Open Access Article
Open Access ArticleEasy access to a self-assembled glycolipid derived from bhilawanol: a promising anti-cancer drug†
Tohira
Banoo
a,
Kajal
Sandhu
b,
S.
Chockalingam
 b and
Subbiah
Nagarajan
b and
Subbiah
Nagarajan
 *a
*a
aAssembled Organic and Hybrid Materials Lab, Department of Chemistry, National Institute of Technology Warangal, Hanumakonda-506004, Telangana, India. E-mail: snagarajan@nitw.ac.in; snrajannpt@yahoo.co.in
bCell Signaling Research Laboratory, Department of Biotechnology, National Institute of Technology Warangal, Hanumakonda-506004, Telangana, India
First published on 3rd April 2025
Abstract
In this study, we synthesised a series of self-assembling glycoconjugates derived from bhilawanol under environmentally friendly conditions in good yields. These self-assembling glycoconjugates displayed anti-cancer activity toward HeLa cells at an IC50 value of 125 μM with minimal side effects, holding immense potential in cancer therapeutics.
Cancer encompasses a range of diseases characterised by uncontrolled cell division and compromised immune system leading to mortality.1 Despite significant strides in cancer therapeutics and diagnostics, the medical community continues to strive for further advancements in combating this disease.2 It has been witnessed that plant extracts have played a predominant role in cancer treatment since ancient times. In this study, we focused on synthesising a class of new glycolipids from a renewable resource, bhilawanol, also known as urushiol, a bioactive molecule obtained from “Semecarpus anacardium”, found in India's sub-Himalayan, tropical, and central regions, that has been used as a traditional Indian medicine for centuries to treat various ailments.3 The seeds of this plant are reported to contain many biologically active molecules displaying potential anticancer, antibacterial, antifungal, and other biological properties.4 Sahoo and co-workers recently presented a mini-review highlighting the potential applications of Semecarpus anacardium Linn. (SCA) in anticancer and anti-inflammatory therapies. The review underscores SCA's therapeutic potential in treating various cancers, including breast cancer, lung cancer, blood cancer, and hepatocellular carcinoma, with advancements reaching clinical trials. It also emphasizes the need for further investigation into the use of SCA extracts in cancer treatment.5 In this report, the conjugation of carbohydrates with bhilawanol resulted in a new class of biologically active glycolipids. The direct conjugation of bhilawanol to carbohydrates or amino acids has not been reported to date. Lepoittevin and co-workers have coupled 3-n-alkyl catechols to the acetate or trichloroimidates of monosaccharides and disaccharides using multistep synthesis.6 Later, in a patent report, amino acids and N-heterocyclic compounds were conjugated to bhilawanol and their biological properties were evaluated.6 Carbohydrates are essential molecules that play crucial roles in various biological processes such as cell development, recognition, cell–cell interaction and communication, growth, and energy storage.7 Hergenrother and coworkers reported the salient features of glycoconjugates in improved cancer targeting and selectivity.8 The tendency of cancer cells to metabolise more sugars than normal cells has been leveraged to target cancer cells using glycoconjugates, which is referred to as the Warburg effect.9–11 To date, 14 distinct glucose transporters (GLUT) have been identified based on their structure and sequence; among these, GLUT-1 is the most extensively studied glucose transporter and is widely recognized for its overexpression in cancer cells.12 Consequently, the other stereoisomers of glucose analogues can be effectively taken up by cancer cells, offering an appealing strategy to broaden the Warburg effect to a broader range of glycoconjugates.13,14 Given their importance in anticancer activity, synthesising glycoconjugates or glycolipids, particularly those derived from renewable sources, is of significant interest.15 Glycolipids are also a key class of amphiphiles prone to self-assembly through non-covalent interactions to furnish the supramolecular architecture, which is fundamental in living organisms, leading to functional structures capable of performing intricate biological functions.16 Recognising the potential of carbohydrates, we investigated the self-assembly of a series of bhilawanol-based glycolipids. This thoughtful design strategy aims to leverage the stabilising role of carbohydrates in complex architectures and explore their promising applications.
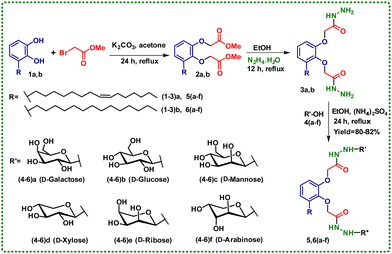
Carbohydrate-based self-assembling systems are integral to the creation of advanced biocompatible materials with versatile functionalities, enabling a wide range of applications.17,18 In this report, one of the biologically significant natural compounds, bhilawanol (1a), has been chemically modified and conjugated with various monosaccharides to synthesize glycoconjugates (BCG).19 This process employed a straightforward and environmentally friendly protocol to create glycolipids. The reaction involves treating bhilawanol hydrazide (3a, b) with various monosaccharides (4a-f) in ethanol, utilising ammonium sulphate as a base under reflux conditions in a single-pot reaction. The established protocol furnished 80–82% of the desired glycolipid without the requirement of column chromatography (Scheme 1).20 The formation of the desired BCG5,6(a-f) was confirmed by nuclear magnetic resonance (NMR), high-resolution mass spectrometry (HRMS), and infrared (IR) spectroscopy. The F2-coupled 1H,13C-HSQC NMR spectrum of BCG6b furnished a 1JC1,H1 coupling constant of 157 Hz, which is less than 168 Hz, clearly revealing the exclusive formation of the 1,2-trans-anomeric product (Fig. S52, ESI†).21
Over the past few decades, self-assembling carbohydrates have gained momentum due to their abundance and accessibility. In addition, easy tuning of the orientation of hydroxyl groups, additional functionalisation and hydrogen bonding are the characteristic features.22 This, in turn, has spurred further exploration into the realms of molecular self-assembly and supramolecular chemistry. Low molecular weight gels (LMWGs) were reported as far back as the 19th century, but the scientific understanding of structure and function was not fully elucidated. Notably, in the recent past, researchers have engineered functional molecules and extensively investigated their self-assembly.22 The “stable to inversion” method identified BCGs’ gelation behaviour. Table S1 (ESI†) summarises the gelation capability of BCG glycolipids in different solvents and oils.23 Interestingly, all the synthesized BCG glycolipids displayed gelation in DMSO. Among the BCG glycolipids 5a-f, which have unsaturation in their hydrophobic unit, BCG5a displays gelation in DMSO + H2O (40%) with a CGC of 1.5% (wt/v). It is worth mentioning that BCG6a, a saturated version of BCG5a, exhibited gelation in DMSO + H2O (40%) with a CGC of 1.0% (wt/v). The better gelation ability of 6a is attributed to the pronounced molecular stacking displayed by the saturated hydrophobic unit. BCG5,6(a-f) show a CGC of 1.0% (wt/v) in linseed oil (Fig. S1, ESI†). The thermal stability of the hydrogel and oleogels formed by BCG5,6(a-f) has been identified by the sol–gel transition temperature (Tg). Hydrogel and oleogel formed by BCG5a and 6a displayed Tg as 117 & 96 °C and 129 & 98 °C, respectively. With an increase in the concentration of the gelator, the Tg also increases, indicating the tunability of gel strength, as shown in Fig. S49 (ESI†).
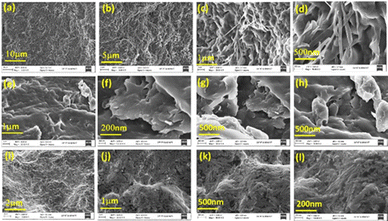
A scanning electronic microscopy (SEM) analysis of the hydrogel and oleogel formed by BCG6a in DMSO + water and Linseed oil and BCG6b in DMSO + water was performed to understand the morphology of the gel. The SEM analysis showed that the hydrogel and oleogel formed by BCG6a reveal a fibrillar, lamellar-like architecture. In contrast, the hydrogel morphology formed by compound BCG6b in DMSO + water reveals a globular structure (Fig. 1). This result clearly demonstrates that the epimers of carbohydrates and the solvent environment both play a crucial role in guiding the supramolecular self-assembly of the gelator into a gel through intermolecular interactions, ultimately influencing its microscopic structure.
Variable temperature NMR (VT-NMR) provides compelling evidence of intermolecular interactions in self-assembly. To our fortune, BCG6a forms a hydrogel in DMSO–H2O, favouring the VT-NMR studies. The VT-NMR spectral studies of the gel displayed a remarkable change compared to the solution state. The disappearance of exchangeable protons and broadening of aromatic signals with minimal shifts is observed (Fig. 2a). In the gel state, a decrease in the intensity of sugar peaks and the disappearance of signals corresponding to aromatic and hydrophobic units is observed. Even up to 60 °C, a similar trend was observed; however, further increase in temperature to 90 °C resulted in well-resolved spectra with the up-field shift of aromatic protons centred at around δ = 7.0 ppm to 6.5 ppm, and protons corresponding to sugar and hydrophobic units were observed. The overall up-field shift of protons of BCG6a is the result of an increase in the electron density during the molecular self-assembly via hydrogen bonding, π–π interactions and van der Waals interactions (Fig. 2a).
Various intermolecular interactions such as hydrogen bonding, hydrophobic interactions, stacking, anionic–π and cationic–π and van der Waals displayed by a molecule play a crucial role in the assembly mechanism. In this study, we explore correlations between molecular structure and self-assembly behaviour, aiming to uncover the fine distinction of these interactions. To gain a deeper insight into the molecular self-assembly of the hydrogel produced from BCG6a, we conducted FTIR studies. The molecular self-assembly brings variations in the intermolecular interaction in the amorphous to assembled state due to the formation of secondary interactions. Fig. 2b illustrates the FTIR spectra of BCG6a in amorphous and assembled states. In the IR spectra, peaks centred at 3256, 3300, 2852, 1680, 1648, 1103 and 1056 cm−1 represent the assembled state. In contrast, the amorphous state displayed a shift and was observed at slightly higher frequencies centred at 3280, 3324, 2846, 1705, 1683, 1081 and 1039 cm−1, respectively, which corresponds to –NH, –OH, –CH (alkanes), carbonyl and alkenes, respectively. The appreciable signal shift displayed by various functional groups of BCG6a is direct evidence of the participation of different functional groups in molecular assembly. The energy-minimised structure is obtained to elucidate the packing arrangement, and their molecular length is calculated and compared with experimental SAXRD diffraction data shown in Fig. 2c.
The xerogel obtained from BCG6a furnished 2θ at 2.21°, 3.37°, 8.72°, 20.81°, 22.59° and 40.80°, corresponding to interplanar d spacing values of 4.31, 2.62, 1.01, 0.43, 0.39, and 0.22 nm. The end-to-end molecular length of BCG6a obtained from computational studies is 3.01 nm, which is lower than the experimental value of 4.31 nm, suggesting the formation of an intercalated double layer stabilised by intermolecular hydrogen bonding (Fig. 2d).
A rheological measurement is performed to understand the practical utility of BCG6a hydrogel in pharmaceutical sectors (Fig. 3). Rheological measurements define the extent of deformation of the material from its original state in terms of storage modulus G′ (elasticity) and loss modulus G′′ (viscous) in response to strain. It was observed from the frequency sweep test that both the values of G′ and G′′ increase linearly in response to applied frequency; the higher value of G′ compared to G′′ reflates the strong tolerance of the gel in response to applied frequency. In the strain sweep test, it was observed that the storage modulus of the gel G′ has higher values than the loss modulus G′′, reflecting the good mechanical strength of the gel. The thixotropy renders fast structural recovery behaviour in response to the applied constant strain, an essential feature in medicine formulation.24
 | ||
| Fig. 3 (a) and (b) Angular frequency and strain amplitude dependence of G′ and G′′ of the hydrogel, and (c) thixotropy of the hydrogel formed by compound BCG6a. | ||
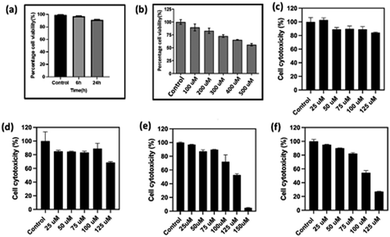
To assess the feasibility of utilizing the compounds for anticancer studies, it is crucial to evaluate their cytotoxicity. The cytotoxicity of compound BCG6a on cellular metabolic activity was tested using immortalized human corneal epithelial cells (HCEC) treated with 500 μM of the compound. Cell viability and cytotoxicity were measured through an MTT assay, as shown in Fig. 4a. The results demonstrated that compound BCG6a exhibited almost 100% cell viability after a 6 h incubation and over 90% viability after 24 h.
Bhilawanol is known to exert significant anti-cancer activity and conversion into glycoconjugates is expected to enhance its potential. Hence, we investigated the anti-cancer activity of the synthesized glycolipids on the HeLa cells.25 It is worth mentioning that low-molecular self-assembled systems play a prominent role in anticancer activity.26 In the present studies, the anticancer potential of the chosen glycolipids was compared with 5-fluorouracil, which is sold under the name of Adrucil, a cytotoxic chemotherapy medication used to treat stomach cancer, oesophageal cancer, pancreatic cancer, breast cancer, and cervical cancer. The anti-cancer studies of 5-fluorouracil against the HeLa cells displayed an IC50 of 500 μM (Fig. 4b). For our investigation, we have selected BCG5a, which is an unsaturated version of BCG6a, and BCG5b, which is the c4 epimer of 5a and an unsaturated version of BCG6b, respectively Fig. 4(c)–(f).
The cytotoxicity of the compounds was examined by using an Alamar blue cell viability assay, which is generally used to quantitatively measure cell proliferation and cytotoxicity involving the oxidation–reduction process. We observed that BCG6a and 6b exhibited a cytotoxic response, as shown in Fig. 4e–f after treatment for 24 h. The cellular morphology was also altered after the treatment as cells began to detach from the plate's surface, as illustrated in Fig. 5 for BCG6a, Fig. 6 for BCG6b, and for BCG5a and BCG5b in Fig. S50 and S51 (ESI†), respectively. In the case of compounds BCG6a and BCG6b, with increasing concentrations of both compounds, more cells detached from the surface and adopted a rounded appearance, indicating cell death. Interestingly, compounds BCG6a and BCG6b demonstrated significant activity, with IC50 values of 125 μM. This is a remarkable result compared to the standard 5-fluorouracil, which exhibits an IC50 value of 500 μM.
 | ||
| Fig. 5 (a)–(g) Microscopic images of the anticancer effect on HeLa cells of BCG6a at different concentrations at a magnification of 10×. | ||
 | ||
| Fig. 6 (a)–(f) Microscopic images of the anticancer effect on HeLa cells of BCG6b at different concentrations at a magnification of 10×. | ||
In conclusion, a new class of glycolipids were synthesised under environmentally friendly conditions from renewable resources in good yields. The bottom-up assembly of glycolipids generated hydrogel and oleogel, which were well characterized by NMR, IR, SEM, XRD, and rheological studies. Self-assembled BCG6a and BCG6b displayed anti-cancer activity toward HeLa cells at IC50 of 125 μM with minimal side effects broadening the application of supramolecular chemistry in medicine.
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors gratefully acknowledge the financial support from SERB (sanction order no. CRG/2023/002466) and DST FIST (SR/FST/CS-II/2018/65). The authors thank the National Institute of Technology Warangal for the infrastructure facilities.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) P. Anand, A. B. Kunnumakara, C. Sundaram, K. B. Harikumar, S. T. Tharakan, O. S. Lai, B. Sung and B. B. Aggarwal, Pharm. Res., 2008, 25, 2097–2116 CrossRef CAS PubMed; (b) D. S. Shewach and R. D. Kuchta, Chem. Rev., 2009, 109, 2859–2861 CrossRef CAS PubMed; (c) S. Fu and X. Yang, J. Mater. Chem. B, 2023, 11, 4584–4599 RSC.
- (a) N. Behtash and N. Mehrdad, J. Cancer Prev., 2006, 7, 683–686 Search PubMed; (b) S. Feng, D. Lin, J. Lin, B. Li, Z. Huang, G. Chen, W. Zhang, L. Wang, J. Pan, R. Chen and H. Zeng, Analyst, 2013, 138, 3967–3974 Search PubMed; (c) P. L. Wantuch and F. Y. Avci, Hum. Vaccines Immunother., 2018, 14, 2303–2309 CrossRef PubMed.
- (a) B. Premalatha, Indian J. Exp. Biol., 2000, 38, 1177–1182 Search PubMed; (b) M. Semalty, A. Semalty, A. Badola, G. P. Joshi and M. S. Rawat, Pharmacogn. Rev., 2010, 4, 88–94 Search PubMed; (c) P. H. Gedam, P. S. Sampathkumaran and M. A. Sivasamban, Phytochemistry, 1974, 13, 513–515 CrossRef CAS; (d) Y. Qi, J. Lang, X. Zhu, J. Huang, L. Li and G. Yi, RSC Adv., 2019, 10, 1132–1141 Search PubMed.
- (a) M. Semalty, A. Semalty, A. Badola, G. P. Joshi and M. S. M. Rawat, Pharmacogn. Rev., 2010, 88–94 CrossRef CAS PubMed; (b) Z. Qi, C. Wang and J. Jiang, Molecules, 2018, 23, 1074 CrossRef PubMed; (c) T. K. Mohanta, J. K. Patra, S. K. Rath, D. K. Pal and H. N. Thatoi, RES Essay, 2007, 2, 486–490 Search PubMed.
- A. K. Mishra, A. Jain, C. Y. Jagtap, G. Dane, S. Paroha and P. K. Sahoo, Fitoterapia, 2024, 175, 105978 CrossRef CAS PubMed.
- (a) S. Mabic, C. Benezra and J. P. Lepoittevin, Tetrahedron Lett., 1993, 34, 4531–4534 CrossRef CAS; (b) M. A. Elsohly, W. Gul, M. K. Ashfaq and S. P. Manly, WO/2009/146131, 2009.
- (a) J. Brand-Miller, J. McMillan-Price, K. Steinbeck and I. Caterson, Asia Pac. J. Clin. Nutr., 2008, 17, 16–19 Search PubMed; (b) F. Yin, J.-J. Li, B. Shi, K. Zhang, X.-L. Li, K.-R. Wang and D.-S. Guo, Mater. Chem. Front., 2023, 7, 5263–5287 Search PubMed.
- E. C. Calvaresi and P. J. Hergenrother, Chem. Sci., 2013, 4(6), 2319–2333 RSC.
- K. H. Tomaszowski, N. Hellmann and V. Ponath, et al. , Sci. Rep., 2017, 7, 13925 CrossRef PubMed.
- (a) D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS PubMed; (b) E. Varghese, S. M. Samuel, A. Líšková, M. Samec, P. Kubatka and D. Büsselberg, Cancers, 2020, 12, 1–34 CrossRef PubMed.
- Z. Wu, S. Chen, Z. Chen, G. Dong, D. Xu and C. Sheng, J. Med. Chem., 2024, 67, 7373–7384 CrossRef CAS PubMed.
- K. C. Carvalho, I. W. Cunha, R. M. Rocha, F. R. Ayala, M. M. Cajaíba, M. D. Begnami, R. S. Vilela, G. R. Paiva, R. G. Andrade and F. A. Soares, Clinics, 2011, 66, 965–972 Search PubMed.
- (a) V. Di Bussolo, E. C. Calvaresi, C. Granchi, L. Del Bino, I. Frau, M. C. Dasso Lang, T. Tuccinardi, M. Macchia, A. Martinelli, P. J. Hergenrother and F. Minutolo, RSC Adv., 2015, 5, 19944–19954 RSC; (b) S. S. Mullapudi, D. Mitra, M. Li, E. T. Kang, E. Neoh and K. G. Chiong, Mol. Syst. Des. Eng., 2020, 5, 772–791 RSC; (c) H. Martin, L. R. Lázaro, T. Gunnlaugsson and E. M. Scanlan, Chem. Soc. Rev., 2022, 51, 9694–9716 RSC.
- L. Su, Y. Feng, K. Wei, X. Xu, R. Liu and G. Chen, Chem. Rev., 2021, 121, 10950–11029 CrossRef CAS PubMed.
- S. I. Elshahawi, K. A. Shaaban, M. K. Kharel and J. S. Thorson, Chem. Soc. Rev., 2015, 44, 7591–7697 RSC.
- (a) F. Raza, H. Zafar, X. You, A. Khan and J. Wu, Cancer Nanomed., 2019, 7639–7655 CAS; (b) F. Yin, J. J. Li, B. Shi, K. Zhang, X. L. Li, K. R. Wang and D. S. Guo, Mater. Chem. Front., 2023, 7, 5263–5287 RSC.
- P. Stallforth, B. Lepenies, A. Adibekian and P. H. Seeberger, J. Med. Chem., 2009, 52, 5561–5577 CrossRef CAS PubMed.
- F. Hossain and P. R. Andreana, Pharmaceuticals, 2019, 12, 84 CrossRef CAS PubMed.
- (a) P. Jain and H. P. Sharma, Int. J. Res. Pharm. Chem., 2013, 3, 564–572 Search PubMed; (b) P. K. Vemula and G. John, Acc. Chem. Res., 2008, 41, 769–782 CrossRef CAS PubMed.
- A. K. Rachamalla, V. P. Rebaka, T. Banoo, R. Pawar, M. Faizan, K. Lalitha and S. Nagarajan, Green Chem., 2022, 24, 2451–2463 RSC.
- C. Fontana and G. Widmalm, Chem. Rev., 2023, 123, 1040–1102 CrossRef CAS PubMed.
- (a) D. K. Smith, Soft Matter, 2023, 20, 10–70 RSC; (b) M. Inès and G. Dhouha, Carbohydrate Res., 2015, 416, 59–69 CrossRef PubMed; (c) A. Thamizhanban, S. Balaji, K. Lalitha, Y. S. Prasad, R. V. Prasad, R. A. Kumar, C. U. Maheswari, V. Sridharan and S. Nagarajan, J. Agric. Food Chem., 2020, 68, 14896–14906 CrossRef CAS PubMed.
- E. R. Draper and D. J. Adams, Chem, 2017, 3, 390–410 CAS.
- A. R. Patel, M. Babaahmadi, A. Lesaffer and K. Dewettinck, J. Agric. Food Chem., 2015, 63, 4862–4869 CrossRef CAS PubMed.
- (a) M. N. Mallick, W. Khan, M. Singh, M. Z. Najm, M. Kashif, S. Ahmad and S. Husain, Drug Dev. Ther., 2016, 7, 55 CrossRef CAS; (b) R. K. Singh, B. Mallik, A. Ranjan, R. Tripathi, S. S. Verma, V. Sharma, S. C. Gupta and A. K. Singh, Nat. Prod. Res., 2024, 38, 1080–1084 CrossRef CAS PubMed; (c) J. Fu, J. Yang, P. H. Seeberger and J. Yin, Carbohydr. Res., 2020, 498, 108195 CrossRef CAS PubMed.
- (a) A. K. Das and P. K. Gavel, Soft Matter, 2020, 16, 10065–10095 RSC; (b) Z. Yang, K. Xu, Z. Guo, Z. Guo and B. Xu, Adv. Mater., 2007, 19, 3152–3156 CrossRef CAS; (c) W. Huang, J. Seo, S. B. Willingham, A. M. Czyzewski, M. L. Gonzalgo, I. L. Weissman and A. E. Barron, PLoS One, 2014, 9, 1–10 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01621e |
| This journal is © The Royal Society of Chemistry 2025 |