Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Halogen–copper redox chemistry as a driving force for spherical Janus microswimmers†
Kelly
Henze
a,
Zuyao
Xiao
a,
Khalifa
Mohamed
 b and
Juliane
Simmchen
b and
Juliane
Simmchen
 *b
*b
aFreigeist Group, Physical Chemistry TU Dresden, Zellescher Weg 19, 01062 Dresden, Germany. Tel: +49 351 463-33886
bPure and Applied Chemistry, University of Strathclyde, Glasgow G1 1BX, UK. E-mail: juliane.simmchen@strath.ac.uk
First published on 21st March 2025
Abstract
Most Janus particle microswimmers use catalytic reaction mechanisms, including hydrogen peroxide or hydrazine degradation, to generate gradients for self-propulsion. However, several alternative reaction mechanisms can also lead to activity, including the redox reactions of noble metals, enzymes and glucose, often using spherical Cu@SiO2 Janus particles. Copper, as a very versatile metal, is involved in reactions, as an oxidant or as an electrode, for example in dilute halogen solutions. This so-called nanobattery system works well for rod-shaped bimetallic and shape-asymmetric rods. To allow comparability with simulations, we present the adaptation of this propulsion reaction for Janus colloids. Altogether, this study provides an overview of the chemical process and swimming behaviour of spherical Cu@SiO2 Janus particles in dilute halogen solutions. We combine an experimental approach that includes the determination of the effect of particle size and cap thickness, supported by simulations of these systems.
The propulsion of artificial active matter has been dominated by H2O2 degradation since its first discovery.1 Given that the degradation of this strongly oxidising chemical provides an excellent and reliable fuel for microscale motion, the combination Pt@SiO2 Janus particle has become a widely used model system to study behaviors. However, continuously expanding applications require more flexibility in fuel selection in order to use more sustainable or biocompatible fuels. Since Sen and his team studied the reaction of copper with halogens using a Pt–Cu bimetallic rod and their self-electrophoretic movement as nanobatteries,2 it is known that suitable reactions are not restricted to catalytic systems. Recent examples include galvanophoretic movement while reacting with noble metal acids,3,4 different enzyme substrates,5,6 and also glucose,7 expanding on the range of potential microswimmer systems. Since the Janus morphology allows better comparison with simulations and theory, this spherical particle type dominates experimental studies. Therefore, in this work we introduce Cu@SiO2 Janus particles in halogen fuels to explore the ‘hydrogen atom’ of active particles in this fuel combination, which allows the study of interesting chemical influences: the interplay between the established gradient, the ionic strength and the superimposed chemical equilibria. We compare microscopic behaviour, chemical analysis and finite element simulation to improve our ability to classify the system under investigation in the ever-expanding field of fuel options. Halogens and copper react to form copper halides, which can be either Cu(I) or Cu(II) halides, depending on the halogen and the reaction conditions. The overall reactivity decreases from fluorine towards iodine, which is the least reactive homologue and frequently forms insoluble CuI. Nevertheless, the halogen is permanently reduced during the reaction while the Cu is continuously oxidised due to its lower electronegativity. Since the reaction of fluorine and chlorine is very vigorous and potentially explosive, which is not ideal in a microscope setup, we restrict our experiments to the less reactive bromine and iodine, highlighting the interesting difference of a soluble product for bromine, while obtaining a highly insoluble precipitating product for iodine. Bromine (Br2) is a reddish brown liquid at room temperature with a strong, pungent odor. It has limited solubility in water and undergoes a self-disproportionation reaction (see eqn (1)).
| Br2+H2O ⇌ HBrO + Br− + H+ Keq = 3.5 × 10−9 M2 (298 K) | (1) |
This process leads to two reactive species, HBrO and Br2. Looking at the equilibrium constant for bromine hydrolysis of 3.5 × 10−9 M2 at 298 K indicates the disproportionation of Br2 in water which results in an initially acidic solution (see Fig. S2, ESI†). In the presence of Cu, the pH increases significantly over time, indicating the development of the reaction.
On this basis, we assume that HBrO is used as a higher oxidant to reduce Cu, acting as a reactive species and shifting the dissociation reaction of Br2 to the side of its disproportionate ions.
| HBrO + H+ + Br− + Cu ⇌ CuBr2(aq) + H2O ΔE = 0.98 V | (2) |
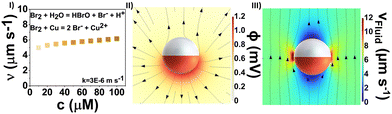
In Fig. 1(I), this reaction is schematically illustrated. For simplification, the Br2 is assumed to dissociate fully into its ions.
 | ||
| Fig. 1 (I) Reaction scheme of Cu with dissociated Br2 (II) mean velocity at different concentrations of Br2 fuel (III) MSD at different concentrations of Br2 solutions. | ||
During the oxidation of the solid Cu surface, the highly soluble CuBr2(aq) is formed (see eqn (2)), releasing ions into the solution. The release of ions creates a concentration gradient that drives the motion of particles through a process known as ionic self-diffusiophoresis. This mechanism is similar to other systems, such as Ag-,8 AgCl-,9 and Ag3PO4 motors.10 However, the halogen fuel system stands out because it exhibits ionic diffusiophoresis at much lower concentrations and does not utilize hydrogen peroxide as a fuel. Additionally, the halogen–copper system operates without the need for an additional power source, such as light, apart from the chemical fuel. Following this, we introduce another type of ionic diffusiophoretic system for spherical Cu@SiO2 microswimmers. Due to the slower diffusion of Cu2+ ions (0.714 × 10−9 m2 s−1) compared to Br− ions (2.08 × 10−9 m2 s−1),11 an outward electric field is established to maintain electroneutrality. This electric field propels the negatively charged particle, causing it to move toward the Cu cap direction. The production of Cu2+ ions during the motion is proven by the conversion with NH3 forming the highly coloured complex of Cu(NH3)42+ and followed by UV-Vis analysis. Fig. S3 (ESI†) depicts the UV-Vis measurement results of the sample with NH3 added after the reaction of Cu@SiO2 with Br2, including samples examining NH3-complexed CuSO4 at varied concentrations.
As copper is consumed during the reaction and the product diffuses into the surrounding liquid, the SEM images of Br2-reacted Cu@SiO2 exhibit no noticeable changes across concentrations from 10 to 250 μM (see Fig. S4, ESI†). At higher concentrations of 500 μM, the copper cap is completely dissolved. Looking at the swimming behaviour of the moving particle in Fig. 1(II) and (III), the shown velocity and MSD curves do not reveal any particular trend from 10 to 100 μM. At 10 μM, the mean velocity of roughly 3 μm s−1 can be attributed to Brownian motion, which is solidified with the linear MSD curve, displaying Brownian diffusivity (MSD(t) ∝ 1).12 A maximum velocity of 6.5 μm s−1 is reached at 25 μM, with an MSD curve indicating super diffusion. However, at higher concentrations, the velocity remains constant around 5 μm s−1, with a lower rise in MSD curve. In contrast, at even higher concentrations of 150 μM, the particles become immediately immobilised due to the large number of ions produced (see Video S1, ESI†), which is related to how these ions affect double-layer interactions and van der Waals forces, as described by the Derjaguin–Landau–Verwey–Overbeek theory.4,13
To understand the correlation between the increasing concentration of Br2 fuel and the increasing ion content, leading to roughly constant swimming speeds, finite element method simulations (COMSOL) were performed. In the simulation of the Br2 system, two major reactions mentioned above were considered: the reaction of Br2 with H2O (eqn (1)) and the reaction of Cu and HOBr (eqn (2)). Since Br2 reacts with H2O to produce HOBr and HOBr will be consumed further by Cu, the overall reaction at the Cu surface was simplified to Cu and Br2 forming CuBr2, which will further dissociate to Cu2+ and Br− (Fig. 2I). We assumed a first order reaction with a constant reaction rate of 3 × 10−6 m s−1 adapted from Sen's work.2 For more details, please refer to the simulation section.
The simulated velocities, as shown in Fig. 2(I), exhibit comparable values ranging from 4 to 6 μm s−1 for Br2 at concentrations between 10 and 100 μM compared to the experimental data. The positive velocity values denote particle propulsion with the Cu cap forward, aligning well with experimental observations. Fig. 2(II) presents the electric potential and electric field lines at a Br2 concentration of 50 μM. In the near field, a slightly higher potential is shown near the Cu surface, along with outward electric field lines extending from Cu to SiO2. These electric field lines, coupled with the negatively charged SiO2, generate a fluid flow, as depicted in Fig. 2(III), moving from the Cu side to the SiO2 side. The velocity does not increase as observed in other chemically active microswimmers at higher fuel concentrations because of the influence of the background ions. At higher concentrations, although more Cu2+ and Br− ions are produced, which would typically generate stronger propulsion, the reaction of Br2 with H2O introduces a significant excess of Br− ions and protons into the solution. These background ions significantly slow down the electrokinetic swimmer due to the increased conductivity of the solution. This occurs in multiple ways, but the primary reason is that the induced electric field E (which is responsible for the propulsion) scales inversely with the solution conductivity δ, as described by Ohm's law: E = i/δ, where i is the current density.14 At higher fuel concentrations, more ions are produced, leading to an increase in the current density i, as it is proportional to the ion concentration. However, the fuel also generates more background ions, causing the solution conductivity δ, to increase proportionally. As a result, the electric field E, remains relatively stable despite the increased ion concentration, which explains why the micromotor speed remains stable even at higher fuel concentrations.
In addition to the Br2 solution, the swimming behavior of Cu@SiO2 Janus particles is observed in I2 fuel, which undergoes an analogous redox reaction with Cu but is less reactive than Br2.
| I2(aq) + 2Cu(s) ⇌ 2CuI(s) | (3) |
At 298 K, the equilibrium constant for the self-disproportion reaction
| I2 + H2O ⇌ HIO + I− + H+ Keq = 5.4 × 10−13 M2 (298 K) | (4) |
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m is further confirmed by XRD (see Fig. S7, ESI†). The formation of insoluble CuI crystals removes most ions from the solution. Nevertheless, the particles swim cap forward, similar to their motion in a Br2 solution. We attribute this propulsion to the presence of intermediate ions, Cu+ and I−, prior to the formation of CuI. Similar to the mechanism in the Br2 system, I− (2.045 × 10−9 m2 s−1) diffuses much faster than Cu+ (0.73 × 10−9 m2 s−1),11 creating an electric field directed from the Cu side to the SiO2 side. This electric field propels the particle forward with the Cu cap leading. Considering the data in Fig. 3(II) and (III) it becomes clear that at a concentration of 10 μM, the movement can be attributed to Brownian motion. At 25 μM, the MSD curve and increased velocity of 5.8 μm s−1 indicate swimming particles, without detectable CuI crystals in SEM and EDX measurements. With increasing fuel concentration, the mean velocities show considerable growth. As a result, the mean velocity with 100 μM I2 is 4 μm s−1 greater than with the same concentration of Br2. Furthermore, the absence of new ion generation in the solution prevents particles from being stuck to the substrate.
3m is further confirmed by XRD (see Fig. S7, ESI†). The formation of insoluble CuI crystals removes most ions from the solution. Nevertheless, the particles swim cap forward, similar to their motion in a Br2 solution. We attribute this propulsion to the presence of intermediate ions, Cu+ and I−, prior to the formation of CuI. Similar to the mechanism in the Br2 system, I− (2.045 × 10−9 m2 s−1) diffuses much faster than Cu+ (0.73 × 10−9 m2 s−1),11 creating an electric field directed from the Cu side to the SiO2 side. This electric field propels the particle forward with the Cu cap leading. Considering the data in Fig. 3(II) and (III) it becomes clear that at a concentration of 10 μM, the movement can be attributed to Brownian motion. At 25 μM, the MSD curve and increased velocity of 5.8 μm s−1 indicate swimming particles, without detectable CuI crystals in SEM and EDX measurements. With increasing fuel concentration, the mean velocities show considerable growth. As a result, the mean velocity with 100 μM I2 is 4 μm s−1 greater than with the same concentration of Br2. Furthermore, the absence of new ion generation in the solution prevents particles from being stuck to the substrate.
 | ||
| Fig. 3 (I) Reaction scheme (II) mean velocity at different concentrations of I2 (III) MSD at different concentrations (IV) SEM and EDX analysis of particles at different I2 concentrations. | ||
To validate the experimental results, we also implemented COMSOL simulations. For the I2 system only one reaction was considered (eqn (3)), since I2 reacts minimally with H2O compared to Br2. The intermediate ions, Cu+ and I−, were considered the major ionic species in the system. The reaction rate constant was set to 1 × 10−7 m s−1, as adapted from Sen et al.2 The simulated velocities mirror the experimental trend, with increasing velocity as the I2 concentration increases from 10 to 100 μM. The lower values observed in the experiments can be attributed to the formation of CuI crystals on the Cu surface, which partially cover the Cu cap and slow down the reaction rate. The distinction between the I2 and Br2 systems can also be understood by examining the generation of background ions. Unlike Br2, I2 does not react with water to produce excess ions. As a result, at higher I2 concentrations, more Cu+ and I− ions are produced in the near field, but the far-field conductivity of the solution remains unchanged (Fig. 4(II)).
Compared to the electric field generated in the Br2 system, the direction of the electric field with I2 is similar. However, the electric potential near the active side of the particle with I2 is significantly higher compared to the Br2 system, which indicates a more vigorous electric field intensity for I2, resulting in a more substantial fluid flow shown in Fig. 4(III). In comparison to a Br2 solution as fuel for the spherical Janus particles, I2 as a fuel exhibits significantly better swimming behavior with increasing fuel concentrations due to the formation of barely soluble CuI crystals rather than ions.
To complete the study we performed experiments to analyse the influence of morphological characteristics of the Janus particle on the swimming. Thus we examined the velocity data for both halogen fuels at a concentration of 50 mM, varying the particle size from 2, to 3, and 5 μm with a constant copper cap thickness of 30 nm and for 5 μm particles we analyse different copper cap thicknesses of 5, 30, and 50 nm, showing the results in Fig. 5.
The smallest particles of 2 μm show for Br2 slightly higher speed (7.7 μm s−1) than for the larger particles, which aligns with studies on the correlation between swimmer diameter and speed, noting that larger particles produce more ions, resulting in a higher screening length.16,17 However, the decrease is not very significant. The data for 50 μM I2 fuel, which show mean velocities in the range of 4 to 7 μm s−1, confirm these findings. The comparison of different layer thicknesses for 5 μm particles shows more interesting tendencies: in a Br2 solution there is no significant difference for the speeds obtained for 5 nm and 30 nm layers (3.7 μm s−1 and 4.9 μm s−1, respectively). However, with a 50 nm cap, the particles become stuck to the substrate immediately after adding the fuel. For the I2 solutions, we observe no apparent trapping even for the thickest layers and their velocities range from 3.8 to 6.4 μm s−1, confirming the crucial importance of the precipitation of CuI.
In conclusion, using a halogen–copper redox fuel system, we investigate the effects of overlaid secondary equilibria on the active motion of spherical Janus particle microswimmers. Our findings reveal that swimming speeds vary with different fuels, showing a peak velocity of 6.5 μm s−1 for Br2 at 25 μM. In contrast, at higher concentrations, the velocity remains approximately constant due to particle-surrounded ions. In contrast, I2 displays a continuous increase in velocity with concentration, driven by the precipitation of insoluble CuI. Simulations support our experimental data, giving insights into the reaction mechanisms and fluid dynamics involved. The differing swimming behaviours are attributed to the distinct disproportionation reactions of the halogens in water and product solubility. This study gives us a clearer perspective on this specific system while also facilitating comparisons with other fuel systems.
JS acknowledges funding from the Cottrell Foundation. Zuyao Xiao acknowledges a CSC PhD fellowship. This work was supported by the Free State of Saxony and by the European Union (ESF Plus) by funding of the research group ‘MultiMOD’.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- J. R. Howse, R. A. Jones, A. J. Ryan, T. Gough, R. Vafabakhsh and R. Golestanian, Phys. Rev. Lett., 2007, 99, 048102 CrossRef PubMed
.
- R. Liu and A. Sen, J. Am. Chem. Soc., 2011, 133, 20064–20067 CrossRef CAS PubMed
.
- L. Feuerstein, C. G. Biermann, Z. Xiao, C. Holm and J. Simmchen, J. Am. Chem. Soc., 2021, 143, 17015–17022 CrossRef CAS PubMed
.
- J. Bastos-Arrieta, C. Bauer, A. Eychmüller and J. Simmchen, J. Chem. Phys., 2019, 150, 144902 CrossRef PubMed
.
- X. Ma, A. Jannasch, U.-R. Albrecht, K. Hahn, A. Miguel-López, E. Schaffer and S. Sánchez, Nano Lett., 2015, 15, 7043–7050 CrossRef PubMed
.
- X. Ma and S. Sánchez, Tetrahedron, 2017, 73, 4883–4886 CrossRef CAS
.
- F. Fessler, M. Wittmann, J. Simmchen and A. Stocco, Soft Matter, 2024, 20, 5904–5914 RSC
.
- Z. H. Shah, S. Wang, L. Xian, X. Zhou, Y. Chen, G. Lin and Y. Gao, Chem. Commun., 2020, 56, 15301–15304 RSC
.
- C. Zhou, H. Zhang, J. Tang and W. Wang, Langmuir, 2018, 34, 3289–3295 CrossRef CAS PubMed
.
- D. Wentao, L. Ran and S. Ayusman, J. Am. Chem. Soc., 2013, 135(4), 1280–1283 CrossRef PubMed
.
-
J. Rumble, CRC Handbook of Chemistry and Physics, Abingdon, 2020 Search PubMed
.
- V. Briane, M. Vimond and C. Kervrann, Briefings Bioinf., 2020, 21, 1136–1150 CrossRef CAS PubMed
.
- O. Malysheva, T. Tang and P. Schiavone, J. Colloid Interface Sci., 2008, 327, 251–260 CrossRef CAS PubMed
.
- J. L. Moran and J. D. Posner, Annu. Rev. Fluid Mech., 2017, 49, 511–540 CrossRef
.
- I. Lengyel, I. R. Epstein and K. Kustin, Inorg. Chem., 1993, 32, 5880–5882 CAS
.
- A. T. Brown, W. C. Poon, C. Holm and J. De Graaf, Soft Matter, 2017, 13, 1200–1222 RSC
.
- S. Ebbens, M.-H. Tu, J. R. Howse and R. Golestanian, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 85, 020401 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc00405e |
| This journal is © The Royal Society of Chemistry 2025 |