 Open Access Article
Open Access ArticleRecent advances in the synthesis and transformations of sulfinate esters
Suguru
Yoshida
 *
*
Department of Biological Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan. E-mail: s-yoshida@rs.tus.ac.jp
First published on 12th March 2025
Abstract
Sulfinate esters (sulfinic acid esters) play a crucial role in various research fields including synthetic organic chemistry and pharmaceutical sciences. In this feature article, I review recent advances in the study of sulfinate esters within the realm of synthetic organic chemistry. First, recent methods for the efficient synthesis of diverse sulfinate esters from readily available starting materials such as thiols, aryl iodides, and sulfinic acids are discussed. Second, I introduce various modern transformations of sulfinate esters, categorized according to their reaction mechanisms.
1. Introduction
Sulfinate esters (sulfinic acid esters) play a crucial role in various research fields including synthetic organic chemistry and pharmaceutical sciences.1 Sulfinate esters are generally shown as two types of resonance structures having polarized S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or S+–O− moiety (Fig. 1A). The good stability of the chiral sp3 sulfur center in sulfinate esters has enabled the efficient asymmetric synthesis of chiral compounds. While RCO2R′ only means esters, RSO2R′ shows sulfones or sulfinate esters (Fig. 1B). Compared to the divergent studies on sulfones, sulfinate ester chemistry is still immature regarding access and utility as synthetic intermediates. Herein, I summarize recent emerging studies on sulfinate esters for significant advances in synthetic organic chemistry.
O or S+–O− moiety (Fig. 1A). The good stability of the chiral sp3 sulfur center in sulfinate esters has enabled the efficient asymmetric synthesis of chiral compounds. While RCO2R′ only means esters, RSO2R′ shows sulfones or sulfinate esters (Fig. 1B). Compared to the divergent studies on sulfones, sulfinate ester chemistry is still immature regarding access and utility as synthetic intermediates. Herein, I summarize recent emerging studies on sulfinate esters for significant advances in synthetic organic chemistry.
 | ||
| Fig. 1 (A) Resonance structures of sulfinate esters. (B) Carboxylic acids, sulfones, and sulfinate esters. | ||
Various sulfinate esters were prepared from sulfinyl chlorides and alcohols under basic conditions similar to the ester synthesis (Fig. 2A).2 Sulfinic acid menthyl esters have played as significant chiral building blocks, which were prepared from sodium sulfinates and (−)-menthol in a diastereoselective manner via the formation of sulfinyl chlorides (Fig. 2B).3 Contrasting to the well-studied ester synthesis from carboxylic acids under acidic conditions, it is not easy to synthesize sulfinate esters from sulfinic acids under acidic conditions due to the disproportionation affording thiosulfonates and sulfonic acids (Fig. 2C, upper).4 Sulfinate ester synthesis was achieved from sulfinic acids and alcohols using condensation reagents including dicyclohexylcarbodiimide (DCC) (Fig. 2C, lower).5 In addition, a wide range of sulfinate esters were synthesized from disulfides by the oxidation with N-bromosuccinimide (NBS) in alcohols (Fig. 2D).6 Although a broad variety of sulfinate esters were synthesized by the classical methods, it is not easy to prepare highly functionalized sulfinate esters from readily available starting materials under mild conditions.
 | ||
| Fig. 2 (A) Synthesis of sulfinate esters from sulfinyl chlorides. (B) Synthesis of sulfinic acid menthyl esters. (C) Condensation of sulfinic acids. (D) Synthesis of sulfinate esters from disulfides. | ||
Diverse transformations of sulfinate esters involving S–O cleavage served in synthesizing sulfoxides and sulfones (Fig. 3). For example, nucleophilic substitutions of sulfinate esters proceed smoothly to furnish organosulfur(IV) compounds (Fig. 3A). In particular, a wide variety of asymmetric syntheses of chiral sulfoxides were enabled by the Andersen method from sulfinic acid menthyl esters with organomagnesium reagents (Fig. 3B).7,8 Isomerization of sulfinate esters easily took place by heating to provide the corresponding sulfones (Fig. 3C).9 A wide range of synthetic methods and transformations of sulfinate esters have been developed recently based on the history described above. A part of recent studies on electrochemical preparation of sulfinate esters from thiols and sulfide synthesis from sulfinate esters were summarized in 20211b and 2023,1c respectively. This feature article shows the modern synthesis of sulfinate esters and a broad range of their transformations categorized by the reaction mechanism.
 | ||
| Fig. 3 (A) Nucleophilic substitution reactions of sulfinate esters. (B) Andersen method. (C) Isomerization of sulfinate esters. | ||
2. Recent advances of sulfinate ester synthesis
2.1. Sulfinate ester synthesis from organosulfur(II) compounds
Oxidative methods were reported for synthesizing sulfinate esters from thiols and alcohols (Fig. 4 and 5). In 2016, Jang and coworkers accomplished the synthesis of sulfinate esters by treating thiols with alcohols in the presence of a catalytic amount of copper iodide and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) at 65 °C under oxygen atmosphere (Fig. 4A).10a The reaction was prohibited by the addition of 2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPO), where the corresponding disulfide was obtained.10a Based on the results obtained from the control experiments and electron paramagnetic resonance (EPR) spectra of the reaction mixture, the authors proposed a reaction pathway involving sulfinyl radicals. In 2024, Taniguchi reported that the copper-catalyzed sulfinate ester synthesis was accomplished under air using CuI and 1,10-phenanthridinone (1,10-phen) monohydrate (Fig. 4B).10b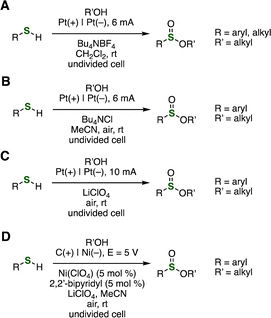 | ||
| Fig. 5 Electrosynthesis of sulfinate esters by (A) Zhong and Ling's group, (B) Wei, Xu, and Zhang's group, (C) X.-C. Wang, Wu, G. Wang, B. Ma, and J. Yang's group, and (D) Kaboudin's group. | ||
A new cobalt nanocatalyst supported on N–SiO2-doped activated carbon (Co/N–SiO2-Ac) was developed by Zhang and coworkers in 2018 (Fig. 4C).11 Various sulfinate esters were synthesized from thiols and alcohols catalyzed by the newly developed reusable catalyst under an oxygen atmosphere. Also, Luu and coworkers reported in 2021 that ultrasound-promoted oxidation of thiols in alcohol with N-bromosuccinimide (NBS) proceeded smoothly to afford a wide variety of sulfinate esters (Fig. 4D).12
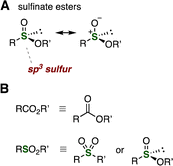
A number of methods were recently developed for electrochemical synthesis of sulfinate esters from thiols and alcohols (Fig. 5).13 For example, in 2019, Zhong, Ling, and coworkers reported that the treatment of a dichloromethane solution of thiols and alcohols with tetrabutylammonium tetrafluoroborate as the electrolyte at room temperature in an undivided cell with platinum plates as electrodes under 6 mA constant current provided the corresponding sulfinate esters (Fig. 5A).13a Various thiols and alcohols participated in the efficient electrosynthesis of sulfinate esters. In 2020, a similar electrosynthetic method of sulfinate esters was reported by Wei, Xu, Zhang, et al. (Fig. 5B)13b and X.-C. Wang, Wu, G. Wang, B. Ma, J. Yang, et al. (Fig. 5C).13c Also, Kaboudin and coworkers found that sulfinate esters were synthesized from thiols and alcohols catalyzed by nickel at room temperature in an undivided cell with nickel foam as a cathode and modified graphite as an anode with lithium perchlorate as an electrolyte at 5.0 V cell potential (Fig. 5D).13d
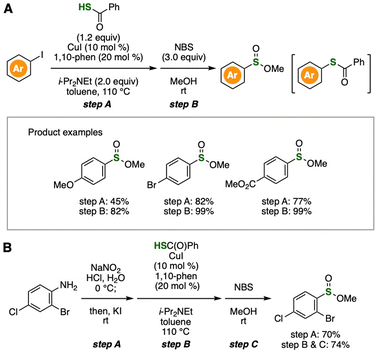
With the inconvenience of thiols as starting materials in terms of their instability under air and unpleasant smell, we recently developed an efficient method to synthesize sulfinate esters from aryl iodides (Fig. 6).14 A wide variety of sulfinate esters were successfully prepared from aryl iodides by copper-catalyzed thiolation with thiobenzoic acid and subsequent oxidation with NBS in alcohols (Fig. 6A). Since the purification of thioester intermediates could be omitted, this 2-step method allowed us to access a wide range of sulfinate esters. Furthermore, we also achieved the synthesis of methyl 2-bromo-4-chlorobenzenesulfinate from the corresponding 2-bromo-4-chloroaniline by diazotization–iodination, copper-catalyzed thiolation, and oxidation to form sulfinate esters (Fig. 6B).
 | ||
| Fig. 6 (A) Sulfinate ester synthesis from aryl iodides. (B) Sulfinate ester synthesis from 2-bromo-3-chloroaniline. 1,10-Phen = 1,10-phenanthroline. | ||
2.2. Sulfinate ester synthesis from organosulfur(IV) compounds
Sulfinate esters were also synthesized from sulfoxides via S–C bond cleavage (Fig. 7). Photoinduced thia-Baeyer–Villiger oxidation was reported by Ma and coworkers in 2020 (Fig. 7A).15 Treatment of dibenzothiophene oxide with tert-butyl hydroperoxide in the presence of a catalytic amount of porphyrin catalyst [TCPPFe]Cl under irradiation conditions afforded sulfinate ester 1 through S–C cleavage. In 2015, Sun et al. reported that tert-butyl sulfoxides reacted with various nucleophiles involving alcohols in the presence of NBS as an oxidant via sulfinyl bromide intermediates (Fig. 7B).16 Instead of alcohols, amines and carbon nucleophiles such as Grignard reagents and indoles also reacted with the sulfinyl bromide intermediates.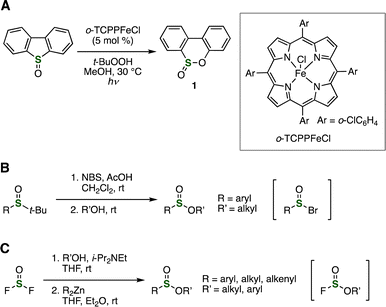 | ||
| Fig. 7 (A) Photoinduced thia-Baeyer–Villiger oxidation. (B) Synthesis of sulfinate esters from tert-butyl sulfoxides. (C) Sulfinate ester synthesis from thionyl fluoride. | ||
Sulfinate esters were synthesized from thionyl fluoride by Sammis and coworkers in 2024 (Fig. 7C).17 Treatment of alcohols with thionyl fluoride facilitated by N-ethyldiisopropylamine resulted in monoalcoholysis. Then, alkylation or arylation of the resulting sulfinyl fluorides took place with dialkyl- or diarylzinc reagents to afford a variety of sulfinate esters.
A number of methods for sulfinate ester synthesis were reported from sulfinic acids and alcohols (Fig. 8). For example, in 2008, Kiełbasiński, Drabowicz, et al. succeeded in the preparation of sulfinate esters catalyzed by ytterbium triflate through sulfinylation of alcohols (Fig. 8A).18 Metal-free sulfinate ester synthesis was developed by Hitchcock et al. in 2021 via pretreatment of sulfinic acids with 1,1′-carbonyldiimidazole (CDI) followed by the addition of alcohols (Fig. 8B).19 In 2021, Wen and coworkers reported isocyanide-induced esterification of sulfinic acids through sulfoxide-type intermediates (Fig. 8C).20
 | ||
| Fig. 8 Sulfinate ester synthesis from sulfinic acids using (A) Yb(OTf)3, (B) CDI, or (C) an isocyanide. | ||
Various methods have been developed this decade to synthesize sulfinate esters from sulfinate anions (Fig. 9). For example, Wu, Li, and coworkers found that trimethylsilyl chloride promoted the formation of sulfinate esters from alcohols and sodium sulfinates through silyl sulfinate intermediates (Fig. 9A).21 Difluoromethanesulfinate ester synthesis was reported by Shibata et al. in 2021 from sodium difluoromethanesulfinate and alcohols with Ph2P(O)Cl as an activator via a phosphinic acid ester intermediate (Fig. 9B).22 A wide variety of enantioselective methods were developed from sulfinate anions and alcohols with organocatalysts and condensation reagents (Fig. 9C–E).23 In 2022, Tan, Zhang, and coworkers achieved an asymmetric synthesis of chiral sulfinate esters from potassium sulfinates and alcohols with ethyl chloroformate in the presence of a catalytic amount of pentadinium (PN) through SN2 reaction of a mixed acid anhydride intermediate (Fig. 9C).23a Enantioselective synthesis of sulfinate esters was reported by Guo, Xie, Tian, et al. in 2024 using pyridine-oxide-type organocatalyst C1 (Fig. 9D).23b In 2024, Chi, Wu's group succeeded in the enantioselective sulfinylation of alcohols with sodium sulfinates catalyzed by quinine (Fig. 9E).23c
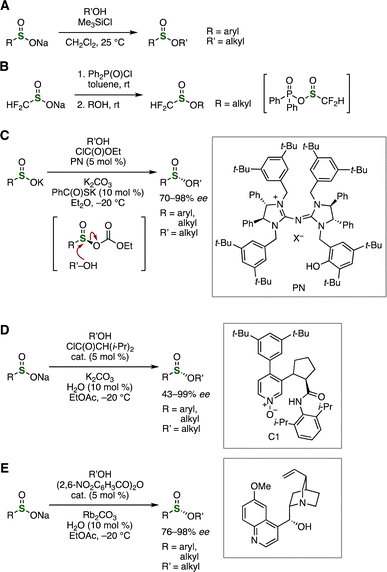 | ||
| Fig. 9 Sulfinate ester synthesis from sulfinate anions using (A) trimethylsilyl chloride, (B) Ph2P(O)Cl, (C) PN, (D) C1, or (E) quinine. | ||
2.3. Sulfinate ester synthesis from organosulfur(VI) compounds
A variety of transformations for sulfinyl esters from sulfonylmethyl isocyanides and alcohols were developed (Fig. 10). In 2015, Wu and coworkers reported that bismuth(III) bromide catalyzed selective formation of sulfinyl esters in the presence of acetic acid through iminoester intermediates, where the corresponding sulfones were not observed (Fig. 10A).24a These reaction conditions were improved for the efficient sulfinylation of alcohols with sulfonylmethyl isocyanides in the presence of α,α,α,-trichloroacetic acid and a catalytic amount of bismuth(III) triflate (Fig. 10B).24b Sulfinylation was also achieved by Prapurna in 2017 using BF3·OEt2 at room temperature, in which sulfones were synthesized at 80 °C (Fig. 10C).24c In 2016, Prapurna et al. found a metal-free protocol for the sulfinyl ester synthesis from sulfonyl isocyanides and alcohols with triphenylphosphine and diisopropyl azodicarboxylate (DIAD) by the Mitsunobu-type mechanism (Fig. 10D).25 | ||
| Fig. 10 Sulfinate ester synthesis from isocyanides using (A) BiBr3, (B) Bi(OTf)3, (C) BF3·OEt2, or (D) PPh3 and DIAD. | ||
Sulfinate esters were also synthesized from sulfonyl hydrazides and alcohols under oxidation conditions (Fig. 11). For example, a copper-catalyzed method for the preparation of sulfinate esters was found by Pan, Han, and coworkers in 2016 (Fig. 11A).26a Treatment of sulfonyl hydrazides with alcohols in the presence of copper(II) triflate under an oxygen atmosphere provided sulfinate esters in good yields. The authors proposed a reaction mechanism involving diazenes and the corresponding sulfinic acids as intermediates. In 2020, Srivastava et al. reported a palladium-catalyzed sulfinyl ester synthesis from quinoline-substituted sulfonyl hydrazides and alcohols in the presence of silver acetate (Fig. 11B).26b Transition-metal-free conditions were found by Ding and coworkers in 2021, in which sulfinate ester synthesis was accomplished from sulfonyl hydrazides in alcohols mediated by NaHSO3 as a reductant under air (Fig. 11C).26c In 2024, Lee et al. found that sulfinate esters were synthesized from sulfonyl hydrazides and ortho esters in an electrochemical manner (Fig. 11D).27
 | ||
| Fig. 11 Sulfinate ester synthesis from N-sulfonylhydrazides with (A) Cu(OTf)2, (B) Pd(OAc)2, (C) NaHSO3 under air, or (D) an electrochemical method. | ||
Base-promoted synthesis of sulfinate esters was developed from sulfonyl hydrazones (Fig. 12A and B). For instance, Wu, Li, et al. reported in 2020 that sulfinate esters were prepared from sulfonyl hydrazones by treatment with N-ethyldiisopropylamine in nitromethane at 90 °C via isomerization and rearrangement with denitrogenation (Fig. 12A).28a Also, K2CO3-catalyzed synthesis of sulfinate esters from sulfonyl hydrazones was reported by Basak et al. in 2020 (Fig. 12B).28b In 2022, Ding, Wu, and coworkers succeeded in the preparation of sulfinyl esters from sulfonyl chlorides and alcohols with rongalite (HOCH2SO2Na) as a reductant via thiosulfonate intermediates (Fig. 12C).29
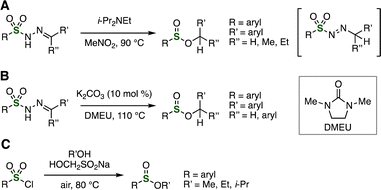 | ||
| Fig. 12 Sulfinate ester synthesis from sulfonyl hydrazones using (A) i-Pr2NEt and (B) K2CO3. (C) Sulfinate ester synthesis from sulfonyl chlorides. DMEU = 1,3-dimethyl-2-imidazolidinone. | ||
3. Recent transformations of sulfinate esters
3.1. Sulfinylation using sulfinate esters
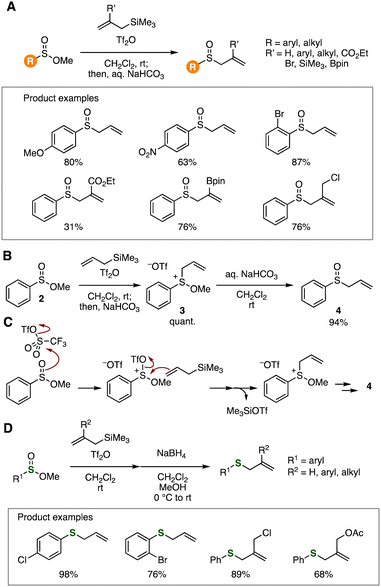
Electrophilic activation of sulfinate esters allowed the sulfinylation of various nucleophiles. In 2011, Ruano, Yuste, and coworkers reported that methyl sulfinates served in the sulfinylation of arenes promoted by aluminum trichloride (Fig. 13).30a For instance, treatment of methyl benzenesulfinate and anisole with aluminum trichloride in 1,2-dichloroethane at 25 °C afforded 4-anisyl phenyl sulfoxide in moderate yield (Fig. 13A). Also, various sulfoxides were synthesized by the Friedel–Crafts-type sulfinylation with sulfinate esters via cationic intermediates. Friedel–Crafts-type sulfinylation of aromatic compounds with recyclable ionic liquid [Bmim]Cl·2AlCl3 (Bmim = 1-butyl-3-methylimidazolium) under ultrasound irradiation was found by Luu et al. in 2017 (Fig. 13B).30bRecently, we have developed a variety of sulfinylation reactions using sulfinate esters (Fig. 14).31–34 In 2020, S-allylation of sulfinate esters was achieved with allylsilanes under Pummerer-type conditions (Fig. 14A).31a For example, allyl 4-anisyl sulfoxide was efficiently synthesized by treating methyl 4-methoxybenzenesulfinate and allyltrimethylsilane with trifluoromethanesulfonic anhydride (Tf2O) in dichloromethane followed by the addition of aqueous sodium bicarbonate. We accomplished the preparation of a wide range of sulfoxides leaving various functional groups untouched. It is worth noting that the sulfoxide moiety remained unreacted despite diverse reports on the transformations of sulfoxides via S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O cleavage to yield sulfides under Pummerer-type conditions.32 We succeeded in the isolation of sulfonium or sulfurane intermediate 3 and subsequent hydrolysis to provide sulfoxides 4 (Fig. 14B). This result supports a plausible reaction mechanism involving electrophilic activation of sulfinate esters with Tf2O, S-allylation with allylsilanes, and following hydrolysis of the resulting sulfonium intermediates (Fig. 14C). We also realized the synthesis of sulfides by the S-allylation–reduction protocol (Fig. 14D).31b The good functional group tolerance in the C–S formation under mild Pummerer-type conditions allowed us to prepare various functionalized sulfoxides and sulfides from various sulfinate esters prepared from the corresponding aryl iodides.
O cleavage to yield sulfides under Pummerer-type conditions.32 We succeeded in the isolation of sulfonium or sulfurane intermediate 3 and subsequent hydrolysis to provide sulfoxides 4 (Fig. 14B). This result supports a plausible reaction mechanism involving electrophilic activation of sulfinate esters with Tf2O, S-allylation with allylsilanes, and following hydrolysis of the resulting sulfonium intermediates (Fig. 14C). We also realized the synthesis of sulfides by the S-allylation–reduction protocol (Fig. 14D).31b The good functional group tolerance in the C–S formation under mild Pummerer-type conditions allowed us to prepare various functionalized sulfoxides and sulfides from various sulfinate esters prepared from the corresponding aryl iodides.
Various dibenzothiophene S-oxides were synthesized by bromide-selective cross-coupling and subsequent intramolecular S-arylation (Fig. 15).31c First, a broad range of sulfinate esters were prepared by the palladium-catalyzed Suzuki–Miyaura cross-coupling with arylboronic acids without damaging sulfinate ester moieties (Fig. 15A). Then, efficient cyclization of the resulting biaryl-substituted sulfinate esters took place with Tf2O followed by the hydrolysis with aqueous sodium bicarbonate to afford various dibenzothiophene S-oxides. We succeeded in the isolation of the intermediate and subsequent hydrolysis (Fig. 15B).
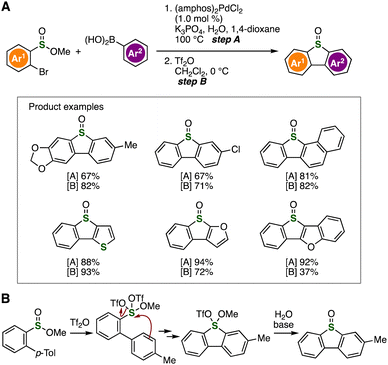 | ||
| Fig. 15 (A) Synthesis of dibenzothiophene S-oxides. (B) Plausible reaction mechanism. amphos = (4-dimethylaminophenyl)di-tert-butylphosphine. | ||
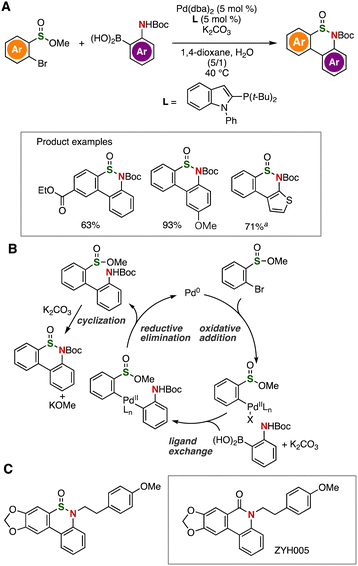
Bromide-selective arylation and following cyclization allowed us to synthesize thiaphenanthridinones (Fig. 16).33 Indeed, various thiaphenanthridinones were prepared from o-bromo-substituted aromatic sulfinate esters and o-borylanilines catalyzed by palladium, in which cyclization also took place under the basic conditions (Fig. 16A). Control experiments support that the thiaphenanthridinone synthesis involved palladium-catalyzed cross-coupling and subsequent intramolecular N-sulfinylation with potassium carbonate (Fig. 16B). We achieved the preparation of thia-analog of bioactive phenanthridinone ZYH005 (Fig. 16C).
We found that sulfinate esters served in the cross-coupling-type S-arylation of arylboronic acids (Fig. 17).34 Treatment of arylboronic acids with sulfinate esters in the presence of potassium carbonate and a catalytic amount of palladium catalyst Xphos Pd G4 provided various sulfoxides (Fig. 17A). At this stage, we proposed two possible pathways to form sulfoxides involving oxidative addition, transmetalation, and reductive elimination, or transmetalation and σ-bond metathesis (Fig. 17B and C).
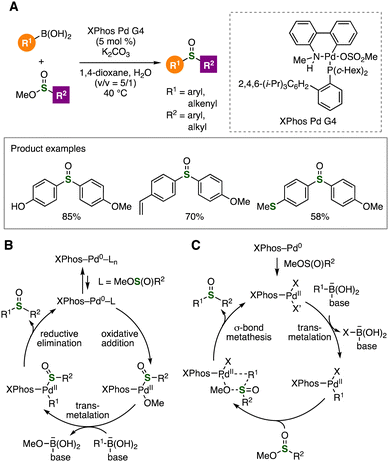 | ||
| Fig. 17 (A) Palladium-catalyzed synthesis of sulfoxides. Plausible reaction mechanisms via (B) oxidative addition of sulfinate esters or (C) σ-bond methasesis. | ||
In 2019, Chen and coworkers reported Ni/NHC-catalyzed synthesis of sulfinamides from sulfinate esters and amines (Fig. 18A).35a A wide variety of sulfinamides were prepared by treating sulfinate esters with amines in the presence of a catalytic amount of nickel dichloride and 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride at 100 °C. In the manuscript on transamidation of sulfinamides with amines reported by Kano et al. in 2023, sulfinamide synthesis was achieved from benzyl 4-toluenesulfinate and piperizine at 110 °C (Fig. 18B).35b
 | ||
| Fig. 18 (A) Nickel-catalyzed sulfinamide synthesis. (B) Synthesis of a sulfinamide from benzyl sulfinate and piperidine. | ||
3.2. Transformations of sulfinate esters through C–S formation via S–C or C–O cleavage
Radical cyclization for the synthesis of cyclic sulfinate esters was reported by Shu and coworkers in 2024 (Fig. 19A).36 Treatment of butenyl trifluoromethanesulfinates having an exo-methylene moiety with a catalytic amount of benzoic peroxide in acetonitrile at 90 °C afforded cyclic sulfinate esters in high yield via S–C cleavage. The authors proposed a radical mechanism through the generation of trifluoromethyl radical from the reaction of butenyl sulfinate esters and phenyl radical. | ||
| Fig. 19 (A) Synthesis of cyclic sulfinate esters via radical cyclization. (B) Synthesis of sulfones via 1,4-sulfinate migration. (C) Synthesis of sulfonylmethyl isocyanides. | ||
Cyclization from sulfinate esters with an enyne moiety was also reported by Li, Xu, and coworkers in 2024 (Fig. 19B).37 The 1,4-sulfinate migration was triggered by furan ring formation catalyzed by copper to afford sulfones via a copper carbene intermediate.
Synthesis of sulfonylmethyl isocyanides under microwave irradiation was achieved by Fleming, Lujan-Montelongo, et al. in 2015 (Fig. 19C).38 Formation of sulfones by treating sulfinate esters with formic acid and paraformaldehyde and following dehydration with phosphoryl chloride enabled the efficient preparation of sulfonylmethyl isocyanides.
3.3. Transformations of sulfinate esters for the synthesis of sulfides
Recently, various methods to synthesize aromatic sulfides have been developed using sulfinate esters and nucleophilic (hetero)arenes (Fig. 20 and 21). For example, Gu, Liu, and coworkers reported sulfenylation of electron-rich aromatic compounds such as 2-aminonaphthalenes and indoles facilitated with tetrabutylammonium iodide (TBAI) (Fig. 20A).39 The authors found that this sulfenylation involved the formation of thiosulfonates from sulfinate esters with TBAI (Fig. 20B). Iodine-catalyzed sulfenylation of isoquinoline-1(2H)-ones with sulfinate esters was disclosed by Tang, Yang, and coworkers in 2021 (Fig. 20C).40a Treatment of isoquinoline-1(2H)-ones with sulfinate esters in the presence of a catalytic amount of iodine in acetonitrile at 140 °C provided 4-sulfenylated isoquinoline-1(2H)-ones in good yield. The formation of di-p-tolyl disulfide from ethyl p-toluenesulfinate catalyzed by iodine in acetonitrile at 140 °C was confirmed by LC-MS analysis (Fig. 20D). Iodine-mediated sulfenylation of imidazo[1,2-a]pyridines with sulfinate esters was found by Tang's group in 2021 (Fig. 21A).40b Various imidazo[1,2-a]pyridines bearing sulfanyl groups were synthesized by the iodine-mediated conditions via the formation of disulfides. In 2019, sulfenylation of indoles with sulfinate esters was reported by Zhang, Liu, and coworkers (Fig. 21B).41 The reaction took place smoothly when indoles were treated with and sulfinate esters in the presence of a catalytic amount of ammonium iodide and 1,10-phenanthroline at 100 °C. The formation of disulfides from sulfinate esters with potassium iodide in the presence of formic acid was reported by Luján-Montelongo and coworkers in 2023 (Fig. 21C).42 | ||
| Fig. 20 (A) Sulfenylation of electron-rich arenes. (B) Formation of a thiosulfonate. (C) Sulfenylation of isoquinoline-1(2H)-ones. (D) Formation of p-tolyl disulfide. | ||
 | ||
| Fig. 21 (A) Sulfenylation of imidazo[1,2-a]pyridines. (B) Sulfenylation of indoles. (C) Synthesis of disulfides from sulfinate esters. | ||
3.4. Transformations of sulfinate esters through the generation of o-quinodimethane intermediates
Cycloaddition of o-quinodimethanes generated from cyclic sulfinate esters was achieved by Ngamnithiporn and coworkers in 2022 and 2024 (Fig. 22A and B).43 For example, treatment of cyclic sulfinate esters with imines catalyzed by copper(II) triflate at 90 °C furnished tetrahydroisoquinolines by aza-Diels–Alder reaction of o-quinodimethane intermediates via the removal of sulfur dioxide (Fig. 22A).43a,b The authors also developed an efficient method to synthesize isochromans by oxa-Diels–Alder reaction of o-quinodimethane intermediates with carbonyl compounds including ethyl glyoxalates (Fig. 22B).43b In 2022, Sparr and coworkers realized enantioselective preparation of o-quinodimethane atropisomers and their stereospecific Diels–Alder reaction from sulfinate esters bearing triyne moieties and dienophiles catalyzed by Rh(cod)2BF4 and (Sa)-BINAP (Fig. 22C).44 The enantioselective formation of o-quinodimethane intermediate 5 proceeded by rhodium-catalyzed [2+2+2] cycloaddition of triyne and subsequent removal of sulfur dioxide.4. Conclusions and future perspectives
This feature article summarizes recent studies on sulfinate esters. The accessibility of sulfinate esters has significantly improved, demonstrating good functional group tolerance when synthesized from readily available starting materials such as thiols, aryl iodides, and sulfinic acids. A wide variety of organosulfur compounds can be synthesized through modern transformations of sulfinate esters, including electrophilic activation and transition-metal-catalyzed processes.Given the growing demand for organosulfur compounds in pharmaceutical sciences, broad synthetic methods that preserve reactive functional groups remain highly sought after. Furthermore, considering great achievements in modern synthetic chemistry using various electrophiles such as amides through a variety of activations involving catalytic methods, the significant potential of sulfinate esters, enabled by diverse activation strategies, is expected to drive the development of novel transformations, contributing to various research fields, including drug discovery. Continued advances in sulfinate ester chemistry will be of great significance across multiple disciplines, including organic chemistry, pharmaceutical sciences, agrochemistry, and materials chemistry.
The authors thank Central Glass Co., Ltd for providing Tf2O and TfOH. This work was supported by JSPS KAKENHI Grant Number JP23K23354 (S. Y.).
Data availability
No primary research results have been included in this feature article.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. Oae and N. Kunieda, Sulfinic Acids and Sulfinic Esters, in Organic Chemistry of Sulfur, ed. S. Oae, Springer, Boston, 1977, pp. 603–647 Search PubMed; (b) N. Amri and T. Wirth, Chem. Rec., 2021, 21, 2526 CrossRef CAS PubMed; (c) O. Pouralimardan, H. Bahir, S. M. Saeed, A. H. Adhab and R. Sadeghzadeh, Chem. Rev. Lett., 2023, 6, 95 CAS.
- (a) I. B. Douglass, Tetrahedron Lett., 1962, 93 Search PubMed; (b) I. B. Douglass, J. Org. Chem., 1965, 30, 633 CrossRef CAS.
- M. Hulce, J. P. Mallamo, L. L. Frye, T. P. Kogan and G. H. Posner, Org. Synth., 1986, 64, 196 CrossRef CAS.
- J. L. Kice and K. W. Bowers, J. Am. Chem. Soc., 1962, 84, 605 Search PubMed.
- A. R. Hajipour, A. R. Falahati and A. E. Ruoho, Tetrahedron Lett., 2006, 47, 2717 CrossRef CAS.
- J. L. G. Ruano, J. Aleman, M. B. Cid and A. Parra, Org. Lett., 2005, 7, 179 CrossRef CAS PubMed.
- (a) K. K. Andersen, Tetrahedron Lett., 1962, 93 CrossRef CAS; (b) H. Gilman, J. Robinson and N. J. Beaber, J. Am. Chem. Soc., 1926, 48, 2715 CrossRef CAS.
- Recent examples for the chiral sulfoxide synthesis, see: (a) Y. Lou, J. Wei, M. Li and Y. Zhu, J. Am. Chem. Soc., 2022, 144, 123 CrossRef CAS PubMed; (b) G.-Y. Zhu, J.-J. Zhou, L.-G. Liu, X. Li, X.-Q. Zhu, X. Lu, J.-M. Zhou and L.-W. Ye, Angew. Chem., Int. Ed., 2022, 61, e202204603 CrossRef CAS PubMed; (c) K. B. Gan, R.-L. Zhong, Z.-W. Zhang and F. Y. Kwong, J. Am. Chem. Soc., 2022, 144, 14864 CrossRef CAS PubMed.
- (a) C. C. Arcus, M. P. Balfe and J. Kenyon, J. Chem. Soc., 1938, 485 RSC; (b) A. H. Wragg, J. S. McFadyen and T. S. Stevens, J. Chem. Soc., 1958, 3603 RSC; (c) A. C. Cope, D. E. Morrison and L. Field, J. Am. Chem. Soc., 1950, 72, 59 CrossRef CAS; (d) D. Darwish and E. A. Preston, Tetrahedron Lett., 1964, 113 CrossRef CAS.
- (a) P. K. Shyam, Y. K. Kim, C. Lee and H.-Y. Jang, Adv. Synth. Catal., 2016, 358, 56 CrossRef CAS; (b) N. Taniguchi, Asian J. Org. Chem., 2024, 13, e202400131 CrossRef CAS.
- C. Zhou, Z. Tan, H. Jiang and M. Zhang, Green Chem., 2018, 20, 1992 RSC.
- L.-A. T. Nguyen, T.-N. Le, C.-T. Duong, C.-T. Vo, F. Duus and T. X. T. Luu, J. Sulfur Chem., 2021, 42, 519 CrossRef CAS.
- (a) C. Ai, H. Shen, D. Song, Y. Li, X. Yi, Z. Wang, F. Ling and W. Zhong, Green Chem., 2019, 21, 5528 RSC; (b) Y. He, J. Zhang, L. Xu and Y. Wei, Tetrahedron Lett., 2020, 61, 151631 CrossRef CAS; (c) H. Zhou, J. Duan, D. Xie, J. Yang, B. Ma, G. Wang, C. Wu and X.-C. Wang, Synthesis, 2020, 2705 CAS; (d) B. Kaboudin, L. Behrouzi, F. Kazemi, M. M. Najafpour and H. Aoyama, ACS Omega, 2020, 5, 17947 CrossRef CAS PubMed.
- K. Nakamura, Y. Kumagai, A. Kobayashi and S. Yoshida, Org. Biomol. Chem., 2023, 21, 6886 RSC.
- (a) X. Ma, Y. Liu, L. Du, J. Zhou and I. E. Markó, Nat. Commun., 2020, 11, 914 CrossRef CAS PubMed; (b) W. Shi, X. Zhou, Y. Kong, J. Li and I. E. Markó, Chem. – Asian J., 2020, 15, 511 CrossRef CAS PubMed.
- J. Wei and Z. Sun, Org. Lett., 2015, 17, 5396 CrossRef CAS PubMed.
- W. P. Chappell, M. Favié and G. M. Sammis, Chem. Commun., 2024, 60, 9765 RSC.
- J. Drabowicz, M. Kwiatkowska and P. Kiełbasiński, Synthesis, 2008, 3563 CrossRef CAS.
- J. L. Shaw, B. J. Austermuehle, J. M. Witte, T. R. Dorsey, C. Delach, C. G. Hamaker and S. R. Hitchcock, Synthesis, 2021, 2693 CAS.
- J. Yang, H. Dong, K. Yan, X. Song, J. Yu and J. Wen, Adv. Synth. Catal., 2021, 363, 5417 CrossRef CAS.
- Y.-Z. Ji, H.-J. Li, J.-Y. Zhang and Y.-C. Wu, Eur. J. Org. Chem., 2019, 1846 CrossRef CAS.
- Y. Sumii, K. Sasaki, O. Matsubara and N. Shibata, Org. Lett., 2021, 23, 2777 CrossRef CAS PubMed.
- (a) X. Zhang, E. C. X. Ang, Z. Yang, C. W. Kee and C.-H. Tan, Nature, 2022, 604, 298 CrossRef CAS PubMed; (b) T. Wei, H.-L. Wang, Y. Tian, M.-S. Xie and H.-M. Guo, Nat. Chem., 2024, 16, 1301 CrossRef CAS PubMed; (c) M. Liao, Y. Liu, H. Long, Q. Xiong, X. Lv, Z. Luo, X. Wu and Y. R. Chi, Chemistry, 2024, 10, 1541 CrossRef CAS.
- (a) H.-J. Li, R. Wang, J. Gao, Y.-Y. Wang, D.-H. Luo and Y.-C. Wu, Adv. Synth. Catal., 2015, 357, 1393 CrossRef CAS; (b) Y.-Z. Ji, M. Wang, H.-J. Li, Y. Liu and Y.-C. Wu, Eur. J. Org. Chem., 2016, 4077 CrossRef CAS; (c) N. Pogaku, P. R. Krishna and Y. L. Prapurna, Synth. Commun., 2017, 47, 1239 CrossRef CAS.
- L. Kadari, P. R. Krisna and Y. L. Prapurna, Adv. Synth. Catal., 2016, 358, 3863 CrossRef CAS.
- (a) B. Du, Z. Li, P. Qing, J. Han and Y. Pan, Chem. – Asian J., 2016, 11, 478 CrossRef CAS PubMed; (b) A. Yadav, M. D. Ambule, R. Kant and A. K. Srivastava, Eur. J. Org. Chem., 2020, 5709 CrossRef CAS; (c) G. Zhang, Q. Fan, H. Wang, Y. Zhao and C. Ding, Adv. Synth. Catal., 2021, 363, 833 CrossRef CAS.
- S. Kim and S. Lee, Org. Biomol. Chem., 2024, 22, 4436 RSC.
- (a) Y.-Z. Ji, Q.-X. Wu, H.-J. Li, D.-H. Luo and Y.-C. Wu, Synthesis, 2020, 755 Search PubMed; (b) H. S. Korawat and A. K. Basak, ACS Omega, 2020, 5, 17818 CrossRef CAS PubMed.
- G. Zhang, Z. Sang, W. Wu, Q. Fan and C. Ding, ChemistrySelect, 2022, 7, e202202833 CrossRef CAS.
- (a) F. Yuste, A. H. Linares, V. M. Mastranzo, B. Ortiz, R. Sánchez-Obregón, A. Fraile, J. Luis and G. Ruano, J. Org. Chem., 2011, 76, 4635 CrossRef CAS PubMed; (b) N.-L. T. Nguyen, H.-T. Vo, F. Duus and T. X. T. Luu, Molecules, 2017, 22, 1458 CrossRef PubMed.
- (a) A. Kobayashi, T. Matsuzawa, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 5429 RSC; (b) A. Kobayashi, T. Matsuzawa, T. Hosoya and S. Yoshida, Chem. Lett., 2020, 49, 813 CrossRef CAS; (c) Y. Kumagai, A. Kobayashi, K. Nakamura and S. Yoshida, Chem. Commun., 2024, 60, 1611 RSC.
- (a) S. K. Bur and A. Padwa, Chem. Rev., 2004, 104, 2401 CrossRef CAS PubMed; (b) K. S. Feldman, Tetrahedron, 2006, 62, 5003 CrossRef CAS; (c) S. Akai and Y. Kita, Top. Curr. Chem., 2007, 274, 35 CrossRef CAS; (d) L. H. S. Smith, S. C. Coote, H. F. Sneddon and D. J. Procter, Angew. Chem., Int. Ed., 2010, 49, 5832 CrossRef CAS PubMed; (e) A. Shafir, Tetrahedron Lett., 2016, 57, 2673 CrossRef CAS; (f) A. P. Pulis and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 9842 CrossRef CAS PubMed; (g) H. Yorimitsu, Chem. Rec., 2017, 17, 1156 CrossRef CAS PubMed; (h) T. Yanagi, K. Nogi and H. Yorimitsu, Tetrahedron Lett., 2018, 59, 2951 CrossRef CAS; (i) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701 CrossRef CAS PubMed; (j) L. Zhang, M. Hu and B. Peng, Synlett, 2019, 2203 CAS.
- K. Nakamura, M. Suzuki and S. Yoshida, Org. Lett., 2024, 26, 9676 CrossRef CAS PubMed.
- M. Suzuki, K. Kanemoto, Y. Nakamura, T. Hosoya and S. Yoshida, Org. Lett., 2021, 23, 3793 CrossRef CAS PubMed.
- (a) G.-J. Li, Y.-L. Pan, Y.-L. Liu, H.-F. Xu and J.-Z. Chen, Tetrahedron Lett., 2019, 60, 151260 CrossRef CAS; (b) S. Tsuzuki and T. Kano, Org. Lett., 2023, 25, 6677 CrossRef CAS PubMed.
- Z. Zhu, Z. Deng, C. Xuan and C. Shu, Org. Lett., 2024, 26, 406 CrossRef CAS PubMed.
- W. Zhang, Z. Li, H. Hu, J. Wang, Z.-F. Xu, M. Yu and C.-Y. Li, Org. Lett., 2024, 26, 5453 CrossRef CAS PubMed.
- J. A. Lujan-Montelongo, A. O. Estevez and F. F. Fleming, Eur. J. Org. Chem., 2015, 1602 CrossRef CAS PubMed.
- Y. Wei, J. He, Y. Liu, L. Xu, L. Vaccaro, P. Liu and Y. Gu, ACS Omega, 2020, 5, 18515 CrossRef CAS PubMed.
- (a) Y. Mu, M. Yang, F. Li, Z. Iqbal, R. Jiang, J. Hou, X. Guo, Z. Yang and D. Tang, New J. Chem., 2021, 45, 4934 RSC; (b) J. Sun, Y. Mu, Z. Iqbal, J. Hou, M. Yang, Z. Yang and D. Tang, Synlett, 2021, 1014 CAS.
- L. Chen, J. Zhang, Y. Wei, Z. Yang, P. Liu, J. Zhang and B. Dai, Tetrahedron, 2019, 75, 130664 CrossRef.
- J. A. Luján-Montelongo, L. J. G. de la Cuesta, A. E. Cruz-Jiménez, P. Hernández and A. Vela, Green Chem., 2023, 25, 7963 RSC.
- (a) A. Ngamnithiporn, P. Chuentragool, P. Ploypradith and S. Ruchirawat, Org. Lett., 2022, 24, 4192 CrossRef CAS PubMed; (b) A. Ngamnithiporn, P. Chuentragool, P. Ploypradith and S. Ruchirawat, Asian J. Org. Chem., 2023, 12, e202300366 CrossRef CAS; (c) A. Ngamnithiporn, P. Chuentragool, P. Songthammawat, S. Punnita, K. Photong, P. Ploypradith and S. Ruchirawat, Synlett, 2024, 1180 CrossRef CAS.
- J. Dong, A. Ostertag and C. Sparr, Angew. Chem., Int. Ed., 2022, 61, e202212627 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The reaction was performed using (
The reaction was performed using (