Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The Lewis basicity of amines on the Legault iodonium Lewis acidity scale†
Soocheta
Jha
,
Souradeep
Basu
,
Madison
Bob
and
David R.
Stuart
 *
*
Department of Chemistry, Portland State University, Portland, Oregon 97201, USA. E-mail: dstuart@pdx.edu
First published on 1st July 2025
Abstract
Diaryliodonium–amine complexes are proposed as key intermediates in metal-free C–N coupling reactions. Herein, 1H NMR titration is used to quantify association constants (Ka) between diaryliodonium triflates and synthetically relevant amines from which the amine Lewis basicity (LBI) parameter is calculated. Compared to anionic Lewis bases, distinct solvent effects are observed.
Aryl amines are prevalent scaffolds in approved drugs and agrochemicals.1 Among the numerous ways to synthesize these motifs, the metal-free arylation of amines with diaryliodonium salts is a convenient method because it merges the simplicity of nucleophilic aromatic substitution (SNAr) with the improved scope of a reductive ligand coupling pathway (Scheme 1a).2–8 The mechanism that has been proposed for these reactions involves the coordination of the amine lone-pair with the iodonium(III) center prior to deprotonation of the amine and reductive ligand coupling (Scheme 1b).4,6 Although logical, there is relatively little support for this type of interaction with primary and secondary amines and anilines that have been the subject of prior synthetic studies. Ochiai and co-workers have quantified the coordination of pyridine and related derivatives (e.g. bipy and terpy) with diphenyliodonium tetrafluoroborate in DCM,9 and Legault and co-workers have quantified the coordination of quinuclidine and 4-pyrrolidylpyridine with several diaryliodonium salts (Scheme 1c).10 Additionally, as part of a synthetic study on the arylation of DABCO, Karchava and co-workers have isolated an X-ray structure suggesting an interaction between DABCO and diphenyliodonium triflate,5 but the strength of this interaction is not known. These pioneering examples have provided evidence for amine–iodonium binding events, but they have been conducted in different solvents and therefore are difficult to compare; and the prior studies represent a small subset of amines with limited relevance to synthetic studies.
Legault and Mayr recently developed an iodonium Lewis acidity scale which is an ideal way to benchmark non-covalent interactions of iodine(III) centers with Lewis basic groups.10 In this Communication we measured the association constants (Ka) between three representative diaryliodonium salts, for which the Lewis acidity (LAI) has been established,10 and 6 amines that have been used in arylation reactions with diaryliodonium salts, including tertiary, secondary, and primary amines, as well as an aromatic amine (Scheme 1d).4–6 Moreover, we calculate the Lewis basicity (LBI) of the amines and discuss the distinct solvent effects observed for interactions of diaryliodonium salts with anionic and neutral Lewis bases.
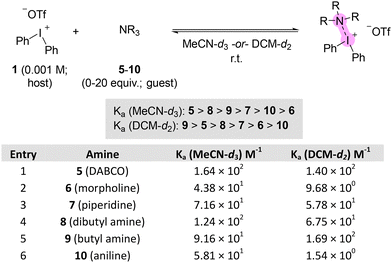
We initiated these studies by selecting three diaryliodonium triflates as reference Lewis acids (1–3; Fig. 1a).10 We also considered the structurally related tertiary amine, quinuclidine 4, not as a reference Lewis base, but rather as a point of comparison with the amines studied in this work because the Lewis basicity (LBI = 2.25) and sensitivity (sI = 0.85) of 4 is known (Fig. 1).10 Although, Ka values for association of heterocyclic amines, such as pyridine, with diaryliodonium salts are known,9,10 we shifted our focus to classes of amines that have precedent in C–N coupling reactions4–7 so that the Lewis basicity (LBI) of these amines could be determined and used as a parameter in future synthetic and mechanistic studies (5–10; Fig. 1b). As part of this study, we also determined the LAI for two additional unsymmetrical diaryliodonium salts, 11 and 12, which are novel arylation reagents (Fig. 1c).
The 1H NMR titration of the diaryliodonium host with increasing equivalents of the amine guest was used to measure the equilibrium constant (Ka) between the reference diaryliodonium salts 1–3 and amines 5–10 in MeCN-d3 at room temperature (Fig. 2 and ESI†). We obtained Ka values that ranged from 30–728 M−1 for aliphatic and aromatic amines 5–10. A plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kavs. LAI revealed linear correlation from which the sI and LBI values were determined for amines 5–10 according to the equation developed by Legault (eqn (1) and Fig. 2).10
Kavs. LAI revealed linear correlation from which the sI and LBI values were determined for amines 5–10 according to the equation developed by Legault (eqn (1) and Fig. 2).10
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = sILAI + LBI Ka = sILAI + LBI | (1) |
The sensitivity, sI, values obtained by this method (i.e., the slope of the correlations) ranged from 0.68–0.78 and were similar to that previously reported for quinuclidine (0.85).10 Moreover, the LBI value obtained in this work for DABCO was 2.24, which is very similar to the LBI value of 2.25 for structurally related quinuclidine (Fig. 2).10 The LBI values calculated for secondary and primary amines were less than that of tertiary DABCO (Fig. 2). Acyclic secondary and primary amines, 8 and 9, followed DABCO with LBI values of 2.09 and 1.97, respectively (Fig. 2). We found that secondary cyclic amines, piperidine 6 and morpholine 7, were less Lewis basic than acyclic amines on the Legault iodonium Lewis acidity scale (Fig. 2). We found that the aromatic amine, aniline 10, had a Lewis basicity of 1.76, which is between that of piperidine and morpholine (Fig. 2). The correlation of observed and calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka values obtained in this study overlay well with the model developed by Legault based on eqn (1) (see Fig. S2 in the ESI†). However, the LBI values obtained for amines 5–10 did not correlate well with other empirically determined parameters for amines, such as pKa of the conjugate acid or Mayr nucleophilicity (N) (see Fig. S3 in the ESI†).11–14
Ka values obtained in this study overlay well with the model developed by Legault based on eqn (1) (see Fig. S2 in the ESI†). However, the LBI values obtained for amines 5–10 did not correlate well with other empirically determined parameters for amines, such as pKa of the conjugate acid or Mayr nucleophilicity (N) (see Fig. S3 in the ESI†).11–14
In addition to diphenyliodonium, unsymmetrical aryl(TMP)iodonium and aryl(DMIX)iodonium reagents have been used in metal-free C–N coupling reactions.3,4,8 The electron-rich TMP and DMIX dummy ligands promote chemoselective aryl transfer of the other aryl group to amine nucleophiles. Legault has previously reported the LAI value for phenyl(TMP)iodonium hexafluorophosphate salt as −0.38, and suggests that the weaker Lewis acidity relative to diphenyliodonium is due to the electron-rich TMP group.10 We used five of the amines 5–9 studied here as reference Lewis bases to determine the LAI values for phenyl(TMP)iodonium and phenyl(DMIX)iodonium triflate salts 11 and 12 (Fig. 3). Based on these experiments and least square linear regression fitting of the data, we obtained an LAI value of −0.35 for 11 with the TMP auxiliary (Fig. 3, blue data points), similar to the value previously obtained by Legault for the corresponding hexafluorophosphate salt.10 However, we were somewhat surprised to find that phenyl(DMIX)iodonium triflate 12 had substantially higher Ka values with all of the amines used as reference Lewis bases and a resulting LAI value of 1.04 (Fig. 3, orange data points). Structurally, the phenyl(DMIX)iodonium 12 and phenyl(Mes)iodonium 3 both have aryl ligands with two ortho-positioned methyl groups, but have very different LAI values (LAI of 12 = 1.04 and LAI of 3 = −0.26). A closer inspection of both steric and electronic effects might reveal a cause for this discrepancy. First, X-ray structure data for a related DMIX compound and 3 reveal C–I bond distances of 2.06 and 2.10 Å, respectively.8,15 However, the 5-membered ring of DMIX actually positions the methyl groups further away from iodine than the 6-membered ring of Mes (3.68 vs. 3.27 Å, respectively) and therefore the DMIX ligand is less sterically encumbering than Mes. Second, the 5-membered imidazole ring of DMIX is inductively withdrawing, whereas the Mes group is inductively donating, and therefore the iodonium of 12 is more electron-deficient than 3.16
 | ||
| Fig. 3 Determination of LAI for unsymmetrical diaryliodoniums 11 and 12. (Grey data points are from Fig. 2.) | ||
Although, the Legault iodonium Lewis acidity scale was developed using acetonitrile as the solvent, dichloroethane and toluene are used more commonly in C–N coupling reactions with diaryliodonium salts,4,6,7 though DMF is also used for aniline arylation.2,3,8 We performed 1H NMR titration experiments with diphenyliodonium triflate 1 and amines 5–10 in dichloromethane-d2 to test the solvent effects on the association of amines with diphenyliodonium triflate. In prior work, Legault observed that association constants for diphenyliodonium and benzoate increase with decreasing solvent polarity,10,17 which is consistent with weaker ion stabilization in less polar solvent and therefore a greater extent of ion-pairing (association) between iodonium and benzoate. Here, we observed that in almost all cases the observed association constants between diphenyliodonium and neutral amines were smaller in magnitude in DCM-d2 than in MeCN-d3 (Fig. 4), which is in contrast to Legault's observation with anionic benzoate. Our rationale for this result is that in DCM-d2, which is less polar than MeCN,17 there is a greater extent of ion-pairing between diphenyliodonium and triflate which attenuates the Lewis acidity of diphenyliodonium group; this trend holds for Ka values obtained in acetone-d6, which has polarity between DCM and acetonitrile (see Fig. S5, in the ESI†). However, it is important to note that the trend in Ka values for the amines was not consistent between the two solvents and may reflect other phenomena taking place during these interactions. For instance, primary butylamine had the highest Ka value (1.69 × 102 M−1) in DCM-d2, but the third highest (Ka = 9.16 × 101 M−1) in MeCN-d3 (Fig. 4). The order of Ka values, from largest to smallest, is 5 > 8 > 7 > 6 was consistent in both solvents. However, aniline 10 had a higher Ka value than morpholine in MeCN-d3 (cf. 58.1 vs. 43.8 M−1), but morpholine had the higher value in DCM-d2 (Fig. 4, entries 2 and 6).
In conclusion, we have determined the Lewis basicity values for six synthetically relevant amines (5–10) on the Legault Lewis acidity scale, including representative tertiary, secondary, and primary amines, aliphatic and aromatic, as well as cyclic and acyclic amines. In the course of these studies, we also determined the Lewis acidity of two unsymmetrical diaryliodonium salts (11 and 12) that have been used in metal-free C–N coupling reactions. Finally, we found that binding events between iodonium and neutral amines generally have smaller association constants in less polar solvent, which is opposite to the observed trend for anionic Lewis bases.
Conceptualization – SB, SJ, and DRS; investigation and methodology – SJ, SB, MB; supervision – SB and DRS; writing (original draft) – DRS; writing (reviewing and editing) – SJ, SB, DRS; funding acquisition, validation, project administration, and resources – DRS.
We are grateful to the National Science Foundation for funding this work under grants no. 1856705 and 2154500. The National Science Foundation provided funding for the BioAnalytical Mass Spectrometry Facility at PSU under grant no. 1828753.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of ESI.†Notes and references
- N. A. McGrath, M. Brichacek and J. T. Njardarson, J. Chem. Educ., 2010, 87, 1348–1349 CrossRef CAS.
- M. A. Carroll and R. A. Wood, Tetrahedron, 2007, 63, 11349–11354 CrossRef CAS.
- J. Malmgren, S. Santoro, N. Jalalian, F. Himo and B. Olofsson, Chem. – Eur. J., 2013, 19, 10334–10342 CrossRef CAS PubMed.
- A. H. Sandtorv and D. R. Stuart, Angew. Chem., Int. Ed., 2016, 55, 15812–15815 CrossRef CAS PubMed.
- D. I. Bugaenko, M. A. Yurovskaya and A. V. Karchava, Org. Lett., 2018, 20, 6389–6393 CrossRef CAS PubMed.
- N. Purkait, G. Kervefors, E. Linde and B. Olofsson, Angew. Chem., Int. Ed., 2018, 57, 11427–11431 CrossRef CAS PubMed.
- G. Kervefors, L. Kersting and B. Olofsson, Chem. – Eur. J., 2021, 27, 5790–5795 CrossRef CAS PubMed.
- Y. Chen, Y. Gu, H. Meng, Q. Shao, Z. Xu, W. Bao, Y. Gu, X.-S. Xue and Y. Zhao, Angew. Chem., Int. Ed., 2022, 61, e202201240 CrossRef CAS PubMed.
- M. Ochiai, T. Suefuji, M. Shiro and K. Yamaguchi, Heterocycles, 2006, 67, 391 CrossRef PubMed.
- R. J. Mayer, A. R. Ofial, H. Mayr and C. Y. Legault, J. Am. Chem. Soc., 2020, 142, 5221–5233 CrossRef CAS PubMed.
- M. R. Crampton and I. A. Robotham, J. Chem. Res., 1997, 22–23 RSC.
- M. Baidya, S. Kobayashi, F. Brotzel, U. Schmidhammer, E. Riedle and H. Mayr, Angew. Chem., Int. Ed., 2007, 46, 6176–6179 CrossRef CAS PubMed.
- T. Kanzian, T. A. Nigst, A. Maier, S. Pichl and H. Mayr, Eur. J. Org. Chem., 2009, 6379–6385 CrossRef CAS.
- R. Tandon, T. Unzner, T. A. Nigst, N. De Rycke, P. Mayer, B. Wendt, O. R. P. David and H. Zipse, Chem. – Eur. J., 2013, 19, 6435–6442 CrossRef CAS PubMed.
- S. S. Karandikar, A. Bhattacharjee, B. E. Metze, N. Javaly, E. J. Valente, T. M. McCormick and D. R. Stuart, Chem. Sci., 2022, 13, 6532–6540 RSC.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- S. Spange, N. Weiß, C. H. Schmidt and K. Schreiter, Chem. Methods, 2021, 1, 42–60 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01711d |
| This journal is © The Royal Society of Chemistry 2025 |