 Open Access Article
Open Access ArticleEfficient direct regeneration of spent LiFePO4 from various degradation states for sustainable battery recycling†
Jintao
Zhang
 a,
Song Lin
Zhang
a,
Nguk Neng
Tham
a,
Hui Ru
Tan
a,
Wanwan
Wang
a,
Song Lin
Zhang
a,
Nguk Neng
Tham
a,
Hui Ru
Tan
a,
Wanwan
Wang
 a,
Yi
Ren
a,
Qing
Wang
a,
Yi
Ren
a,
Qing
Wang
 *b and
Zhaolin
Liu
*b and
Zhaolin
Liu
 *a
*a
aInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Innovis #08-03, Singapore 138634, Republic of Singapore. E-mail: zl-liu@imre.a-star.edu.sg
bDepartment of Materials Science and Engineering, National University of Singapore, Singapore 117576, Republic of Singapore. E-mail: msewq@nus.edu.sg
First published on 8th May 2025
Abstract
Efficient regeneration of spent LiFePO4 is essential for the sustainable management of end-of-life electric vehicle batteries, given their significant market share. A universal method capable of directly regenerating spent LiFePO4 from cells with varying states of health is crucial for practical implementation. Herein, we developed an oxalic acid/lithium hydroxide-based regeneration approach that effectively restores mixed spent LiFePO4, achieving a high specific capacity of 163.1 mA h g−1 at 0.1C.
With the rapid expansion of the EV market, the volume of retired EV batteries has grown significantly in recent years, a trend expected to accelerate globally in the coming decades.1–3 Effective management of end-of-life (EoL) EV batteries is crucial not only for environmental sustainability but also for economic viability due to the high value and potential toxicity of battery materials.2,4–6 Battery recycling represents the final stage of a battery's lifecycle, though repurposing or remanufacturing may precede it.5,7 Recycling methods are generally classified based on their end products: if the process yields raw materials for lithium-ion battery (LIB) production, it is termed recovery;8–12 if the end product is active materials suitable for direct reuse in LIBs, it is termed direct regeneration.13–20
Conventional recovery, primarily based on extracting key valuable materials (e.g., Li, Co, Ni), has already been industrialized due to its operational simplicity.11,12,21 This method is particularly advantageous for lithium cobalt oxide (LCO)22,23 and nickel manganese cobalt (NMC) batteries.10,24,25 However, recovering lithium iron phosphate (LFP) batteries is less economically attractive, as the only recoverable high-value products are lithium-based salts (e.g., lithium hydroxide or lithium carbonate). While lithium salts have higher unit mass values than LFP (Fig. 1(a)), direct regeneration of 1 ton of spent LFP (denoted as S-LFP) batteries can double the product value (Fig. 1(b) and Table S1, ESI†), making this approach significantly more viable (Fig. S1, ESI†).
Existing direct regeneration strategies for LFP batteries primarily focus on replenishing lithium deficiencies in spent LFP.19,26 These methods typically utilize a reductant—often an organic acid, such as citric acid,27L-ascorbic acid,18,28,29 or tartaric acid,16,17 along with a lithium source, typically lithium hydroxide. While these methods have shown promise, their application remains largely confined to laboratory-scale studies, with several factors limiting their practical feasibility. One key challenge is whether a given method is suitable for mixed spent LFP derived from different battery sources. To address this issue, we developed a rapid microwave-assisted hydrothermal method (Table S2, ESI†) utilizing oxalic acid (OA) as a reducing and chelating agent, along with lithium hydroxide as the lithium source. This method was applied to mixed spent LFP from various batteries with different states of health (SOH). Additionally, we systematically investigated the effects of reaction temperature and the molar ratios of OA, lithium hydroxide (LiOH), and spent LFP on the regeneration efficiency.
We cycled 1.5 A h LFP cylindrical cells to different states of health (SOH), ranging from 55% to 70% (Fig. S2, ESI†). The spent LFP (S-LFP) was obtained from these cells and mixed together. The separation and purification processes are detailed in the ESI.† The XRD pattern of S-LFP (Fig. 2(d)) exhibits specific peaks similar to reported result,27 with impurity peaks of FePO4 appearing around 18 degrees.
We first examined the impact of reaction temperature on direct LFP regeneration. XRD analysis (Fig. 2(a)–(c)) revealed that excessively high temperatures (180 °C) led to the formation of Fe5(PO4)4(OH)3·2H2O (PDF: 45-1436), an undesirable impurity. Meanwhile, FePO4 residues persisted in R-LFP-120, likely due to the lower reaction rate at 120 °C. The optimal temperature was 150 °C, as R-LFP-150 exhibited the best match with simulated LFP (PDF: 04-013-4453).
Based on literature findings, organic acids play a critical role in the direct regeneration of spent LFP, acting as reducing agent.16,18,27 To assess the impact of increasing OA concentration, we added 20 wt% more OA to the reactor while maintaining constant amounts of S-LFP and LiOH. The XRD pattern of R-LFP-150-2 (Fig. 2(e)) indicated that excessive OA resulted in the formation of the Fe5(PO4)4(OH)3·2H2O phase, similar to that observed in R-LFP-180 (Fig. 2(c)). Overall, a LiOH/OA molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was determined to be optimal for lithium replenishment in spent LFP at 150 °C.
1 was determined to be optimal for lithium replenishment in spent LFP at 150 °C.
Considering the LiOH/OA molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as a regeneration agent (RA), the ratio of S-LFP to RA is critical for practical applications. Doubling the amount of RA compared to R-LFP-150 resulted in R-LFP-150-3, where the XRD pattern (Fig. 2(f)) showed the presence of Li3PO4 impurities. These findings suggest that excessive RA promotes the formation of the Li3PO4 phase. To further optimize the process, we doubled the concentration of all reactants, leading to R-LFP-150-4. The XRD pattern (Fig. 2(j)) displayed distinct LFP-specific peaks, indicating that adjusting feedstock concentrations while maintaining the correct molar ratios is a promising strategy to reduce wastewater production during LFP direct regeneration.
1 as a regeneration agent (RA), the ratio of S-LFP to RA is critical for practical applications. Doubling the amount of RA compared to R-LFP-150 resulted in R-LFP-150-3, where the XRD pattern (Fig. 2(f)) showed the presence of Li3PO4 impurities. These findings suggest that excessive RA promotes the formation of the Li3PO4 phase. To further optimize the process, we doubled the concentration of all reactants, leading to R-LFP-150-4. The XRD pattern (Fig. 2(j)) displayed distinct LFP-specific peaks, indicating that adjusting feedstock concentrations while maintaining the correct molar ratios is a promising strategy to reduce wastewater production during LFP direct regeneration.
We investigated the chemical valence changes of regenerated LFP (denoted as R-LFP) using X-ray photoelectron spectroscopy (XPS) (Fig. 3(a)). The results indicate that S-LFP exhibited Fe3+ peaks at approximately 712.00 eV and Fe2+ peaks at around 710.19 eV in the Fe 2p3/2 region. After regeneration, R-LFP showed only Fe2+ peaks (710.35 eV), confirming that most Fe3+ was reduced during the hydrothermal process under the reductive influence of oxalic acid. The valence states of Fe were further verified by electron energy loss spectroscopy (EELS) (Fig. 4(b)). Changes in Fe valence states were evident from the energy loss shifts of the Fe L2-edge and L3-edge.30 The lower shift of the Fe L3-edge in R-LFP-150 compared to S-LFP indicates the successful reduction of Fe3+ to Fe2+ during regeneration.
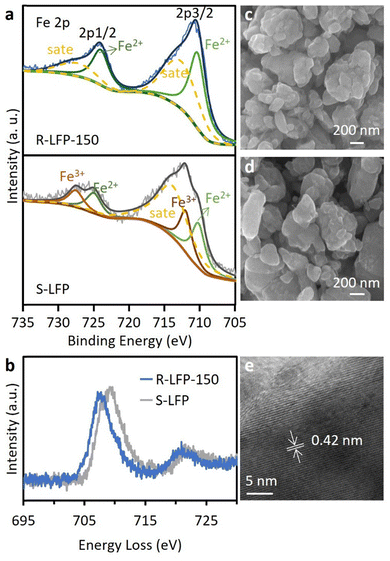 | ||
| Fig. 3 (a) Fe 2p spectra of R-LFP-150 and S-LFP. (b) Fe L-edge ELLS spectra of a representative R-LFP-150 and S-LFP. FESEM images of (c) R-LFP-150 and (d) S-LFP. (e) HRTEM of R-LFP-150. | ||
The morphology of R-LFP-150 and S-LFP was examined using scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM). The SEM images of R-LFP-150 (Fig. 3(c) and Fig. S3a, b (ESI†)) revealed a particle size comparable to that of S-LFP (Fig. 3(d) and Fig. S3c, d (ESI†)). The HRTEM image of R-LFP-150 (Fig. 3(e)) displayed well-defined lattice fringes with an interplanar spacing of 0.42 nm, corresponding to the (101) crystal plane of LFP.20 The elemental composition of R-LFP-150 and S-LFP was analyzed via energy-dispersive X-ray spectroscopy (EDX) (Fig. S4, ESI†). Both materials exhibited signals for C, O, Fe, and P, while no detectable signals of fluorine (F) or aluminum (Al) were observed in S-LFP. This indicates that the regeneration process was highly effective, ensuring the complete removal of Al fragments, binder, and electrolyte salt from S-LFP.
To evaluate the electrochemical performance and regeneration efficacy of our method, we assembled 2032-type coin cells using R-LFP-120, R-LFP-150, R-LFP-180, and S-LFP as electrodes. As shown in Fig. 4(a), the R-LFP-150 exhibited an initial capacity of 163.1 mA h g−1 at a 0.1C rate, which is significantly higher than the 123.1 mA h g−1 of S-LFP. Additionally, R-LFP-150 demonstrated a smaller potential interval compared to S-LFP (Fig. S5, ESI†) and a lower charge transfer resistance (Rct) (Fig. S6, ESI†), highlighting the superior effect of the regeneration process.
The low-rate cycling performance was evaluated at a 0.2C rate (Fig. 4(b)). R-LFP-150 exhibited an initial capacity of 159.2 mA h g−1 and maintained stable performance over 200 cycles, retaining 147.5 mA h g−1 at the end. R-LFP-120 showed an initial capacity of 138.8 mA h g−1, stabilizing at 130.4 mA h g−1 after 200 cycles. Both R-LFP-150 and R-LFP-120 demonstrated significant capacity improvements compared to the S-LFP electrode, which delivered 116.8 mA h g−1 in the first cycle and 103.5 mA h g−1 after 200 cycles. The slightly lower capacity of R-LFP-120 compared to R-LFP-150 is attributed to the residual impurity phase of FePO4 (as shown in Fig. 2(a)), which does not contribute to capacity.27 In contrast, the R-LFP-180 electrode exhibited an initial capacity of 113.1 mA h g−1, lower than that of S-LFP, which might be due to the formation of the new Fe5(PO4)4(OH)3·2H2O phase (Fig. 2(c)).
Fig. 4(c) and Fig. S7 (ESI†) illustrate the rate performance of R-LFP and S-LFP at various current rates (0.1, 0.2, 0.5, and 1C). The reversible specific capacities of R-LFP-150 were 163.1, 160.2, 151.6, and 141.7 mA h g−1, respectively. Upon returning to a 0.1C rate, a capacity of 162.5 mA h g−1 was recovered, demonstrating superior rate performance compared to S-LFP, which exhibited specific capacities of 123.1, 122.2, 117.2, and 109.7 mA h g−1. Similarly, R-LFP-120 showed enhanced performance over S-LFP, delivering capacities of 146.0, 143.1, 135.9, and 127.5 mA h g−1. However, for R-LFP-180, the low-rate capacity was comparable to that of S-LFP, but at higher charging/discharging rates, the capacity declined more significantly, indicating that excessively high regeneration temperatures adversely affect lithium replenishment and induce new phase generation, as demonstrated by the XRD pattern in Fig. 2(c).
We further investigated the high-rate (1C) cycling performance of R-LFP-150 (Fig. 4(d)). The electrode delivered 142.9 mA h g−1 in the first 1C discharge. After 500 cycles, it retained a capacity of 133.2 mA h g−1, with an exceptionally low degradation rate of 0.013%.
In summary, this study demonstrates the effectiveness of oxalic acid and lithium hydroxide as both reducing agents and lithium sources for the efficient regeneration of spent LiFePO4 materials. The effects of reaction temperature, reagent dosage, structural composition, and electrochemical performance were systematically investigated and optimized. The regenerated LiFePO4 (R-LFP-150) exhibited enhanced electrochemical performance, delivering an initial capacity of 159.2 mA h g−1 at 0.2C and maintaining stability over 200 cycles. This method provides a viable approach for directly regenerating spent LiFePO4 from various degradation states, significantly enhancing its feasibility for practical applications.
J. Z. Q. W. and Z. L. conceived the original concept and initiated the project. J. Z. mainly performed the cycling of cylindrical cells, chemical treatment, SEM, XRD, and electrochemical performance. S. Z. and N. T. helped partial experiments. H. T. carried out TEM and EELS test. W. W. carried out XPS test. J. Z. wrote the manuscript.
Data availability
All the data supporting this article have been included in the main text and the ESI.†This work was financially supported by a grant for Programmatic Funds (MTC Domain, grant no. M24N5b0037, project reference no. SC25/24-843926) from the Agency for Science, Technology and Research (A*STAR), Singapore.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Hua, X. Liu, S. Zhou, Y. Huang, H. Ling and S. Yang, Resour., Conserv. Recycl., 2021, 168, 105249 CrossRef.
- Z. Yang, H. Huang and F. Lin, Adv. Energy Mater., 2022, 12, 2200383 CrossRef.
- C. Y. Yang, Z. Jiang, X. Y. Chen, W. Luo, T. F. Zhou and J. P. Yang, Chem. Commun., 2024, 60, 10245 RSC.
- D. Kamath, R. Arsenault, H. C. Kim and A. Anctil, Environ. Sci. Technol., 2020, 54, 6878 CrossRef PubMed.
- R. Ma, S. Tao, X. Sun, Y. Ren, C. Sun, G. Ji, J. Xu, X. Wang, X. Zhang, Q. Wu and G. Zhou, Nat. Commun., 2024, 15, 7641 CrossRef PubMed.
- A. Nurdiawati and T. K. Agrawal, Resour., Conserv. Recycl., 2022, 185, 106484 CrossRef.
- M. Y. Chen, X. T. Ma, B. Chen, R. Arsenault, P. Karlson, N. Simon and Y. Wang, Joule, 2019, 3, 2622 CrossRef.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. S. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75 CrossRef PubMed.
- J. Z. Yu, X. Wang, M. Y. Zhou and Q. Wang, Energy Environ. Sci., 2019, 12, 2672 RSC.
- J. T. Hu, J. L. Zhang, H. X. Li, Y. Q. Chen and C. Y. Wang, J. Power Sources, 2017, 351, 192 CrossRef.
- Z. Xing and M. Srinivasan, Chem. Eng. J., 2023, 474, 145306 CrossRef.
- H. Y. Wang, K. Huang, Y. Zhang, X. Chen, W. Jin, S. L. Zheng, Y. Zhang and P. Li, ACS Sustainable Chem. Eng., 2017, 5, 11489 CrossRef.
- P. P. Xu, D. H. S. Tan, B. L. Jiao, H. P. Gao, X. L. Yu and Z. Chen, Adv. Funct. Mater., 2023, 33, 2213168 CrossRef.
- G. H. Jiang, Y. N. Zhang, Q. Meng, Y. J. Zhang, P. Dong, M. Y. Zhang and X. Yang, ACS Sustainable Chem. Eng., 2020, 8, 18138 CrossRef.
- R. Shi, N. Zheng, H. Ji, M. Zhang, X. Xiao, J. Ma, W. Chen, J. Wang, H. M. Cheng and G. Zhou, Adv. Mater., 2024, 36, e2311553 CrossRef PubMed.
- B. B. Chen, M. Liu, S. Cao, H. Hu, G. R. Chen, X. W. Guo and X. Y. Wang, J. Alloys Compd., 2022, 924, 166487 CrossRef.
- S. P. Hao, Y. L. Lv, Y. Zhang, S. W. Liu, Z. L. Tan, W. Liu, Y. G. Xia, W. Yin, Y. Q. Liao, H. J. Ji, Y. L. Kong, Y. D. Shao, Y. H. Huang and L. X. Yuan, Energy Environ. Sci., 2025, 18, 3750 RSC.
- J. Tang, H. T. Qu, C. B. Sun, X. Xiao, H. C. Ji, J. X. Wang, J. F. Li, G. J. Ji, X. Zhang, H. M. Cheng and G. M. Zhou, Adv. Mater., 2025, 37, 2420238 CrossRef PubMed.
- K. Jia, G. Yang, Y. He, Z. Cao, J. Gao, H. Zhao, Z. Piao, J. Wang, A. M. Abdelkader, Z. Liang, R. V. Kumar, G. Zhou, S. Ding and K. Xi, Adv. Mater., 2024, 36, e2313273 CrossRef PubMed.
- Y. Cao, J. Li, D. Tang, F. Zhou, M. Yuan, Y. Zhu, C. Feng, R. Shi, X. Wei, B. Wang, Y. Song, H. M. Cheng and G. Zhou, Adv. Mater., 2024, 36, e2414048 CrossRef PubMed.
- Z. T. Fei, Y. Y. Su, Y. C. Zha, X. H. Zhao, Q. Meng, P. Dong and Y. J. Zhang, Chem. Eng. J., 2023, 464, 142534 CrossRef.
- B. Zhang, Y. Xu, B. Makuza, F. Zhu, H. Wang, N. Hong, Z. Long, W. Deng, G. Zou, H. Hou and X. Ji, Chem. Eng. J., 2023, 452, 139258 CrossRef.
- J. F. Xiao, J. Li and Z. M. Xu, Environ. Sci. Technol., 2017, 51, 11960 CrossRef PubMed.
- X. Chang, M. Fan, C. F. Gu, W. H. He, Q. H. Meng, L. J. Wan and Y. G. Guo, Angew. Chem., Int. Ed., 2022, 61, e202202558 CrossRef PubMed.
- L. Li, Y. F. Bian, X. X. Zhang, Y. B. Guan, E. S. Fan, F. Wu and R. J. Chen, Waste Manage., 2018, 71, 362 CrossRef.
- T. Yingnakorn, J. Hartley, J. S. Terreblanche, C. H. Lei, W. M. Dose and A. P. Abbott, RSC Sustainability, 2023, 1, 2341 RSC.
- P. P. Xu, Q. Dai, H. P. Gao, H. D. Liu, M. H. Zhang, M. Q. Li, Y. Chen, K. An, Y. S. Meng, P. Liu, Y. R. Li, J. S. Spangenberger, L. Gaines, J. Lu and Z. Chen, Joule, 2020, 4, 2609 CrossRef.
- Z. Y. Jiang, J. Sun, P. S. Jia, W. L. Wang, Z. L. Song, X. Q. Zhao and Y. P. Mao, Sustainable Energy Fuels, 2022, 6, 2207 RSC.
- T. Ouaneche, L. Stievano, F. Rabuel, A. Jamali, C. Guery, L. Monconduit, M. T. Sougrati and N. Recham, Batteries Supercaps, 2025, e202400765 Search PubMed.
- D. Tang, G. J. Ji, J. X. Wang, Z. Liang, W. Chen, H. C. Ji, J. Ma, S. Liu, Z. F. Zhuang and G. M. Zhou, Adv. Mater., 2024, 36, e2309722 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01308a |
| This journal is © The Royal Society of Chemistry 2025 |



