 Open Access Article
Open Access ArticlePeptide catalyzed regio- and enantioselective ε-alkylation of γ-branched 2,4-dienals via trienamine activation†
Qian
Liu
,
Kengo
Akagawa
and
Kazuaki
Kudo
 *
*
Institute of Industrial Science, The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo 153-8505, Japan. E-mail: kkudo@iis.u-tokyo.ac.jp
First published on 7th March 2025
Abstract
The regio- and enantioselective ε-alkylation of γ-branched 2,4-dienals was successfully achieved via trienamine catalysis. N-Terminal prolyl pentapeptide with a turn structure was effective as a catalyst for this transformation.
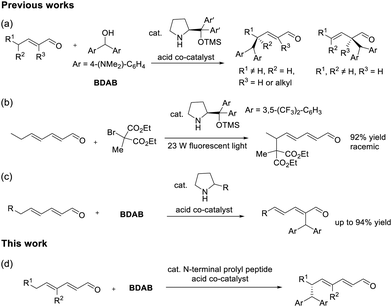
The vinylogous reactivity of enolate equivalents derived from α,β-unsaturated aldehydes and their higher conjugated analogues is of interest from the perspective of regio- and stereoselectivity.1 Enamine catalysis is a powerful method for generating such nucleophiles from a variety of aldehydes, including unsaturated ones.2 The merit of enamine catalysis lies in its ability to elevate the HOMO energy level and provide efficient stereocontrol due to the nature of covalent catalysis. In this context, numerous regio- and enantioselective reactions have been reported for chiral dienamines derived from enals (Fig. 1, left).3
In 2006, Jørgensen's group reported the dienamine catalysis for the regio- and enantioselective γ-amination of enals using azodicarboxylate esters.4 This finding prompted researchers to study the reaction of dienamines with a variety of substrates. In some cases, the reaction preferentially proceeds at the α-position,5 yielding products as either α,β-unsaturated6 or β,γ-unsaturated aldehydes.7 For the former type of the products, while the dienamine mechanism is plausible, the possibility of a Morita–Baylis–Hillman (MBH)-type mechanism cannot be excluded.5 γ-Selectivity for the reaction of enals via dienamine catalysis has also been observed in alkylation, aldol reaction, and the reaction with metal-activated electrophiles.3b,8 For the alkylation of dienamines with a cation precursor 4,4′-bis(dimethylamino)benzhydrol (BDAB), regioselectivity is influenced by the substituents on the dienamine (Scheme 1a).7b,9 The enantiofacial selectivity of dienamines can be controlled either by steric repulsion from the bulky substituent on the amine catalyst9b,10 or through attracting interactions between the electrophile and the catalyst molecule.11 Through theoretical calculation, Jørgensen's group demonstrated that the mechanism of the γ-selective amination of dienamine is the consecutive Diels–Alder reaction and the hydrolysis of the resulting aminals rather than the simple nucleophilic addition.4 Inspired by this finding, the cycloaddition chemistry of dienamines has also been widely explored.12
In the study of trienamine reactivity (Fig. 1, right), early investigations focused on [4+2] cycloadditions occurring at the β- and ε-positions.13 As a result, the cycloaddition chemistry of trienamines with various dienophiles has been extensively investigated.12,14 In contrast, studies on one-bond-forming reactions remain relatively limited. In 2015, Albrecht's group demonstrated that 5-alkyl furfurals undergo dearomative trienamine formation, which then reacts with electrophiles in an ε-selective manner.15 Several variants of this reaction have since been developed.16 Two studies have explored one-bond-forming reaction of simple linear trienamines derived from dienals. Melchiorre's group demonstrated a photocatalytic C–C bond formation of aldehydes via enamine catalysis, where a trienamine derived from diarylprolinol silyl ether and 2,4-heptadienal reacted with complete ε-selectivity but no enantioselectivity (Scheme 1b).17 Later, Appayee's group showed that, using pyrrolidine or its derivatives, the alkylation of the trienamine via an SN1 mechanism selectively occurred at the α-position, yielding MBH-type products without creating a chiral center (Scheme 1c).18 Currently, there are no reported examples of a reaction that achieves both regio- and enantioselective one bond formation in trienamines.
Our group previously reported a regioselective 1,6-addition to 2,4-dienals via an iminium ion intermediate, utilizing an N-terminal prolyl peptide with a turn structure as a catalyst.19 We envisaged that a similar directing effect of the peptide catalyst could facilitate the regioselective reaction of dienal-derived trienamines. Herein, we report the regio- and enantioselective ε-alkylation of 2,4-dienals via trienamine activation using a peptide catalyst under mild conditions (Scheme 1d).
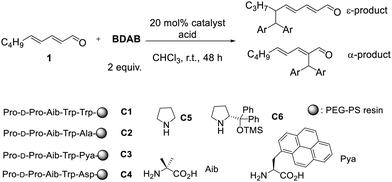
According to our previous work, we initially attempted the alkylation of linear dienal 1 with BDAB using the peptide catalyst C1, H-Pro-D-Pro-Aib-Trp-Trp-PEG-PS resin,20 in the presence of an acid co-catalyst.9a,18 Although the yield was low, ε-product was predominantly obtained (Table 1, entry 1).21 The minor component was the α-product with an MBH-type structure, which is consistent with a previous report by Appayee's group.18
| Entry | Catalyst | Acid (equiv.) | Yielda (%) | ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αa αa |
|---|---|---|---|---|
| a Determined by 1H NMR spectra of the crude mixture. | ||||
| 1 | C1 | AcOH (0.2) | 18 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 2 | C1 | AcOH (1.0) | 30 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 3 | C1 | TFA (1.0) | 21 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
| 4 | C1 | PhCO2H (1.0) | 59 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 5 | C2 | PhCO2H (1.0) | 22 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 64 |
| 6 | C3 | PhCO2H (1.0) | 27 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
| 7 | C4 | — | 29 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 8 | C5 | PhCO2H (1.0) | 42 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 9 | C6 | PhCO2H (1.0) | 24 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
Encouraged by this result, we carried out catalyst screening. As for the co-catalyst, an excess of weak acid was required for optimal selectivity (Table 1, entries 1–4). The fifth amino acid residue from the N-terminus of the peptide was scanned because, according to our previous results,20 the residue is likely to be positioned near the ε-position of the trienamine intermediate. Substituting Trp with either a small (Ala) or bulky (Pya) residue reduced ε-selectivity (entries 5 and 6). Notably, the acid co-catalyst remained effective when it was incorporated within the peptide chain (entry 7), showing only a slightly lower ε-selectivity. Catalysts C5 and C6 only gave α-products (entries 8 and 9). The low yields are primarily attributed to self-condensation of substrate 1, leading to multiple side products. In all cases, no γ-substituted product was detected.
From the results of catalyst screening, it is suggested that the fifth Trp residue plays an important role for the ε-selectivity. As the alkylation with BDAB is known to proceed through the intermediacy of benzylic cation species,9b attractive interaction of the cation and the electron rich indole ring on the Trp residue could be the driving force.22 DFT calculations, using 3-methylindole (a model for the catalyst substructure) and the BDAB-derived cation, show a significant stabilization energy of 32 kJ mol−1 upon complex formation. This stabilization is accompanied by a close contact (3.42 Å) between the aromatic rings of the two molecules (Fig. S1, ESI†). This strong intermolecular interaction may facilitate the delivery of the cation to the ε-position.23
As for the enantioselectivity, despite extensive screening of the peptide catalysts and the reaction conditions, the enantiomeric excess (ee) value remained low at the maximum of 13%. A previous NMR study indicated that the trienamine made of diphenylprolinol trimethylsilyl ether and nona-2,4-dienal adopts a 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of (1E,3E,5E) and (1E,3E,5Z) isomers.24 The result of DFT calculations on a model trienamine, 1-(hepta-1,3,5-trien-1-yl)-N-methyl prolinamide were in accordance with the NMR observation (Table S2, ESI†). This configurational heterogeneity may be responsible for the low enantioselectivity.
1 mixture of (1E,3E,5E) and (1E,3E,5Z) isomers.24 The result of DFT calculations on a model trienamine, 1-(hepta-1,3,5-trien-1-yl)-N-methyl prolinamide were in accordance with the NMR observation (Table S2, ESI†). This configurational heterogeneity may be responsible for the low enantioselectivity.
Related to this, an NMR study on the configuration of dienamines has been reported, where a similar configurational isomerism was observed.25 In this context, Melchiorre's research group demonstrated that the introduction of α-branching to the enal induced the preference for the (1E,3E) isomer of the dienamine.9b Accordingly, we presumed that the γ-branching structure could similarly impact trienamine configurations, potentially enhancing enantioselectivity.
To investigate this hypothesis, we selected dienal 2a as a nucleophile precursor. To our delight, the regioselectivity remained largely unchanged, while the ee value increased to 72% under the same reaction conditions (Table 2, entry 1). The major enantiomer of the ε-product was determined to have (R) configuration (Scheme S2, ESI†). Screening on peptide sequences revealed that the highest selectivity was achieved when Trp or its derivatives occupied the fifth position (Table S3, ESI†). Moreover, regioselectivity correlated positively with the electron density of the indole ring on the Trp derivatives. Conducting the reaction in toluene significantly improved outcomes (76% yield for 2 days at room temperature), affording the ε-product with an ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α ratio of 97
α ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 89% ee (entry 6). Interestingly, the α-product was obtained as 3,5-dienals.18
3 and 89% ee (entry 6). Interestingly, the α-product was obtained as 3,5-dienals.18
Subsequently, we applied the peptide catalyst to the ε-alkylation of several γ-branched 2,4-dienals. As shown in Table 3, the ε-products were obtained with high enantio- and regioselectivity. Furthermore, we also investigated the feasibility of extending regioselective ε-alkylation to 3-(1-cyclohexen-1-yl)-2-propenal 2f (Table 3, entry 6).
DFT calculations on 1-(4-methylhepta-1,3,5-trien-1-yl)-N-methyl prolinamide, which is a model compound of the trienamine from 2a, revealed that the energy difference of (1E,3E,5E) and (1E,3E,5Z) isomers was 19.3 kJ mol−1 and this value is significantly larger than that of the non-γ-methylated counterpart (4.4 kJ mol−1, see Table S2, ESI†). This configurational preference for the trienamine, along with the conformational advantage for the (s-trans, s-trans, s-trans) structure, might serve as the enhancement of enantioselectivity.
It is interesting to mention that the present peptide catalyzed ε-alkylation of the trienamines showed an opposite sense for the enantiofacial selectivity compared to the γ-alkylation of dienamines brought by diphenylprolinol silyl ether, even though both catalysts are derived from L-proline.9b This might be attributed to the difference in the interaction of the electrophile and the catalyst; in the reaction of dienamine, the stereochemical course was controlled by the steric repulsion with the substituent on the catalyst, whereas the peptide chain behaved as a carrier of the electrophile based on the strong interaction between the Trp and the cation (Fig. 2).11
In this study, we have successfully developed the first catalytic asymmetric ε-alkylation of 2,4-dienals. This novel transformation provides functionalized compounds amenable to further modifications at α to δ positions, including intriguing cascade sequences. Our research highlights the unique capability of peptide catalysis in imparting distinctive reactivity profiles to challenging compound classes, exemplified by γ-branched 2,4-dienals via the trienamine intermediate.
This work was partially supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (15K05495 for K. K.). We thank Dr. Akihiro Sakama for proofreading the draft manuscript and Mr. Kohsaku Okuyama for his experimental assistance. MS spectral data were obtained at the Komaba Analysis Core, Institute of Industrial Science, The University of Tokyo. Computational resources were provided by the Research Center for Computational Science, Okazaki, Japan (Project: 24-IMS-C186).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Curti, L. Battistini, A. Sartori and F. Zanardi, Chem. Rev., 2020, 120, 2448 CrossRef CAS PubMed.
- (a) S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef CAS PubMed; (b) I. D. Jurberg, I. Chatterjee, R. Tannert and P. Melchiorre, Chem. Commun., 2013, 49, 4869 RSC.
- (a) D. B. Ramachary and Y. V. Reddy, Eur. J. Org. Chem., 2012, 865 CrossRef CAS; (b) V. Marcos and J. Alemán, Chem. Soc. Rev., 2016, 45, 6812 RSC.
- S. Bertelsen, M. Marigo, S. Brandes, P. Dinér and K. A. Jørgensen, J. Am. Chem. Soc., 2006, 128, 12973 CrossRef CAS PubMed.
- Z. H. Wang, Y. You, J. Q. Zhao, Y. P. Zhang, J. Q. Yin and W. C. Yuan, Org. Chem. Front., 2023, 10, 3130 RSC.
- (a) J. Vesely, P. Dziedzic and A. Córdova, Tetrahedron Lett., 2007, 48, 6900 CrossRef CAS; (b) N. Utsumi, H. Zhang, F. Tanaka and C. F. Barbas, Angew. Chem., Int. Ed., 2007, 46, 1878 CrossRef CAS PubMed; (c) J. Vesely, R. Rios and A. Córdova, Tetrahedron Lett., 2008, 49, 1137 CrossRef CAS; (d) B. C. Hong, M. F. Wu, H. C. Tseng and J. H. Liao, Org. Lett., 2008, 10, 3651 CrossRef CAS; (e) S. Číhalová, M. Remeš, I. Císařová and J. Veselý, Eur. J. Org. Chem., 2009, 6277 CrossRef; (f) S. Číhalová, P. Dziedzic, A. Cõrdova and J. Veselý, Adv. Synth. Catal., 2011, 353, 1096 CrossRef; (g) E. Marqués-López, R. P. Herrera, T. Marks, W. C. Jacobs and M. Christmann, Synthsis, 2013, 45, 1016 CrossRef.
- (a) B. Han, Y. C. Xiao, Z. Q. He and Y. C. Chen, Org. Lett., 2009, 11, 4660 CrossRef CAS PubMed; (b) J. Stiller, E. Marqués-López, R. P. Herrera, R. Fröhlich, C. Strohmann and M. Christmann, Org. Lett., 2011, 13, 70 CrossRef CAS PubMed.
- (a) M. S. Kutwal and C. Appayee, Eur. J. Org. Chem., 2017, 4230 CrossRef CAS; (b) Y. S. Huang, S. G. Song, L. Ren, Y. G. Li and X. Wu, Eur. J. Org. Chem., 2019, 6838 CrossRef CAS; (c) M. Vargiu, L. Favero, A. Menichetti, V. Di Bussolo, F. Marchetti, G. Pescitelli, S. Di Pietro and M. Pineschi, Chirality, 2019, 31, 522 CrossRef CAS PubMed; (d) M. Balletti, T. Wachsmuth, A. Di Sabato, W. C. Hartley and P. Melchiorre, Chem. Sci., 2023, 14, 4923 RSC.
- (a) G. Bergonzini, S. Vera and P. Melchiorre, Angew. Chem., Int. Ed., 2010, 49, 9685 CrossRef CAS PubMed; (b) M. Silvi, C. Cassani, A. Moran and P. Melchiorre, Helv. Chim. Acta, 2012, 95, 1985 CrossRef CAS.
- C. Cassani and P. Melchiorre, Org. Lett., 2012, 14, 5590 CrossRef CAS PubMed.
- E. Reyes, L. Prieto, U. Uria, L. Carrillo and J. L. Vicario, Catalysts, 2023, 13, 1091 CrossRef CAS.
- (a) H. B. Hepburn, L. Dell’Amico and P. Melchiorre, Chem. Rec., 2016, 1787 CrossRef CAS PubMed; (b) L. Klier, F. Tur, P. H. Poulsen and K. A. Jørgensen, Chem. Soc. Rev., 2017, 46, 1080 RSC.
- Z. J. Jia, H. Jiang, J. L. Li, B. Gschwend, Q. Z. Li, X. Yin, J. Grouleff, Y. C. Chen and K. A. Jørgensen, J. Am. Chem. Soc., 2011, 133, 5053 CrossRef CAS PubMed.
- (a) I. Kumar, P. Ramaraju and N. A. Mir, Org. Biomol. Chem., 2013, 11, 709 RSC; (b) T. J. Pawar, S. B. Mitkari, E. Peña-Cabrera, C. Villegas Gómez and D. Cruz Cruz, Eur. J. Org. Chem., 2020, 6044 CrossRef CAS.
- A. Skrzyńska, A. Przydacz and Ĺ. Albrecht, Org. Lett., 2015, 17, 5682 CrossRef PubMed.
- (a) M. Sayed, H. C. Shen, L. Zhang and L. Z. Gong, Synthsis, 2020, 52, 703 CrossRef CAS; (b) H. Akutsu, M. Ito, M. Kawada, K. Nakashima, S. Ichi Hirashima and T. Miura, Tetrahedron Lett., 2020, 61, 151478 CrossRef CAS; (c) C. J. Xu, H. W. Li, X. L. He, W. Du and Y. C. Chen, Asian J. Org. Chem., 2019, 8, 1037 CrossRef CAS; (d) G. J. Yang, W. Du and Y. C. Chen, J. Org. Chem., 2016, 81, 10056 CrossRef CAS PubMed; (e) Y. L. Su, Z. Y. Han, Y. H. Li and L. Z. Gong, ACS Catal., 2017, 7, 7917 CrossRef CAS; (f) M.-H. Xu, Y.-H. Yuan, D.-D. Liang, X.-M. Zhang, F.-M. Zhang, Y.-Q. Tu, A.-J. Ma, K. Zhang and J.-B. Peng, Org. Chem. Front., 2021, 8, 3292 RSC; (g) M. Dyguda, A. Przydacz, A. Krzemińska and Ł. Albrecht, Org. Biomol. Chem., 2019, 17, 6025 RSC.
- M. Silvi, E. Arceo, I. D. Jurberg, C. Cassani and P. Melchiorre, J. Am. Chem. Soc., 2015, 137, 6120 CrossRef CAS PubMed.
- V. M. D. Padmaja, S. Jangra and C. Appayee, Org. Biomol. Chem., 2019, 17, 1714 RSC.
- K. Akagawa, J. Sen and K. Kudo, Angew. Chem., Int. Ed., 2013, 52, 11585 CrossRef CAS PubMed.
- K. Akagawa and K. Kudo, Acc. Chem. Res., 2017, 50, 2429 CrossRef CAS PubMed.
- Regioselective, non-asymmetric C–C bond formation at the ε-position of linear 2,4-dienals has been reported by two groups; (a) S. Saito, M. Shiozawa, M. Ito and H. Yamamoto, J. Am. Chem. Soc., 1998, 120, 813 CrossRef CAS; (b) I. Franzoni, L. Guénée and C. Mazet, Chem. Sci., 2013, 4, 2619 RSC.
- (a) D. A. Dougherty, Science, 1996, 271, 163 CrossRef CAS PubMed; (b) D. A. Dougherty, Acc. Chem. Res., 2013, 46, 885 CrossRef CAS PubMed; (c) L. R. Rutledge, H. F. Durst and S. D. Wetmore, Phys. Chem. Chem. Phys., 2008, 10, 2801 RSC.
- S. Yamada, Chem. Rev., 2018, 118, 11353 CrossRef CAS PubMed.
- B. Łągiewka and Ł. Albrecht, Asian J. Org. Chem., 2017, 6, 516 CrossRef.
- A. Seegerer, J. Hioe, M. M. Hammer, F. Morana, P. J. W. Fuchs and R. M. Gschwind, J. Am. Chem. Soc., 2016, 138, 9864 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc00805k |
| This journal is © The Royal Society of Chemistry 2025 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:

