Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electroorganocatalytic asymmetric Diels–Alder cycloaddition of hydroquinones with α,β-unsaturated aldehydes†
Dominika
Pomikło
 a,
Krzysztof
Romaniuk
a,
Lesław
Sieroń
a,
Krzysztof
Romaniuk
a,
Lesław
Sieroń
 b and
Anna
Albrecht
b and
Anna
Albrecht
 *b
*b
aInstitute of Organic Chemistry, Faculty of Chemistry, Lodz University of Technology, Żeromskiego 116, 90-924 Łódź, Poland
bInstitute of General and Ecological Chemistry, Faculty of Chemistry, Lodz University of Technology, Żeromskiego 116, 90-924 Łódź, Poland. E-mail: anna.albrecht@p.lodz.pl
First published on 8th April 2025
Abstract
The concept of combining asymmetric aminocatalysis with electrochemistry remains underexplored. Herein, we report an electrochemically driven Diels–Alder cycloaddition reaction of substituted hydroquinones with a series of enals activated by a TMS-protected prolinol catalyst, leading to optically active products with high yields and perfect enantiomeric ratios up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 e.r.
1 e.r.
Quinones and their derivatives constitute versatile building blocks commonly employed in organic chemistry for the preparation of diverse polycyclic systems.1 They undergo various additions, cycloadditions and cascades, leading to products of synthetic or biological relevance.2 They have also been employed as starting materials in asymmetric transformations. In this context, organocatalytic approaches have become increasingly prominent, encompassing various catalytic systems such as aminocatalysis, phosphoric acid catalysis and bifunctional catalysis. These catalysts not only enhance the reaction efficiency, but also provide precise control over enantioselectivity and regioselectivity. This enables the synthesis of complex quinone derivatives with high yields and excellent enantioselectivities, under mild reaction conditions.3 Notably, quinone and related hydroquinone ring systems are present in natural products exhibiting a wide range of structural complexity. They possess significant biological activity ranging from simple antibiotic to anticancer properties.4 Electrochemistry is a multidisciplinary science applied across various fields within the physical, chemical, and biological sciences. It is a powerful method, particularly in organic synthesis, for the efficient functionalization of organic molecules.5 Electrochemical reaction, or electrosynthesis, involves conducting chemical transformations using an electrochemical cell.6 This technique allows for heterogeneous redox reactions, avoiding the use of stoichiometric amounts of redox reagents and the formation of stoichiometric by-products. One of the key advantages of organic electrochemistry is its ability to offer more sustainable and environmentally friendly alternatives to traditional synthetic methods. By leveraging electricity as a clean reagent, it reduces the reliance on hazardous chemicals and minimizes waste production. This makes electrochemistry a valuable tool in the pursuit of greener and more efficient chemical processes.
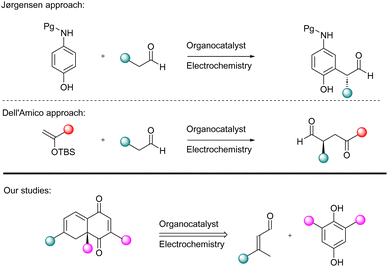
Despite advancements in the field of electrochemical organic synthesis, the application of electrochemical methods in asymmetric organocatalysis still remains limited.7 In 2010, Jørgensen and co-workers demonstrated that electrochemistry is compatible with asymmetric aminocatalysis. They performed an enamine-mediated Michael addition/hemi-acetalization reaction cascade with the corresponding electrophile generated via anodic oxidation.8 In 2024, Dell’Amico performed electrochemical asymmetric radical functionalization of aldehydes in the presence of a redox shuttle.9 In this context, it is worth noting that the combination of organocatalysis with electrochemistry has been proven to be possible with the existing examples showing the potential of such strategies as a useful tool in the stereocontrolled synthesis of functionalized organic compounds (Fig. 1).
Herein, we present our studies on enantioselective, asymmetric Diels–Alder cycloaddition of hydroquinone with α,β-unsaturated aldehydes. The corresponding dienophile is generated electrochemically, providing the opportunity to perform Diels–Alder cycloaddition. Anodic oxidation of hydroquinones results in the formation of appropriate para-quinones, which constitute a group of highly reactive electron-poor dienophiles able to react with electron-rich dienamine intermediates derived from α,β-unsaturated aldehydes.10
The feasibility of the reaction was assessed by applying 2,6-dimethylhydroquinone 1a and 3-methyl-2-butenal 2a in a CH3CN/H2O/HFIP solvent mixture in the presence of organocatalyst A (20 mol%), employing tetrabutylammonium perchlorate as the supporting electrolyte, an RVC electrode as the anode and stainless steel (SS) as the cathode in an undivided electrochemical cell under constant current conditions (2.5 mA, 3.8 F mol−1). The desired product was formed in 52% yield and with excellent stereoselectivity 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 e.r. (Table 1, entry 1).
1 e.r. (Table 1, entry 1).
| Entry | Deviation from standard conditions | Yield (%) | e.r. |
|---|---|---|---|
a Standard reaction conditions: 1a (0.217 mmol, 1.0 eq.) and 2a (0.651 mmol, 3 eq.) added portionwise (1 eq. every 3 hours) in the presence of [nBu4N]+[ClO4]− (0.217 mmol, 1 eq.) and organocatalyst A (20 mol%) in CH3CN/H2O/HFIP (0.1 M).
b First value refers to the duration of electrolysis. Total reaction time is given in the brackets.
c Reactions conducted in the solvent mixture 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1.
d Reaction conducted in the solvent mixture 1 0.1.
d Reaction conducted in the solvent mixture 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05.
e Reaction conducted in the solvent mixture 0.9 0.05.
e Reaction conducted in the solvent mixture 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05.
f Reaction conditions: 1a (1.0 mmol, 1.0 eq.) and 2a (3.0 mmol, 3 eq.), 2.5 mA, 3.8 F mol−1, 40 h (4 days) (see footnote b). 0.05.
f Reaction conditions: 1a (1.0 mmol, 1.0 eq.) and 2a (3.0 mmol, 3 eq.), 2.5 mA, 3.8 F mol−1, 40 h (4 days) (see footnote b).
|
|||
| 1c | None | 52 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2c | 2.5 mA, 5 h 30 min (24 h)b, 2.2 F mol−1 | 37 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 3c | C(graphite) instead of RVC | 46 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 4c | C(glass) instead of RVC | 47 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 5c | nBu4NBF4 as electrolyte | 43 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 6c | nBu4NPF6 as electrolyte | 51 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 7c | Reaction degassed | 44 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 8c | Reaction degassed, 2 eq. of electrolyte | 38 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 9c | 0.75 eq. of electrolyte | 51 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10c | Reaction degassed, 2 eq. of electrolyte, 6 h (24 h) | 6 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 11d | CH3CN/HFIP | 63 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 12e | CHCl3/CH3CN/HFIP | 81 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13e | Catalyst B, CHCl3/CH3CN/HFIP | 24 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14e | Catalyst C, CHCl3/CH3CN/HFIP | 54 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15e | Catalyst A (10 mol%), CHCl3/CH3CN/HFIP | 85 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 16e | Catalyst A (10 mol%), benzoic acid (10 mol%), CHCl3/CH3CN/HFIP | 85 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17ef | Catalyst A (10 mol%), benzoic acid (10 mol%), CHCl3/CH3CN/HFIP | 56 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 18e | No electricity | No reaction | — |
| 19e | No catalyst | No reaction | — |
Notably, the anodic oxidation process of 2,6-dimethylhydroquinone 1a using the above electrochemical setup was achieved in 9 h (monitored by 1H NMR) and the reaction was left overnight for full conversion due to the presence of the oxidized analogue of 1a in the crude reaction mixture. In the next stage of research, we tested other carbon-based electrodes (C(graphite) and C(glass), Table 1, entries 3 and 4) as the anode and stainless steel (SS) as the cathode. Disappointingly, both the yield and enantiomeric excess deteriorated under these conditions.
The influence of different electrolytes on the chemical efficiency and stereoselectivity of the model reaction was also investigated. The reaction was observed to proceed less efficiently in the case of nBu4NBF4, NaClO4·H2O and LiClO4 (43–49% yield, Table 1, entry 5 and Table S1, ESI†), and with comparable efficiency applying other quaternary ammonium salts such as nBu4NPF6 or nBu4NBr (50–51% yield, Table 1, entry 6 and ESI†). Furthermore, the reaction stereocontrol slightly decreased.
Disappointingly, screening of different supporting electrolytes did not bring any significant improvement in the reaction yield. Therefore, we decided to investigate the influence of degassing (Table 1, entry 7), and increased (Table 1, entry 8) or decreased (Table 1, entry 9) concentration of the supporting electrolyte (for more details see Table S2, ESI†). In general degassing and increased loading of the electrolyte were highly unbeneficial in terms of reaction yield, while reducing the amount of electrolyte gave a slight decrease in the yield of the desired product with the persistence of high enantioselectivity (Table 1, entry 9).
To our delight, evaluation of the solvent mixture showed it to be crucial for the chemical efficiency of the process. Firstly, we identified that the reaction proceeded more efficiently without the water additive (Table 1, entry 11), while the CHCl3/CH3CN/HFIP solvent mixture allowed the desired product to be obtained in 81% yield and with excellent stereoselectivity of 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 e.r. (Table 1, entry 12). Organocatalyst A proved to be the best in this electrochemical protocol, as the use of a more sterically hindered silyl protecting group as in catalyst B or the less nucleophilic aminocatalyst C highly suppressed the chemical efficiency of the optimized process (Table 1, entries 13 and 14). Finally, a decreased loading of organocatalyst A to 10 mol% (Table 1, entry 15) and addition of benzoic acid, which facilitates dienamine formation (Table 1, entry 16), was evaluated in the model reaction. The latter protocol was the most efficient for the developed process. Furthermore, the model reaction was performed at a 1.0 mmol scale to demonstrate the utility of the method, and the desired product 3a was obtained in a slightly lower yield and stereoselectivity (Table 1, entry 17). The necessity of the applied current and organocatalyst was confirmed by a control experiment as the cycloaddition product 3a was not observed in their absence (Table 1, entries 18 and 19).
1 e.r. (Table 1, entry 12). Organocatalyst A proved to be the best in this electrochemical protocol, as the use of a more sterically hindered silyl protecting group as in catalyst B or the less nucleophilic aminocatalyst C highly suppressed the chemical efficiency of the optimized process (Table 1, entries 13 and 14). Finally, a decreased loading of organocatalyst A to 10 mol% (Table 1, entry 15) and addition of benzoic acid, which facilitates dienamine formation (Table 1, entry 16), was evaluated in the model reaction. The latter protocol was the most efficient for the developed process. Furthermore, the model reaction was performed at a 1.0 mmol scale to demonstrate the utility of the method, and the desired product 3a was obtained in a slightly lower yield and stereoselectivity (Table 1, entry 17). The necessity of the applied current and organocatalyst was confirmed by a control experiment as the cycloaddition product 3a was not observed in their absence (Table 1, entries 18 and 19).
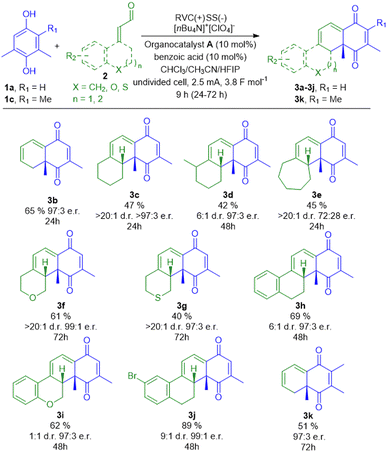
With the optimized protocol in hand, we next investigated the generality of the process (Fig. 2). A series of structurally diverse α,β-unsaturated aldehydes were tested in the reaction, categorized as follows: uncyclic 2b, cyclic 2c–2e, heterocyclic 2f–2g and functionalized polycyclic 2h–2j. Notably, the application of enals 2c and 2e–2j resulted in the formation of products possessing two chiral carbon atoms, while 2d provided the structure with 3 stereogenic centers.
Initially, trans-crotonaldehyde (2b) was tested in the model reaction. The desired product 3b was obtained in 65% yield and a high enantiomeric ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 e.r. (Fig. 2). A group of cyclic enals 2c–2e delivered the appropriate cycloaddition products 3c–3e with moderate yields in the range of 42–47% (Fig. 2). Reactions of unsubstituted derivatives (2c, cyclohexyl) and (2e, cycloheptyl) resulted in the formation of products 2c and 2e as single diastereoisomers, while 2-(2-methylcyclohexylidene)acetaldehyde (2d) gave a mixture of two diastereoisomers 3d due to the presence of an additional stereogenic centre at position 2 (Fig. 2). In the next stage of research, two heterocyclic aldehydes 2f–2g were tested and delivered the desired products 3f and 3g in 61% and 40% yields, respectively, with high diastereo- and enantioselectivities. Finally, a series of polycyclic precursors of dienes 2h–2j were evaluated in the model electroorganocatalytic approach to achieve further extension of the cyclic system. Successfully, 1,2,3,4-tetrahydronaphthalene-based aldehyde 2h furnished the desired cycloaddition product 3h in 62% yield with high diastereo- and enantioselectivities. Surprisingly, stereocontrol of the process was highly diminished when applying heteroaromatic enal 2i (Fig. 2). Application of 7-bromo derivative 2j in the described protocol resulted in the formation of bromo-tetrahydrochrysene-1,4-dione product 3j in the highest yield of 89% with an excellent enantiomeric ratio of 99
3 e.r. (Fig. 2). A group of cyclic enals 2c–2e delivered the appropriate cycloaddition products 3c–3e with moderate yields in the range of 42–47% (Fig. 2). Reactions of unsubstituted derivatives (2c, cyclohexyl) and (2e, cycloheptyl) resulted in the formation of products 2c and 2e as single diastereoisomers, while 2-(2-methylcyclohexylidene)acetaldehyde (2d) gave a mixture of two diastereoisomers 3d due to the presence of an additional stereogenic centre at position 2 (Fig. 2). In the next stage of research, two heterocyclic aldehydes 2f–2g were tested and delivered the desired products 3f and 3g in 61% and 40% yields, respectively, with high diastereo- and enantioselectivities. Finally, a series of polycyclic precursors of dienes 2h–2j were evaluated in the model electroorganocatalytic approach to achieve further extension of the cyclic system. Successfully, 1,2,3,4-tetrahydronaphthalene-based aldehyde 2h furnished the desired cycloaddition product 3h in 62% yield with high diastereo- and enantioselectivities. Surprisingly, stereocontrol of the process was highly diminished when applying heteroaromatic enal 2i (Fig. 2). Application of 7-bromo derivative 2j in the described protocol resulted in the formation of bromo-tetrahydrochrysene-1,4-dione product 3j in the highest yield of 89% with an excellent enantiomeric ratio of 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 e.r. Two different quinone precursors were also tested in the described protocol. Trimethylhydroquinone (1c) resulted in the formation of the product 3k in 51% yield as a single diastereoisomer with a high enantiomeric ratio of 97
1 e.r. Two different quinone precursors were also tested in the described protocol. Trimethylhydroquinone (1c) resulted in the formation of the product 3k in 51% yield as a single diastereoisomer with a high enantiomeric ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 e.r. The reaction time was found to be dependent on the structure of the starting enals. Unsuccessful experiments are included in the ESI.† For simple aldehydes such as 2a, 2b, 2c, and 2e, a reaction time of 24 hours was sufficient to achieve full conversion and to obtain the desired products. However, enals 2f and 2g required an extended reaction time up to 72 hours to complete the organocatalytic transformation. In contrast, aldehydes 2h, 2i, and 2j underwent full conversion within 48 hours, affording the corresponding products 3h, 3i, and 3j.
3 e.r. The reaction time was found to be dependent on the structure of the starting enals. Unsuccessful experiments are included in the ESI.† For simple aldehydes such as 2a, 2b, 2c, and 2e, a reaction time of 24 hours was sufficient to achieve full conversion and to obtain the desired products. However, enals 2f and 2g required an extended reaction time up to 72 hours to complete the organocatalytic transformation. In contrast, aldehydes 2h, 2i, and 2j underwent full conversion within 48 hours, affording the corresponding products 3h, 3i, and 3j.
The structure and relative configuration of the product 3c were unambiguously determined by single crystal X-ray diffraction analysis (Fig. S26, ESI†). The stereochemistry of products 3a–3b and 3d–3j was assigned by analogy and was in accordance with the absolute configuration assigned by Jørgensen and coworkers.2f,11
Having demonstrated the synthetic utility of the reaction, cycloadduct 3j was subjected to Suzuki coupling, resulting in its transformation to 7-phenyl tetrahydrochrysene-1,4-dione derivative 4 in 55% yield. Importantly, the formation of 4 proceeds without deterioration of the enantiomeric ratio of 3j (Scheme 1).
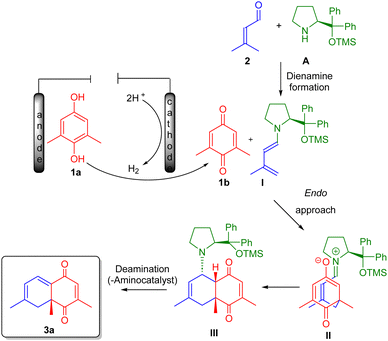
After experimental confirmation of the structure and relative stereochemistry of the final products 3, a plausible mechanism for the studied [4+2]-cycloaddition was proposed (Scheme 2). The stereo- and regioselective anodic oxidation and organocatalytic formation of 1,4-diones 3 is proposed to proceed through a sequential process (Scheme 2). The first step involves the electrochemical oxidation of 2,6-dimethylhydroquinone 1a to generate the dienophilic intermediate 1b. In the second step, an endo-selective vinylogous nucleophilic addition of an electron-rich dienamine intermediate I, formed via the condensation of aldehyde 2 with organocatalyst A, to quinone 1b furnishes the zwitterionic intermediate II. A second bond-forming event generates the cyclized intermediate III. Subsequent deprotonation eventually triggers the regeneration of the aminocatalyst and yields product 3a. The regio- and endo-selectivity were previously confirmed by Jørgensen et al.'s DFT calculations.2b
In summary, we have demonstrated a simple and concise access to a series of polycyclic enantiorich products 3 employing an electro/organocatalytic cycloaddition process. The developed reactivity constitutes a facile means for electrosynthesis of 3 in a diastereo- and enantioselective manner. Transformation of the cycloadduct 3j to 7-phenyl analogue 4 without deterioration of the enantioselectivity confirmed the utility of the developed methodology.
This work was supported by a grant from the National Science Centre, Poland (UMO-2022/46/E/ST4/00338).
Data availability
Data for this article, including NMR FIDs and crystallographic data, are available at https://rdb.p.lodz.pl/dataverse/W3 and https://doi.org/10.34658/RDB.ZOXWZA. Supplementary crystallographic data can be found at The Cambridge Crystallographic Data Centre under CCDC number 2383376.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) in The Chemistry of the Quinoid Compounds, ed. S. Patai and Z. Rapoport, Wiley, New York, 1988 Search PubMed; (b) A. Kutyrev, Tetrahedron, 1991, 47, 8043 CrossRef CAS; (c) M. Tisler and A. R. Katritzky, Advances in Heterocyclic Chemistry, Academic Press, London, 1989, vol. 45, p. 37 Search PubMed.
- (a) J. Aleman, S. Cabrera, E. Maerten, J. Overgaard and K. A. Jørgensen, Angew. Chem., Int. Ed., 2007, 46, 5520–5523 CrossRef CAS PubMed; (b) T. K. Johansen, C. V. Gómez, J. R. Bak, R. L. Davis and K. A. Jørgensen, Chem. – Eur. J., 2013, 19, 16518–16522 CrossRef CAS PubMed; (c) J. Escorihuela, W. J. E. Looijen, X. Wang, A. J. A. Aquino, H. Lischka and H. Zuilhof, J. Org. Chem., 2020, 85, 13557–13566 CrossRef CAS PubMed; (d) J. Blom, T. K. Johansen, F. Jensen and K. A. Jørgensen, Chem. Commun., 2016, 52, 7153–7156 RSC; (e) Ł. Albrecht, C. V. Gómez, C. B. Jacobsen and K. A. Jørgensen, Org. Lett., 2013, 15, 3010–3013 CrossRef PubMed; (f) K. S. Halskov, B. S. Donslund, S. Barfüsser and K. A. Jørgensen, Angew. Chem., Int. Ed., 2014, 53, 4137–4141 CrossRef CAS PubMed.
- (a) B. Hosamani, M. F. Ribeiro, E. N. da Silva Júnior and I. N. N. Namboothiri, Org. Biomol. Chem., 2016, 14, 6913–6931 RSC; (b) X. Zhang, Y.-H. Chen and B. Tan, Tetrahedron Lett., 2018, 59, 473–486 CrossRef CAS.
- (a) A. R. Mehendale and R. H. Thomson, Phytochemistry, 1975, 14, 801–802 CrossRef CAS; (b) J. Yin and L. S. Liebeskind, J. Org. Chem., 1998, 63, 5726–5727 CrossRef CAS PubMed; (c) T. Dunlap, R. E. Chandrasena, Z. Wang, V. Sinha, Z. Wang and G. R. Thatcher, Chem. Res. Toxicol., 2007, 20, 1903–1912 Search PubMed; (d) M. Chigr, H. Fillion, A. Rougny, M. Berlion, J. Riondel and H. Beriel, Chem. Pharm. Bull., 1990, 38, 688–691 CrossRef CAS PubMed; (e) K. Haruna, H. Kanezaki, K. Tanabe, W. M. Dai and S. Nishimoto, Bioorg. Med. Chem., 2006, 14, 4427–4432 CrossRef CAS PubMed; (f) E. J. Lana, F. Carazza and J. A. Takahashi, J. Agric. Food Chem., 2006, 54, 2053–2056 CrossRef CAS PubMed; (g) C. H. Sun, Y. Wang, Z. Wang, J. Q. Zhou, W. Z. Jin, X. F. You, H. Gao, L. X. Zhao, S. Y. Si and X. Li, J. Antibiot., 2007, 60, 211–215 CrossRef CAS PubMed; (h) L. Mendoza, A. R. Maturana, W. Cardona, T. Delgado-Castro, C. Garcia, C. Lagos and M. Cotoras, J. Agric. Food Chem., 2005, 53, 10080–10084 CrossRef CAS PubMed; (i) I. Ali, F. G. Khan, K. A. Suri, B. D. Gupta, N. K. Satti, P. Dutt, F. Afrin, G. N. Qaziand and I. A. Khan, Ann. Clin. Microbiol. Antimicrob., 2010, 9, 7 CrossRef PubMed; (j) S. K. Berezin and J. T. Davis, J. Am. Chem. Soc., 2009, 131, 2458–2459 CrossRef CAS PubMed.
- (a) H. A. Lund, J. Electrochem. Soc., 2002, 149, 21–33 CrossRef; (b) A. G. A. Volta, Nat. Philos. Chem. Arts, 1800, 4, 179–187 Search PubMed; (c) M. Faraday, Ann. Phys., 1834, 47, 438 Search PubMed.
- (a) M. D. Karkas, Chem. Soc. Rev., 2018, 47, 5786 RSC; (b) D. Pollok and S. R. Waldvoge, Chem. Sci., 2020, 11, 12386 RSC; (c) C. Margarita and H. Lundberg, Catalysts, 2020, 10, 982 CrossRef CAS; (d) J. M. Lassaletta, Nat. Commun., 2020, 11, 3787 CrossRef CAS PubMed; (e) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230 CrossRef CAS PubMed; (f) C. Kingston, M. D. Palkowitz, Y. Takahira, J. C. Vantourout, B. K. Peters, Y. Kawamata and P. S. Baran, Acc. Chem. Res., 2020, 53, 72 CrossRef CAS PubMed; (g) C. Zhu, N. W. J. Ang, T. H. Meyer, Y. Qiu and L. Ackermann, ACS Cent. Sci., 2021, 7, 415–431 CrossRef CAS PubMed.
- (a) J.-Y. He, N.-N. Wang, R. Zhao, L.-X. Kong, C. Zhu, T.-S. Mei and H. Xu, Eur. J. Org. Chem., 2024, e202400817 CrossRef CAS; (b) A. Krech, M. Laktsevich-Iskryk, N. Deil, M. Fokin, M. Kimm and M. Ošeka, Chem. Commun., 2024, 60, 14026–14029 RSC; (c) N. Fu, L. Li, Q. Yang and S. Luo, Org. Lett., 2017, 19, 2122 CrossRef CAS PubMed; (d) X.-H. Ho, S.-l Mho, H. Kang and H.-Y. Jang, Eur. J. Org. Chem., 2010, 4436 CrossRef CAS.
- K. L. Jensen, P. T. Franke, L. T. Nielsen, K. Daasbjerg and K. A. Jørgensen, Angew. Chem., Int. Ed., 2010, 49, 129 CrossRef CAS PubMed.
- D. Mazzarella, C. Qi, M. Vanzella, A. Sartorel, G. Pelosi and L. Dell’Amico, Angew. Chem., Int. Ed., 2024, 63, e202401361 CrossRef CAS PubMed.
- (a) M. M. Heravi, V. Zadsirjan, E. Kouhestania and B. Alimadadi Jani, Chem. Rec., 2020, 20, 273 CrossRef CAS PubMed; (b) K. Chiba, M. Jinno, R. Kuramoto and M. Tada, Tetrahedron Lett., 1998, 39, 5527 CrossRef CAS.
- CCDC 2383376 contains the supplementary crystallographic data for this paper†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2383376. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc00505a |
| This journal is © The Royal Society of Chemistry 2025 |