Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A BNAlP-heterocycle†
Tim
Wellnitz
ab,
Leonie
Wüst
ab,
Holger
Braunschweig
 *ab and
Christian
Hering-Junghans
*ab and
Christian
Hering-Junghans
 *c
*c
aInstitute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: h.braunschweig@uni-wuerzburg.de
bInstitute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
cLeibniz Institute for Catalysis e.V. (LIKAT), A.-Einstein-Str. 29a, 18059 Rostock, Germany. E-mail: christian.hering-junghans@catalysis.de
First published on 5th February 2025
Abstract
We report the [2+2]-cyclization reaction of a phosphaalumene with its lighter analog, an iminoborane, to give a species with a four-membered BNAlP-heterocycle with alternating Lewis basic and acidic centers in the ring. The reactivity of this heterocycle towards alkenes and alkynes is described, giving chain molecules and ring-expanded species.
Multiple bonds of heavier main group elements1 are the focus of contemporary research for their potential in the activation of small, unreactive molecules, the design of new materials, and for their potential applications in catalysis.2,3 Among these, Pn–Al (Pn = P, As) multiple bonds have long evaded synthetic realization.4 In 2021 the first phospha- and arsaalumenes were synthesized as thermally robust, violet or blue crystalline compounds,5 respectively, with a strongly shielded 31P NMR signal in the phosphaalumene. DippTerPn = AlCp* (Pn = P (1), As; DippTer = 2,6-(2,6-iPr2C6H3)-C6H3) show E-configured Pn–Al multiple bonds, which are strongly polarized towards the pnictogen center, especially the π-component. Despite its strong polarization the P–Al bond in 1 readily engages in [2+2]-cycloaddition reactions, for example with disubstituted alkynes, giving so-called 1,2-phospaalumetes (Scheme 1, A). With acetylene two additional molecules insert into the P–Al bond giving 1,4-phosphaaluminabarrelenes (Scheme 1, B).6 Iminoboranes of the general form R–N
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–R′ are isosters of alkynes and are the isovalence electronic lighter analogs of 1. The triple bond character in iminoboranes differs from phosphaborenes,7,8-alumenes5,9 and -gallenes10–13 with negligible non-classical triple bond character.
B–R′ are isosters of alkynes and are the isovalence electronic lighter analogs of 1. The triple bond character in iminoboranes differs from phosphaborenes,7,8-alumenes5,9 and -gallenes10–13 with negligible non-classical triple bond character.
 | ||
| Scheme 1 Reactivity of phosphaalumene DippTerPAlCp* (1) and iminoboranes (C) towards different alkynes (TMP = 2,2,6,6-tetramethylpyrolidyl; Pip = piperidyl; Ar* = 2,6-(Ph2CH)-4-tBu-C6H2). | ||
By contrast to alkynes the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond in iminoboranes is strongly polarized (similar to 1); thus, its chemistry is dominated by oligomerization processes. Steric protection, or Lewis bases can significantly stabilize the B
N bond in iminoboranes is strongly polarized (similar to 1); thus, its chemistry is dominated by oligomerization processes. Steric protection, or Lewis bases can significantly stabilize the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and suppress oligomerization.14 The parent iminoborane HNBH has only been stabilized in push–pull fashion through FLP-type stabilization.15tBu–N
N bond and suppress oligomerization.14 The parent iminoborane HNBH has only been stabilized in push–pull fashion through FLP-type stabilization.15tBu–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–tBu (Scheme 1, C) is a moderately stable variant (t1/2 at 50 °C ca. 3 d) and was shown to engage in dipolar [3+2]-cycloaddition reactions with organic azides and diazomethane derivatives even at low temperatures.16 In the coordination sphere of rhodium, tBu–N
B–tBu (Scheme 1, C) is a moderately stable variant (t1/2 at 50 °C ca. 3 d) and was shown to engage in dipolar [3+2]-cycloaddition reactions with organic azides and diazomethane derivatives even at low temperatures.16 In the coordination sphere of rhodium, tBu–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–tBu has been shown to selectively react with alkynes to give 1,2- and 1,4-azaborinines (Scheme 1, D).17–19 In the case of 1,4-azaborinines this process involves B
B–tBu has been shown to selectively react with alkynes to give 1,2- and 1,4-azaborinines (Scheme 1, D).17–19 In the case of 1,4-azaborinines this process involves B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond scission, which has also been observed in the reaction of tBu–N
N bond scission, which has also been observed in the reaction of tBu–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–tBu with silylenes or when the sterically crowded iminoborane (TMP)B
B–tBu with silylenes or when the sterically crowded iminoborane (TMP)B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NAr* (Scheme 1, E; TMP = 2,2,6,6-tetramethylpyrolidyl; Ar* = 2,6-(Ph2CH)-4-tBu-C6H2) reacted with the activated alkyne C2Pip2 (Pip = piperidyl).20 In this study we investigate the reactivity of phosphaalumene 1 towards its formal “smaller sibling” iminoborane tBu–N
NAr* (Scheme 1, E; TMP = 2,2,6,6-tetramethylpyrolidyl; Ar* = 2,6-(Ph2CH)-4-tBu-C6H2) reacted with the activated alkyne C2Pip2 (Pip = piperidyl).20 In this study we investigate the reactivity of phosphaalumene 1 towards its formal “smaller sibling” iminoborane tBu–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–tBu, giving the first example of a four-membered ring containing B, N, Al and P. This ring species was further treated with alkenes and alkynes, revealing insertion into the P–B-bond, in stark contrast to previously reported PAlC2-ring species.
B–tBu, giving the first example of a four-membered ring containing B, N, Al and P. This ring species was further treated with alkenes and alkynes, revealing insertion into the P–B-bond, in stark contrast to previously reported PAlC2-ring species.
When 1 and tBu–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–tBu were combined in benzene no immediate reaction was detected (Scheme 2), however after heating at 80 °C for 14 h the characteristic violet color of 1 had faded into a dull yellow to green solution. NMR spectroscopy revealed full conversion of 1 and formation of a new species with a rather sharp singlet in the 31P NMR spectrum at −59.9 ppm (cf. (Mes*P(H2CO)BTmp)2δ(31P) = −61.0 ppm)21 and a broad singlet at 56.0 ppm in the 11B NMR spectrum, indicating a three-coordinate boron atom. Single crystal X-ray diffraction (SCXRD) experiments revealed the formation of compound 2 (Fig. 1), a species with a BNAlP four-membered ring, which can be classified as cyclo-1-alumina-3-borata-4-phospha-2-azene. 2 is the product of a formal [2+2]-cycloaddition between 1 and tBu-N
B–tBu were combined in benzene no immediate reaction was detected (Scheme 2), however after heating at 80 °C for 14 h the characteristic violet color of 1 had faded into a dull yellow to green solution. NMR spectroscopy revealed full conversion of 1 and formation of a new species with a rather sharp singlet in the 31P NMR spectrum at −59.9 ppm (cf. (Mes*P(H2CO)BTmp)2δ(31P) = −61.0 ppm)21 and a broad singlet at 56.0 ppm in the 11B NMR spectrum, indicating a three-coordinate boron atom. Single crystal X-ray diffraction (SCXRD) experiments revealed the formation of compound 2 (Fig. 1), a species with a BNAlP four-membered ring, which can be classified as cyclo-1-alumina-3-borata-4-phospha-2-azene. 2 is the product of a formal [2+2]-cycloaddition between 1 and tBu-N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B-tBu with the expected P,B- and Al,N-connectivity and a puckered ring (P1–B1–N1–Al1 13.21(9)°) with the substituents on P and Al being minimally trans-oriented with respect to the P–Al axis.
B-tBu with the expected P,B- and Al,N-connectivity and a puckered ring (P1–B1–N1–Al1 13.21(9)°) with the substituents on P and Al being minimally trans-oriented with respect to the P–Al axis.
The B (∑(<B) = 356.62°) and N (∑(<N) = 358.86°) atoms are in distorted trigonal planar coordination environments with a short B–N distance (1.425(2) Å) as expected for aminoboranes (cf. ∑rcov(B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) = 1.38 Å;22 (IDipp)(Mes)B
N) = 1.38 Å;22 (IDipp)(Mes)B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(tBu)Dipp 1.420(2) Å).23 The Cp*-substituent is η5-coordinated to an aluminium atom that deviates from an ideal trigonal planar coordination (Σ(<Al) = 349.5°) with Al–N (1.8751(12) Å) and Al–P (2.3380(5) Å) distances that are in line with polarized single bonds (cf. ∑rcov(Al–P) = 2.37, (Al–N) = 1.97 Å).22 The phosphorus atom is trigonal pyramidally coordinated with a rather long P–B distance (2.005(2) Å; cf. Mes*P(PhCCH)B(TMP)24 1.987(2) Å). According to NBO analysis at the PBE0-D3/def2-TZVP level of theory the P–B bond is delocalized into a lone valence (LV) at the aluminium center, which is further accepting electron density from a p-type lone pair of electrons on the nitrogen atom, as well as from the s-type LP at the P center. This results in a ring with alternating natural charges (P: −1.28; Al 1.79; N −1.28; B 0.85 e) and Wiberg Bond Indexes that are in line with polarized single bonds (P–Al 0.65, N–Al 0.30, P–B 0.97), with a high ionic character in the case of the Al–N bond. The B–N bond is strongly polarized towards the N atom (σ 81%, π 85%), thus resulting in a WBI close to one (B–N 1.04). Next the reactivity of 2 towards C–C multiple bond systems was investigated. As a first entry a benzene solution of 2 was stirred under an atmosphere of C2H2 for 1 h (Scheme 3), which, according to 31P NMR spectroscopy, resulted in the formation of a mixture of products (δ(31P) = −73.0 (16%), −78.1 (67%), −81.0 (16%) ppm), while in the 11B NMR spectrum a broad singlet at 50.4 ppm was detected.
N(tBu)Dipp 1.420(2) Å).23 The Cp*-substituent is η5-coordinated to an aluminium atom that deviates from an ideal trigonal planar coordination (Σ(<Al) = 349.5°) with Al–N (1.8751(12) Å) and Al–P (2.3380(5) Å) distances that are in line with polarized single bonds (cf. ∑rcov(Al–P) = 2.37, (Al–N) = 1.97 Å).22 The phosphorus atom is trigonal pyramidally coordinated with a rather long P–B distance (2.005(2) Å; cf. Mes*P(PhCCH)B(TMP)24 1.987(2) Å). According to NBO analysis at the PBE0-D3/def2-TZVP level of theory the P–B bond is delocalized into a lone valence (LV) at the aluminium center, which is further accepting electron density from a p-type lone pair of electrons on the nitrogen atom, as well as from the s-type LP at the P center. This results in a ring with alternating natural charges (P: −1.28; Al 1.79; N −1.28; B 0.85 e) and Wiberg Bond Indexes that are in line with polarized single bonds (P–Al 0.65, N–Al 0.30, P–B 0.97), with a high ionic character in the case of the Al–N bond. The B–N bond is strongly polarized towards the N atom (σ 81%, π 85%), thus resulting in a WBI close to one (B–N 1.04). Next the reactivity of 2 towards C–C multiple bond systems was investigated. As a first entry a benzene solution of 2 was stirred under an atmosphere of C2H2 for 1 h (Scheme 3), which, according to 31P NMR spectroscopy, resulted in the formation of a mixture of products (δ(31P) = −73.0 (16%), −78.1 (67%), −81.0 (16%) ppm), while in the 11B NMR spectrum a broad singlet at 50.4 ppm was detected.
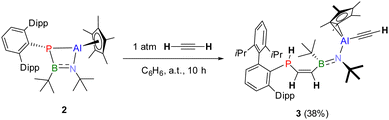
SCXRD-quality crystals were obtained by slow evaporation of a saturated n-pentane solution, which revealed formation of the chain-molecule DippTerP(H)–C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)–B(tBu)=N(tBu)–Al(η2-Cp*)(C
C(H)–B(tBu)=N(tBu)–Al(η2-Cp*)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) (3) (Fig. 2). We hypothesize that 3 results from initial acetylene insertion into the strongly polarized P–B bond (Fig. S31, ESI†), with subsequent deprotonation of acetylene by the P atom and Al capturing the released acetylenide anion resulting in P–Al bond cleavage. A reverse order of addition, first across the P–Al bond with C–H bond cleavage and subsequent insertion of the second C2H2 into the P–B bond cannot be ruled out. The presence of three different isomers in solution is due to conformers of the alternating C
CH) (3) (Fig. 2). We hypothesize that 3 results from initial acetylene insertion into the strongly polarized P–B bond (Fig. S31, ESI†), with subsequent deprotonation of acetylene by the P atom and Al capturing the released acetylenide anion resulting in P–Al bond cleavage. A reverse order of addition, first across the P–Al bond with C–H bond cleavage and subsequent insertion of the second C2H2 into the P–B bond cannot be ruled out. The presence of three different isomers in solution is due to conformers of the alternating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1.338(3) Å) and B
C (1.338(3) Å) and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1.408(3) Å) double bonds, with the Z(CC)/E(BN) isomer being present in the solid state. This reactivity clearly differs from 1, which reacted with acetylene through threefold insertion into the P–Al bond.6
N (1.408(3) Å) double bonds, with the Z(CC)/E(BN) isomer being present in the solid state. This reactivity clearly differs from 1, which reacted with acetylene through threefold insertion into the P–Al bond.6
The conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–B
C–B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N unit is terminated by P–C (1.821(2) Å) and N–Al (1.855(2) Å) single bonds. The B (∑(<B) = 359.4°), N (∑(<N) = 360.0°) and Al (∑(<Al) = 357.6°) atoms are distorted trigonally planar coordinated with a terminal C
N unit is terminated by P–C (1.821(2) Å) and N–Al (1.855(2) Å) single bonds. The B (∑(<B) = 359.4°), N (∑(<N) = 360.0°) and Al (∑(<Al) = 357.6°) atoms are distorted trigonally planar coordinated with a terminal C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH unit (the asymmetric CH stretching vibration is detected in the IR at 3258 cm−1) on the Al atom (d(C3–C4) = 1.198(3) Å) and an η2-coordinated Cp*-substituent (d(Al–CCp* = 2.120(2), 2.133(2) Å)). Interestingly, the trigonal pyramidal P atom (∑(<P) = 301.0°) is protonated. In the 1H NMR spectrum of 3 the main isomer can be clearly characterized with a ddd-signature for the P–CH proton at 6.80 ppm and a doublet at 6.38 ppm for the B–CH proton, while the P–H gives a doublet of doublets at 4.40 ppm (1JPH = 226.6 Hz, 3JPH = 2.6 Hz) and the terminal CCH proton is detected as a singlet at 1.59 ppm. 1H DOSY experiments revealed similar diffusion coefficients for all three isomers detected in the 31P NMR spectrum, supporting the notion that these only differ in the configuration of the double bonds.
CH unit (the asymmetric CH stretching vibration is detected in the IR at 3258 cm−1) on the Al atom (d(C3–C4) = 1.198(3) Å) and an η2-coordinated Cp*-substituent (d(Al–CCp* = 2.120(2), 2.133(2) Å)). Interestingly, the trigonal pyramidal P atom (∑(<P) = 301.0°) is protonated. In the 1H NMR spectrum of 3 the main isomer can be clearly characterized with a ddd-signature for the P–CH proton at 6.80 ppm and a doublet at 6.38 ppm for the B–CH proton, while the P–H gives a doublet of doublets at 4.40 ppm (1JPH = 226.6 Hz, 3JPH = 2.6 Hz) and the terminal CCH proton is detected as a singlet at 1.59 ppm. 1H DOSY experiments revealed similar diffusion coefficients for all three isomers detected in the 31P NMR spectrum, supporting the notion that these only differ in the configuration of the double bonds.
When 1 was treated with an excess of styrene at ambient temperature in C6D6 no reaction occurred. Heating to 80 °C for at least 2 days, gave three new signals in the 31P NMR spectrum at −10.4, −19.3 and −20.9 ppm, which correspond to the different regio-isomers of twofold styrene insertion into the P–Al bond in 1, giving 1,4-aluminaphosphorinanes (5, Scheme 5), which we had previously reported.6 The 11B NMR spectrum of the reaction mixture clearly indicated the release of tBuN![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) BtBu, foreshadowing that 2 can act as a disguised form of 1. When first treating DippTerPAlCp* with styrene (1.6 eq.), giving the previously reported phosphaalumate 1Phin situ (Scheme 4),6 followed by the addition of tBu–N
BtBu, foreshadowing that 2 can act as a disguised form of 1. When first treating DippTerPAlCp* with styrene (1.6 eq.), giving the previously reported phosphaalumate 1Phin situ (Scheme 4),6 followed by the addition of tBu–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–tBu (1 eq.) the formation of a new species with a 31P NMR signal at −39.2 ppm was detected, shielded compared to 1Ph (cf. δ(31P) = 76.0 ppm).6 SCXRD experiments revealed the formation of a six-membered PC2BNAl-heterocycle (4, Fig. 3), formed through insertion of the iminoborane into the Al–C bond in 1Ph. The six-membered ring shows an envelope conformation, being folded ca. 115° along the Al1–C1 axis. Both the P1–C2 (1.908(1) Å) and P1–Al1 (2.3695(4) Å) distances are similar to those in 1Ph (cf. 1.920(1), 2.3992(5) Å),6 with the Al1–N1 (1.842(1) Å) distance being minimally shorter compared to 2. The Al atom is in a distorted trigonal planar coordination environment with an η1-coordinated Cp*-substituent (d(Al1–C17) 2.044(1) Å). The C2–C1 (1.575(2) Å) and C1–B1 distances (1.654(2) Å) are also in line with single bonds (cf. ∑rcov(B–C) = 1.60, (C–C) = 1.50 Å),22 while the N1–B1 (1.403(2) Å) is similar to that in 2 and in line with the formulation of 4 as a cyclic aminoborane, which is further corroborated by the BN stretching vibration at 1361 cm−1 in the IR spectrum.
B–tBu (1 eq.) the formation of a new species with a 31P NMR signal at −39.2 ppm was detected, shielded compared to 1Ph (cf. δ(31P) = 76.0 ppm).6 SCXRD experiments revealed the formation of a six-membered PC2BNAl-heterocycle (4, Fig. 3), formed through insertion of the iminoborane into the Al–C bond in 1Ph. The six-membered ring shows an envelope conformation, being folded ca. 115° along the Al1–C1 axis. Both the P1–C2 (1.908(1) Å) and P1–Al1 (2.3695(4) Å) distances are similar to those in 1Ph (cf. 1.920(1), 2.3992(5) Å),6 with the Al1–N1 (1.842(1) Å) distance being minimally shorter compared to 2. The Al atom is in a distorted trigonal planar coordination environment with an η1-coordinated Cp*-substituent (d(Al1–C17) 2.044(1) Å). The C2–C1 (1.575(2) Å) and C1–B1 distances (1.654(2) Å) are also in line with single bonds (cf. ∑rcov(B–C) = 1.60, (C–C) = 1.50 Å),22 while the N1–B1 (1.403(2) Å) is similar to that in 2 and in line with the formulation of 4 as a cyclic aminoborane, which is further corroborated by the BN stretching vibration at 1361 cm−1 in the IR spectrum.
In solution the description of 4 as a cyclic aminoborane is supported by a broad singlet in the 11B NMR spectrum (tol-d8) at 44.5 ppm. The 1H NMR spectrum of 4 is complex, indicating C1 symmetry in solution. A singlet for the Cp* substituent indicates a fast sigmatropic rearrangement in solution, while two characteristic signals for the diasterotopic ring CH2 protons are detected at −0.41 and ca. 1.55 ppm. Compared to 1Ph (PhCHCH2δ(1H) = 1.76, 2.04 ppm) one of the CH2 protons is significantly shielded (−0.41 ppm) with a complex coupling pattern, that also includes through space JPH coupling (6.8 Hz). This shielding is indicative of an agostic interaction of the C–H bond with the Al center.25 This is further supported by a short C1–Al1 distance (2.294(1) Å) and an Al1⋯H–C1 angle of 89.1(8)° determined with Hirshfeld atom refinement (HAR).26 2nd order perturbation theory analysis at the PBE0-D3/def2-TZVP level of theory reveals a donor–acceptor interactions between the C–H bond and a lone valence at aluminium of ca. 26 kcal mol−1. This significant interaction is further supported by an interaction region indicator (IRI) analysis (Fig. 3, right).27 Interestingly, when a C6D6 solution of 4 was heated to 60 °C the formation of 2 was detected with release of styrene.
Additionally, the reactivity of 2 towards 3-hexyne was probed. To in situ generated 2 in C6D6, 3-hexyne was added and the mixture was heated at 80 °C for 24 h, showing partial conversion of 2 into a new species with a 31P NMR signal at 40.6 ppm with the concomitant release of tBuN![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) BtBu. This indicated the formation of a four-membered species, phosphaalumete DippTerP(EtC
BtBu. This indicated the formation of a four-membered species, phosphaalumete DippTerP(EtC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CEt)AlCp* (6, Scheme 5), by comparison with the 31P NMR shifts of previously reported derivatives (cf.DippTerP(PhC = CPh)AlCp* 35.3 ppm).6 A rather clean reaction was observed when a n-pentane solution of 2 was exposed to CO2, resulting in a color change to orange. A singlet in the 31P NMR spectrum at −201.8 ppm shows conversion into the previously reported phosphaketene DippTerPCO (7, Scheme 5),12 while a broad signal in the 11B NMR spectrum at ca. 36 ppm indicated further reaction of the iminoborane with CO2 to an unidentified product, along with signals for oligomeric [Cp*AlO]x in the 1H NMR spectrum.28 These reactivities underline that 2 in some cases acts as a disguised source of 1.
CEt)AlCp* (6, Scheme 5), by comparison with the 31P NMR shifts of previously reported derivatives (cf.DippTerP(PhC = CPh)AlCp* 35.3 ppm).6 A rather clean reaction was observed when a n-pentane solution of 2 was exposed to CO2, resulting in a color change to orange. A singlet in the 31P NMR spectrum at −201.8 ppm shows conversion into the previously reported phosphaketene DippTerPCO (7, Scheme 5),12 while a broad signal in the 11B NMR spectrum at ca. 36 ppm indicated further reaction of the iminoborane with CO2 to an unidentified product, along with signals for oligomeric [Cp*AlO]x in the 1H NMR spectrum.28 These reactivities underline that 2 in some cases acts as a disguised source of 1.
In summary the synthesis of the unique BNAlP heterocycle 2 has been described. While 2 reacted with acetylene via insertion into the P–B bond to give conjugated aminoborane 3, the reaction with styrene resulted in iminoborane release. However, when the order of addition was changed the 6-membered aminoborane 4 was afforded, which shows an intramolecular agostic Al⋯(C–H) interaction. Studies harnessing the lability of the tBuNBtBu unit in 2 are currently underway. Moreover, the reactivity of 2 towards E–H bonds (E = H, B, Si, N, etc.) will be explored, as well as that of aryl phosphaketene DippTerPCO.
T. W. designed and carried out the experimental work, collected and analysed the data and assembled the ESI.† T. W. and L. W carried out the SCXRD experiments. T. W. and C. H.-J. did the DFT calculations. H. B. and C. H.-J. supervised the work and drafted the manuscript. All authors have given approval to the final version of the manuscript.
C. H.-J. wishes to thank the ITMZ at the University of Rostock for access to the Cluster Computer and especially Malte Willert for technical support. T. W. thanks Krzysztof Radacki for help with X-ray analysis and calculations. H. B. wishes to acknowledge financial support by the DFG.
Data availability
The data supporting this article, including synthetic procedures, spectral data, and crystallographic and computational details have been included as part of the ESI.† Crystallographic data has been deposited at the CCDC under identification numbers 2417544 (2), 2417545 (3) and 2417546 (4), respectively.Conflicts of interest
There are no conflicts to declare.Notes and references
- P. P. Power, Organometallics, 2020, 39, 4127–4138 CrossRef CAS.
- C. Weetman, Chem. – Eur. J., 2021, 27, 1941–1954 CrossRef CAS PubMed.
- F. Hanusch, L. Groll and S. Inoue, Chem. Sci., 2021, 12, 2001–2015 RSC.
- F. Dankert and C. Hering-Junghans, Chem. Commun., 2022, 58, 1242–1262 RSC.
- M. Fischer, S. Nees, T. Kupfer, J. T. Goettel, H. Braunschweig and C. Hering-Junghans, J. Am. Chem. Soc., 2021, 143, 4106–4111 CrossRef CAS PubMed.
- S. Nees, T. Wellnitz, F. Dankert, M. Härterich, S. Dotzauer, M. Feldt, H. Braunschweig and C. Hering-Junghans, Angew. Chem., Int. Ed., 2023, 62, e202215838 CrossRef CAS PubMed.
- J. Li, Z. Lu and L. L. Liu, J. Am. Chem. Soc., 2022, 144, 23691–23697 CrossRef CAS PubMed.
- E. A. LaPierre, B. O. Patrick and I. Manners, J. Am. Chem. Soc., 2023, 145, 7107–7112 CrossRef CAS PubMed.
- L. S. Szych, L. Denker, J. Feld and J. M. Goicoechea, Chem. – Eur. J., 2024, 30, e202401326 CrossRef CAS PubMed.
- D. W. N. Wilson, J. Feld and J. M. Goicoechea, Angew. Chem., Int. Ed., 2020, 59, 20914–20918 CrossRef CAS PubMed.
- M. K. Sharma, C. Wölper, G. Haberhauer and S. Schulz, Angew. Chem., Int. Ed., 2021, 60, 6784–6790 CrossRef CAS PubMed.
- T. Taeufer, F. Dankert, D. Michalik, J. Pospech, J. Bresien and C. Hering-Junghans, Chem. Sci., 2023, 14, 3018–3023 RSC.
- Á. García-Romero, C. Hu, M. Pink and J. M. Goicoechea, J. Am. Chem. Soc., 2025, 147, 1231–1239 CrossRef PubMed.
- Y. Fan, J. Cui and L. Kong, Eur. J. Org. Chem., 2022, e202201086 CrossRef.
- (a) A. K. Swarnakar, C. Hering-Junghans, K. Nagata, M. J. Ferguson, R. McDonald, N. Tokitoh and E. Rivard, Angew. Chem., Int. Ed., 2015, 54, 10666–10669 CrossRef CAS PubMed; (b) B. L. Frenette, A. A. Omana, M. J. Ferguson, Y. Zhou and E. Rivard, Chem. Commun., 2021, 57, 10895–10898 RSC.
- P. Paetzold, C. V. Plotho, G. Schmid, R. Boese, B. Schrader, D. Bougeard, U. Pfeiffer, R. Gleiter and W. Schüfer, Chem. Ber., 1984, 117, 1089–1102 CrossRef CAS.
- H. Braunschweig, K. Geetharani, J. O. C. Jimenez-Halla and M. Schäfer, Angew. Chem., Int. Ed., 2014, 53, 3500–3504 CrossRef CAS PubMed.
- M. Heß, I. Krummenacher, T. Dellermann and H. Braunschweig, Chem. – Eur. J., 2021, 27, 9503–9507 CrossRef PubMed.
- M. Schäfer, N. A. Beattie, K. Geetharani, J. Schäfer, W. C. Ewing, M. Krahfuß, C. Hörl, R. D. Dewhurst, S. A. Macgregor, C. Lambert and H. Braunschweig, J. Am. Chem. Soc., 2016, 138, 8212–8220 CrossRef PubMed.
- L. Winner, A. Hermann, G. Bélanger-Chabot, O. F. González-Belman, J. O. C. Jiménez-Halla, H. Kelch and H. Braunschweig, Chem. Commun., 2018, 54, 8210–8213 RSC.
- A. M. Borys, E. F. Rice, G. S. Nichol and M. J. Cowley, J. Am. Chem. Soc., 2021, 143, 14065–14070 CrossRef CAS PubMed.
- P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 12770–12779 CrossRef PubMed.
- L. Winner, G. Bélanger-Chabot, M. A. Celik, M. Schäfer and H. Braunschweig, Chem. Commun., 2018, 54, 9349–9351 RSC.
- A. N. Price, G. S. Nichol and M. J. Cowley, Angew. Chem., Int. Ed., 2017, 56, 9953–9957 CrossRef CAS PubMed.
- M. Brookhart, M. L. H. Green and G. Parkin, Proc. Nat. Acad. Sci. U. S. A., 2007, 104, 6908–6914 CrossRef CAS PubMed.
- F. Kleemiss, O. V. Dolomanov, M. Bodensteiner, N. Peyerimhoff, L. Midgley, L. J. Bourhis, A. Genoni, L. A. Malaspina, D. Jayatilaka, J. L. Spencer, F. White, B. Grundkötter-Stock, S. Steinhauer, D. Lentz, H. Puschmann and S. Grabowsky, Chem. Sci., 2021, 12, 1675–1692 RSC.
- T. Lu and Q. Chen, Chem.: Methods, 2021, 1, 231–239 CAS.
- A. C. Stelzer, P. Hrobárik, T. Braun, M. Kaupp and B. Braun-Cula, Inorg. Chem., 2016, 55, 4915–4923 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of compounds, NMR spectra, crystallographic, and computational details. CCDC 2417544–2417546. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc00308c |
| This journal is © The Royal Society of Chemistry 2025 |