Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Geminal homologative fluorination of carbonyl derivatives en route to 1-fluoro-2-haloethyl skeletons†
Margherita
Miele
*a,
Davide
Castiglione
a,
Alexander
Prado-Roller
b,
Laura
Castoldi
*c and
Vittorio
Pace
 *ad
*ad
aDepartment of Chemistry, University of Turin, Via Giuria 7, 10125 Turin, Italy. E-mail: margherita.miele@unito.it; vittorio.pace@unito.it
bInstitute of Inorganic Chemistry, University of Vienna, Waehringerstrasse 42, 1090, Vienna, Austria
cDepartment of Pharmaceutical Sciences, General and Organic Chemisty Section “A. Marchesini” – Via Venezian 21, University of Milan, 20133 Milan, Italy. E-mail: laura.castoldi@unimi.it
dDepartment of Pharmaceutical Sciences, Division of Pharmaceutical Chemistry, University of Vienna, Josef-Holaubek-Platz 2, 1090, Vienna, Austria. E-mail: vittorio.pace@univie.ac.at
First published on 6th June 2025
Abstract
Carbonyl groups undergo the sequential installation of two nucleophilic elements, halomethyl and fluoride moieties. This formal gem-difunctionalization enables the preparation–under full chemocontrol – of vic-fluorohaloethanes by simply defining the C1 nucleophile, thus enabling access to all combinations of the four halogens.
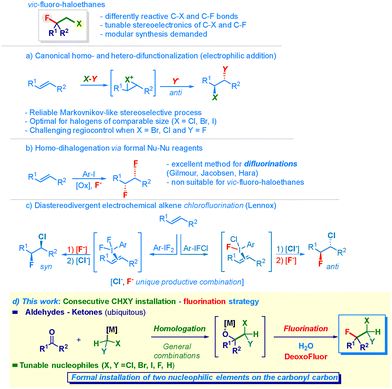
The unique ability of the fluorine atom to modulate the structural editing of organic skeletons constitutes a robust tool for the fine-tuning of pivotal physical–chemical parameters.1 This is mainly due to the following constitutive aspects: (a) local polarity inversion at the competent connective carbon; (b) minimal steric variation as a consequence of comparable van der Waals radii, inter alia.2,3 Positioning an (additional) distinct halogen on the vicinal carbon further amplifies the modular control of the stereoelectronic and conformational features of the resulting backbone exhibiting two adjacent sites amenable for regioselective diversification (Scheme 1, upper panel).4 Indeed, the selective insertion of one or two halogen atoms enables the precise design of bioisosters, and thus the generation of new entities suitable for undergoing pharmacokinetic and dynamic analysis, as well as for uncovering novel materials or agrochemicals.5 Historically, olefins have been used to forge 1,2-dihaloethyl units using different strategies based – inter alia – on a formal electrophilic addition with an X–Y type reagent (Scheme 1, path a).6 While documenting outstanding levels of stereocontrol in the case of preparing chloro- and bromo-analogues,5,7 as also shown in the preparation of halogenated natural products,6c,8 the access to 1-halo-2-fluoroethyl clusters still remains critical. The origin of this shortcoming is attributed to regioselective aspects arising from the inherent difficulty of controlling the attack of two halogens with opposite polarity and sensitively different radii (e.g. iodine vs. bromine or chlorine compared to fluorine).4a,9 Moreover, it is instructive to mention some substrate dependence that was noticed by O’Hagan when synthesizing 1-bromo-2-fluoro systems.10 Notably, the adoption of catalytic alkene-activation strategies9b pioneered in fluorinative chemistry by Jacobsen,11 Gilmour12 and Lennox13 enabled the productive attack of two F− anions, thus realizing the hitherto elusive 1,2-difluorination of olefins (Scheme 1, path b).12c,14 The concept was elegantly translated to a diastereodivergent assembly of chloro–fluoro–ethanes by Lennox in 2024 (Scheme 1, path c):15 indeed, the tunable electrochemical oxidative formation of distinct λ3-iodanes for activating the olefin allows the controlled installation of the inherently reactive (i.e. nucleophilic) fluoride and chloride anions by modulating their relative concentrations. Accordingly, both anti and syn addition products can be directly prepared. Unfortunately, this benchmark tactic is not expansive. Thus, designing a route enabling access to all the possible distinct fluorohaloethanes (chloro, bromo, iodo) persists as an unmet goal in current synthesis.16 Inspired by our interest in forging functionalized C–C bonds via electrophilic–nucleophilic reactivity,17 we questioned whether the release of halomethyl synthons (–CH2–Hal) to a competent recipient electrophilic acceptor (i.e. carbonyl moiety)–followed by direct deoxyfluorination18 of the addition intermediate–could supply a reliable preparative protocol for 1-halo-2-fluoroethyl chains (Scheme 1, path d). Indeed, the proposed approach relies on the documented flexibility of delivering the methylene unit featuring the exact degree of functionalization through the use of a proper (nucleophilic) lithium halocarbenoid (i.e. LiCHXY).19 Thus, after selecting the desired (tunable) CH2–Hal element to attack the carbonyl group, the subsequent fluorinative event conducted on the addition intermediate produces the targeted vic-fluorohaloethyl chain. Collectively, the transformation can be conceptualized as a carbonyl-geminal difunctionalization,20 with the initial carbonyl linchpin being the site of attack of the tunable CH2–Hal element (Cl, Br, F, I) and the subsequently introduced fluorine. Whenever productive, the strategy would de facto overcome the risk of low regiocontrol affecting dihalofunctionalizations of olefins.
ω-Chlorobutyrophenone 1 was selected as the model substrate featuring two reactive electrophilic sites that are potentially amenable to modification under the employed nucleophilic conditions (Table 1). The success of the ketone homologation was deeply influenced by the correct genesis of the carbenoid through I/Li exchange conducted on chloroiodomethane and MeLi–LiBr.21 In particular, the rate of addition of the latter played a critical role in maximizing the process (Table 1, green box). The controlled delivery (0.20 mL min−1) via a syringe pump enabled the precise generation of LiCH2Cl (1.4 equiv.) in THF at −78 °C, thus furnishing the lithiated tetrahedral intermediate adduct 1a – almost quantitatively within 30 min – as judged by the conversion into chlorohydrin 2a after acidic quenching (NH4Cl). Having established the feasibility of the initial event of the transformation occurring with promising chemoselective control (no change at the ω-chloro pendant functionality), we then focused on the direct nucleophilic fluorination of 1a. Accordingly, by adding DAST22 (1.5 equiv.) at −78 °C and leaving the reaction to slowly reach rt, the desired chloro–fluoro compound 2 was obtained in 21% yield together with halohydrin 2a (65%) and an appreciable amount of (unidentified) decomposition material (entry 1). Presumably, the structural characteristics of the tertiary alkoxide render the system primed for suffering competing side reactions such as eliminations and rearrangements.23 Considering that the carbenoid generation event might produce collateral entities altering the expected outcome,24 we were pleased to note that–upon washing with water, followed by re-solubilizing in DCM–the fluorination occurred with a significantly higher yield (46%) without detectable side products (entry 2). The effect of the temperature was remarkable: keeping it at −78 °C was detrimental and only traces of 2 were recovered after 24 h (entry 3), whereas increasing to 0 °C gave a modest 13% yield (entry 4), suggesting a beneficial thermal activation during the C–O breaking step. Additional implementation – augmenting the yield up to 55% – was secured by employing 2.2 equiv. of DAST (entry 5). The screening of distinct S–F-type deoxyfluorinating agents known to be applicable to carbinols (entries 6–9), such as DeoxoFluor,25 XtalFluor,26 Pyfluor,27 and SulfoxFluor,28 evidenced the optimal performance of the former (entry 6), furnishing chloro–fluoro derivative 2 in an excellent 92% yield.
| Entry | Fluorinating agent (equiv.) | Temperature [°C] | Yield of 2b (%) | Yield of 2aa (%) |
|---|---|---|---|---|
| a Isolated yield after the homologation/fluorination sequence (o/n). Unless otherwise stated, after completing the addition of MeLi–LiBr, the reaction mixture was washed with purified water, evaporated and redissolved in DCM to reach a concentration of 0.5 M. b DAST was added directly to the mixture without water washing. c Unidentified products (10% NMR). d The treatment with DAST was prolonged for 24 h. | ||||
| 1bc | DAST (1.5) | −78 to rt | 21 | 65 |
| 2 | DAST (1.5) | −78 to rt | 46 | 39 |
| 3d | DAST (1.5) | −78 | — | 90 |
| 4d | DAST (1.5) | 0 | 13 | 74 |
| 5e | DAST (2.2) | −78 to rt | 55 | 31 |
| 6 | DeoxoFluor (2.2) | −78 to rt | 92 | — |
| 7 | XtalFluor M (2.2) | −78 to rt | 82 | 13 |
| 8 | PyFluor (2.2) | −78 to rt | 77 | 17 |
| 9 | SulfoxFluor (2.2) | −78 to rt | 71 | 21 |
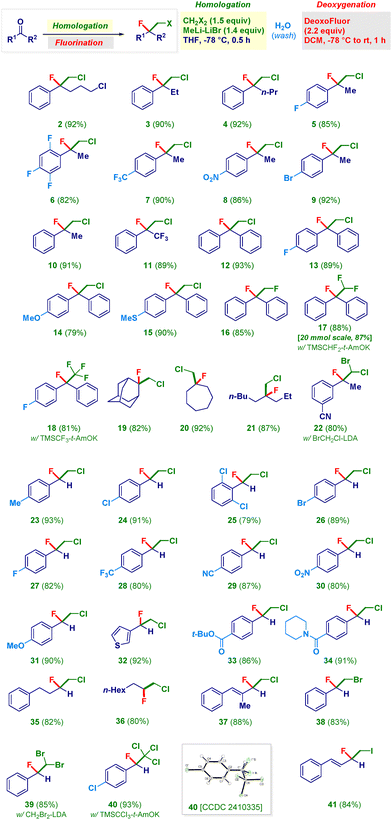
With the optimal conditions in hand, we undertook a study of the scope of the sequential homologation–nucleophilic fluorinative transformation (Scheme 2). Four- and three-carbon phenones gave chloro–fluoro ethyl-units 2–4 in excellent yields. Acetophenones featuring a variety of different substituents on the aromatic ring [fluoro-(5, 6), trifluoromethyl (7), nitro (8), bromine (9), hydrogen (i.e. unsubstituted, 10)] provided the targeted manifolds in comparable high efficiency. Notably, α,α,α-trifluoromethyl-acetophenone could be used for assembling rare chloro-tetrafluoro-analogue 11 through a conceptually simple approach. Benzophenones (12–15) were equally compatible with the methodology: again, no significant difference was found when installing functionalities such as a second fluorine atom (13), an ether (14) or a thioether (15). To our great delight, the delivery of diverse C1 units exhibiting one, two or three fluorine atoms enabled the smooth preparation of extremely challenging poly-fluoro alkyl chains 16–18.29 In particular, for the 1,2-difluoroethyl analogue 16, LiCH2F30 was used, whereas the CHF2 residue – for constructing 17 – was delivered upon the activation of the commercially available TMSCHF2 (in the presence of a Lewis base such as KO-t-Am).31 This latter reaction could be run at 20 mmol scale without affecting the efficiency. The release of the formal CF3 carbanion from the Ruppert–Prakash reagent (TMSCF3)32 guaranteed access to compound 18. Aliphatic ketones also underwent the consecutive transformation, as documented both in the case of cyclic analogues, such as the highly sterically hindered33 adamantyl (19) or cycloheptyl (20) derivatives, and the acyclic analogue 21. Moreover, the synthesis of bromo–chloro–fluoro analogue 22 was accomplished by using LiCHBrCl34 as the competent (first) nucleophile. It should be noted that no modification occurred at the (electrophilic) nitrile functionality. The protocol could be advantageously applied for converting aldehydes into the corresponding fluorohaloethyl motifs. Thus, a series of benzaldehydes furnished products characterized by the presence of substituents of different chemical behavior, ranging from alkyl (23) to halogens (24–27) and trifluoromethyl (28), as well as nitrile (29), nitro (30) and ether (31). It is important to note that substitution at positions 2 and 6 of the phenyl ring (25) does not affect the effectiveness of the transformation. Analogously, by reacting a heteroaromatic aldehyde (3-thienyl) under the usual conditions, structure 32 was formed. The overall reaction exhibited a truly chemoselective profile: in fact, not only could the aforementioned nitrile groups be conveniently placed on the aromatic rings (22, 29) but also when an ester (33) or a piperidyl-amide (34) were present, the unique reactive site was the aldehyde carbonyl. The latter case is significant since nucleophilic acyl substitutions on these substrates are known.35 Furthermore, aliphatic and α,β-unsaturated aldehydes produced the chloro–fluoro-homologated adducts (35–37) under identical reaction conditions. As illustrated above, the selection of the first nucleophilic element to be added enables diversification of the substitution pattern of the resulting fluorohaloethyl chain. Therefore, LiCH2Br21c or LiCHBr234 generated platforms 38 and 39, whereas by employing the formal CCl3− anion (see ESI†), the trichloro–fluoro system 40 was prepared. Finally, the addition of LiCH2I21a provided a convenient route to the fluoro–iodo backbone 41 in which the styryl moiety maintained its chemical integrity.
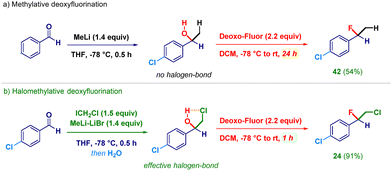
To rationalize the process, we conducted the gem-functionalization of an aldehyde in the absence of a halomethyl-releasing agent. To this end, upon the addition of simple MeLi to p-chlorobenzaldehyde followed by the usual treatment with DeoxoFluor, we noticed a dramatic increase in the reaction time (24 h) required to furnish the expected fluorinated compound 42 (Scheme 3, path a). Presumably, the constitutive lack of a halogen does not offer the possibility of creating a five-membered halogen-bond linchpin36 for activating the putative alkoxide towards the reaction with DeoxoFluor. This outcome is evidently not observed with LiCH2Cl (Scheme 3, path b) which guarantees the productive triggering of the subsequent fluorinative event, as also documented by the different isolated yields (54% for 42vs. 91% for 24).
In summary, we have documented the formal geminal difunctionalization of carbonyl linchpins (aldehydes and ketones) with a (poly)-halomethyl fragment and a fluoride anion en route to 1-fluoro-2-haloethyl skeletons. The transformation is based on the chemoselective nucleophilic attack of the halogenated C1-synthon followed by the straightforward deoxyfluorination of the putative alcohol with DeoxoFluor. Through the judicious selection of the first nucleophile (M-CXYZ), a high degree of flexibility can be imparted to the protocol.
We thank the University of Vienna, the University of Turin, the University of Milan and All4Labels Group (Hamburg, Germany) for generous funding. Financial support from PRIN projects no. 20228W9TBL (L. C.) and no. 2022JLSZMY (V. P.), and FWF-Austria Project no. P 37068-B (V. P.) is gratefully acknowledged. The authors thank Prof. W. Holzer (University of Vienna) for NMR elucidations.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Britton, V. Gouverneur, J.-H. Lin, M. Meanwell, C. Ni, G. Pupo, J.-C. Xiao and J. Hu, Nat. Rev. Methods Primers, 2021, 1, 47 CrossRef CAS
.
- T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed
.
- C. Thiehoff, Y. P. Rey and R. Gilmour, Isr. J. Chem., 2017, 57, 92 CrossRef CAS
.
-
(a) B. Marciniak, J. Walkowiak-Kulikowska and H. Koroniak, J. Fluorine Chem., 2017, 203, 47 CrossRef CAS
; (b) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed
; (c) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed
.
- A. E. Lubaev, M. D. Rathnayake, F. Eze and L. Bayeh-Romero, J. Am. Chem. Soc., 2022, 144, 13294 CrossRef CAS PubMed
.
-
(a) W.-j Chung and C. D. Vanderwal, Angew. Chem., Int. Ed., 2016, 55, 4396 CrossRef CAS PubMed
; (b) C. Nilewski, R. W. Geisser, M.-O. Ebert and E. M. Carreira, J. Am. Chem. Soc., 2009, 131, 15866 CrossRef CAS PubMed
; (c) S. Patra, V. Valsamidou, B. N. Nandasana and D. Katayev, ACS Catal., 2024, 14, 13747 CrossRef CAS
.
-
(a) D. Zhang, M. Pu, Z. Liu, Y. Zhou, Z. Yang, X. Liu, Y.-D. Wu and X. Feng, J. Am. Chem. Soc., 2023, 145, 4808 CrossRef CAS PubMed
; (b) M. Zhang, J. Zhang and M. Oestreich, Nat. Synth., 2023, 2, 439 CrossRef CAS
; (c) Y. Li, Y. Gao, Z. Deng, Y. Cao, T. Wang, Y. Wang, C. Zhang, M. Yuan and W. Xie, Nat. Commun., 2023, 14, 4673 CrossRef CAS PubMed
; (d) X. Dong, J. L. Roeckl, S. R. Waldvogel and B. Morandi, Science, 2021, 371, 507 CrossRef CAS PubMed
; (e) H. Moon, J. Jung, J.-H. Choi and W.-J. Chung, Nat. Commun., 2024, 15, 3710 CrossRef CAS PubMed
; (f) D. X. Hu, F. J. Seidl, C. Bucher and N. Z. Burns, J. Am. Chem. Soc., 2015, 137, 3795 CrossRef CAS PubMed
; (g) A. J. Cresswell, S. T. C. Eey and S. E. Denmark, Nat. Chem., 2015, 7, 146 CrossRef CAS PubMed
; (h) J. Bock, S. Guria, V. Wedek and U. Hennecke, Chem. – Eur. J., 2021, 27, 4517 CrossRef CAS PubMed
.
- W.-J. Chung and C. D. Vanderwal, Acc. Chem. Res., 2014, 47, 718 CrossRef CAS PubMed
.
-
(a) G. W. Gribble, Acc. Chem. Res., 1998, 31, 141 CrossRef CAS
; (b) A. J. Cresswell, S. T.-C. Eey and S. E. Denmark, Angew. Chem., Int. Ed., 2015, 54, 15642 CrossRef CAS PubMed
; (c) R. Zeng, L. Zhang and D. Huang, Eur. J. Org. Chem., 2024, e202400506 CrossRef CAS
.
- C. J. Thomson, Q. Zhang, N. Al-Maharik, M. Bühl, D. B. Cordes, A. M. Z. Slawin and D. O’Hagan, Chem. Commun., 2018, 54, 8415 RSC
.
- S. M. Banik, J. W. Medley and E. N. Jacobsen, J. Am. Chem. Soc., 2016, 138, 5000 CrossRef CAS PubMed
.
-
(a) I. G. Molnár and R. Gilmour, J. Am. Chem. Soc., 2016, 138, 5004 CrossRef PubMed
; (b) F. Scheidt, M. Schäfer, J. C. Sarie, C. G. Daniliuc, J. J. Molloy and R. Gilmour, Angew. Chem., Int. Ed., 2018, 57, 16431 CrossRef CAS PubMed
; (c) I. G. Molnár, C. Thiehoff, M. C. Holland and R. Gilmour, ACS Catal., 2016, 6, 7167 CrossRef
.
-
(a) S. Doobary, A. T. Sedikides, H. P. Caldora, D. L. Poole and A. J. J. Lennox, Angew. Chem., Int. Ed., 2020, 59, 1155 CrossRef CAS PubMed
; (b) S. Doobary and A. J. J. Lennox, Synlett, 2020, 1333 CAS
; (c) D. M. Heard, S. Doobary and A. J. J. Lennox, ChemElectroChem, 2021, 8, 2070 CrossRef CAS
.
- S. Hara, J. Nakahigashi, K. Ishi-i, M. Sawaguchi, H. Sakai, T. Fukuhara and N. Yoneda, Synlett, 1998, 495 CrossRef CAS
.
- S. Doobary, A. J. D. Lacey, S. G. Sweeting, S. B. Coppock, H. P. Caldora, D. L. Poole and A. J. J. Lennox, Nat. Chem., 2024, 16, 1647 CrossRef CAS PubMed
.
-
(a) T. Kitamura, R. Komoto, J. Oyamada, M. Higashi and Y. Kishikawa, J. Org. Chem., 2021, 86, 18300 CrossRef CAS PubMed
; (b) S. Yano and S. Hara, Synthesis, 2015, 2839 CAS
; (c) G. Haufe, G. Alvernhe and A. Laurent, Tetrahedron Lett., 1986, 27, 4449 CrossRef CAS
; (d) M. Stangier, A. Scheremetjew and L. Ackermann, Chem. Eur. J., 2022, 28, e202201654 CrossRef CAS PubMed
; (e) A. Le Darz, U. Castelli, N. Mokhtari, A. Martin-Mingot, J. Marrot, F. Bouazza, O. Karam and S. Thibaudeau, Tetrahedron, 2016, 72, 674 CrossRef CAS
; (f) Z.-X. Wang, Y. Xu and R. Gilmour, Nat. Commun., 2024, 15, 5770 CrossRef CAS PubMed
.
- L. Castoldi, S. Monticelli, R. Senatore, L. Ielo and V. Pace, Chem. Commun., 2018, 54, 6692 RSC
.
- J. Jelen and G. Tavčar, Synthesis, 2025, 1517 CAS
.
- V. Pace, W. Holzer and N. De Kimpe, Chem. Rec., 2016, 16, 2061 CrossRef CAS PubMed
.
-
(a) D. Seebach, Angew. Chem., Int. Ed., 2011, 50, 96 CrossRef CAS PubMed
; (b) M. Miele, A. Citarella, T. Langer, E. Urban, M. Zehl, W. Holzer, L. Ielo and V. Pace, Org. Lett., 2020, 22, 7629 CrossRef CAS PubMed
; we recently reported the sequential treatment of carbonyls with carbenoids and thionyl chloride for preparing haloolefins, see: ; (c) M. Miele, D. Castiglione, W. Holzer, L. Castoldi and V. Pace, Chem. Commun., 2025, 61, 1180 RSC
.
-
(a) L. Ielo, L. Castoldi, S. Touqeer, J. Lombino, A. Roller, C. Prandi, W. Holzer and V. Pace, Angew. Chem., Int. Ed., 2020, 59, 20852 CrossRef CAS PubMed
; (b) L. Ielo, S. Touqeer, A. Roller, T. Langer, W. Holzer and V. Pace, Angew. Chem., Int. Ed., 2019, 58, 2479 CrossRef CAS PubMed
; (c) L. Ielo, V. Pillari, M. Miele, W. Holzer and V. Pace, Adv. Synth. Catal., 2020, 362, 5444 CrossRef CAS
.
- W. J. Middleton, J. Org. Chem., 1975, 40, 574 CrossRef CAS
.
-
P. Kirsch, Modern Fluorooganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2013 Search PubMed
.
- V. Pace, L. Castoldi, E. Mazzeo, M. Rui, T. Langer and W. Holzer, Angew. Chem., Int. Ed., 2017, 56, 12677 CrossRef CAS PubMed
.
- G. S. Lal, G. P. Pez, R. J. Pesaresi, F. M. Prozonic and H. Cheng, J. Org. Chem., 1999, 64, 7048 CrossRef CAS
.
- A. L’Heureux, F. Beaulieu, C. Bennett, D. R. Bill, S. Clayton, F. LaFlamme, M. Mirmehrabi, S. Tadayon, D. Tovell and M. Couturier, J. Org. Chem., 2010, 75, 3401 CrossRef PubMed
.
- M. K. Nielsen, C. R. Ugaz, W. Li and A. G. Doyle, J. Am. Chem. Soc., 2015, 137, 9571 CrossRef CAS PubMed
.
- J. Guo, C. Kuang, J. Rong, L. Li, C. Ni and J. Hu, Chem. – Eur. J., 2019, 25, 7259 CrossRef CAS PubMed
.
-
(a) S. Meyer, J. Häfliger and R. Gilmour, Chem. Sci., 2021, 12, 10686 RSC
; (b) S. Meyer, J. Häfliger, M. Schäfer, J. J. Molloy, C. G. Daniliuc and R. Gilmour, Angew. Chem., Int. Ed., 2021, 60, 6430 CrossRef CAS PubMed
.
- G. Parisi, M. Colella, S. Monticelli, G. Romanazzi, W. Holzer, T. Langer, L. Degennaro, V. Pace and R. Luisi, J. Am. Chem. Soc., 2017, 139, 13648 CrossRef CAS PubMed
.
- M. Miele, R. D’Orsi, V. Sridharan, W. Holzer and V. Pace, Chem. Commun., 2019, 55, 12960 RSC
.
-
(a) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS PubMed
; (b) M. Miele, L. Castoldi, E. Beccalli and V. Pace, Adv. Synth. Catal., 2024, 366, 2277 CrossRef CAS
.
- M. Aoyama, T. Fukuhara and S. Hara, J. Org. Chem., 2008, 73, 4186 CrossRef CAS PubMed
.
- S. Touqeer, R. Senatore, M. Malik, E. Urban and V. Pace, Adv. Synth. Catal., 2020, 362, 5056 CrossRef CAS
.
- V. Pace, W. Holzer and B. Olofsson, Adv. Synth. Catal., 2014, 356, 3697 CrossRef CAS
.
- L. Castoldi, W. Holzer, T. Langer and V. Pace, Chem. Commun., 2017, 53, 9498 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2410335. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc01542a |
| This journal is © The Royal Society of Chemistry 2025 |