Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A [CuII24] truncated octahedron and its [CuII8] building block†
Lucinda R. B.
Wilson
 a,
Gary S.
Nichol
a,
Gary S.
Nichol
 a,
Scott J.
Dalgarno
a,
Scott J.
Dalgarno
 *b and
Euan K.
Brechin
*b and
Euan K.
Brechin
 *a
*a
aEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, Scotland, UK. E-mail: ebrechin@ed.ac.uk
bInstitute of Chemical Sciences, Heriot-Watt University, Riccarton, Edinburgh EH14 4AS, UK. E-mail: S.J.Dalgarno@hw.ac.uk
First published on 25th February 2025
Abstract
Reaction of CuCl2·2H2O with p-tert-butylthiacalix[4]arene (H4TC[4]A) affords a [CuII24] cage whose metallic skeleton conforms to a truncated octahedron in which the metal ions are strongly antiferromagnetically coupled. A structurally related [CuII8] cluster can be made using CuBr2 in an otherwise identical reaction.
Polymetallic complexes of CuII represented the gateway to the field of molecular magnetism, beginning with studies of copper acetate1 in the 1950s through to the development of magneto-structural correlations in hydroxide- and halide-bridged dimers in the 1970s and 80s.2 Later research revealed the presence of spin frustration in equilateral CuII triangles and prompted detailed studies into the mechanisms of magnetic exchange.3 Indeed, high symmetry molecules are often ideal model complexes to examine geometric spin frustration, and for larger nuclearity cages this can lead to some exotic behaviour including enhanced ground-state degeneracy, low-lying singlets, non-collinear ground states and unusual magnetisation plateaus/jumps.4 The synthesis of high symmetry molecules is however not trivial.5 One approach is to build small nuclearity, high symmetry complexes such as triangles or squares, since these are the building blocks of certain Archimedean and Platonic (or Keplerate) polyhedra, and assemble them into larger species often at high temperatures and pressures.6 An additional challenge comes in designing an organic ligand capable of stabilising such building blocks, and an excellent candidate is p-tert-butylthiacalix[4]arene (H4TC[4]A, Fig. 1). This versatile molecule was first synthesised in 1997 and has since proved to be a highly successful platform for a breadth of supramolecular and coordination chemistry.7–13 In the latter area, the [S4O4] donor atom set typically leads to the formation of square [M4] building blocks that can self-assemble to form aesthetically pleasing molecular cages exhibiting a variety of nuclearities and topologies.14 This versatility is particularly appealing to chemists interested in constructing high nuclearity cages of paramagnetic metal ions with a view to developing magneto-structural correlations and/or uncovering novel magnetic phenomena. Surprisingly however, a search of the Cambridge structural database for TC[4]A-supported CuII cages reveals just five hits: four [Cu4] squares and one [Cu13Na2] cluster.15 Herein, we outline the synthesis, structure and magnetic behaviour of a [CuII24] cage and a structurally related [CuII8] building block.
 | ||
| Fig. 1 The structure of p-tert-butylthiacalix[4]arene (H4TC[4]A). Colour code: C = grey, O = red, S = yellow, H = white. Only the phenolic H atoms are shown. | ||
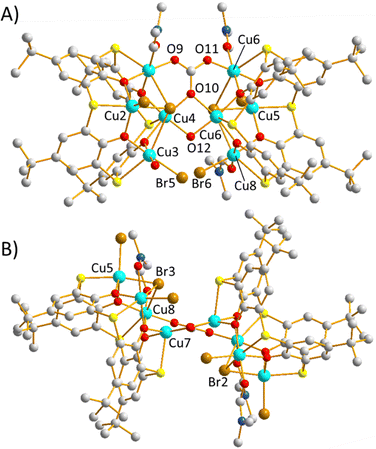
Reaction of CuCl2·2H2O with H4TC[4]A in a basic dmf/MeOH solution (see ESI,† for full details) leads to the formation of brown single crystals after 3 days, upon slow evaporation of the mother liquor. Crystals of [Cu24(μ4-TC[4]A)6(μ4-Cl)6(μ6-CO3)6(μ-OH)6(dmf)6]·22dmf (1·22dmf) were found to be in a triclinic cell and structure solution was performed in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (see Fig. S1, ESI,† shows the PXRD). The asymmetric unit (ASU) contains two distinct half clusters (i.e. the unit cell contains two [Cu24] clusters which lie about independent inversion centres) and symmetry expansion affords the cage shown in Fig. 2. The metallic skeleton (Fig. 3) of 1 describes a [CuII24] truncated octahedron. The fully deprotonated TC[4]A ligands sit atop the six square faces, bonding to the four Cu ions through four μ-O atoms and four terminal S atoms, as expected. Below the square lies a μ4-Cl1− ion, creating the [Cu4(TC[4]A)Cl] building block. Six of these building blocks are self-assembled into the [Cu24] unit by a disordered combination of six μ6-CO32− ions and six μ-OH1− ions (see the CIF file/ESI,† for full details), which lie in the hexagonal faces of the truncated octahedron. The carbonate anions originate from CO2 fixation. Perhaps counter-intuitively, the deliberate addition of CO32− ions to the reaction mixture, in the form of Na2CO3/NaHCO3, prevents crystal formation.
space group (see Fig. S1, ESI,† shows the PXRD). The asymmetric unit (ASU) contains two distinct half clusters (i.e. the unit cell contains two [Cu24] clusters which lie about independent inversion centres) and symmetry expansion affords the cage shown in Fig. 2. The metallic skeleton (Fig. 3) of 1 describes a [CuII24] truncated octahedron. The fully deprotonated TC[4]A ligands sit atop the six square faces, bonding to the four Cu ions through four μ-O atoms and four terminal S atoms, as expected. Below the square lies a μ4-Cl1− ion, creating the [Cu4(TC[4]A)Cl] building block. Six of these building blocks are self-assembled into the [Cu24] unit by a disordered combination of six μ6-CO32− ions and six μ-OH1− ions (see the CIF file/ESI,† for full details), which lie in the hexagonal faces of the truncated octahedron. The carbonate anions originate from CO2 fixation. Perhaps counter-intuitively, the deliberate addition of CO32− ions to the reaction mixture, in the form of Na2CO3/NaHCO3, prevents crystal formation.
The Cu ions are all six coordinate and in Jahn–Teller (JT) distorted {CuO4SCl} environments with the JT axis directed along the S–Cu–Cl vector (Cu–O, ∼1.60–2.10 Å, Cu–S, ∼2.59–2.63 Å, Cu–Cl, ∼2.65–2.67 Å). A dmf molecule sits in each of the calixarene cavities, although two were handled using a solvent mask during structure refinement. The self-assembly of the six [Cu4(TC[4]A)Cl] moieties gives rise to a small encapsulated space at the centre of the [Cu24] cluster that is occupied by three disordered H2O molecules. These are H-bonded to both the μ4-Cl1− ions and the O-atoms of the μ6-CO32−/μ-OH1− ions (O⋯O/Cl ≤ 3 Å) that line the interior wall of the cage. Closest intermolecular interactions occur between the tBu groups of the TC[4]A ligands (C⋯C, ≥ 3.5 Å) and between the tBu groups and the dmf solvate (C⋯O, ≥ 3.7 Å). Examination of the extended structure (Fig. S2, ESI†) confirms that the cluster cores are very well isolated from symmetry equivalents due to the TC[4]A ligands, with the closest Cu⋯Cu distance found being >12.6 Å.
We note that TC[4]A-stabilised truncated octahedra are known for CoII, NiII and MnII,16 but 1 represents the first example obtained with CuII. Interestingly a [Cu24I] cage was recently synthesised using tetramercaptotetrathiacalix[4]arene,17 which suggests that control over metal oxidation state can be achieved through choice/variation of lower rim substituent, or through the use of different calix[n]arenes within the same reaction, routes not yet exploited.
A [CuII8] cluster representing approximately one third of the structure of 1 can be isolated by replacing CuCl2·2H2O with CuBr2 in an otherwise identical reaction. Crystals of [CuII8(μ4-TC[4]A)2(μ4-CO3)(μ-OH)(Br)5(dmf)3(H2O)]·2dmf (2·2dmf, Fig. S1, ESI,† shows the PXRD) were in an orthorhombic cell with structure solution performed in the space group Pbca; the ASU contains the whole formula (Fig. 4). The structure of 2 describes two [Cu4(TC[4]A)Br] metalloligands bridged by one CO32− ligand and one OH1− ion. The [Cu4] squares are asymmetric, with one Cu–Br bond length in the range ∼2.43–2.44 Å and three in the range ∼2.98–3.08 Å. As a result, the Cu⋯Cu distances are also asymmetric, and in the range ∼3.21–3.45 Å. The [CO3]2− ion (which as with 1 originates from CO2 fixation) acts as a μ4-bridge between the two [Cu4] squares, linking Cu1 and Cu4 on one square with Cu6 and Cu7 on the second square. Cu4 and Cu7 are also bridged by the sole μ-OH1− ion (Cu4–O12–Cu7, ∼105°) which itself is H-bonded to the terminal Br1− ion (Br6) attached to Cu8 with 50% occupancy (O(H)⋯Br, ∼3.15 Å). Charge-balance is afforded through the 50% occupancy of another terminal Br1− ion (Br5) attached to Cu3, but at an angle that does not allow for H-bonding with the OH1− ion. The remaining coordination sites on the Cu ions are filled with a dmf molecule (Cu1, Cu3, Cu6), a H2O molecule (Cu8), and a terminal Br1− ion with full occupancy (Cu2, Cu5). The Cu ions adopt four different geometries/coordination spheres. Cu3 and Cu8 are five-/six-coordinate as the Br1− ion is disordered across the two positions and thus have distorted square pyramidal/octahedral {CuO3Br1.5S} geometries. Cu2 and Cu5 are five-coordinate and are in distorted square pyramidal {CuO2Br2S} geometries, and Cu1/Cu4/Cu6/Cu7 are all six-coordinate and in distorted octahedral {CuO4BrS} geometries. In each case the JT axis of the CuII ion lies along the S–Cu–Br vector. A molecule of dmf occupies each TC[4]A cavity. Deliberate introduction of CO32− ions to the reaction mixture, in the form of Na2CO3/NaHCO3 results in the formation of insoluble powder.
Examination of the extended structure of 2 reveals that symmetry equivalent clusters pack to form a bi-layer type structure (Fig. S3, ESI†). This type of self-assembled bi-layer structure is commonly observed18 in a variety of calixarene solvates and coordination compounds in which the ligands are not forced to pack in a parallel manner, e.g. as in the structure of 1. The result of this is that the closest crystallographically unique Cu⋯Cu distance between neighbouring clusters is found to be significantly shorter than in 1, in this case at ∼8.4 Å as shown in Fig. S3 (ESI†).
Dc magnetic susceptibility data were measured on powdered polycrystalline samples of 1–2 in the T = 300–2.0 K temperature range, in a field of B = 0.1 T (Fig. 5). The χT values of 1/2 at 300 K are 4.29/2.49 cm3 K mol−1, both well below the Curie constant expected for 24/8 uncoupled S = ½ ions (9/3 cm3 K mol−1 for g = 2.00). As the temperature is decreased the value of χT decreases rapidly and reaches a value of 0 cm3 K mol−1 at T = 15/5 K in both cases. This behaviour is clearly indicative of very strong antiferromagnetic exchange between neighbouring CuII ions in both 1 and 2, and the presence of well isolated diamagnetic ground states, as corroborated by magnetisation data (Fig. S4, ESI†).
 | ||
| Fig. 5 Experimental χT versus T data for 1 (○) and 2 (□) measured in the T = 300–2.0 K temperature range in an applied field, B = 0.1 T. The experimental data for 1 is divided by two and modelled using spin-Hamiltonian (1) and model 1 shown in Fig. S5 (ESI†). The solid red lines are the best fits to the experimental data. See text for details. | ||
The presence of two different clusters of 1 in the unit cell, the disordered [CO3]/[OH] and a total of twenty four CuII ions per cluster poses some problems (over-parameterisation, computational limits) for modelling the magnetic behaviour. In order to overcome these issues, we have constructed three [Cu12] model compounds (models 1–3, Fig. S5, ESI†) that describe half the cluster, and assume the presence of not more than three distinct exchange interactions based on the different bridging atoms and angles present. These are J1 = Cu–O/Cl–Cu along the sides of the square faces (Cu–O–Cu = ∼109–112°, Cu–Cl–Cu = ∼75°); J2 = Cu–Cl–Cu across the diagonal of the square faces (Cu–Cl–Cu = ∼118°); and J3 = Cu–O–Cu along the edges of hexagonal faces (Cu–Ohydroxide–Cu = ∼94°, Cu–Ocarbonate–Cu = ∼120°) through the disordered OH/CO3 bridges. We ignore any diagonal interactions across the face of the hexagon through the three atom Cu–O–C–O–Cu carbonate bridges.
 | (1) |
The best fit parameters using these models and spin-Hamiltonian (1), where the indices refer to the constituent CuII ions, μB is the Bohr magneton, g is the g-factor fixed at g = 2.00, Ŝ is a spin operator and Jij is the pairwise isotropic exchange interaction parameter, are collected in Fig. S4 (ESI†). What is clear is that each model matches the experimental data (Fig. 5 and Fig. S6, ESI†) well but does so with different sets of J values. In other words, there is no unique fit of the susceptibility data and we can only conclude that the J values in 1 range between −56 < J < −139 cm−1. We disregard model 2 on account of the erroneously large J2 value. Models based on just 2 J values led to poorer fits and/or erroneously large J values.
The data for 2 can fitted using the model shown in Fig. S7 (ESI†), which contains two J values, one within each [Cu4] square (J1 = Cu–O/Br–Cu), and one between the two squares through the two single O-atom bridges (J2 = Cu–OCO3/OOH–Cu). The best fit parameters are J1 = –98.6 cm−1 and J2 = –40.6 cm−1, with g = 2.00. The J values obtained for 1 and 2 are in accordance with previous magneto-structural correlations developed for O/Cl-bridged CuII dimers with similar Cu–O/Cl–Cu angles.1,2
In conclusion, reaction of CuCl2·2H2O or CuBr2 with H4TC[4]A in basic dmf/MeOH solutions affords [CuII24] and [CuII8] clusters, respectively. The former possesses a metallic skeleton conforming to a truncated octahedron containing six [Cu4(TC[4]A)] metalloligands, and the latter a dimer of [Cu4(TC[4]A)] squares. In each case the self-assembly process has been facilitated by the presence of carbonate ions, originating from the fixation of CO2. Both 1 and 2 are novel structure types in Cu-TC[4]A coordination chemistry, the former joining the family of known TC[4]A-supported [M24] cages of NiII, CoII and MnII. Surprisingly, 1 and 2 are just the sixth and seventh known polymetallic Cu-TC[4]A clusters, with 1 being by far the largest yet reported. Given the clear affinity between MII ions and TC[4]A, one would expect many more such compounds await discovery. Magnetic susceptibility measurements reveal the presence of very strong antiferromagnetic interactions between neighbouring CuII ions in 1 and 2, resulting in well isolated S = 0 ground states in both cases.
The work has been supported financially by the Leverhulme Trust (RPG-2021-176).
Data availability
Crystallographic data for compounds 1–2 have been deposited at the CCDC under numbers 2417304 and 2417305 and can be obtained from https://www.ccdc.cam.ac.uk/structures/.† Further data supporting this manuscript have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- B. Bleaney and K. D. Bowers, Proc. R. Soc. London, 1952, 214, 451–465 Search PubMed
.
-
(a) W. H. Crawford, H. W. Richardson, J. R. Wasson, D. J. Hodgson and W. E. Hatfield, Inorg. Chem., 1976, 15, 2107–2110 Search PubMed
; (b) W. E. Marsh, K. C. Patel, W. E. Hatfield and D. J. Hodgson, Inorg. Chem., 1983, 22, 511–515 CrossRef
.
-
(a) S. Ferrer, F. Lloret, E. Pardo, J. M. Clemente-Juan, M. Liu-González and S. García-Granda, Inorg. Chem., 2012, 51, 985–1001 CrossRef PubMed
; (b) M.-A. Bouammali, N. Suaud, N. Guihéry and R. Maurice, Inorg. Chem., 2022, 61, 12138–12148 CrossRef
.
- J. Schnack, Dalton Trans., 2010, 39, 4677–4686 RSC
.
- M. A. Palacios, E. Moreno Pineda, S. Sanz, R. Inglis, M. B. Pitak, S. J. Coles, M. Evangelisti, H. Nojiri, C. Heesing, E. K. Brechin, J. Schnack and R. E. P. Winpenny, ChemPhysChem, 2016, 17, 55–60 CrossRef
.
- L. R. B. Wilson, M. Coletta, M. Evangelisiti, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Dalton Trans., 2022, 51, 4213–4226 RSC
.
-
N. Iki, Thiacalixarenes, in Calixarenes and Beyond, ed. P. Neri, J. Sessler and M. X. Wang, Springer, Cham, 2016 Search PubMed
.
- T. Sone, Y. Ohba, K. Moriya, H. Kumada and K. Ito, Tetrahedron, 1997, 53, 10689–10698 CrossRef
.
- H. Kumagai, M. Hasegawa, S. Miyanari, Y. Sugawa, Y. Sato, T. Hori, S. Ueda, H. Kamiyama and S. Miyano, Tetrahedron Lett., 1997, 38, 3971–3972 Search PubMed
.
- N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291–5316 CrossRef PubMed
.
- R. Kumar, Y. O. Lee, V. Bhalla, M. Kumar and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4824–4870 RSC
.
- M. Yamada, M. R. Gandhi, U. Maheswara, R. Kunda and F. Hamada, J. Inclusion Phenom. Macrocyclic Chem., 2016, 85, 1–18 CrossRef
.
- Y. Bi, S. Du and W. Liao, Coord. Chem. Rev., 2014, 276, 61–72 CrossRef
.
- See for example:
(a) A. Bilyk, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, B. W. Skelton and A. H. White, Inorg. Chem., 2001, 40, 672–686 CrossRef PubMed
; (b) A. Bilyk, J. W. Dunlop, R. O. Fuller, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, G. A. Koutsantonis, I. W. Murray, B. W. Skelton, A. N. Sobolev, R. L. Stamps and A. H. White, Eur. J. Inorg. Chem., 2010, 2127–2152 CrossRef CAS
.
-
(a) A. Bilyk, J. W. Dunlop, R. O. Fuller, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, G. A. Koutsantonis, I. W. Murray, B. W. Skelton, R. L. Stamps and A. H. White, Eur. J. Inorg. Chem., 2010, 2106–02126 CrossRef CAS
; (b) G. Mislin, E. Graf, M. W. Hosseini, A. Bilyk, A. K. Hall, J. M. Harrowfield, B. W. Skelton and A. H. White, Chem. Commun., 1999, 373–374 RSC
; (c) Y. Bi, W. Liao, X. Wang, R. Deng and H. Zhang, Eur. J. Inorg. Chem., 2009, 4989–4994 CrossRef CAS
; (d) C. Zhang, Z. Wang, W.-D. Si, L. Wang, J.-M. Dou, C.-H. Tung and D. Sun, ACS Nano, 2022, 6, 9598–9607 CrossRef
.
-
(a) M. Liu, W. Liao, C. Hu, S. Du and H. Zhang, Angew. Chem., Int. Ed., 2012, 51, 1585–1588 CrossRef CAS
; (b) K. Xiong, F. Jiang, Y. Gai, D. Yuan, L. Chen, M. Wu, K. Sua and M. Hong, Chem. Sci., 2012, 3, 2321–2325 RSC
; (c) K. Su, F. Jiang, J. Qian, Y. Gai, M. Wu, S. M. Bawaked, M. Mokhtar, S. A. AL-Thabaiti and M. Hong, Cryst. Growth Des., 2014, 14, 3116–3123 CrossRef CAS
; (d) K.-C. Xiong, F.-L. Jiang, Y.-L. Gai, D.-Q. Yuan, D. Han, J. Ma, S.-Q. Zhang and M. C. Hong, Chem. – Eur. J., 2012, 18, 5536–5540 CrossRef CAS PubMed
.
- N. Frank, A. Dallmann, B. Braun-Cula, C. Herwig and C. Limberg, Angew. Chem., Int. Ed., 2020, 59, 6735–6739 CrossRef CAS PubMed
.
- See for example:
(a) K. Su, F. Jiang, J. Qian, M. Wu, K. Xiong, Y. Gai and M. Hong, Inorg. Chem., 2013, 52, 3780–3786 CrossRef CAS
; (b) D. Yuan, W.-X. Zhu, S. Ma and X. Yan, J. Mol. Struct., 2002, 616, 241–246 CrossRef
; (c) N. Morohashi, K. Nanbu, A. Tonosaki and T. Hattori, CrystEngComm, 2015, 17, 4799–4808 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, XRD and magnetometry collection/analysis details, additional figures. CCDC 2417304 and 2417305. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc00532a |
| This journal is © The Royal Society of Chemistry 2025 |