | ||
| Fig. 10 Schematic representation of the crystallisation of CPOS-7 under flow conditions. Adapted with permission from ref. 52 (copyright 2023 Wiley-VCH). | ||
 | ||
| Fig. 11 Schematic representation of the microfluidic device for synthesis of silver acetylides and microphotographs of distribution and morphology of crystals formed along the microchannel. Adapted with permission from ref. 85 (copyright 2015 Wiley-VCH). | ||
Two other aspects that can control behaviour in application are particle size and morphology, which can both significantly influence material performance. Flow dynamics can also play a crucial role in shaping these properties, affecting the way particles aggregate, disperse, or interact within a given system.
2.4 Control of particle size and morphology in self-assembled structures
Precise control over the size and morphology of self-assemblies remains a significant challenge due to kinetic (nucleation rates, diffusion limitations, and reaction times) and thermodynamic factors (intermolecular interactions, solvent effects, and temperature). Balancing these factors is essential for achieving well-defined, reproducible structures with tailored properties. Flow chemistry, with its ability to finely tune reaction conditions such as concentration gradients, mixing efficiency, and residence time, offers a promising approach to overcoming these limitations. Unlike conventional batch methods, the laminar flow regime in microfluidic channels establishes well-defined reaction interfaces, facilitating precise control over nucleation and growth processes. This approach allows for the tailored synthesis of self-assemblies with desired sizes and properties, overcoming limitations inherent in traditional synthesis techniques.109,113–115 Here, reactor design is frequently used to control particle nucleation and growth, using sheath flow, droplet flow, or flow cells to generate solvent–solvent interfaces and influence mixing regimes. | ||
| Fig. 12 Schematic representation of the formation of TTF assembly and prepared hybrid ribbons at varied flow-rate ratios under microfluidic conditions. Adapted with permission from ref. 113 (copyright 2010 Wiley-VCH). | ||
Microfluidic systems can also provide deeper insights into reaction mechanisms. In 2016 Knowles, Gazit, and co-workers reported the controllable distribution of nanotube lengths by adjusting the molecular ratio of diphenylalanine and its end-capped analogue (Fig. 13).116 Using a microfluidic chip with micron-scale pillars, they successfully tuned the length of individual peptide nanotubes formed from diphenylalanine (FF) building blocks. These nanotubes were subsequently exposed to monomer solutions of varying concentrations, allowing real-time visualisation and analysis of their growth or shrinkage through electron microscopy—an approach that would be challenging in batch reactions due to uncontrolled aggregation.
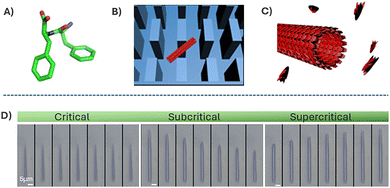 | ||
| Fig. 13 (A) Chemical structure of FF. (B) Schematic illustration of a single FF tube trapped between the micro-scale pillars. (C) Illustration of the FF nanotube growth. (D) Imaging of FF nanotubes at different times under critical (2.43 mM, 60 s interval), subcritical (1.6 mM, 10 s interval), and supercritical (3.20 mM, 100 s interval) concentration conditions. Adapted with permission from ref. 116 (copyright 2016 Springer Nature). | ||
In 2014, a 3D hydrodynamic focusing device was developed by Huang and co-workers to protect the channel wall from the precursors to avoid precipitation (Fig. 14).117 Using Dean vortices in curved channels, it vertically focused solutions of TTF and HAuCl4, optimising spatial control during self-assembly. This innovation facilitated the selective formation of diverse morphologies, such as branched and hexagonal structures.
 | ||
| Fig. 14 (A) Schematic representation of the formation of 3D hydrodynamic flow focusing device used for the shape control synthesis of TTF-Au structures under microfluidic conditions and SEM images of the structures collected at a FRR of (B) 20 and (C) 0.2, respectively. Adapted with permission from ref. 117 (copyright 2014 American Chemical Society). | ||
In 2014, Nair and co-workers synthesised ZIF-8 MOF membranes with exceptional separation performance, achieving H2/C3H8 and C3H6/C3H8 separation factors of 370 and 12 respectively, by changing flow parameters to tune the membrane thickness (Fig. 15).118 Using a microfluidic device with polymeric hollow fibre supports, they introduced Zn2+ and methylimidazole ligand solutions into separate layers to achieve controlled membrane production.
 | ||
| Fig. 15 Schematic representation of the formation of thickness controlled ZIF-8 MOF membranes under microfluidic conditions. Adapted with permission from ref. 118 (copyright 2014 The American Association for the Advancement of Science). | ||
MOF film growth in batch processes typically relies on a supported liquid interface, where undesired nucleation or overgrowth on preformed crystals often occurs, resulting in uneven membrane thickness. In 2011, De Vos and co-workers synthesized the MOF HKUST-1 by introducing a Cu(II) solution dropwise into a 1,3,5-benzenetricarboxylic acid (BTC) ligand solution under continuous flow, restricting the reaction to the phase interface (Fig. 16).121,122 The diffusion of MOF precursors occurs more rapidly at remaining defects than through the already-formed layer, leading to the preferential formation of new crystallites at these defect sites, effectively sealing the gaps, resulting in the formation of a uniformly thick film. Moreover, by adjusting the ratio of flow rates, size tuneable crystalline capsules are achievable.
 | ||
| Fig. 16 Schematic representation of the formation of size-controlled MOF HKUST-1, under droplets-based microfluidic conditions. Adapted with permission from ref. 121 (copyright 2011 Springer Nature). | ||
Similarly, in 2013, Kim and co-workers reported the biphasic droplet-based microfluidic synthesis of size- and morphology-controlled MOFs, including HKUST-1, MOF-5, IRMOF-3, and UiO-66.120 Notably, MOF crystals were assembled within minutes under microfluidic conditions, whereas conventional batch synthesis required several hours to days to achieve comparable results.54 In the same year, Coronas and co-workers reported synthesis of three dicarboxylate based MOFs (Fe-MIL-88B-NH2, Fe-MIL-88B, and Fe-MIL-88B-Br) by using droplet-based microfluidics, allowing control of the crystal size of MOFs in the range of 90–900 nm.123 In 2015, Falcaro and co-workers synthesised bio-MOF MIL-88A hollow spheres with controlled sizes using a droplet-based microfluidic system.124 By adjusting the FRR of the two phases and the reaction tube diameter, MIL-88A hollow spheres ranging from 35 to 2000 μm in size were successfully obtained. In 2019, Sun and co-workers reported a continuous synthetic process for MOF capsules with a hierarchical pore structure using a droplet-based microfluidic strategy.125 By precisely tuning the flow rates of the organic and aqueous phases, they were able to control the size distribution of the microcapsules with high precision. These studies highlight the significant advantages of microfluidic droplet-based systems in accelerating MOF crystallisation while ensuring precise control over particle characteristics, although scalability can be challenging in such systems.
The application of droplet-based microfluidics extends beyond MOFs to hydrogel and polymer microcapsule synthesis. In 2009, Shen and co-workers fabricated supramolecular hydrogel microspheres via hydrogen bonding between 1,2,4,5-benzene tetracarboxylic acid and 4-hydroxy pyridine, achieving precise size control by regulating flow rates.88 In 2019, we developed a continuous synthesis of polyurea microcapsules (Fig. 17).119 Using a flow system with a droplet sizing method based on video recording and Python-based analysis, precise control over microcapsule diameters was achieved (27–35 μm) with narrow size distributions (±2 μm).
 | ||
| Fig. 17 Size control of limonene droplets produced in a microfluidic chip (A) Synthesis of the polyurea microcapsules and an example of still from microscope video that has undergone processing to detect and measure droplet diameter via Python code. (B) Optical microscope images of o/w emulsions produced at varied FRR. Adapted with permission from ref. 119 (copyright 2019 Springer Nature). | ||
 | ||
| Fig. 18 Schematic representation of the formation of thickness-controlled 3D COF films under cell-based flow conditions. Adapted with permission from ref. 126 (copyright 2016 American Chemical Society). | ||
Other microfluidic techniques have also been employed for the controlled synthesis of self-assembled structures with precise size and morphology. In 2012, Witters and co-workers developed a high-throughput digital microfluidic method for synthesising single MOF crystals (Fig. 19).128 Using a modular two-plate device with an electronically controlled bottom plate and a hydrophilic-patterned top plate, they achieved precise spatial control over HKUST-1 crystal formation. Crystal size was finely tuned by adjusting the hydrophilic micro-patch dimensions, offering a scalable and efficient approach for tailored MOF synthesis.
 | ||
| Fig. 19 (A) Schematic representation of the formation of MOF crystals under a digital microfluidic chip. (B) By transporting mother droplets over arrays of hydrophilic-in-hydrophobic micropatches, large arrays of femtoliter droplets are printed due to the selective wettability of the hydrophilic micropatches. (C) And crystals are formed from these droplets upon evaporation. Adapted with permission from ref. 128 (copyright 2012 Wiley-VCH). | ||
As these examples demonstrate, flow reactors offer significant advantages over traditional batch processes in the control of size and morphology during self-assembly. The ability to precisely regulate flow rates, temperature, and concentration gradients in a flow reactor enables more uniform and reproducible formation of self-assembled structures. This level of control minimizes the variability in particle size and shape, which is often a challenge in batch processes due to the uncontrolled nature of solvent evaporation and mixing. Furthermore, the ability to fine-tune the micro-environment in flow systems extends beyond size and morphology control, offering broader opportunities for tailoring the structural and functional properties of self-assembled materials.
2.5 Other improved properties
 | ||
| Fig. 20 (A) Schematic representation of the formation of chirality biased H2TPPS4 porphyrin. (B) CD showing the effect of microfluidic versus flask-based mixing on the chirality of the formed H2TPPS4/CTAB heteroaggregates. Adapted with permission from ref. 129 (copyright 2016 American Chemical Society). | ||
2.6 Summarised comparison of different methodology
In summary, the selected cases presented in this review underscore the versatility of flow chemistry in both the investigation and scalable production of self-assembled materials. A range of flow techniques has been introduced, each offering unique advantages tailored to specific stages of research and development—from fundamental mechanistic studies to pilot-scale synthesis (Fig. 21). | ||
| Fig. 21 A comparative overview of batch reactors, microfluidic systems, and general tubing flow reactors. | ||
2.7 The challenges faced in self-assembly under continuous flow conditions
Despite the growing interest in integrating flow chemistry with self-assembling systems, several critical challenges remain that warrant focused future research.3. Conclusions and prospects
In this review, we have highlighted how the unique conditions provided by flow reactors can be effectively leveraged to optimize the synthesis of various self-assembled structures compared to conventional batch methods. Key advantages include: (1) scalable production with reduced solvent and reagent consumption, enabling large-scale production while maintaining uniformity and reproducibility; (2) the ability to tune selectivity of kinetically trapped species vs. thermodynamically controlled structures; (3) improved crystallinity and (4) precise control over crystal size and morphology by modulating key reaction parameters such as flow rate, reagent concentration, and temperature. These studies demonstrate that flow chemistry serves as a powerful toolbox for uncovering, studying, and designing self-assembly mechanisms that are inaccessible in batch reactors, thereby facilitating the development of novel functional materials.Although there are few examples of hierarchical or far-from-equilbrium self-assembly demonstrated in flow, the methods described above, such as controlled and sequential mixing in telescoped reactions, may be particularly suited to control and scale such processes. As previously noted, most non-covalent-based self-assemblies are constrained to production scales in the g h−1 range. To assist in bridging the gap between microfluidic control and typically millifluidic scalability, flow chemistry combined with inline analysis offers opportunities for real-time characterization of self-assembly processes, helping to optimise reactions136 or, for example, control particle size.137 Combining continuous flow processes with high-throughput platforms could greatly accelerate discovery and optimisation in materials science by enabling large-scale parallel synthesis and characterization,98,138 and would help provide the missing datasets that are needed to effectively use approaches such as machine learning. However, developments in analytical technology, high-throughput data handling, and methods for interrogating complex data will be essential to elucidate the complex mixtures of products often present during self-assembly processes. Further research should also focus on developing novel reactor designs that maximize efficiency while minimizing energy consumption and waste.
By bridging fundamental principles with engineering innovations, self-assembly under flow conditions holds significant potential for applications ranging from fundamental understanding of nanomaterials synthesis to advanced manufacturing. We anticipate that such approaches will find use in ever-broader classes of supramolecular systems, reflecting the diversity and breadth of the field and lowering the barriers to translation and industrial use of functional supramolecular architectures.
Author contributions
Conceptualization: Liqun Guo, Qiang Zhu, Anna Slater; writing – original draft: Liqun Guo; writing – review and editing: Liqun Guo, Anna Slater; supervision: Anna Slater; funding acquisition: Anna Slater.Data availability
This Feature Article titled “Self-assembly under continuous flow conditions” is a review summarizing recent developments in the field of self-assembly under continuous flow conditions, and no original experimental data were generated or analysed during the preparation of this manuscript. As such, there are no data sets associated with this work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AGS gratefully acknowledges receipt of a Royal Society University Research Fellowship (URF\R1\201168).Notes and references
- B. J. Holliday and C. A. Mirkin, Strategies for the Construction of Supramolecular Compounds through Coordination Chemistry, Angew. Chem., Int. Ed., 2001, 40(11), 2022–2043 CrossRef CAS PubMed.
- J.-M. Lehn, Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture), Angew. Chem., Int. Ed. Engl., 1988, 27(1), 89–112 CrossRef.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles, Chem. Rev., 2011, 111(11), 6810–6918 CrossRef CAS PubMed.
- E. Gazit, Diversity for Self-Assembly, Nat. Chem., 2010, 2(12), 1010–1011 CrossRef CAS PubMed.
- G. Vantomme and E. W. Meijer, The Construction of Supramolecular Systems, Science, 2019, 363(6434), 1396–1397 CrossRef CAS PubMed.
- S. Datta, M. L. Saha and P. J. Stang, Hierarchical Assemblies of Supramolecular Coordination Complexes, Acc. Chem. Res., 2018, 51(9), 2047–2063 CrossRef CAS PubMed.
- J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, Mastering Molecular Matter. Supramolecular Architectures by Hierarchical Self-Assembly, J. Mater. Chem., 2003, 13(11), 2661–2670 RSC.
- F. B. L. Cougnon, A. R. Stefankiewicz and S. Ulrich, Dynamic Covalent Synthesis, Chem. Sci., 2024, 15(3), 879–895 RSC.
- A. I. Cooper, Porous Molecular Solids and Liquids, ACS Cent. Sci., 2017, 3(6), 544–553 CrossRef CAS PubMed.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Introduction to Metal–Organic Frameworks, Chem. Rev., 2012, 112(2), 673–674 CrossRef CAS PubMed.
- C. Liu, J. Wang, J. Wan and C. Yu, MOF-on-MOF Hybrids: Synthesis and Applications, Coord. Chem. Rev., 2021, 432, 213743 CrossRef CAS.
- H. C. Zhou and S. “Joe” Kitagawa, Metal–Organic Frameworks (MOFs), Chem. Soc. Rev., 2014, 43(16), 5415–5418 RSC.
- J. Fonseca, T. Gong, L. Jiao and H.-L. Jiang, Metal–Organic Frameworks (MOFs) beyond Crystallinity: Amorphous MOFs, MOF Liquids and MOF Glasses, J. Mater. Chem. A, 2021, 9(17), 10562–10611 RSC.
- S. Y. Ding and W. Wang, Covalent Organic Frameworks (COFs): From Design to Applications, Chem. Soc. Rev., 2012, 42(2), 548–568 RSC.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Covalent Organic Frameworks: Design, Synthesis, and Functions, Chem. Rev., 2020, 120(16), 8814–8933 CrossRef CAS PubMed.
- S. Ge, K. Wei, W. Peng, R. Huang, E. Akinlabi, H. Xia, M. Wakil Shahzad, X. Zhang, B. Bin Xu and J. Jiang, A Comprehensive Review of Covalent Organic Frameworks (COFs) and Their Derivatives in Environmental Pollution Control, Chem. Soc. Rev., 2024, 53(23), 11259–11302 RSC.
- S. Kitagawa, R. Kitaura and S. Noro, Functional Porous Coordination Polymers, Angew. Chem., Int. Ed., 2004, 43(18), 2334–2375 CrossRef CAS PubMed.
- Z. Lin, J. J. Richardson, J. Zhou and F. Caruso, Direct Synthesis of Amorphous Coordination Polymers and Metal–Organic Frameworks, Nat. Rev. Chem., 2023, 7(4), 273–286 CrossRef CAS PubMed.
- T. Uemura, N. Yanai and S. Kitagawa, Polymerization Reactions in Porous Coordination Polymers, Chem. Soc. Rev., 2009, 38(5), 1228–1236 RSC.
- L. Yang, X. Tan, Z. Wang and X. Zhang, Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions, Chem. Rev., 2015, 115(15), 7196–7239 CrossRef CAS PubMed.
- X. Yang, Z. Ullah, J. F. Stoddart and C. T. Yavuz, Porous Organic Cages, Chem. Rev., 2023, 123(8), 4602–4634 CrossRef CAS PubMed.
- H. Wang, Y. Jin, N. Sun, W. Zhang and J. Jiang, Post-Synthetic Modification of Porous Organic Cages, Chem. Soc. Rev., 2021, 50(16), 8874–8886 RSC.
- C. J. T. Cox, J. Hale, P. Molinska and J. E. M. Lewis, Supramolecular and Molecular Capsules, Cages and Containers, Chem. Soc. Rev., 2024, 53(21), 10380–10408 RSC.
- J. Dong, Y. Liu and Y. Cui, Supramolecular Chirality in Metal–Organic Complexes, Acc. Chem. Res., 2021, 54(1), 194–206 CrossRef CAS PubMed.
- C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Supramolecular Catalysis in Metal–Ligand Cluster Hosts, Chem. Rev., 2015, 115(9), 3012–3035 CrossRef CAS PubMed.
- S. D. P. Fielden, D. A. Leigh and S. L. Woltering, Molecular Knots, Angew. Chem., Int. Ed., 2017, 56(37), 11166–11194 CrossRef CAS PubMed.
- R. S. Forgan, J. P. Sauvage and J. F. Stoddart, Chemical Topology: Complex Molecular Knots, Links, and Entanglements, Chem. Rev., 2011, 111(9), 5434–5464 CrossRef CAS PubMed.
- O. Lukin and F. Vögtle, Knotting and Threading of Molecules: Chemistry and Chirality of Molecular Knots and Their Assemblies, Angew. Chem., Int. Ed., 2005, 44(10), 1456–1477 CrossRef CAS PubMed.
- Y. Zhou, K. Jie, R. Zhao and F. Huang, Supramolecular-Macrocycle-Based Crystalline Organic Materials, Adv. Mater., 2020, 32(20), 1904824 CrossRef CAS PubMed.
- X. Y. Lou, S. Zhang, Y. Wang and Y.-W. Yang, Smart Organic Materials Based on Macrocycle Hosts, Chem. Soc. Rev., 2023, 52(19), 6644–6663 RSC.
- E. M. Ahmed, Hydrogel: Preparation, Characterization, and Applications: A Review, J. Adv. Res., 2015, 6(2), 105–121 CrossRef CAS PubMed.
- C. Yang and Z. Suo, Hydrogel Ionotronics, Nat. Rev. Mater., 2018, 3(6), 125–142 CrossRef CAS.
- Z. Q. Wang, X. Wang and Y. W. Yang, Pillararene-Based Supramolecular Polymers for Adsorption and Separation, Adv. Mater., 2024, 36(4), 2301721 CrossRef CAS PubMed.
- G. Zhang, W. Lin, F. Huang, J. Sessler and N. M. Khashab, Industrial Separation Challenges: How Does Supramolecular Chemistry Help?, J. Am. Chem. Soc., 2023, 145(35), 19143–19163 CrossRef CAS PubMed.
- D. Wu, P. F. Zhang, G. Yang, L. Hou, W. Y. Zhang, Y. F. Han, P. Liu and Y. Y. Wang, Supramolecular Control of MOF Pore Properties for the Tailored Guest Adsorption/Separation Applications, Coord. Chem. Rev., 2021, 434, 213709 CrossRef CAS.
- X. Ma and Y. Zhao, Biomedical Applications of Supramolecular Systems Based on Host–Guest Interactions, Chem. Rev., 2015, 115(15), 7794–7839 CrossRef CAS PubMed.
- Q. D. Hu, G. P. Tang and P. K. Chu, Cyclodextrin-Based Host–Guest Supramolecular Nanoparticles for Delivery: From Design to Applications, Acc. Chem. Res., 2014, 47(7), 2017–2025 CrossRef CAS PubMed.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Applications of Supramolecular Anion Recognition, Chem. Rev., 2015, 115(15), 8038–8155 CrossRef CAS PubMed.
- Y. N. Gong, D. C. Zhong and T. B. Lu, Porous Supramolecular Crystalline Materials for Photocatalysis, Angew. Chem., Int. Ed., 2025, 137(11), e202424452 CrossRef.
- X. Sun and T. D. James, Glucose Sensing in Supramolecular Chemistry, Chem. Rev., 2015, 115(15), 8001–8037 CrossRef CAS PubMed.
- L. You, D. Zha and E. V. Anslyn, Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing, Chem. Rev., 2015, 115(15), 7840–7892 CrossRef CAS PubMed.
- L. Camps, L. Moens, U. Groth, L. Braeken, S. Kuhn and L. C. J. Thomassen, Characterization Method for Mass Mixing in Batch Reactors Based on Temperature Profiles, Chem. Eng. Res. Des., 2020, 156, 300–310 CrossRef CAS.
- L. Zhou, C. Yang, W. Dou, T. Jin, H. Yang and L. Xu, Supramolecular Flow Chemistry: Construction of Multiscale Supramolecular Assemblies by Micro/Nanofluidic Techniques, Chin. Chem. Lett., 2024, 35(1), 108669 CrossRef CAS.
- M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, New Synthetic Routes towards MOF Production at Scale, Chem. Soc. Rev., 2017, 46(11), 3453–3480 RSC.
- L. Capaldo, Z. Wen and T. Noël, A Field Guide to Flow Chemistry for Synthetic Organic Chemists, Chem. Sci., 2023, 14(16), 4230–4247 RSC.
- M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, The Hitchhiker's Guide to Flow Chemistry, Chem. Rev., 2017, 117(18), 11796–11893 CrossRef CAS PubMed.
- X. Hou, Y. S. Zhang, G. T. Santiago, M. M. Alvarez, J. Ribas, S. J. Jonas, P. S. Weiss, A. M. Andrews, J. Aizenberg and A. Khademhosseini, Interplay between Materials and Microfluidics, Nat. Rev. Mater., 2017, 2(5), 1–15 Search PubMed.
- K. S. Elvira, X. C. i Solvas, R. C. R. Wootton and A. J. deMello, The Past, Present and Potential for Microfluidic Reactor Technology in Chemical Synthesis, Nat. Chem., 2013, 5(11), 905–915 CrossRef CAS PubMed.
- T. Beatus, I. Shani, R. H. Bar-Ziv and T. Tlusty, Two-Dimensional Flow of Driven Particles: A Microfluidic Pathway to the Non-Equilibrium Frontier, Chem. Soc. Rev., 2017, 46(18), 5620–5646 RSC.
- A. I. Alfano, J. García-Lacuna, O. M. Griffiths, S. V. Ley and M. Baumann, Continuous Flow Synthesis Enabling Reaction Discovery, Chem. Sci., 2024, 15(13), 4618–4630 RSC.
- Y. Xin, S. Peng, J. Chen, Z. Yang and J. Zhang, Continuous Flow Synthesis of Porous Materials, Chin. Chem. Lett., 2020, 31(6), 1448–1461 CrossRef CAS.
- M. O’Shaughnessy, A. C. Padgham, R. Clowes, M. A. Little, M. C. Brand, H. Qu, A. G. Slater and A. I. Cooper, Controlling the Crystallisation and Hydration State of Crystalline Porous Organic Salts, Chem. – Eur. J., 2023, 29(64), e202302420 CrossRef PubMed.
- D. Khoeini, T. F. Scott and A. Neild, Microfluidic Enhancement of Self-Assembly Systems, Lab Chip, 2021, 21(9), 1661–1675 RSC.
- S. Sevim, A. Sorrenti, C. Franco, S. Furukawa, S. Pané, A. J. deMello and J. Puigmartí-Luis, Self-Assembled Materials and Supramolecular Chemistry within Microfluidic Environments: From Common Thermodynamic States to Non-Equilibrium Structures, Chem. Soc. Rev., 2018, 47(11), 3788–3803 RSC.
- Z. Wei, S. Wang, J. Hirvonen, H. A. Santos and W. Li, Microfluidics Fabrication of Micrometer-Sized Hydrogels with Precisely Controlled Geometries for Biomedical Applications, Adv. Healthcare Mater., 2022, 11(16), 2200846 CrossRef CAS PubMed.
- F. Esteve, B. Altava, S. V. Luis and E. García-Verdugo, Tools and New Metric (Macrocyclization Environmental Impact – MEI) to Tackle the Sustainability of Macrocyclization Reactions, Catal. Today, 2024, 426, 114407 CrossRef CAS.
- A. Laybourn, K. Robertson and A. G. Slater, Quid Pro Flow, J. Am. Chem. Soc., 2023, 145(8), 4355–4365 CrossRef CAS PubMed.
- F. Parveen, N. Watson, A. M. Scholes and A. G. Slater, Continuous Flow as an Enabling Technology for Sustainable Supramolecular Chemistry, Curr. Opin. Green Sustainable Chem., 2024, 48, 100935 CrossRef.
- K. Ollerton, R. L. Greenaway and A. G. Slater, Enabling Technology for Supramolecular Chemistry, Front. Chem., 2021, 9, 774987 CrossRef PubMed.
- C. J. Taylor, A. Pomberger, K. C. Felton, R. Grainger, M. Barecka, T. W. Chamberlain, R. A. Bourne, C. N. Johnson and A. A. Lapkin, A Brief Introduction to Chemical Reaction Optimization, Chem. Rev., 2023, 123(6), 3089–3126 CrossRef CAS PubMed.
- M. Guidi, S. Moon, L. Anghileri, D. Cambié, P. H. Seeberger and K. Gilmore, Combining Radial and Continuous Flow Synthesis to Optimize and Scale-up the Production of Medicines, React. Chem. Eng., 2021, 6(2), 220–224 RSC.
- G. M. Martins, M. F. A. Magalhães, T. J. Brocksom, V. S. Bagnato and K. T. de Oliveira, Scaled up and Telescoped Synthesis of Propofol under Continuous-Flow Conditions, J. Flow Chem., 2022, 12(3), 371–379 CrossRef CAS PubMed.
- I. Rossetti and M. Compagnoni, Chemical Reaction Engineering, Process Design and Scale-up Issues at the Frontier of Synthesis: Flow Chemistry, Chem. Eng. J., 2016, 296, 56–70 CrossRef CAS.
- Z. Dong, Z. Wen, F. Zhao, S. Kuhn and T. Noël, Scale-up of Micro- and Milli-Reactors: An Overview of Strategies, Design Principles and Applications, Chem. Eng. Sci: X., 2021, 10, 100097 CAS.
- S. Wang, M. Wahiduzzaman, C. Martineau-Corcos, G. Maurin and C. Serre, A Microporous Zirconium Metal–Organic Framework Based on Trans-Aconitic Acid for Selective Carbon Dioxide Adsorption, Eur. J. Inorg. Chem., 2019,(22), 2674–2679 CrossRef CAS.
- L. Garzón-Tovar, M. Cano-Sarabia, A. Carné-Sánchez, C. Carbonell, I. Imaz and D. Maspoch, A Spray-Drying Continuous-Flow Method for Simultaneous Synthesis and Shaping of Microspherical High Nuclearity MOF Beads, React. Chem. Eng., 2016, 1(5), 533–539 RSC.
- A. Carné-Sánchez, I. Imaz, M. Cano-Sarabia and D. Maspoch, A Spray-Drying Strategy for Synthesis of Nanoscale Metal–Organic Frameworks and Their Assembly into Hollow Superstructures, Nat. Chem., 2013, 5(3), 203–211 CrossRef PubMed.
- A. Polyzoidis, T. Altenburg, M. Schwarzer, S. Loebbecke and S. Kaskel, Continuous Microreactor Synthesis of ZIF-8 with High Space–Time-Yield and Tunable Particle Size, Chem. Eng. J., 2016, 283, 971–977 CrossRef CAS.
- Y. Wang, L. Li, L. Yan, L. Cao, P. Dai, X. Gu and X. Zhao, Continuous Synthesis for Zirconium Metal–Organic Frameworks with High Quality and Productivity via Microdroplet Flow Reaction, Chin. Chem. Lett., 2018, 29(6), 849–853 CrossRef CAS.
- M. Taddei, D. A. Steitz, J. A. van Bokhoven and M. Ranocchiari, Continuous-Flow Microwave Synthesis of Metal–Organic Frameworks: A Highly Efficient Method for Large-Scale Production, Chem. Eur. J., 2016, 22(10), 3245–3249 CrossRef CAS PubMed.
- S. Waitschat, M. T. Wharmby and N. Stock, Flow-Synthesis of Carboxylate and Phosphonate Based Metal–Organic Frameworks under Non-Solvothermal Reaction Conditions, Dalton Trans., 2015, 44(24), 11235–11240 RSC.
- M. Taddei, N. Casati, D. A. Steitz, K. C. Dümbgen, J. A. Bokhoven and M. van Ranocchiari, In Situ High-Resolution Powder X-Ray Diffraction Study of UiO-66 under Synthesis Conditions in a Continuous-Flow Microwave Reactor, CrystEngComm, 2017, 19(23), 3206–3214 RSC.
- A. S. Munn, P. W. Dunne, S. V. Y. Tang and E. H. Lester, Large-Scale Continuous Hydrothermal Production and Activation of ZIF-8, Chem. Commun., 2015, 51(64), 12811–12814 RSC.
- M. Rubio-Martinez, T. D. Hadley, M. P. Batten, K. Constanti-Carey, T. Barton, D. Marley, A. Mönch, K.-S. Lim and M. R. Hill, Scalability of Continuous Flow Production of Metal–Organic Frameworks, ChemSusChem, 2016, 9(9), 938–941 CrossRef CAS PubMed.
- C. McKinstry, R. J. Cathcart, E. J. Cussen, A. J. Fletcher, S. V. Patwardhan and J. Sefcik, Scalable Continuous Solvothermal Synthesis of Metal Organic Framework (MOF-5) Crystals, Chem. Eng. J., 2016, 285, 718–725 CrossRef CAS.
- M. Rubio-Martinez, M. P. Batten, A. Polyzos, K. C. Carey, J. I. Mardel, K. S. Lim and M. R. Hill, Versatile, High Quality and Scalable Continuous Flow Production of Metal–Organic Frameworks, Sci. Rep., 2014, 4(1), 5443 CrossRef CAS PubMed.
- H. Vardhan, G. Rummer, A. Deng and S. Ma, Large-Scale Synthesis of Covalent Organic Frameworks: Challenges and Opportunities, Membranes, 2023, 13(8), 696 CrossRef CAS PubMed.
- Y. Peng, W. K. Wong, Z. Hu, Y. Cheng, D. Yuan, S. A. Khan and D. Zhao, Room Temperature Batch and Continuous Flow Synthesis of Water-Stable Covalent Organic Frameworks (COFs), Chem. Mater., 2016, 28(14), 5095–5101 CrossRef CAS.
- M. Traxler and W. R. Dichtel, Continuous Flow Synthesis and Post-Synthetic Conversion of Single-Crystalline Covalent Organic Frameworks, Chem. Sci., 2024, 15(20), 7545–7551 RSC.
- D. Rodríguez-San-Miguel, A. Abrishamkar, J. A. R. Navarro, R. Rodriguez-Trujillo, D. B. Amabilino, R. Mas-Ballesté, F. Zamora and J. Puigmartí-Luis, Crystalline Fibres of a Covalent Organic Framework through Bottom-up Microfluidic Synthesis, Chem. Commun., 2016, 52(59), 9212–9215 RSC.
- M. Kitchin, K. Konstas, C. J. Sumby, M. L. Czyz, P. Valente, M. R. Hill, A. Polyzos and C. J. Doonan, Continuous Flow Synthesis of a Carbon-Based Molecular Cage Macrocycle via a Three-Fold Homocoupling Reaction, Chem. Commun., 2015, 51(75), 14231–14234 RSC.
- M. E. Briggs, A. G. Slater, N. Lunt, S. Jiang, M. A. Little, R. L. Greenaway, T. Hasell, C. Battilocchio, S. V. Ley and A. I. Cooper, Dynamic Flow Synthesis of Porous Organic Cages, Chem. Commun., 2015, 51(98), 17390–17393 RSC.
- B. D. Egleston, M. C. Brand, F. Greenwell, M. E. Briggs, S. L. James, A. I. Cooper, D. E. Crawford and R. L. Greenaway, Continuous and Scalable Synthesis of a Porous Organic Cage by Twin Screw Extrusion (TSE), Chem. Sci., 2020, 11(25), 6582–6589 RSC.
- H. Lee, J. U. Joo, A. Dhamija, A. Gunnam, J. Koo, P. Giri, Y. Ho Ko, I. C. Hwang, D. P. Kim and K. Kim, Flow Synthesis of Gigantic Porphyrinic Cages: Facile Synthesis of P12L24 and Discovery of Kinetic Product P9L18, Chem. – Eur. J., 2023, 29(34), e202300760 CrossRef CAS PubMed.
- X. Liu, Q. Yi, Y. Han, Z. Liang, C. Shen, Z. Zhou, J. Sun, Y. Li, W. Du and R. Cao, A Robust Microfluidic Device for the Synthesis and Crystal Growth of Organometallic Polymers with Highly Organized Structures, Angew. Chem., Int. Ed., 2015, 54(6), 1846–1850 CrossRef CAS PubMed.
- S. Ogi, A. Takamatsu, K. Matsumoto, S. Hasegawa and S. Yamaguchi, Biomimetic Design of a Robustly Stabilized Folded State Enabling Seed-Initiated Supramolecular Polymerization under Microfluidic Mixing, Angew. Chem., Int. Ed., 2023, 62(34), e202306428 CrossRef CAS PubMed.
- M. Rubio-Martinez, I. Imaz, N. Domingo, A. Abrishamkar, T. S. Mayor, R. M. Rossi, C. Carbonell, A. J. deMello, D. B. Amabilino, D. Maspoch and J. Puigmartí-Luis, Freezing the Nonclassical Crystal Growth of a Coordination Polymer Using Controlled Dynamic Gradients, Adv. Mater., 2016, 28(37), 8150–8155 CrossRef CAS PubMed.
- W. Chen, Y. Yang, C. Rinadi, D. Zhou and A. Q. Shen, Formation of Supramolecular Hydrogel Microspheres via Microfluidics, Lab Chip, 2009, 9(20), 2947–2951 RSC.
- N. Weigel, Y. Li, J. Thiele and A. Fery, From Microfluidics to Hierarchical Hydrogel Materials, Curr. Opin. Colloid Interface Sci., 2023, 64, 101673 CrossRef CAS.
- A. M. Garcia, J. A. Garcia-Romero, S. H. Mejias, P. Prieto, V. Saggiomo, A. H. Velders, M. Laura Soriano, V. Ruiz-Díez, J. Cabanillas-González and M. Victoria Gomez, Microfluidic-Driven Short Peptide Hydrogels with Optical Waveguiding Properties, J. Mater. Chem. C, 2024, 12(17), 6027–6034 RSC.
- Z. Dong, Z. Wen, F. Zhao, S. Kuhn and T. Noël, Scale-up of Micro- and Milli-Reactors: An Overview of Strategies, Design Principles and Applications, Chem. Eng. Sci: X., 2021, 10, 100097 CAS.
- P. A. Bayliss, I. A. Ibarra, E. Pérez, S. Yang, C. C. Tang, M. Poliakoff and M. Schröder, Synthesis of Metal–Organic Frameworks by Continuous Flow, Green Chem., 2014, 16(8), 3796–3802 RSC.
- A. Laybourn, A. M. López-Fernández, I. Thomas-Hillman, J. Katrib, W. Lewis, C. Dodds, A. P. Harvey and S. W. Kingman, Combining Continuous Flow Oscillatory Baffled Reactors and Microwave Heating: Process Intensification and Accelerated Synthesis of Metal–Organic Frameworks, Chem. Eng. J., 2019, 356, 170–177 CrossRef CAS.
- P. Martinez-Bulit, A. Sorrenti, D. Rodriguez San Miguel, M. Mattera, Y. Belce, Y. Xia, S. Ma, M.-H. Huang, S. Pané and J. Puigmartí-Luis, In Flow-Based Technologies: A New Paradigm for the Synthesis and Processing of Covalent-Organic Frameworks, Chem. Eng. J., 2022, 435, 135117 CrossRef CAS.
- C. Du, A. C. Padgham, A. G. Slater and L. Zhang, Efficient Flow Synthesis of a Star of David [2]Catenane and a Pentafoil Knot, Chem, 2024, 11(1), 102328 Search PubMed.
- J. Casaban, Y. Zhang, R. Pacheco, C. Coney, C. Holmes, E. Sutherland, C. Hamill, J. Breen, S. L. James, D. Tufano, D. Wong, E. Stavrakakis, H. Annath and A. Moore, Towards MOFs’ Mass Market Adoption: MOF Technologies’ Efficient and Versatile One-Step Extrusion of Shaped MOFs Directly from Raw Materials, Faraday Discuss., 2021, 231(0), 312–325 RSC.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Synthesis by Extrusion: Continuous, Large-Scale Preparation of MOFs Using Little or No Solvent, Chem. Sci., 2015, 6(3), 1645–1649 RSC.
- W. Zhang, M. Yu, T. Liu, M. Cong, X. Liu, H. Yang, Y. Bai, Q. Zhu, S. Zhang, H. Gu, X. Wu, Z. Zhang, Y. Wu, H. Tian, X. Li, W.-H. Zhu and A. I. Cooper, Accelerated Discovery of Molecular Nanojunction Photocatalysts for Hydrogen Evolution by Using Automated Screening and Flow Synthesis, Nat. Synth., 2024, 3(5), 595–605 CrossRef CAS.
- J. Y. Han, J. N. La Fiandra and D. L. DeVoe, Microfluidic Vortex Focusing for High Throughput Synthesis of Size-Tunable Liposomes, Nat. Commun., 2022, 13(1), 6997 CrossRef CAS PubMed.
- B. A. Grzybowski, C. E. Wilmer, J. Kim, K. P. Browne and K. J. M. Bishop, Self-Assembly: From Crystals to Cells, Soft Matter, 2009, 5(6), 1110–1128 RSC.
- M. Numata, R. Nogami and A. Kitamura, Enhanced Self-Assembly Abilities Coupled with Nano- and Microscale Non-Equilibrium Phenomena in Flowing Microfields, Chem. Nanomater., 2018, 4(2), 175–182 CAS.
- L. Buglioni, F. Raymenants, A. Slattery, S. D. A. Zondag and T. Noël, Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry, Chem. Rev., 2022, 122(2), 2752–2906 CrossRef CAS PubMed.
- F. Helmich and E. W. Meijer, Controlled Perturbation of the Thermodynamic Equilibrium by Microfluidic Separation of Porphyrin-Based Aggregates in a Multi-Component Self-Assembling System, Chem. Commun., 2013, 49(18), 1796–1798 RSC.
- M. Numata and T. Kozawa, Supramolecular Polymerization in Microfluidic Channels: Spatial Control Over Multiple Intermolecular Interactions, Chem. – Eur. J., 2013, 19(38), 12629–12634 CrossRef CAS PubMed.
- M. Numata, Y. Nishino, Y. Sanada and K. Sakurai, Creation of Kinetically Stabilized Porphyrin Microfilms through Synchronized Hydrogen-Bonding Interactions in Microflow, Chem. Lett., 2015, 44(6), 861–863 CrossRef CAS.
- M. Numata, A. Sato and R. Nogami, Energy-Dissipative Self-Assembly Driven in Microflow: A Time-Programmed Self-Organization and Decomposition of Metastable Nanofibers, Chem. Lett., 2015, 44(7), 995–997 CrossRef CAS.
- C. Kanzaki, T. Nakadozono and M. Numata, Creation of Discrete 1D Microstructures: Directional Dissociation from the Ends of a Metastable Supramolecular Polymer, ChemPlusChem, 2020, 85(1), 74–78 CrossRef CAS.
- C. D. Jones, L. J. Kershaw Cook, D. Marquez-Gamez, K. V. Luzyanin, J. W. Steed and A. G. Slater, High-Yielding Flow Synthesis of a Macrocyclic Molecular Hinge, J. Am. Chem. Soc., 2021, 143(19), 7553–7565 CrossRef CAS PubMed.
- E. Amstad, M. Gopinadhan, C. Holtze, C. O. Osuji, M. P. Brenner, F. Spaepen and D. A. Weitz, Production of Amorphous Nanoparticles by Supersonic Spray-Drying with a Microfluidic Nebulator, Science, 2015, 349(6251), 956–960 CrossRef CAS PubMed.
- B. K. H. Yen, A. Günther, M. A. Schmidt, K. F. Jensen and M. G. Bawendi, A Microfabricated Gas–Liquid Segmented Flow Reactor for High-Temperature Synthesis: The Case of CdSe Quantum Dots, Angew. Chem., Int. Ed., 2005, 44(34), 5447–5451 CrossRef CAS PubMed.
- B. Zheng, J. D. Tice and R. F. Ismagilov, Formation of Arrayed Droplets by Soft Lithography and Two-Phase Fluid Flow, and Application in Protein Crystallization, Adv. Mater., 2004, 16(15), 1365–1368 CrossRef CAS PubMed.
- C. Hu, Y. Bai, M. Hou, Y. Wang, L. Wang, X. Cao, C. W. Chan, H. Sun, W. Li, J. Ge and K. Ren, Defect-Induced Activity Enhancement of Enzyme-Encapsulated Metal–Organic Frameworks Revealed in Microfluidic Gradient Mixing Synthesis, Sci. Adv., 2020, 6(5), eaax5785 CrossRef CAS PubMed.
- J. Puigmartí-Luis, D. Schaffhauser, B. R. Burg and P. S. Dittrich, A Microfluidic Approach for the Formation of Conductive Nanowires and Hollow Hybrid Structures, Adv. Mater., 2010, 22(20), 2255–2259 CrossRef PubMed.
- K. F. Jensen, Microreaction Engineering—Is Small Better?, Chem. Eng. J., 2001, 56(2), 293–303 CAS.
- J. Puigmartí-Luis, M. Rubio-Martínez, U. Hartfelder, I. Imaz, D. Maspoch and P. S. Dittrich, Coordination Polymer Nanofibers Generated by Microfluidic Synthesis, J. Am. Chem. Soc., 2011, 133(12), 4216–4219 CrossRef PubMed.
- Z. A. Arnon, A. Vitalis, A. Levin, T. C. T. Michaels, A. Caflisch, T. P. J. Knowles, L. Adler-Abramovich and E. Gazit, Dynamic Microfluidic Control of Supramolecular Peptide Self-Assembly, Nat. Commun., 2016, 7(1), 13190 CrossRef CAS PubMed.
- M. q Lu, S. k Yang, Y. P. Ho, C. L. Grigsby, K. W. Leong and T. J. Huang, Shape-Controlled Synthesis of Hybrid Nanomaterials via Three-Dimensional Hydrodynamic Focusing, ACS Nano, 2014, 8(10), 10026–10034 CrossRef CAS PubMed.
- A. J. Brown, N. A. Brunelli, K. Eum, F. Rashidi, J. R. Johnson, W. J. Koros, C. W. Jones and S. Nair, Interfacial Microfluidic Processing of Metal–Organic Framework Hollow Fiber Membranes, Science, 2014, 345(6192), 72–75 CrossRef CAS PubMed.
- M. F. Thorne, F. Simkovic and A. G. Slater, Production of Monodisperse Polyurea Microcapsules Using Microfluidics, Sci. Rep., 2019, 9(1), 17983 CrossRef PubMed.
- M. Faustini, J. Kim, G. Y. Jeong, J. Y. Kim, H. R. Moon, W. S. Ahn and D. P. Kim, Microfluidic Approach toward Continuous and Ultrafast Synthesis of Metal–Organic Framework Crystals and Hetero Structures in Confined Microdroplets, J. Am. Chem. Soc., 2013, 135(39), 14619–14626 CrossRef CAS PubMed.
- R. Ameloot, F. Vermoortele, W. Vanhove, M. B. J. Roeffaers, B. F. Sels and D. E. De Vos, Interfacial Synthesis of Hollow Metal–Organic Framework Capsules Demonstrating Selective Permeability, Nat. Chem., 2011, 3(5), 382–387 CrossRef CAS PubMed.
- S. Furukawa, J. Reboul, S. Diring, K. Sumida and S. Kitagawa, Structuring of Metal–Organic Frameworks at the Mesoscopic/Macroscopic Scale, Chem. Soc. Rev., 2014, 43(16), 5700–5734 RSC.
- L. Paseta, B. Seoane, D. Julve, V. Sebastián, C. Téllez and J. Coronas, Accelerating the Controlled Synthesis of Metal–Organic Frameworks by a Microfluidic Approach: A Nanoliter Continuous Reactor, ACS Appl. Mater. Interfaces, 2013, 5(19), 9405–9410 CrossRef CAS PubMed.
- G. Y. Jeong, R. Ricco, K. Liang, J. Ludwig, J. O. Kim, P. Falcaro and D. P. Kim, Bioactive MIL-88A Framework Hollow Spheres via Interfacial Reaction In-Droplet Microfluidics for Enzyme and Nanoparticle Encapsulation, Chem. Mater., 2015, 27(23), 7903–7909 CrossRef CAS.
- S. Wu, Z. Xin, S. Zhao and S. Sun, High-Throughput Droplet Microfluidic Synthesis of Hierarchical Metal–Organic Framework Nanosheet Microcapsules, Nano Res., 2019, 12(11), 2736–2742 CrossRef CAS.
- R. P. Bisbey, C. R. DeBlase, B. J. Smith and W. R. Dichtel, Two-Dimensional Covalent Organic Framework Thin Films Grown in Flow, J. Am. Chem. Soc., 2016, 138(36), 11433–11436 CrossRef CAS PubMed.
- Y. Yang, C. Schäfer and K. Börjesson, Detachable All-Carbon-Linked 3D Covalent Organic Framework Films for Semiconductor/COF Heterojunctions by Continuous Flow Synthesis, Chem, 2022, 8(8), 2217–2227 CrossRef CAS PubMed.
- D. Witters, N. Vergauwe, R. Ameloot, S. Vermeir, D. De Vos, R. Puers, B. Sels and J. Lammertyn, Digital Microfluidic High-Throughput Printing of Single Metal–Organic Framework Crystals, Adv. Mater., 2012, 24(10), 1316–1320 CrossRef CAS PubMed.
- A. Sorrenti, R. Rodriguez-Trujillo, D. B. Amabilino and J. Puigmartí-Luis, Milliseconds Make the Difference in the Far-from-Equilibrium Self-Assembly of Supramolecular Chiral Nanostructures, J. Am. Chem. Soc., 2016, 138(22), 6920–6923 CrossRef CAS PubMed.
- J. Zhao, K. Zeng, W.-T. Dou, X. Zhao, W. Wang, X. Shi, X. Li, J. Fang, X. Qian, H.-B. Yang and L. Xu, Boosted Circularly Polarized Luminescence of a Chiral Metallacage Through Co-Assembly in Confined Microfluidic Environments, Adv. Funct. Mater., 2024, 35(3), e2207333 Search PubMed.
- F. M. Akwi and P. Watts, Continuous Flow Chemistry: Where Are We Now? Recent Applications, Challenges and Limitations, Chem. Commun., 2018, 54(99), 13894–13928 RSC.
- H. L. D. Hayes and C. J. Mallia, Continuous Flow Chemistry with Solids: A Review, Org. Process Res. Dev., 2024, 28(5), 1327–1354 Search PubMed.
- R. L. Hartman, Flow Chemistry Remains an Opportunity for Chemists and Chemical Engineers, Curr. Opin. Chem. Eng., 2020, 29, 42–50 CrossRef.
- A. Mata, C. de Fraipont, C. Hervieux, L. Giacchetti, O. Hadj-Sassi, A. Bogicevic, V. Marichez and T. M. Hermans, Nonclogging Liquid-Walled Continuous Flow Reactors, Org. Process Res. Dev., 2025, 29(2), 472–478 Search PubMed.
- E. Gavi, D. L. Marchisio and A. A. Barresi, On the Importance of Mixing for the Production of Nanoparticles, J. Dispers. Sci. Technol., 2008, 29(4), 548–554 CrossRef CAS.
- F. Parveen, H. J. Morris, H. West and A. G. Slater, Continuous Flow Synthesis of Meso-Substituted Porphyrins with Inline UV-Vis Analysis, J. Flow Chem., 2024, 14(1), 23–31 CrossRef CAS.
- C. Ferreira, J. Cardona, O. Agimelen, C. Tachtatzis, I. Andonovic, J. Sefcik and Y.-C. Chen, Quantification of Particle Size and Concentration Using In-Line Techniques and Multivariate Analysis, Powder Technol., 2020, 376, 1–11 CrossRef CAS.
- P. Zhou, J. He, L. Huang, Z. Yu, Z. Su, X. Shi and J. Zhou, Microfluidic High-Throughput Platforms for Discovery of Novel Materials, Nanomaterials, 2020, 10(12), 2514 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |




