Open Access Article
Open Access ArticleOrganocatalytic atroposelective fluorooxindole addition to coumarin Michael acceptors†
Maria
Bouda
 ,
Grace E.
Hana
,
Dea
Xhili
,
Archita
Sripada
,
Jeffery A.
Bertke
,
Grace E.
Hana
,
Dea
Xhili
,
Archita
Sripada
,
Jeffery A.
Bertke
 and
Christian
Wolf
and
Christian
Wolf
 *
*
Department of Chemistry, Georgetown University, Washington, D. C. 20057, USA. E-mail: cw27@georgetown.edu
First published on 29th April 2025
Abstract
Organocatalytic atropisomeric synthesis with fluorinated oxindoles and 4-halo-3-nitrocoumarins gives congested structures displaying a Csp2–Csp3 chirality axis and an adjacent tetrasubstituted stereogenic carbon center with good yields, up to 97% ee and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The scalable dehalogenative C–C bond formation is achieved under mild conditions with a commercially available urea catalyst.
1 dr. The scalable dehalogenative C–C bond formation is achieved under mild conditions with a commercially available urea catalyst.
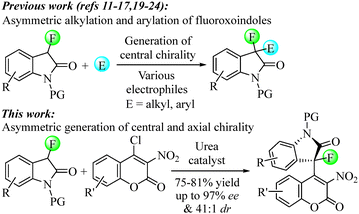
The widespread interest in fluorinated organic compounds stems at least in part from their favorable lipophilicity, bioavailability and metabolic stability, which among other advantageous properties have been widely recognized and pursued in the drug development realm. The general demand for new fluorinated compounds with ever-increasing molecular complexity has been a steady driving force in the intense search of synthetic methods that can address this need and conquer new chemical space.1–3 Today, a large pool of fluorinated building blocks is readily available but the small size and high electronegativity of fluorine continue to render stereocontrolled C–C bond formation challenging, in particular when highly substituted stereocenters or more than one element of chirality needs to be introduced.4–7 The discovery of Maxipost and analogues thereof8–10 has inspired numerous drug development initiatives and a surge of catalytic asymmetric protocols that use fluorooxindoles to produce new structures with potential medicinal use.11–24 Like oxindoles, coumarins are known for their wide-ranging biological activities and therapeutic value, for example, as antimicrobial, anticoagulant, anti-inflammatory and anticancer agents.25 It is not surprising that chiral drugs combining these two prevalent pharmacophores have also evolved as promising leads with various medical applications. New synthetic methods that generate such hybrid scaffolds have therefore emerged and become remarkably sought-after in recent years.26–30
While the focus on medicinally relevant scaffolds in asymmetric catalysis follows the obvious rationale to generate promising libraries of future drug candidates, many research groups have begun to pay increasing attention to the construction of complex molecules exhibiting not only central but also axial, planar or helical chirality.31 To this end, the steadily growing significance of axially chiral drugs32 has initiated numerous efforts that have significantly expanded the breadth of atroposelective catalysis and an impressive variety of fascinating atropisomeric architectures can nowadays be accessed.33–49
By contrast, examples of atropisomerselective synthesis including either oxindole or coumarin molecules to forge a chiral axis are very rare.50 We now wish to report an organocatalytic method that generates atropisomeric fluorooxindole-coumarin hybrids exhibiting an adjacent tetrasubstituted stereogenic carbon center. This reaction uses a commercially available urea catalyst to achieve stereocontrolled construction of axial and central chirality in congested, multifunctional structures that are obtained in high enantiomeric and diastereomeric ratios and good yields at room temperature (Scheme 1).
At the onset of this study we chose to explore the possibility of asymmetric C–C bond formation with N-methyl-3-fluorooxindole, 1a, and 4-chloro-3-nitrocoumarin, 2a, in the presence of various organocatalysts. Extensive screening of 15 different chiral urea, thiourea, squaramide and phase-transfer catalysts in several solvents at varying temperatures revealed that the axially chiral structure 3a can be obtained with high conversion and high diastereomeric ratios while ee's varied substantially (Table 1 and ESI†). Our initial attempts with phase-transfer catalysts like PTC-1 were disappointing showing slow conversion to racemic 3a albeit with high diastereoselectivity (entry 1). We therefore resorted to urea, thiourea and squaramide catalysts to exploit activation of the coumarin electrophile via hydrogen bonding with the nitro group. This proved successful and we observed smooth reaction to the desired product which was obtained in 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and with promising enantiomeric excess, in particular when SQ-1 and Urea-1 were used (entries 3 and 6). We then tested a variety of low polarity solvents and combinations thereof in the presence of commercially available Urea-1 (entries 7–15) and found that the use of 10 mol% of this catalyst in a toluene/pentane solvent mixture allows formation of a chiral axis and a chiral center with excellent enantio- and diastereoselectivity at room temperature after 48 hours (entry 15). It is noteworthy that the organocatalytic reaction typically requires 1–2 days for completion but the long reaction times do not favor formation of by-products. Interestingly, a decrease in the reaction temperature to −40 °C only slightly improved ee's while the conversion to 3a was still incomplete after 72 hours (compare entries 12 and 13).
1 dr and with promising enantiomeric excess, in particular when SQ-1 and Urea-1 were used (entries 3 and 6). We then tested a variety of low polarity solvents and combinations thereof in the presence of commercially available Urea-1 (entries 7–15) and found that the use of 10 mol% of this catalyst in a toluene/pentane solvent mixture allows formation of a chiral axis and a chiral center with excellent enantio- and diastereoselectivity at room temperature after 48 hours (entry 15). It is noteworthy that the organocatalytic reaction typically requires 1–2 days for completion but the long reaction times do not favor formation of by-products. Interestingly, a decrease in the reaction temperature to −40 °C only slightly improved ee's while the conversion to 3a was still incomplete after 72 hours (compare entries 12 and 13).
| Entry | Time (h) | Temp. (°C) | Solvent | Catalyst | Conv. (%) | dr | ee |
|---|---|---|---|---|---|---|---|
| Reaction conditions: N-methyl-3-fluoro-2-oxindole (0.09 mmol), 4-chloro-3-nitrocoumarin (0.1 mmol), potassium carbonate (3 eq.) and 10 mol% of the catalyst were dissolved in 0.8 mL of the indicated solvent. Conversion determined by 1H NMR spectroscopy. dr determined by 19F NMR spectroscopy. ee determined by chiral HPLC. n.d. = not determined. | |||||||
| 1 | 24 | 25 | Toluene | PTC-1 | 17 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 2 | 24 | 25 | Toluene | TU-1 | 3 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
n.d. |
| 3 | 24 | 25 | Dichloromethane | SQ-1 | 83 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 |
| 4 | 24 | 25 | Dichloromethane | SQ-2 | 39 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 |
| 5 | 48 | −40 | Dichloromethane | SQ-2 | 38 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
| 6 | 24 | 25 | Toluene | Urea-1 | 99 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79 |
| 7 | 24 | 25 | Mesitylene | Urea-1 | 45 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 |
| 8 | 24 | 25 | Chlorobenzene | Urea-1 | 79 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77 |
| 9 | 48 | −40 | Xylene | Urea-1 | 73 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 |
| 10 | 24 | 25 | Trifluorotoluene | Urea-1 | 37 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72 |
| 11 | 24 | 25 | Dichloromethane | Urea-1 | 83 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 |
| 12 | 24 | 25 | Toluene/ether (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Urea-1 | 99 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71 |
| 13 | 72 | −40 | Toluene/ether (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Urea-1 | 50 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 |
| 14 | 24 | 25 | Toluene/pentane (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Urea-1 | 82 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
| 15 | 48 | 25 | Toluene/pentane (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Urea-1 | 99 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
Having developed an optimized protocol, we continued with the evaluation of the reaction scope by testing the suitability of different fluorooxindoles using stoichiometric amounts of the coumarin and 10 mol% of Urea-1 in a toluene/pentane solution at room temperature. We were pleased to find that 3a was formed in 81% yields, 97% ee and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 2). Excellent results were also obtained with N-phenyl and N-benzyl fluorooxindoles and we isolated 3b and 3c, respectively, in similar yields, 84–95% ee and high dr's. However, the reaction with unprotected oxindole resulted in low yields due to by-product formation and the use of N-Boc-3-fluorooxindole gave racemic product. The presence of fluoride, chloride and bromide substituents at C-5 in the oxindole ring is well tolerated. The corresponding atropisomers 3d–f were produced in 75–81% yield and up to 90% ee and 26
1 dr (Scheme 2). Excellent results were also obtained with N-phenyl and N-benzyl fluorooxindoles and we isolated 3b and 3c, respectively, in similar yields, 84–95% ee and high dr's. However, the reaction with unprotected oxindole resulted in low yields due to by-product formation and the use of N-Boc-3-fluorooxindole gave racemic product. The presence of fluoride, chloride and bromide substituents at C-5 in the oxindole ring is well tolerated. The corresponding atropisomers 3d–f were produced in 75–81% yield and up to 90% ee and 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio. Finally, three fluoroxindoles carrying a bromide, cyano or methyl substituent at position-6 were employed in the same protocol which gave the corresponding atropisomeric fluorooxindole-coumarin hybrids 3g–i in comparable yields and stereoselectivities. The reaction can be performed at larger scale without erosion of the yield and enantioselectivity while the diastereomeric ratio is reduced but still very high. In fact, a 10 times increase of the reaction scale gave 3a in 82% yield, 98% ee, and 24
1 diastereomeric ratio. Finally, three fluoroxindoles carrying a bromide, cyano or methyl substituent at position-6 were employed in the same protocol which gave the corresponding atropisomeric fluorooxindole-coumarin hybrids 3g–i in comparable yields and stereoselectivities. The reaction can be performed at larger scale without erosion of the yield and enantioselectivity while the diastereomeric ratio is reduced but still very high. In fact, a 10 times increase of the reaction scale gave 3a in 82% yield, 98% ee, and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (see ESI†).
1 dr (see ESI†).
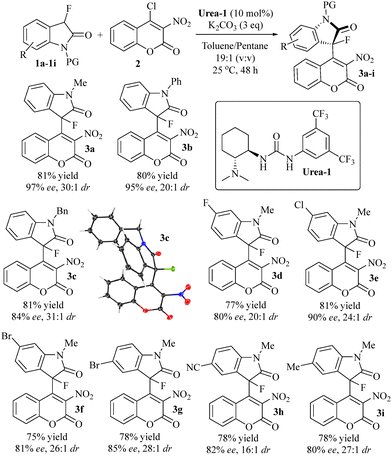 | ||
Scheme 2 Atroposelective catalysis with different fluorooxindoles. General conditions: fluorooxindole (0.09 mmol), coumarin (0.1 mmol), K2CO3 (0.27 mmol), and Urea-1 (10 mol%) were stirred in anhydrous toluene/pentane (0.4 mL, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) under nitrogen at 25 °C for 48 hours. See ESI† for details. 1 v/v) under nitrogen at 25 °C for 48 hours. See ESI† for details. | ||
Next, the coumarin scaffold was varied and several derivatives were employed in the urea catalyzed reaction (Scheme 3). Accordingly, we prepared chlorocoumarins exhibiting substituents at the C-6 or C-7 positions and identified noticeable effects on the stereoselectivities. Incorporation of a methyl or halogen moiety at C-6 did not compromise the product formation but both ee and dr values were slightly lower in most cases. Compound 4a–4d were isolated in good yields ranging from 75 to 79% which is very close to the results discussed above, however, ee's decreased to 68–75% and the diastereomeric ratios varied substantially between 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 28
1 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Interestingly, better results were obtained with coumarins carrying a methyl or methoxy group at C-7. Although the fluoride 4e was produced in only 73% ee and 17
1. Interestingly, better results were obtained with coumarins carrying a methyl or methoxy group at C-7. Although the fluoride 4e was produced in only 73% ee and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, we found that the formation of the atropisomers 4f–j occurred with excellent central and axial chirality control resulting in 87–93% ee and diastereomeric ratios between 19
1 dr, we found that the formation of the atropisomers 4f–j occurred with excellent central and axial chirality control resulting in 87–93% ee and diastereomeric ratios between 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 41
1 and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
 | ||
| Scheme 3 Atroposelective catalysis with different coumarins. General conditions: see Scheme 2 and ESI,† for details. | ||
The atropisomeric scaffold of these oxindole-coumarin adducts is very crowded and does not show any sign of diastereomerization via rotation about the chiral axis even at increased temperatures. Chiral HPLC analysis of a sample of 3b exhibiting 95% ee and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr did not show any change in its stereochemical composition after heating in refluxing acetonitrile for 7 hours. The presence of the nitro group is considered important to provide sufficient steric hindrance to rotation about the chiral axis but it also is essential to the coumarin reactivity and the stereoselectivity of the reaction. Replacement with a formyl or a trifluoroacetyl group led to decreased asymmetric induction, i.e. significantly reduced enantio- and diastereoselectivity, and lower yields due to incomplete conversion as well as competing 1,2-addition of the fluorooxindole enolate to the aldehyde.
1 dr did not show any change in its stereochemical composition after heating in refluxing acetonitrile for 7 hours. The presence of the nitro group is considered important to provide sufficient steric hindrance to rotation about the chiral axis but it also is essential to the coumarin reactivity and the stereoselectivity of the reaction. Replacement with a formyl or a trifluoroacetyl group led to decreased asymmetric induction, i.e. significantly reduced enantio- and diastereoselectivity, and lower yields due to incomplete conversion as well as competing 1,2-addition of the fluorooxindole enolate to the aldehyde.
We found that some compounds display a high propensity to form racemic crystals even when the enantiomeric excess is higher than 90%. The single crystals of 3c and 4d obtained by layering of a dichloromethane solution with pentane were indeed racemic (see ESI†). This tendency offers practical means to increase the enantiopurity of the atropisomers by crystallization and removal of racemic precipitate (see ESI,† for several examples) but it rendered the determination of the absolute configuration of the asymmetric reaction product challenging. After many attempts with several compounds, we were able to increase the enantiopurity of 3e to 95% ee and grew an enantiopure single crystal by slow evaporation of a dichloromethane solution. X-ray crystallography together with chiral HPLC analysis of this crystal revealed that the major enantiomer formed is (S,Ra)-3e (Scheme 4 and ESI†). Interestingly, the C–F bond is in the same plane as the neighboring nitro group while the oxindole and coumarin rings point in almost perpendicular directions.
The stereochemical outcome of the Urea-1 catalyzed reaction is in agreement with a Si-face attack of the fluoroxoindole enolate on the 4-chlorocoumarin Re-face which was expected as this approach minimizes the overall dipole moments of the two substrates. The corresponding intermediate 5 exhibiting two halogenated tetrasubstituted stereocenters then undergoes chloride elimination and counterclockwise rotation of the oxindole moiety to adopt the favored and crystallographically verified axially chiral conformation with the C–F bond and the nitro group residing in a synperiplanar orientation.
In summary, we have introduced an organocatalytic atropisomeric synthesis protocol that produces unprecedented fluorooxindole-coumarin hybrid structures displaying a stable chirality axis and an adjacent tetrasubstituted stereogenic carbon center. The reaction proceeds with good yields, up to 97% ee and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, can be scaled up, and tolerates various substituents in the oxindole and coumarin rings. The urea catalyst used is commercially available and the starting materials are readily prepared following known literature protocols. The products are thermally stable to atropisomerization and no sign of rotation about the highly congested chiral Csp2–Csp3 axis was observed after heating at 80 °C for 7 hours. It is expected that this reaction will attract increasing attention to the development of other catalytic atropisomeric methods that achieve stereocontrolled formation of multifunctional scaffolds with two or more different elements of chirality.
1 dr, can be scaled up, and tolerates various substituents in the oxindole and coumarin rings. The urea catalyst used is commercially available and the starting materials are readily prepared following known literature protocols. The products are thermally stable to atropisomerization and no sign of rotation about the highly congested chiral Csp2–Csp3 axis was observed after heating at 80 °C for 7 hours. It is expected that this reaction will attract increasing attention to the development of other catalytic atropisomeric methods that achieve stereocontrolled formation of multifunctional scaffolds with two or more different elements of chirality.
We gratefully acknowledge financial support from the US National Institutes of Health (GM106260).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Notes and references
- K. Müller and C. Faeh, Diederich Sci., 2007, 317, 1881 CrossRef PubMed.
- Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Acena, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS PubMed.
- B. R. Smith, C. M. Eastman and J. Njardarson, J. Med. Chem., 2014, 57, 9764 CrossRef CAS PubMed.
- A. M. Cook and C. Wolf, Angew. Chem., Int. Ed., 2016, 55, 2929 CrossRef CAS PubMed.
- R. Ding and C. Wolf, Org. Lett., 2018, 20, 892 CrossRef CAS PubMed.
- R. Ding and Z. A. De Los Santos, ACS Catal., 2019, 9, 2169 CrossRef CAS PubMed.
- A. Sripada and C. Wolf, J. Org. Chem., 2022, 87, 11880 CrossRef CAS PubMed.
- V. K. Gribkoff, J. E. Starrett, S. L. Dworetzky, P. Hewawasam, C. G. Boissard, D. A. Cook, S. W. Frantz, K. Heman, J. R. Hibbard, K. Huston, G. Johnson, B. S. Krishnan, G. G. Kinney, L. A. Lombardo, N. A. Meanwell, P. B. Molinoff, R. A. Myers, S. L. Moon, A. Ortiz, L. Pajor, R. L. Pieschl, D. J. Post-Munson, L. J. Signor, N. Srinivas, M. T. Taber, G. Thalody, J. T. Trojnacki, H. Wiener, K. Yeleswaram and S. W. Yeola, Nat. Med., 2001, 7, 471 CrossRef CAS PubMed.
- P. Hewawasam, V. K. Gribkoff, Y. Pendri, S. I. Dworetzky, N. A. Meanwell, E. Martinez, C. G. Boissard, D. J. Post-Munson, J. T. Trojnacki, K. Yeleswaram, L. M. Pajor, J. Knipe, Q. Gao, R. Perrone and J. E. Starrett Jr., Bioorg. Med. Chem. Lett., 2002, 12, 1023 CrossRef CAS PubMed.
- P. L. N. Ranganath and A. V. Narsaiah, J. Fluorine Chem., 2023, 268, 110134 CrossRef CAS.
- T. Wang, D. L. Hoon and Y. Lu, Chem. Commun., 2015, 51, 10186 RSC.
- X. Chen, Y. Li, J. Zhao, B. Zheng, Q. Lu and X. Ren, Adv. Synth. Catal., 2017, 359, 3057 CrossRef CAS.
- Y. Zhu, H. Mei, J. Han, V. A. Soloshonok, J. Zhou and Y. Pan, J. Org. Chem., 2017, 82, 13663 CrossRef CAS PubMed.
- Y. Jin, M. Chen, S. Ge and J. F. Hartwig, Org. Lett., 2017, 19, 1390 CrossRef CAS PubMed.
- S. Paladhi, S. Y. Park, J. W. Yang and C. E. Song, Org. Lett., 2017, 19, 5336 CrossRef CAS PubMed.
- K. Balaraman and C. Wolf, Angew. Chem., Int. Ed., 2017, 56, 1390 CrossRef CAS PubMed.
- Y. Zhu, Y. Mao, H. Mei, Y. Pan, J. Han, V. A. Soloshonok and T. Hayashi, Chem. – Eur. J., 2018, 24, 8994 CrossRef CAS PubMed.
- S. Hajra, A. Hazra and P. Mandal, Org. Lett., 2018, 20, 6471 CrossRef CAS PubMed.
- J. Zhao, Y. Li, L.-Y. Chen and X. Ren, J. Org. Chem., 2019, 84, 5099 CrossRef CAS PubMed.
- B.-Y. Li, Y. Lin and D.-M. Du, J. Org. Chem., 2019, 84, 11752 CrossRef CAS PubMed.
- Z.-B. Qiu, L.-Y. Chen, J. Ji, X. Ren and Y. Li, J. Fluorine Chem., 2020, 236, 109594 CrossRef CAS.
- T. W. Butcher, W. M. Amberg and J. F. Hartwig, Angew. Chem., Int. Ed., 2022, 61, e202112251 CrossRef CAS PubMed.
- Y.-L. Liu, X.-P. Wang, J. Wei and Y. Li, Org. Biomol. Chem., 2022, 20, 538 RSC.
- M. Bouda, J. A. Bertke and C. Wolf, J. Org. Chem., 2024, 89, 6100 CrossRef CAS PubMed.
- A. Khursheed and V. Jain, J. Nat. Prod., 2021, 11, 648 CAS.
- B. Zhou, Z. Luo and Y. Li, Chem. – Eur. J., 2013, 19, 4428 CrossRef CAS PubMed.
- K. Parthasarathy, C. Praveen, J. C. Jeyaveeran and A. A. M. Prince, Bioorg. Med. Chem. Lett., 2016, 26, 4310 CrossRef CAS PubMed.
- C.-W. Lei, C.-B. Zhang, Z.-H. Wang, K.-X. Xie, J.-Q. Zhao, M.-Q. Zhou, X.-M. Zhang, X.-Y. Xua and W.-C. Yuan, Org. Chem. Front., 2020, 7, 499 RSC.
- C.-W. Lei, C.-B. Zhang, Z.-H. Wang, K.-X. Xie, J.-Q. Zhao, M.-Q. Zhou, X.-M. Zhang, X.-Y. Xua and W.-C. Yuan, Org. Biomol. Chem., 2020, 18, 845 RSC.
- V. P. R. Gajulapalli, N. Kumarswamyreddy, K. Lokesh and V. Kesavan, ChemistrySelect, 2021, 6, 7855 CrossRef.
- H.-H. Zhang, T.-Z. Li, S.-J. Liu and F. Shi, Angew. Chem., Int. Ed., 2024, 63, e202311053 CrossRef CAS PubMed.
- S. R. LaPlante, L. D. Fader, K. R. Fandrick, D. R. Fandrick, O. Hucke, R. Kemper, S. P. F. Miller and P. J. Edwards, J. Med. Chem., 2011, 54, 7005 CrossRef CAS PubMed.
- L. Wang, S. Li, M. Blümel, A. R. Philipps, A. Wang, R. Puttreddy, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2016, 55, 11110 CrossRef CAS PubMed.
- K. Yamanomoto, K. Yamamoto, S. Yoshida, S. Sato and T. Akiyama, Chem. Commun., 2024, 60, 582 RSC.
- Y. Yang, C. Wu, J. Xing and X. Dou, J. Am. Chem. Soc., 2024, 146, 6283 CrossRef CAS PubMed.
- Z. Zhang, M. M. Simon, S. Yu, S. Li, X. Chen, S. Cattani, X. Hong and L. Ackermann, J. Am. Chem. Soc., 2024, 146, 9172 CrossRef CAS PubMed.
- B. Ye, L. Su, K. Zheng, S. Gao and J. Liu, Angew. Chem., Int. Ed., 2025, 64, e202413949 CrossRef CAS PubMed.
- D. Xu, G. Zhou, B. Liu, S. Jia, Y. Liu and H. Yan, Angew. Chem., Int. Ed., 2025, 64, e202416873 CrossRef CAS PubMed; C. Ma, F. Sheng, H. Wang, S. Deng, Y. Zhang, Y. Jiao, W. Tan and F. Shi, J. Am. Chem. Soc., 2020, 142, 15686 CrossRef PubMed.
- W. Zhan, J. Hu, X. Chen, G. Luo and X. Song, Chem. Commun., 2024, 60, 14984 RSC.
- S. He, B. Shen, L. Zuo, S. Xiang, H. Liu, P. Yu and B. Tan, J. Am. Chem. Soc., 2024, 146, 19137 CrossRef CAS PubMed.
- C. Weng, L. Liu, M. Sun, X. Lu, X. Hong, L. Ye and B. Zhou, Angew. Chem., Int. Ed., 2024, e202418254 Search PubMed.
- X. Tang, Y. Tang, J. Peng, H. Du, L. Huang, J. Jiahui Gao, S. Liu, D. Wang, W. Wang, L. Gao, Y. Lan and Z. Song, J. Am. Chem. Soc., 2024, 146, 26639 CrossRef CAS PubMed.
- Y. Xiao, Z. Zhao, E. Irran and M. Oestreich, Angew. Chem., Int. Ed., 2024, 63, e202414005 CrossRef CAS PubMed.
- Y. Gao, Z. Liu, S. Tian, Y. Min, X. Li, Y. Chen, X. Hong, W. Zhang and L. Wang, Angew. Chem., Int. Ed., 2024, e202418888 Search PubMed.
- Y. Du, Q. Lu, Y. Cui, K. Wu, Y. Wang, Y. Zhang, Z. Zhao, J. Hou and Q. Cai, Angew. Chem., Int. Ed., 2024, e202421060 Search PubMed.
- Y. Wang, W. Liu, T. Li, Y. Lu, Y. Yu, H. Liu, M. Liu, P. Li, P. Qian, H. Tang, J. Guan, L. Ye and L. Li, J. Am. Chem. Soc., 2024, 146, 33804 CrossRef CAS PubMed.
- J. Zhang, Y. Zhang and Y. Huang, Angew. Chem., Int. Ed., 2025, 64, e202413102 CrossRef CAS PubMed.
- B. Ye, L. Su, K. Zheng, S. Gao and J. Liu, Angew. Chem., Int. Ed., 2025, 64, e202413949 CrossRef CAS PubMed.
- D. Xu, G. Zhou, B. Liu, S. Jia, Y. Liu and H. Yan, Angew. Chem., Int. Ed., 2025, 64, e202416873 CrossRef CAS PubMed.
- C. Ma, F. Sheng, H. Wang, S. Deng, Y. Zhang, Y. Jiao, W. Tan and F. Shi, J. Am. Chem. Soc., 2020, 142, 15686 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2426004, 2426005 and 2426009. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc01166c |
| This journal is © The Royal Society of Chemistry 2025 |