Open Access Article
Open Access ArticleContinuous selective oxidation of methane to methanol on H+-ferrierite having a sheet-like morphology†
A.
Yilmaz‡
 a,
M. O.
Ozbek
a,
M. O.
Ozbek
 b,
F.
Al
b,
F.
Al
 a,
G.
Celik
a,
G.
Celik
 a and
B.
Ipek
a and
B.
Ipek
 *a
*a
aDepartment of Chemical Engineering, Middle East Technical University, Ankara, 06800, Turkey. E-mail: bipek@metu.edu.tr
bDepartment of Chemical Engineering, Gebze Technical University, Kocaeli 41400, Turkey
First published on 11th June 2025
Abstract
This study reports continuous and selective catalytic methanol production from methane on H+-FER with a sheet-like morphology using N2O as an oxidant. At 325 °C, total selectivity for methanol and dimethyl ether reaches up to 97% via trace Cu addition or thermal treatment.
Direct methane to methanol conversion (DMTM) with high methanol selectivity has long been regarded as the Holy Grail of C1 chemistry and catalysis. High methanol selectivity (>95%) has been reported on Cu- or Fe-exchanged zeolites via a stepwise reaction, where a pre-treatment forms active CuxOyHz and FexOy sites at high temperatures (∼450 °C) with O2, or at room temperature with N2O.1,2 Spectroscopic and theoretical analyses identified these sites as [CuOH]+,3 mono-(μ-oxo)dicopper(II) ([Cu–O–Cu]2+),1,4,5trans-μ-1,2-peroxo dicopper(II),6 [Cu3(μ-O)3]2+,7,8 or [Fe
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]2+,9 depending on the zeolite framework, Al density and arrangement and the type of oxidant.10,11 Once formed, these active sites react stoichiometrically with methane at temperatures lower than 300 °C (Trxn ∼ 200 °C) while preventing overoxidation of methane to CO2. After the reaction, surface-bound intermediates (e.g. methoxy or methanol12) must be removed from the surface, typically using water vapor as a solvent.13 In this reaction, active site formation and methane conversion occur at different temperatures because generating active sites requires more energy than methane activation, which is prone to over-oxidation. On the other hand, industry prefers continuous catalytic methanol production over 3-step stoichiometric processes due to fewer temperature and pressure shifts and more stable gas feed conditions. Thus, several groups have investigated continuous catalytic conversion of methane to methanol using O2,3,14 N2O15,16 or H2O217 as oxidants. However, the main limitation of these processes is the low methanol selectivity. Higher methanol selectivity (>80%) can be achieved with limited O2 (ppm level) or higher N2O concentrations; however, it is typically accompanied by very low methane conversion (generally below 0.1%).18,19 Recently, methanol formation rates as high as 2448 μmol g−1 h−1 have been reported on Cu-SSZ-39 at 350 °C and a WHSV of 15
O]2+,9 depending on the zeolite framework, Al density and arrangement and the type of oxidant.10,11 Once formed, these active sites react stoichiometrically with methane at temperatures lower than 300 °C (Trxn ∼ 200 °C) while preventing overoxidation of methane to CO2. After the reaction, surface-bound intermediates (e.g. methoxy or methanol12) must be removed from the surface, typically using water vapor as a solvent.13 In this reaction, active site formation and methane conversion occur at different temperatures because generating active sites requires more energy than methane activation, which is prone to over-oxidation. On the other hand, industry prefers continuous catalytic methanol production over 3-step stoichiometric processes due to fewer temperature and pressure shifts and more stable gas feed conditions. Thus, several groups have investigated continuous catalytic conversion of methane to methanol using O2,3,14 N2O15,16 or H2O217 as oxidants. However, the main limitation of these processes is the low methanol selectivity. Higher methanol selectivity (>80%) can be achieved with limited O2 (ppm level) or higher N2O concentrations; however, it is typically accompanied by very low methane conversion (generally below 0.1%).18,19 Recently, methanol formation rates as high as 2448 μmol g−1 h−1 have been reported on Cu-SSZ-39 at 350 °C and a WHSV of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml g−1 h−1, using 40% N2O as the oxidant, with CH4 conversion approaching 2%.20 This methanol formation rate approaches the industrially viable benchmark of 3000 μmol g−1 h−1, as reported by Lange et al.,21 although N2O remains a more expensive oxidant compared to air. They also reported unexpectedly high methanol formation rates (reaching 18 mmol g−1 h−1 at 350 °C with an optimized WHSV and H2O ratio) on a transition-metal-free (i.e., Cu- or Fe-free) ferrierite.22 A clear increase in methanol formation rate and selectivity has been previously reported with decreasing Cu concentration in zeolites,14,20 linking the decline in methanol formation to increased over-oxidation to CO and CO2 products.23 However, N2O activation of H+-zeolites such as H+-ferrierite, H+-ZSM-5 and H+-MOR for methane partial oxidation has not been previously reported and is an intriguing discovery. Although several dealumination and characterization techniques were implemented by Xiao et al. to identify the active sites in H+-ferrierite,22 the precise position and coordination of Al atoms still require further investigation to better understand this unexpected activity.
000 ml g−1 h−1, using 40% N2O as the oxidant, with CH4 conversion approaching 2%.20 This methanol formation rate approaches the industrially viable benchmark of 3000 μmol g−1 h−1, as reported by Lange et al.,21 although N2O remains a more expensive oxidant compared to air. They also reported unexpectedly high methanol formation rates (reaching 18 mmol g−1 h−1 at 350 °C with an optimized WHSV and H2O ratio) on a transition-metal-free (i.e., Cu- or Fe-free) ferrierite.22 A clear increase in methanol formation rate and selectivity has been previously reported with decreasing Cu concentration in zeolites,14,20 linking the decline in methanol formation to increased over-oxidation to CO and CO2 products.23 However, N2O activation of H+-zeolites such as H+-ferrierite, H+-ZSM-5 and H+-MOR for methane partial oxidation has not been previously reported and is an intriguing discovery. Although several dealumination and characterization techniques were implemented by Xiao et al. to identify the active sites in H+-ferrierite,22 the precise position and coordination of Al atoms still require further investigation to better understand this unexpected activity.
This study investigates methanol formation activity on sheet-like H+-ferrierite (Nano-FER) compared to commercial ferrierite (Com-FER), and examines Al distribution, Cu locations, and N2O binding using in situ diffuse reflectance infra-red spectroscopy (DRIFTS), nuclear magnetic resonance (NMR) and density functional theory (DFT) analyses.
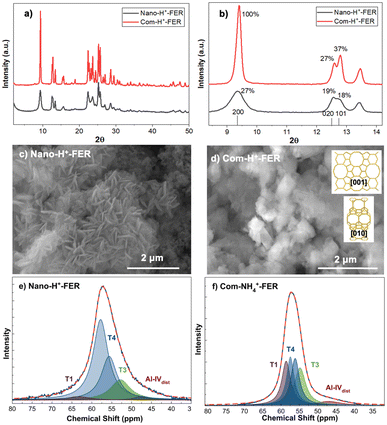
XRD analysis of Nano-H+-FER and Com-H+-FER reveals characteristic diffraction peaks of the FER-type zeolite, whose crystal structure is orthorhombic and belongs to the Immm space group. The relative XRD intensity values of Nano-H+-FER on the (200), (020), and (101) planes indicate enhanced crystal growth in the [010] direction, along with reduced thickness in the [100] direction. Hence, the crystals grow predominantly in the bc plane, which reduces the concentration of straight 10MR channels (Fig. 1a and b). This orientation is further confirmed by TEM analysis of Nano-H+-FER, which shows a measured d-spacing of 0.94 nm (Fig. S2d, ESI†), consistent with the (200) plane d-spacing and indicative of crystal alignment along the bc plane.24 SEM analysis showed that the thickness of the nano-sheets ranged from approximately 40 to 60 nm (Fig. 1c), while TEM imaging revealed that individual sheets can be as thin as ∼10 nm (Fig. S2, ESI†). Com-FER crystals exhibit more uniform crystal lengths in all three directions, with sizes varying between 500 nm and 1 μm (Fig. 1d). The 27Al MAS NMR spectra of Com-NH4+-FER showed that only 2% of Al atoms were in octahedral coordination (Fig. S3, ESI†). Among the tetrahedrally coordinated Al atoms, 53% were located at the T4 site (56–58 ppm25), and 21% at the T3 site (55 ppm, Fig. 1e). In contrast, Nano-H+-FER exhibited 13% extra-framework Al (octahedrally coordinated), attributed to NH4+-exchange and subsequent heat treatment (Fig. S3c, ESI†). Of the tetrahedrally coordinated Al atoms, 82% were located at the T4 site and 14% at the T3 site (Fig. 1e and Table S1, ESI†).
At 325 °C, Nano-H+-FER exhibited a slightly higher direct methanol formation rate (2061 μmol g−1 h−1, or 34 μmol g−1 min−1) compared to Com-H+-FER (1957 μmol g−1 h−1, or 32.6 μmol g−1 min−1) (Fig. 2). These values are similar to the methanol production rates reported by Xiao et al. under similar conditions (2820 μmol g−1 h−1 (47 μmol g−1 min−1) on H+-FER, at 350 °C, WHSV 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml g−1 h−1, 40 kPa CH4, 40 kPa N2O, 8% H2O22). Due to lower coke formation rate and selectivity on Nano-H+-FER, the total carbon-based selectivity for methanol and dimethyl ether increases to 89% (with 74% methanol selectivity), compared to 86% (69% methanol selectivity) observed on Com-H+-FER (Table S3, ESI†). On Nano-H+-FER, increasing the water vapor concentration to 11% further suppressed carbon formation and increased the total carbon-based selectivity for methanol and dimethyl ether to 94% (with methanol selectivity reaching 82%) (Table S4, ESI†). As a result of suppressed carbon formation, Nano-H+-FER experienced only a 7% drop in methanol formation activity over 11 hours of testing under 11% water vapor concentration. For the case of Com-H+-FER, the loss in methanol formation activity was 11%. On the other hand, Cu-exchanged Com-FER and Nano-FER showed no deactivation during 11 hours of testing (Fig. S4, ESI†).
000 ml g−1 h−1, 40 kPa CH4, 40 kPa N2O, 8% H2O22). Due to lower coke formation rate and selectivity on Nano-H+-FER, the total carbon-based selectivity for methanol and dimethyl ether increases to 89% (with 74% methanol selectivity), compared to 86% (69% methanol selectivity) observed on Com-H+-FER (Table S3, ESI†). On Nano-H+-FER, increasing the water vapor concentration to 11% further suppressed carbon formation and increased the total carbon-based selectivity for methanol and dimethyl ether to 94% (with methanol selectivity reaching 82%) (Table S4, ESI†). As a result of suppressed carbon formation, Nano-H+-FER experienced only a 7% drop in methanol formation activity over 11 hours of testing under 11% water vapor concentration. For the case of Com-H+-FER, the loss in methanol formation activity was 11%. On the other hand, Cu-exchanged Com-FER and Nano-FER showed no deactivation during 11 hours of testing (Fig. S4, ESI†).
 | ||
Fig. 2 Methanol formation rates and carbon-based product selectivity values of Com-H+-FER, Com-Cu-FER, Nano-H+-FER, Nano-Cu-FER, and Nano-H+-FER-850 obtained at 325 °C and 40.5 kPa CH4, 15.2 kPa N2O, 9 kPa H2O, and balance He (∼300 mg catalyst, 100 sccm total flow, GHSV = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml g−1 h−1, see Table S3 for all selectivity values, ESI†). 000 ml g−1 h−1, see Table S3 for all selectivity values, ESI†). | ||
To investigate methanol selectivity and activity, small amounts of Cu were ion-exchanged into Nano-FER (Si/Al = 12, Cu/Al = 0.027, 0.20 wt% Cu) and Com-FER (Si/Al = 9, Cu/Al = 0.024, 0.26 wt% Cu, Table S2, ESI†). Despite the increased over-oxidation observed on Com-Cu+-FER compared to Com-H+-FER, the total selectivity for methanol and dimethyl ether rose from 86% to 91%, due to the suppression of olefin and coke formation by Cu addition. When 0.20 wt% Cu is ion-exchanged to Nano-FER, the total selectivity for methanol and dimethyl ether increased to 96% (Nano-Cu-FER, Fig. 2a). Cu exchange of the two Brønsted acid sites located in the 10MR can explain the reduced olefin and coke formation on Cu-zeolites, as this region is where DME and olefin production primarily occurs.22,26 This assignment is further supported by vibrational spectroscopy: the framework frequency values at 912 cm−1 and 955 cm−1 observed on dehydrated Com-Cu-FER and Nano-Cu-FER (Fig. 3a) agree well with the calculated Cu–O bending vibrations for Cu located at site I (α-site in the 10MR)27 near two Al atoms at the T3 positions (Fig. S8, ESI†). The additional 971 cm−1 band observed for Nano-Cu-FER indicates that some of the Cu cations are located at site II (β-site in 8MR cage27) near two Al atoms at T4 positions, which is theoretically calculated to be more favorable (Table S8, ESI†).
Heating the Nano-H+-FER sample at 850 °C for 5 hours increases the total selectivity for methanol and dimethyl ether to 97%, with a corresponding methanol formation rate of 2270 μmol g−1 h−1 (Nano-H+-FER-850, Table S3, ESI†). This heat treatment reduces the proportion of tetrahedrally coordinated Al atoms—and thus the Brønsted acid concentration—resulting in increased AlV and AlVI coordination (5% and 24%, respectively), which correlates with lower olefin and coke production, despite an increase in Al distribution at the T3 positions (Table S1, ESI†).
To probe the location of active sites, pyridine was adsorbed at 150 °C and partially desorbed at 325 °C—conditions under which pyridine remains in the 10MRs (Fig. S6 and S7, ESI†). When methane activation was then performed, the methanol formation rate on Nano-H+-FER dropped by nearly half (Table S3, ESI†). This implies that methane activation predominantly occurs in the 8MRs, as pyridine blocks 10MRs and may also obstruct methanol diffusion through them. The dimethyl ether formation rate (55 μmol g−1 h−1) is one quarter of that observed on pyridine-free Nano-H+-FER (211 μmol g−1 h−1), and the near absence of C2–C4 (olefin and alkane) products further indicates pyridine occupancy in the 10MRs. Removing coke from the list of products—since calculated coke increases due to pyridine combustion—raises methanol selectivity from 78% to 91% in the presence of pyridine and increases the total selectivity for methanol and dimethyl ether to a remarkable 99%. However, adsorbed pyridine does not affect methanol activity on Nano-H+-FER-850, while it further reduces olefin formation due to its binding to H+ sites in the 10MRs (Table S3, ESI†).
In the DRIFT spectra of dehydrated Com-H+-FER, O–H stretching bands appear at 3745 cm−1 for external silanol groups and at 3600 cm−1 for Brønsted acid sites (Fig. S5a, ESI†). Brønsted acid sites were not discernible in Nano-H+-FER, consistent with its sheet-like morphology. Instead, absorption increases at 3650 cm−1 and 3745 cm−1. The absorption at 3650 cm−1 is mostly attributed to the extra-framework Al groups.28 However, Ravi et al. assigned a similar absorption band at 3660 cm−1, observed on MOR, to the O–H stretching vibration of a distorted tetrahedrally coordinated Al species ((SiO)3–Al–OH).29 The presence of such species is also evident in the deconvoluted 27Al MAS NMR spectra of Nano-H+-FER and Com-H+-FER (Fig. 1 and Table S1, ESI†). The DFT-computed O–H vibrational frequency for a surface Al–OH species with distorted tetrahedral geometry was 3653 cm−1 (Table S7, ESI†), and aligns well with the experimentally observed 3650 cm−1 band. In addition, the T–O–T region of dehydrated Nano-H+-FER shows absorption bands at 971 cm−1 and 885 cm−1 (Fig. 3a). These features can be attributed to Al–OH bending modes involving Al at the T4 site (site II, 8MR), as well as T–O bending from distorted tetrahedral Si and Al atoms located along the ac and bc planes (Table S7, ESI†). Collectively, the NMR, DRIFTS, and DFT results indicate that Al atoms are primarily located at T4 sites near the 8MR channels in Nano-FER, with additional distorted tetrahedral Al species likely present at the surface or within the 8MR regions of the FER crystals.
N2O adsorption on Nano-H+-FER and Nano-Cu-FER showed absorption features at 2231 and 2219 cm−1, and at 1299 and 1274 cm−1, attributed to the asymmetric and symmetric stretching of the N–N–O molecule, respectively (Fig. 3b and Fig. S9, ESI†). N2O molecules are weakly bound to zeolites at 25 °C as desorption of the molecule under inert flow is observed (Fig. 3b). Computational investigations were conducted to identify possible N2O binding sites on Nano-H+-FER and Nano-Cu-FER. For Cu-exchanged FER, four candidate sites—sites I, II, III and IV—were examined, with N2O modelled to coordinate through either the nitrogen or oxygen atom. Among these, N2O binding to site IV yielded the highest adsorption energy (−85 kJ mol−1), compared to sites I and II (−53 to −46 kJ mol−1 and −44 to −40 kJ mol−1, respectively; Fig. S10, ESI†). However, the calculated vibrational modes at these Cu-bound sites do not match the experimentally observed values (Table S8, ESI†). Further DFT simulations were performed to explain the vibrational features observed on Nano-H+-FER (2231 and 2219 cm−1; 1299 and 1274 cm−1), by modelling N2O adsorption near distorted tetrahedral and trigonal Al sites at site II (Fig. S11, ESI†). The resulting frequencies—2259 cm−1 and 1275 cm−1—for a distorted tetrahedral Al coordination showed the closest match to the experimentally observed values of 2231 cm−1 and 1274 cm−1.
In summary, Nano-H+-FER catalysts with a sheet-like morphology were prepared, exhibiting suppressed coke formation and achieving a total carbon-based selectivity of 89% for methanol and dimethyl ether at 325 °C and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml g−1 h−1 WHSV. Methanol and dimethyl ether selectivity was further enhanced to 96% through the addition of a very low amount of Cu (0.20 wt%) or to 97% through heat treatment at 850 °C. Cu addition reduces olefin formation by reducing Brønsted acid density in the 10MR channels, while heat treatment suppresses primarily olefin and coke formation. Occlusion of pyridine in 10MRs led to a partial loss of activity in Nano-H+-FER, which is attributed to an active centre partly in 8MRs and partly at the crystal surface.
000 ml g−1 h−1 WHSV. Methanol and dimethyl ether selectivity was further enhanced to 96% through the addition of a very low amount of Cu (0.20 wt%) or to 97% through heat treatment at 850 °C. Cu addition reduces olefin formation by reducing Brønsted acid density in the 10MR channels, while heat treatment suppresses primarily olefin and coke formation. Occlusion of pyridine in 10MRs led to a partial loss of activity in Nano-H+-FER, which is attributed to an active centre partly in 8MRs and partly at the crystal surface.
A. Y.: data curation, formal analysis, investigation, writing – original draft. M. O. O: data curation, formal analysis, investigation. F. A.: data curation, formal analysis. G.C.: data curation, formal analysis, investigation. B. I.: conceptualization, investigation, funding acquisition, project administration, writing – original draft, writing – review and editing.
This project is funded by the Middle East Technical University Research Grant No. HDESP-304-2021-10806. B. I. acknowledges support from The Science Academy of Turkey, Young Scientist Awards Program. The DFT computations reported in this paper were fully performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- J. S. Woertink, P. J. Smeets, M. H. Groothaert, M. A. Vance, B. F. Sels, R. A. Schoonheydt and E. I. Solomon, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18908–18913 Search PubMed
.
- E. V. Starokon, M. V. Parfenov, L. V. Pirutko, S. I. Abornev and G. I. Panov, J. Phys. Chem. C, 2011, 115, 2155–2161 Search PubMed
.
- J. Ohyama, Y. Tsuchimura, H. Yoshida, M. Machida, S. Nishimura and K. Takahashi, J. Phys. Chem. C, 2022, 126, 19660–19666 Search PubMed
.
- P. Vanelderen, B. E. R. Snyder, M.-L. Tsai, R. G. Hadt, J. Vancauwenbergh, O. Coussens, R. A. Schoonheydt, B. F. Sels and E. I. Solomon, J. Am. Chem. Soc., 2015, 137, 6383–6392 CrossRef CAS PubMed
.
- G. Brezicki, J. D. Kammert, T. B. Gunnoe, C. Paolucci and R. J. Davis, ACS Catal., 2019, 9, 5308–5319 Search PubMed
.
- B. Ipek, M. J. Wulfers, H. Kim, F. Göltl, I. Hermans, J. P. Smith, K. S. Booksh, C. M. Brown and R. F. Lobo, ACS Catal., 2017, 7, 4291–4303 Search PubMed
.
- G. Li, P. Vassilev, M. Sanchez-Sanchez, J. A. Lercher, E. J. M. Hensen and E. A. Pidko, J. Catal., 2016, 338, 305–312 CrossRef CAS
.
- S. Grundner, M. A. C. Markovits, G. Li, M. Tromp, E. A. Pidko, E. J. M. Hensen, A. Jentys, M. Sanchez-Sanchez and J. A. Lercher, Nat. Commun., 2015, 6, 7546 Search PubMed
.
- B. E. R. Snyder, P. Vanelderen, M. L. Bols, S. D. Hallaert, L. H. Böttger, L. Ungur, K. Pierloot, R. A. Schoonheydt, B. F. Sels and E. I. Solomon, Nature, 2016, 536, 317–321 Search PubMed
.
- H. Zhang, J. Li, D. Wang, Y. Wang and H. Xiong, Coord. Chem. Rev., 2024, 503, 215637 CrossRef CAS
.
- M. H. Mahyuddin, Y. Shiota and K. Yoshizawa, Catal. Sci. Technol., 2019, 9, 1744–1768 RSC
.
- V. L. Sushkevich, R. Verel and J. A. van Bokhoven, Angew. Chem., Int. Ed., 2020, 59, 910–918 Search PubMed
.
- M. A. Newton, A. J. Knorpp, V. L. Sushkevich, D. Palagin and J. A. Van Bokhoven, Chem. Soc. Rev., 2020, 49, 1449–1486 Search PubMed
.
- K. T. Dinh, M. M. Sullivan, K. Narsimhan, P. Serna, R. J. Meyer, M. Dincă and Y. Román-Leshkov, J. Am. Chem. Soc., 2019, 141, 11641–11650 Search PubMed
.
- O. Memioglu and B. Ipek, Chem. Commun., 2021, 57, 1364–1367 RSC
.
- Y. Chen, N. Liu, C. Dai, R. Xu, G. Yu, N. Wang and B. Chen, Catal. Today, 2024, 442, 114934 CrossRef CAS
.
- J. Xu, R. D. Armstrong, G. Shaw, N. F. Dummer, S. J. Freakley, S. H. Taylor and G. J. Hutchings, Catal. Today, 2016, 270, 93–100 Search PubMed
.
- J. Ohyama, A. Hirayama, Y. Tsuchimura, N. Kondou, H. Yoshida, M. Machida, S. Nishimura, K. Kato, I. Miyazato and K. Takahashi, Catal. Sci. Technol., 2021, 11, 3437–3446 RSC
.
- A. Hirayama, Y. Tsuchimura, H. Yoshida, M. Machida, S. Nishimura, K. Kato, K. Takahashi and J. Ohyama, Catal. Sci. Technol., 2021, 11, 6217–6224 RSC
.
- P. Xiao, Y. Wang, K. Nakamura, Y. Lu, T. De Baerdemaeker, A. N. Parvulescu, U. Müller, D. De Vos, X. Meng, F. S. Xiao, W. Zhang, B. Marler, U. Kolb, R. Osuga, M. Nishibori, H. Gies and T. Yokoi, ACS Catal., 2023, 13, 11057–11068 CrossRef CAS
.
- J. P. Lange, V. L. Sushkevich, A. J. Knorpp and J. A. Van Bokhoven, Ind. Eng. Chem. Res., 2019, 58, 8674–8680 CrossRef CAS
.
- P. Xiao, Y. Wang, Y. Lu, K. Nakamura, N. Ozawa, M. Kubo, H. Gies and T. Yokoi, J. Am. Chem. Soc., 2024, 146, 10014–10022 CrossRef CAS PubMed
.
- M. A. Artsiusheuski, R. Verel, J. A. van Bokhoven and V. L. Sushkevich, ACS Catal., 2021, 11, 12543–12556 CrossRef CAS
.
- A. Bolshakov, R. van de Poll, T. van Bergen-Brenkman, S. C. C. Wiedemann, N. Kosinov and E. J. M. Hensen, Appl. Catal., B, 2020, 263, 118356 Search PubMed
.
- Z. Xiong, G. Qi, L. Bai, E. Zhan, Y. Chu, J. Xu, N. Ta, A. Hao, F. Deng and W. Shen, Catal. Sci. Technol., 2022, 12, 4993–4997 Search PubMed
.
- M. Zhang, S. Xu, Y. Wei, J. Li, J. Chen, J. Wang, W. Zhang, S. Gao, X. Li, C. Wang and Z. Liu, RSC Adv., 2016, 6, 95855–95864 RSC
.
- S. D. Hallaert, M. L. Bols, P. Vanelderen, R. A. Schoonheydt, B. F. Sels and K. Pierloot, Inorg. Chem., 2017, 56, 10681–10690 CrossRef CAS PubMed
.
- R. Rachwalik, M. Hunger and B. Sulikowski, Appl. Catal., A, 2012, 427–428, 98–105 CrossRef CAS
.
- M. Ravi, V. L. Sushkevich and J. A. van Bokhoven, Chem. Sci., 2021, 12, 4094–4103 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc00719d |
| ‡ Present address: Department of Chemical and Biomolecular Engineering, University of Delaware, Newark, DE, 19716, USA. |
| This journal is © The Royal Society of Chemistry 2025 |