Scaling-up green chemistry: bridging innovation and industrial applications
Gregory
Chatel

Univ. Savoie Mont Blanc, CNRS, EDYTEM, 73000 Chambéry, France. E-mail: gregory.chatel@univ-smb.fr
First published on 19th May 2025
Abstract
This Feature Article reviews recent advances in green chemistry, with a focus on scaling up both conceptually and industrially. We particularly discuss the contributions to circular economy principles through solvent replacement, non-conventional activation technologies, and the valorization of waste and biomass. The article also outlines strategies to transition lab-scale innovations into widespread and impactful industrial applications, highlighting opportunities and challenges associated with this transition.
1. Introduction – green chemistry in the context of global challenges
The modern world faces unprecedented environmental and societal challenges, ranging from climate change and resource depletion to the pressing need for a circular economy.1,2 In this context, green chemistry has emerged as a pivotal discipline aimed at addressing these global issues by promoting sustainable practices in chemical science and industry.3 Central to this approach are the twelve principles (Fig. 1) that prioritize, for examples, waste reduction, energy efficiency and the minimization of hazardous substances, thereby offering solutions that align environmental stewardship with economic viability.4–7 | ||
| Fig. 1 24 principles of green chemistry and green engineering under “IMPROVEMENTS PRODUCTIVITY” format.8 | ||
A core principle of green chemistry is its integration into industrial processes, as the chemical industry serves both as a significant contributor to environmental impacts and a key driver of global innovation (Fig. 1).8,9 This integration involves designing bio-based and recyclable intermediates, developing durable catalysts and implementing closed-loop recycling systems to minimize waste and optimize resource use. These efforts are essential to advancing sustainable development, as highlighted in the 17 sustainable development goals (SDGs) of the United Nations, which call for balancing environmental preservation with economic and social priorities.10
However, achieving this balance presents significant challenges. How can the chemical industry adopt green practices while remaining competitive? The path forward requires industries to recognize that embracing green chemistry offers a strategic advantage, positioning them to access new markets driven by sustainability demands. Furthermore, evolving legislative frameworks increasingly compel large corporations to prioritize sustainability, creating both pressure and opportunity for industrial innovation. Scaling up green chemistry is not just an environmental necessity; it is an economic and strategic imperative for businesses aiming to thrive in a rapidly evolving global landscape.
Energy consumption in chemical processes exemplifies this urgency. As one of the 12 principles of green chemistry, reducing energy use has become increasingly critical in today's economic climate, where energy costs and availability are pivotal.11,12 Innovative technologies such as microwave-assisted synthesis and sonochemistry address these challenges by enhancing process efficiency and reducing energy inputs. Yet, broader adoption of such technologies of unconventional activation requires significant investment and collaboration to overcome industrial and economic barriers.
Solvent substitution further illustrates the practical challenges and opportunities within green chemistry.13,14 For instance, replacing hexane—a solvent with known environmental and health hazards—requires redesigning processes and operational workflows. While alternative solutions exist, they often demand significant technical adjustments, underscoring the necessity for continuous innovation and interdisciplinary collaboration.
Societal acceptance and integration are equally vital for the widespread adoption of green chemistry. Public understanding and support for decarbonization efforts play a crucial role in driving meaningful change. Social sciences can help address consumer resistance and misconceptions about sustainability, particularly in cases where bio-based materials compete with food supplies.15 Aligning green chemistry with societal priorities is both complex and essential for normalizing it as an industrial and academic standard.
Green chemistry is not merely a scientific pursuit but a multidimensional response to global challenges. It integrates technological advancements with societal priorities, aiming to redefine how chemistry is practiced and perceived. By fostering collaboration among academia, industry and society, green chemistry has the potential to become a foundation for a sustainable future. These goals are intricately aligned with the broader framework of the SDGs, which emphasize the interconnectedness of environmental, social and economic sustainability.
Christian Römlein, CEO of Intelligent Fluids, highlights the necessity of scaling up green chemistry, noting the growing global attention on sustainability beyond traditional CO2 emissions.16 Römlein underscores the importance of a holistic approach to sustainability that moves beyond “end-of-pipe” solutions, emphasizing proactive prevention and comprehensive life cycle integration. Similarly, Natalie Runyon argues that scaling green chemistry is essential to mitigating biodiversity loss, a pressing financial and ecological risk for many organizations.17
These perspectives reinforce the global imperative to scale green chemistry in two dimensions: the technical transition from lab-scale innovations to industrial applications and the broader diffusion of green chemistry principles across disciplines and industries. This Feature Article builds on these foundations to explore the transformative potential of green chemistry, offering strategies to advance its role as a catalyst for environmental, economic and societal change.
2. Bridging chemistry and sustainability
Sustainability has become an imperative for addressing the mounting environmental and societal challenges of our time. Chemistry, as a central science, plays a crucial role in this transition, particularly through its integration with circular economy principles.4,18,19 The circular economy, centered on resource efficiency, waste minimization and material cycling, offers a compelling framework for sustainable development. Within this paradigm, chemists contribute significantly by designing recyclable materials, developing innovative valorization pathways, and advancing biorefinery concepts to maximize the use of renewable resources.18,20One of the critical challenges in realizing a circular economy is ensuring the recyclability of materials designed for specific applications. Chemical recycling offers a promising solution, particularly in addressing the growing problem of plastic waste.21 However, thermoset composites—widely used for their durability—pose significant challenges due to their complex structures, requiring advanced chemical methods for effective recycling. Clear criteria for what constitutes green chemistry in recycling processes are essential for making meaningful progress. Waste, once considered merely a by-product, is now increasingly seen as a valuable raw material. Chemists are at the forefront of this transformation, pioneering new approaches to convert waste into high-value products, thereby redefining its role in industrial systems.
Biorefineries epitomize the principles of a circular economy by integrating processes that convert biomass into chemicals, fuels and materials while minimizing waste.22–24 This concept, inspired by traditional oil refineries, demonstrates the potential of renewable resources to replace fossil-based feedstocks. For example, microalgae serve as a promising biomass source, offering bioactive compounds for applications in cosmetics, pharmaceuticals and food industries.25 These processes highlight the synergy between green chemistry and biorefineries in achieving economic and environmental sustainability (Fig. 2).26
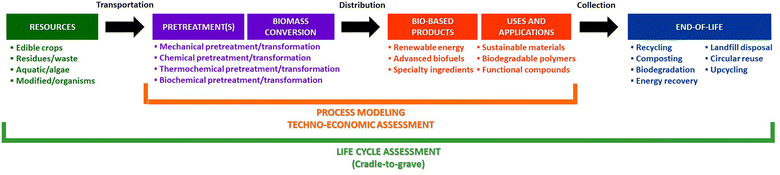 | ||
| Fig. 2 Methodology for integrated environmental and techno-economic assessment. This figure was entirely redrawn for the present article and is conceptually inspired by ref. 26, without reusing any visual or graphical element from the original publication. | ||
Despite the promise of biorefineries and circular economy practices, significant techno-economic challenges remain.27 Many initiatives struggle to scale due to the lack of robust economic models and insufficient governance frameworks. Effective waste collection and sorting systems, for instance, are often underdeveloped because they lack clear funding mechanisms. This raises critical questions about who bears the costs of implementing these systems and how financial incentives can be structured to promote their adoption. To address these barriers, life-cycle analysis (LCA) has emerged as a crucial tool for assessing the economic and environmental trade-offs of new processes and materials. Incorporating LCA at the earliest stages of project design enables chemists and decision-makers to identify opportunities for improving efficiency, reducing environmental impact, and enhancing economic viability.26
The food industry exemplifies the potential of circular economy practices, particularly through the valorization of byproducts. Agrifood chemistry, focused on transforming waste streams into valuable products, has garnered significant academic and industrial attention. For instance, food waste biorefineries have demonstrated how platform molecules like fructose can serve as precursors for producing widely used polyesters such as polyethylene terephthalate (PET) and polyethylene 2,5-furandicarboxylate (PEF).28 These examples underscore the importance of linking chemistry with sustainability to create new markets and reduce dependence on fossil resources.
However, the successful implementation of circular economy practices in chemistry requires a multidisciplinary approach.18 Chemists alone cannot achieve circularity; collaboration with engineers, economists, and policymakers is essential to develop holistic solutions. This multidimensional effort extends across five levels of contribution that I proposed few years ago: optimizing resource use, simplifying product design, innovating new technologies, performing global assessments, and fostering multidisciplinary integration (Fig. 3)18 These contributions highlight the breadth of chemistry's role in advancing sustainability.
As the challenges of climate change and resource depletion intensify, bridging chemistry and sustainability becomes not just an opportunity but a necessity. By innovating recycling technologies, advancing biorefinery processes, and addressing techno-economic barriers, chemistry can serve as a cornerstone of the circular economy. Achieving these goals will require concerted efforts to integrate technical expertise, policy frameworks and societal support.
3. Cascading biomass valorization for zero-waste chemistry
The valorization of biomass and waste is a cornerstone of green chemistry, with cascading valorization strategies offering a pathway to zero-waste solutions. In this approach, each stage of biomass processing maximizes resource utilization by extracting valuable compounds while minimizing residual waste.29 Despite significant advancements, the development of efficient waste valorization chains remains incomplete, with critical gaps in collection systems, economic models, and logistical frameworks. These barriers highlight the need for integrated solutions that combine innovative chemistry with sustainable economic and societal practices.A major challenge lies in waste collection and sorting. Insufficient infrastructure, combined with the absence of robust financial incentives, hampers the establishment of effective valorization chains. Without clear economic models to support these systems, stakeholders often lack the resources or motivation to invest in sustainable waste processing. For example, agricultural waste, textile residues and municipal byproducts demonstrate the potential for valorization, but inconsistent demand and limited financial backing restrict their scalability. Lessons from these sectors underscore the importance of creating replicable and economically viable models that can drive the broader adoption of waste valorization practices.
Biorefineries provide a promising framework for cascading biomass valorization. Inspired by traditional oil refineries, biorefineries employ a series of integrated processes to convert biomass into high-value products, including biofuels, chemicals, and materials, while minimizing waste. This concept not only aligns with green chemistry principles but also addresses the pressing need for sustainable resource management. For example, agricultural residues such as lignocellulosic biomass can be fractionated to produce bioethanol, while secondary processes recover additional compounds for pharmaceutical or cosmetic applications. Such approaches exemplify how cascading valorization enhances the economic and environmental value of biomass.
The success of biorefineries depends on leveraging innovative extraction and processing techniques. Fractionation processes allow the separation of biomass into distinct components, each with specific properties and applications. Advanced technologies such as supercritical CO2 extraction and microwave-assisted processing have proven effective in isolating high-value compounds while maintaining environmental integrity. At lab for example, the valorization of Japanese knotweed demonstrates the potential of cascading approaches.30,31 By extracting resveratrol for industrial applications and subsequently developing economic models with stakeholders, the project highlights how targeted valorization can transform invasive plants into valuable resources. This initiative led to the creation of a start-up, Rhizomex, which integrates economic and ecological objectives into a single valorization chain (Fig. 4).
The cascading model has also been applied to viticultural byproducts, such as grapevine prunings. Historically burned in the fields, these residues posed environmental and regulatory challenges. Through collaborative efforts between scientists, local authorities and farmers, the VITIVALO project established a valorization chain that includes the extraction of molecules with pharmaceutical and cosmetic potential, as well as the development of biocontrol applications (Fig. 5).32,33 Residual biomass has been further processed into cardboard materials, ensuring a zero-waste approach. However, long-term success requires the engagement of economic actors to scale these solutions to industrial levels. Addressing logistical challenges such as transport, storage and variability in biomass quality remains critical for achieving broader adoption.
At lab, we also studied the valorization of coffee grounds and apple residues. Coffee grounds, often discarded as waste, are a rich source of bioactive compounds. Using supercritical CO2 extraction, researchers have developed clean processes to recover valuable molecules for pharmaceutical and cosmetic applications.34,35 Similarly, apple residues have been explored for their antimicrobial properties, with potential applications in hospital cleaning products.36 These initiatives highlight the potential for cross-sectoral collaborations to enhance biomass valorization. However, challenges such as regulatory hurdles, economic scalability and logistical constraints continue to limit the implementation of these projects on an industrial scale.
Ethical and logistical considerations are equally important in biomass valorization. Competition between biomass use for food, feed and industrial applications necessitates careful planning to avoid exacerbating food security issues. Additionally, the seasonal availability of biomass and the impacts of climate change on crop yields must be factored into valorization strategies. Collaborations with biologists are essential to understanding the cellular mechanisms and properties of plant biomass, enabling more efficient and sustainable valorization processes. Indicators such as the cell disintegration index can provide valuable insights into the efficiency of biomass processing.37
Addressing these barriers requires a multi-pronged approach. Establishing charters, labels and partnerships with agricultural stakeholders can promote the sustainable use of biomass while ensuring ethical practices. We currently work with PROXIPEL company to integrate their mobile units capable of processing and pelletizing biomass on-site in order to offer a practical solution to transportation and storage issues, facilitating the adoption of cascading valorization models.38 Furthermore, integrating life cycle analysis (LCA) into project planning can help identify bottlenecks and optimize resource use from the outset.
In conclusion, cascading biomass valorization represents a powerful strategy for achieving zero-waste chemistry. By extracting maximum value at each stage of processing, this approach aligns with the principles of green chemistry while addressing economic and societal challenges. However, realizing its full potential requires the development of robust economic models, interdisciplinary collaboration, and the engagement of stakeholders across the value chain.
4. Revolutionizing processes with non-conventional activation
Green chemistry aims to revolutionize chemical processes, integrating sustainability principles with technological innovation. Among the most impactful advances are non-conventional activation technologies —such as sonochemistry, microwave-assisted synthesis, plasma, and mechanochemistry— as well as unconventional solvents, including supercritical and subcritical fluids, ionic liquids, and deep eutectic solvents. Far from being mere auxiliary tools, these approaches serve as genuine drivers of innovation when they are carefully applied, optimized, and understood at both fundamental and mechanistic levels.39Sonochemistry, which utilizes ultrasound, exemplifies the potential of non-conventional activation technologies in green chemistry.40,41 This approach leverages cavitational phenomena, where the collapse of microscopic bubbles generates localized extreme conditions, leading to enhanced reactivity.42 Applications span from sonocatalysis and organic synthesis to material preparation and biomass conversion.43–46 For instance, ultrasound-assisted extraction (UAE) has demonstrated significant advantages in green chemistry, such as increased yields, reduced reaction times and the ability to use eco-friendly solvents like water. Additionally, UAE enables the extraction of bioactive compounds from lignocellulosic biomass, presenting a promising avenue for sustainable biofuel production.47,48
Microwave-assisted synthesis (MW) offers another compelling example of innovative activation technologies.49,50 Microwaves provide rapid, uniform heating, often leading to reduced reaction times, enhanced yields, and improved selectivity. By coupling MW with other activation methods such as sonochemistry, synergistic effects can be achieved, further advancing green chemistry processes.51 For example, microwave-assisted biomass conversion has shown promise in efficiently breaking down complex lignocellulosic structures, enabling the extraction of high-value platform molecules such as hydroxymethylfurfural (HMF).52
Supercritical and subcritical fluids also exemplify the transformative potential of non-conventional activation technologies.53,54 Supercritical carbon dioxide (SC-CO2) and subcritical water (SCW) have been effectively employed as solvents, catalysts and reagents in sustainable processes. Their tunable properties enable selective extraction of bioactive compounds, such as sterols and terpenoids from coffee by-products, with reduced environmental impact compared to traditional methods.34 For example, SC-CO2 extraction can achieve similar or superior yields of high-value molecules while eliminating the need for toxic solvents. Subcritical water, on the other hand, offers the unique ability to function as a solvent, hydrolyzing agent, and catalyst under specific conditions, making it ideal for the valorization of biomass.
Ionic liquids (ILs) represent another class of alternative solvents that have garnered significant attention for their chemical and thermal stability, tunable properties, and potential alignment with green chemistry principles. These neoteric solvents have shown promise in various applications such as catalysis, extraction, electrochemistry, and even pharmaceutical formulation. However, their sustainability remains debated due to concerns about synthesis routes, toxicity, and limited biodegradability. As highlighted by Jesus and Filho, the full life cycle of ILs—from design to disposal—reveals cumulative environmental challenges that question their “green” status.55 Nevertheless, progress has been made through the use of computational modeling to rationally design ILs with improved environmental profiles. Beyond general applications, ILs have shown unique potential in niche, high-value sectors such as the development of active pharmaceutical ingredient ionic liquids (API-ILs). As mention by Shamshina and Rogers, these API-ILs offer novel solutions to issues like drug polymorphism and solubility, yet challenges such as stability, trace impurity control, and regulatory acceptance currently limit their broader industrial implementation.56 While promising, the future of ILs likely lies in their integration within highly specific applications where their unique properties can be fully leveraged.
More recently, deep eutectic solvents (DES) have emerged as a key class of green solvents with notable advantages in terms of synthesis simplicity, low cost, and reduced environmental impact.57–59 DESs are typically formed by mixing naturally derived components, such as choline chloride and urea, to produce eutectic mixtures with unique solvent properties. These solvents are increasingly applied in sustainable processes, including biomass valorization and pharmaceutical synthesis, and recent developments highlight their scalability. For instance, continuous mechanochemical processes have enabled the production of various DESs, such as choline chloride–urea and choline chloride–oxalic acid, without external heating, reaching productivities up to 254 g min−1 and maintaining physicochemical properties comparable to conventionally produced DESs.60 Moreover, DESs have shown efficacy in organic synthesis, as demonstrated by a recent two-step protocol for the antihistamine drug Thenfadil.61 The process, which uses bio-based DESs under mild and aerobic conditions, allowed for scale-up to 50 grams of substrate with consistent yields and simplified purification. These advancements position DESs as scalable, green alternatives capable of overcoming some of the limitations that have hindered the industrial application of other neoteric solvents like ILs.
Plasma and mechanochemistry further illustrate the innovative potential of non-conventional activation technologies.62,63 Plasma-induced processes generate highly reactive species under mild conditions, facilitating reactions that are otherwise challenging under conventional methods. Similarly, mechanochemistry leverages mechanical energy to drive chemical transformations without the need for solvents, aligning perfectly with the principles of green chemistry. These technologies enable unique reactivity and open avenues for sustainable and scalable chemical processes.
Despite their immense potential, the industrial application of these technologies faces significant challenges. Scaling up non-conventional activation methods requires the development of tailored reactors and pilot platforms to validate industrial feasibility. For instance, while laboratory-scale sonochemistry demonstrates remarkable efficiency, replicating these results at larger scales is a persistent challenge. The design of reactors capable of operating continuously and efficiently at an industrial scale is crucial for wider adoption. Additionally, the capital investment required for these technologies can only be justified when their added value is demonstrably significant.40
Custom-designed reactors are pivotal for advancing non-conventional activation technologies. These reactors must integrate real-time data collection to enable continuous process optimization and facilitate scale-up. For example, ultrasonic reactors with improved cavitation control or microwave reactors with precise thermal monitoring can significantly enhance efficiency and reproducibility. Rigorous reporting of experimental parameters, such as frequency, power, and reactor geometry, is essential for reproducibility and understanding the underlying mechanisms. Standardizing these parameters will also enable meaningful comparisons across studies, fostering broader adoption of these technologies.40
Energy efficiency is a critical consideration for the broader adoption of non-conventional activation technologies. These methods often lead to reduced energy consumption compared to traditional processes due to shorter reaction times and lower operating temperatures. For instance, microwave-assisted processes have demonstrated significant energy savings in biomass valorization and organic synthesis. However, comprehensive life-cycle assessments (LCA) are necessary to quantify the overall environmental benefits of these technologies and to address potential trade-offs, such as energy costs associated with specialized equipment.
Looking forward, the integration of these technologies with emerging fields offers exciting opportunities. Combining ultrasound or microwave irradiation with ionic liquids, enzymes, or photochemistry can create synergies that enhance process efficiency and expand the scope of applications.64–66 Such combinations exemplify the innovative potential of non-conventional activation technologies to drive green chemistry forward.
In conclusion, non-conventional activation technologies are revolutionizing chemical processes by offering sustainable, efficient, and innovative alternatives to traditional methods. Their successful implementation requires a comprehensive understanding of their mechanisms, the development of tailored equipment, and rigorous reporting of experimental conditions. As these technologies advance, they promise to transform green chemistry by enabling scalable, energy-efficient, and environmentally friendly processes. However, addressing challenges in scale-up, standardization and industrial integration remains critical for their broader adoption and impact.
5. Measuring sustainability metrics and life cycle analysis (LCA)
As green chemistry continues to gain prominence, defining and quantifying what constitutes a “green” chemical process or product remains a fundamental challenge. A systematic assessment of sustainability metrics is essential to ensure that claims of environmental friendliness are grounded in rigorous and reproducible methodologies. However, the field currently lacks standardized criteria, making it difficult to universally evaluate and compare the sustainability of chemical processes and products.Central to assessing sustainability is the concept of eco-design, which emphasizes a multi-criteria approach spanning the entire life cycle of a product or process. Life-cycle analysis (LCA) serves as a powerful tool to evaluate environmental impacts from raw material extraction to end-of-life disposal. LCA considers factors such as resource consumption, energy use, emissions, and waste generation, providing a holistic view of a product's environmental footprint.67–69 For instance, the development of bio-based plastics often raises the question of whether they are inherently more sustainable than conventional plastics. While bio-based plastics reduce dependence on fossil resources, their production may involve significant water use, land competition, and energy consumption, necessitating a thorough LCA to validate their environmental advantages.18
Similarly, the sustainability of durable plastics and composites requires careful scrutiny. Thermoset composites, known for their strength and resilience, pose significant challenges for end-of-life management due to their non-recyclable nature.70,71 Developing sustainable alternatives or recycling technologies for such materials is critical for achieving circularity in the chemical industry. Furthermore, the debate over whether to produce less plastic, transition to bio-based plastics, or focus on improving recyclability highlights the need for objective sustainability metrics to guide decision-making.
Despite the growing body of research on sustainable and green processes, many publications fail to report consistent or comprehensive indicators of sustainability. Often, green metrics are designed to evaluate specific processes or products, limiting their applicability to broader contexts. For example, metrics for green extraction processes, such as ultrasound-assisted or supercritical CO2 extraction, may focus on energy efficiency and solvent reduction but lack consideration of upstream impacts, such as raw material sourcing and equipment energy consumption. While these metrics provide valuable insights, their arbitrary selection criteria prevent generalization and hinder the development of universal standards.
To advance the field, the establishment of standardized green chemistry metrics is imperative. Such metrics should integrate environmental, economic and social dimensions of sustainability while remaining adaptable to various contexts. A systematic comparison of processes—e.g., evaluating which is “greener” between two methods—can provide relative insights but does not establish an absolute threshold for what qualifies as “green.” Defining this threshold is critical for industrial adoption and regulatory frameworks, as it allows clear differentiation between sustainable and unsustainable practices.
Indicators for green chemistry must also evolve to encompass multi-dimensional aspects of sustainability. For example, metrics such as E-factor (mass of waste generated per unit of product)72 or atom economy (proportion of reactants incorporated into the final product)73 are widely used but fail to capture broader life-cycle impacts.74 Incorporating LCA alongside traditional green chemistry metrics can provide a more comprehensive assessment, enabling the comparison of processes across their entire life cycles.18
Moreover, the development of standardized tools for green chemistry assessments would benefit both academic and industrial stakeholders. Companies often create internal metrics to evaluate sustainability, but these frameworks are rarely shared, limiting their broader applicability. Open-access databases and tools could help bridge this gap, facilitating the exchange of best practices and enabling consistent benchmarking across the field.
In conclusion, measuring sustainability in green chemistry requires a shift toward standardized, holistic, and reproducible metrics. The integration of LCA with green chemistry indicators offers a pathway to more robust assessments, ensuring that environmental claims are scientifically substantiated. By addressing these challenges, the field can progress toward establishing clear criteria for defining and validating green chemical processes and products, ultimately supporting their industrial adoption and global sustainability goals.
6. Training future chemists to make green chemistry the norm
As the world grapples with environmental challenges and sustainability imperatives, the role of education in shaping the next generation of chemists is more critical than ever. To make green chemistry the norm rather than the exception, it is essential to integrate sustainability principles into chemistry education at every level. This requires not only equipping students with foundational knowledge but also fostering innovation, interdisciplinary thinking and a commitment to addressing global challenges.10The foundation of green chemistry education lies in teaching the principles and metrics that define sustainable processes. Future chemists must understand the criteria for assessing whether a chemical process or product aligns with green chemistry goals, such as minimizing waste, reducing energy use and utilizing renewable resources. This understanding should extend beyond theoretical frameworks to include practical tools like life-cycle analysis (LCA) and green chemistry metrics. These skills enable chemists to critically evaluate the environmental and economic impacts of their work and to develop solutions that are both innovative and sustainable.
In response to this need, we have recently launched the Chimie Verte Academy in France to transform chemistry education at the national level.75 This program unites universities, industry stakeholders and public institutions to develop a robust educational framework for green chemistry. Its mission is clear: to train students and professionals to address the challenges of sustainability, promote public awareness of green chemistry, and foster a network of excellence in research and education. By aligning its vision with long-term sustainability goals, the Chimie Verte Academy aims to revolutionize how chemistry is taught, practiced and perceived in France (Fig. 6).
One of the Academy's primary objectives is to address the evolving needs of the chemical industry by preparing graduates with the skills required for future employment. This includes training students in emerging technologies such as bio-based product development, biodegradable materials and digital innovation. The integration of digital tools, for instance, can enhance the efficiency of green chemistry processes and enable more precise environmental assessments through LCA. These innovations are critical for meeting the sustainability challenges of the 21st century and must be central to any comprehensive green chemistry curriculum.
Continuous education is equally important for fostering a culture of sustainability within the existing workforce. Professionals at all levels, from technicians to senior executives, must be equipped to navigate the rapidly changing landscape of green chemistry. Lifelong learning programs, such as those offered by the Chimie Verte Academy, aim to upskill employees, enabling them to adopt sustainable practices and drive innovation within their organizations. This emphasis on continuous learning ensures that green chemistry remains a dynamic and evolving discipline, capable of responding to new challenges and opportunities.
International initiatives, such as Beyond Benign's green chemistry education network, further demonstrate the global importance of training future chemists.76 Beyond Benign provides educators with resources, training and support to make green chemistry an integral part of their teaching. By engaging students, educators and the broader community, these programs create a continuum of education that extends from primary school to higher education and professional development. This global approach underscores the need for collaboration and the sharing of best practices to make green chemistry a universal standard.
The importance of aligning green chemistry education with industry needs was eloquently expressed by John Warner, a co-founder of green chemistry. At the launch of the Chimie Verte Academy, he emphasized the importance of teaching not only the “why” and “what” of green chemistry but also the “how”. Without the skills to implement sustainable practices, even the most well-intentioned efforts will fall short. This insight underscores the need for education systems to focus on practical applications, ensuring that graduates are equipped to turn sustainability goals into reality.
In addition to technical skills, green chemistry education must address the broader societal and regulatory context. Future chemists need to understand the regulatory frameworks that govern sustainability practices, as well as the economic and social factors that influence the adoption of green technologies. By incorporating these elements into the curriculum, educational programs can produce well-rounded chemists who are not only technically proficient but also capable of driving systemic change.
Ultimately, training future chemists to make green chemistry the norm requires a holistic approach that combines technical education, interdisciplinary collaboration, and societal engagement. Programs such as the Chimie Verte Academy and Beyond Benign exemplify the potential of this approach, demonstrating how education can be a catalyst for innovation and sustainability. By equipping students and professionals with the skills, knowledge, and mindset needed to lead in this field, green chemistry education can pave the way for a more sustainable and equitable future.
9. Scaling green chemistry from lab to industry
Green chemistry has seen remarkable progress in research laboratories over the past decades, driven by its potential to address pressing environmental challenges. However, the transition from laboratory-scale innovations to widespread industrial adoption remains a significant bottleneck. While green chemistry is often celebrated for its principles and promise, it is now imperative to industrialize these concepts, transforming them from theoretical frameworks into practical, scalable solutions.77 This step is crucial not only to prevent greenwashing but also to ensure meaningful environmental and economic impacts.Collaboration between academia and industry is pivotal in accelerating this transition. Eco-partnerships, which unite the research expertise of academic institutions with the operational capacities of industrial stakeholders, have demonstrated their effectiveness in overcoming technological barriers. For instance, shared laboratories, industrial research chairs, and collaborative projects create environments where innovations can be rapidly tested, refined, and scaled. These models of partnership are essential for translating green chemistry principles into industrial applications.
In our case, we developed a unique continuous-flow extraction and functionalization process for bioactive compounds derived from Fallopia japonica, using a patented radial ultrasound irradiation prototype. This prototype, now commercialized by the start-up Rhizomex, demonstrates the feasibility of transitioning laboratory innovations to real-world applications.30 Such initiatives exemplify how start-ups can drive the scale-up of advanced green technologies by targeting specific biomass sources and integrating process intensification strategies. Start-ups also play a crucial role in driving innovation, particularly in upcycling and sustainable technologies.78 By focusing on niche applications and agile development processes, start-ups can bridge the gap between academic discoveries and market-ready solutions. Their ability to commercialize emerging technologies provides a critical link in the green chemistry value chain, fostering both economic growth and environmental benefits.
Recent literature further supports the industrial relevance of microwave-assisted technologies. Pilot- and field-scale microwave-assisted biorefineries have been experimentally validated, particularly for solid-phase processes like pyrolysis.79 Techno-economic assessments and life-cycle analyses suggest that microwave processing may offer lower costs and carbon footprints than conventional techniques, although independent evaluations remain necessary to confirm these findings across broader contexts. In the food industry, microwave applications are already widely implemented in processes such as drying, sterilization, and nutrient extraction.80 These techniques not only reduce processing times and operational costs but also minimize solvent use and water consumption, making them attractive for sustainable development. Despite their promise, the scale-up of such technologies remains constrained by the high cost of pilot platforms, specialized infrastructure, and the need for highly trained personnel. This stage—often referred to as the “valley of death” in innovation management—requires dedicated support to bridge the gap between proof-of-concept and industrial implementation.81
There is also an urgent need to further integrate green chemistry into corporate governance structures.82 Embedding green chemistry considerations at the decision-making level ensures that sustainability is prioritized in industrial policies and practices. Companies that align their strategic goals with green chemistry principles not only enhance their environmental credentials but also position themselves competitively in markets increasingly driven by sustainability.
To accelerate the industrialization of green chemistry, more robust mechanisms are needed to bridge the gap between research and application. Investment in pilot-scale facilities, shared platforms for testing industrial feasibility, and interdisciplinary training programs can address this gap. Scaling up green chemistry also requires clear regulatory frameworks that incentivize sustainable practices while providing guidance on compliance. These measures ensure that laboratory innovations are not only scalable but also economically viable and aligned with global sustainability goals.
By fostering stronger collaborations, empowering start-ups and embedding green chemistry into governance structures, the transition from lab to industry can be accelerated. This transformation is not merely an opportunity but a necessity, ensuring that green chemistry fulfills its promise of creating a sustainable future.
10. Conclusion and outlook: scaling-up green chemistry – a call to action
Throughout this Feature Article, a strategic roadmap has been sketched by connecting major advances in green chemistry, identifying current gaps, and outlining key directions for future development. Green chemistry has evolved from a conceptual framework into a crucial response to global environmental, economic, and societal challenges. However, its successful industrialization and widespread adoption require concerted efforts to address persistent barriers and seize emerging opportunities.Clarifying terminology and objectives is essential for aligning stakeholders. Terms such as eco-design, green chemistry and decarbonization often confuse public and professional discourse. Establishing actionable definitions through global collaboration, as highlighted by Cannon et al., ensures coherent communication and shared objectives.83
Strengthening public policies and governance is critical. Transparent regulatory frameworks must incentivize innovation, support financing mechanisms and ensure accountability. Addressing challenges such as high energy costs and waste valorization requires targeted legislation that balances competitiveness with sustainability. Effective governance can fast-track the adoption of green chemistry across industries, ensuring both environmental and economic gains.
Fostering partnerships between academia and industry accelerates the transition from laboratory research to industrial application. Eco-partnerships, shared research facilities and industrial chairs are pivotal for overcoming technological and economic barriers. Start-ups and SMEs also play a vital role by translating niche innovations into scalable solutions, creating synergies between research and commercialization.
Investing in education and training is essential to build a workforce capable of addressing the complexities of green chemistry. Continuous learning initiatives must equip professionals with skills in life-cycle analysis, digital tools and sustainable technologies. Programs such as the Chimie Verte Academy or Beyond Benign exemplify the potential of targeted education to foster innovation and drive systemic change.
Developing robust economic models for circular economy practices, particularly waste valorization, is non-negotiable. Financial incentives, coupled with clear governance, will enable scalable and replicable models that transform waste into resources.
In conclusion, scaling up green chemistry requires a multifaceted approach encompassing clear definitions, robust governance, collaborative partnerships, targeted education and sustainable economic frameworks. By addressing these priorities, we can transform green chemistry from a promising concept into a transformative force for a sustainable future. The time for action is now, more than ever!
Data availability
This Feature Article does not present new experimental data. No new data were generated or analysed in this study. All data supporting the discussions and conclusions drawn in this article are available in the references cited throughout the text.Conflicts of interest
The author declares no financial conflicts of interest. The author is the director of the Chimie Verte Academy and collaborates with Rhizomex through an institutional research partnership. These affiliations do not influence the content or conclusions of this work.Acknowledgements
This research has been financially supported since 2017 by a wide range of institutions and companies through collaborative projects and research initiatives. The author gratefully acknowledges the support of Université Savoie Mont Blanc, the Conseil Savoie Mont Blanc (and now the Département de la Savoie), the Fondation Université Savoie Mont Blanc, ADEME, SATT Linksium Grenoble Alpes, France 2030 and its funding operated by the French National Research Agency (ANR), the companies Rhizomex and PEP's/Proxipel, ANRT, the Auvergne-Rhône-Alpes Region, and the French Ministry of Higher Education and Research. The author also warmly thanks John Warner and the Beyond Benign team for their inspiring support in advancing green chemistry education.References
- S. A. Matlin, S. E. Cornell, A. Krief, H. Hopf and G. Mehta, Chem. Sci., 2022, 13, 11710 RSC.
- J. C. Slootweg, One Earth, 2024, 7, 754–758 CrossRef.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998 Search PubMed.
- C. Silvestri, L. Silvestri, A. Forcina, G. Di Bona and D. Falcone, J. Cleaner Prod., 2021, 294, 126137 CrossRef CAS.
- I. T. Horvath and P. T. Anastas, Chem. Rev., 2007, 107, 2169–2173 CrossRef CAS PubMed.
- J. H. Clark, Green Chem., 1999, 1, 1–8 Search PubMed.
- R. A. Sheldon, Green Chem., 2016, 18, 3180–3183 RSC.
- S. Y. Tang, R. A. Bourne, R. L. Smith and M. Poliakoff, Green Chem., 2008, 10, 268–269 RSC.
- M. J. Mulvihill, E. S. Beach, J. B. Zimmerman and P. T. Anastas, Annu. Rev. Environ. Resour., 2011, 36, 271–293 Search PubMed.
- D. Cole-Hamilton, Chem. – Eur. J., 2020, 26, 1894–1899 Search PubMed.
- F. Meng, A. Wagner, A. B. Kremer, D. Kanazawa, J. J. Leung, P. Goult, M. Guan, S. Herrmann, E. Speelman, P. Sauter, S. Lingeswaran, M. M. Stuchtey, K. Hansen, E. Masanet, A. C. Serrenho, N. Ishii, Y. Kikuchi and J. M. Cullen, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2218294120 Search PubMed.
- D. S. Mallapragada, Y. Dvorkin, M. A. Modestino, D. V. Esposito, W. A. Smith, B.-M. Hodge, M. P. Harold, V. M. Donnelly, A. Nuz, C. Bloomquist, K. Baker, L. C. Grabow, Y. Yan, N. N. Rajput, R. L. Hartman, E. J. Biddinger, E. S. Aydil and A. D. Taylor, Joule, 2023, 7, 23–41 CrossRef CAS.
- V. Hessel, N. N. Tran, M. R. Asrami, Q. D. Tran, N. V. D. Long, M. Escribà-Gelonch, J. O. Tejada, S. Linke and K. Sundmacher, Green Chem., 2022, 24, 410–437 RSC.
- B. Nanda, M. S. Sailu, P. Mohapatra, R. K. Pradhan and B. B. Nanda, Mater. Today Proc., 2021, 47, 1234–1240 CrossRef CAS.
- L. Biber-Freudenberger, C. Ergeneman, J. J. Förster, T. Dietz and J. Börner, Sustainable Dev., 2020, 28, 1220–1235 CrossRef.
- C. Römlein, Open Access Government, 2024, available at: https://www.openaccessgovernment.org/scaling-green-chemistry-a-catalyst-for-change/165702/ Search PubMed.
- N. Runyon, Thomson Reuters, 2023, available at: https://www.thomsonreuters.com/en-us/posts/esg/green-chemistry/ Search PubMed.
- G. Chatel, Chem. – Eur. J., 2020, 26, 9665–9673 Search PubMed.
- A. Ncube, S. Mtetwa, M. Bukhari, G. Fiorentino and R. Passaro, Energies, 2023, 16, 1752 CrossRef CAS.
- K. Kümmerer, J. H. Clark and V. G. Zuin, Science, 2020, 367, 6476 Search PubMed.
- A. Schade, M. Melzer, S. Zimmermann, T. Schwarz, K. Stoewe and H. Kuhn, ACS Sustainable Chem. Eng., 2024, 12, 12270–12288 CrossRef CAS.
- J. H. Clark, R. Luque and A. S. Matharu, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 183–207 CrossRef CAS PubMed.
- J. H. Clark, V. Budarin, F. E. I. Deswarte, J. J. E. Hardy, F. M. Kerton, A. J. Hunt, R. Luque, D. J. Macquarrie, K. Milkowski, A. Rodriguez, O. Samuel, S. J. Tavener, R. J. White and A. J. Wilson, Green Chem., 2006, 8, 853–860 RSC.
- J. H. Clark, F. E. I. Deswarte and T. J. Farmer, Biofuels, Bioprod. Bioref., 2009, 3, 72–90 CrossRef CAS.
- L. Vernès, Y. Li, F. Chemat and M. Abert-Vian, Plant-Based Green Chemistry 2.0, Springer, 2020 Search PubMed.
- D. Pérez-Almada, Á. Galán-Martín, M. del Mar Contreras and E. Castro, Sustainable Energy Fuels, 2023, 7, 4031–4050 RSC.
- N. E. Sammons Jr., W. Yuan, M. R. Eden, B. Aksoy and H. T. Cullinan, Chem. Eng. Res. Des., 2008, 86, 800–808 CrossRef.
- G. Kaur, K. Uisan, K. L. Ong and C. S. K. Lin, Curr. Opin. Green Sustainable Chem., 2018, 9, 30–39 CrossRef.
- R. A. Sheldon, J. Mol. Catal. A: Chem., 2016, 422, 3–12 CrossRef CAS.
- G. Chatel, R. Duwald, M. Draye, P. Fanget and C. Piot, patent FR20 06171, 2020, WO2021250251A1 Search PubMed.
- V. Quinty, C. Colas, R. Nasreddine, R. Nehmé, C. Piot, M. Draye, E. Destandau, D. Da Silva and G. Chatel, Plants, 2023, 12, 83 CrossRef CAS PubMed.
- M. Zwingelstein, M. Draye, J.-L. Besombes, C. Piot and G. Chatel, Waste Manage., 2020, 102, 782–794 CrossRef CAS PubMed.
- M. Zwingelstein, M. Draye, J.-L. Besombes, C. Piot and G. Chatel, ACS Sustainable Chem. Eng., 2019, 7, 8310–8316 CrossRef CAS.
- A. Vandeponseele, M. Draye, C. Piot and G. Chatel, Green Chem., 2020, 22, 8544–8571 RSC.
- A. Vandeponseele, M. Draye, C. Piot and G. Chatel, Clean Technol., 2021, 3, 335–350 CrossRef.
- L. Bruna, M. Draye, G. Cravotto and G. Chatel, Waste Biomass Valorization, 2024, 15, 4541–4555 CrossRef CAS.
- L. Drévillon, M. Koubaa and E. Vorobiev, Biomass Bioenergy, 2018, 115, 143–150 CrossRef.
- Proxipel, https://proxipel.com/technologie/?utm (accessed December 2024) Search PubMed.
- F. Jérôme, G. Chatel and K. de Oliveira Vigier, Green Chem., 2016, 18, 3903–3913 RSC.
- G. Chatel, Ultrason. Sonochem., 2018, 40, 117–122 CrossRef CAS PubMed.
- G. Chatel, Sonochemistry: New Opportunities for Green Chemistry, World Scientific, 2017, p. 188 Search PubMed.
- K. S. Suslick, Science, 1990, 247, 1439–1445 CrossRef CAS PubMed.
- J.-L. Luche, Synthetic Organic Sonochemistry, Springer Science, 1998, p. 425 Search PubMed.
- P. N. Amaniampong, A. Karam, Q. T. Trinh, K. Xu, H. Hirao, F. Jérôme and G. Chatel, Sci. Rep., 2017, 7, 40650 CrossRef PubMed.
- P. N. Amaniampong, J.-L. Clément, D. Gigmes, C. Ortiz Mellet, J. M. Garcia Fernandez, Y. Blériot, G. Chatel, K. De Oliveira Vigier and F. Jérôme, ChemSusChem, 2018, 16, 2673–2676 CrossRef PubMed.
- G. Chatel, K. De Oliveira Vigier and F. Jérôme, ChemSusChem, 2014, 7, 2774–2787 CrossRef CAS PubMed.
- F. Chemat, N. Rombaut, A.-G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier and M. Abert-Vian, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- G. Cravotto and A. Binello, Low-Frequency, High-Power Ultrasound-Assisted Food Component Extraction, in Innovative Food Processing Technologies, Woodhead Publishing Series in Food Science, Technology and Nutrition, 2016, pp. 3–29 Search PubMed.
- G. Chatel and R. S. Varma, Green Chem., 2019, 21, 6043–6050 RSC.
- S. Tiwari and S. Talreja, J. Pharm. Res. Int., 2022, 34(39A), 74–79 CrossRef.
- K. Martina, S. Tagliapietra, A. Barge and G. Cravotto, Review, 2016, 374, 79 Search PubMed.
- A. Kumar and S. Sharma, Bioresour. Technol., 2017, 245, 518–525 CrossRef PubMed.
- Green Chemistry Series, ed. A. J. Hunt and T. M. Attard, RSC, Cambridge, 2018, p. 678 Search PubMed.
- Y. Xu, V. Musumeci and C. Aymonier, React. Chem. Eng., 2019, 4, 2030–2054 RSC.
- S. S. de Jesus and R. M. Filho, Renewable Sustainable Energy Rev., 2022, 157, 112039 CrossRef CAS.
- J. L. Shamshina and R. D. Rogers, Chem. Rev., 2023, 123(20), 11894–11953 CrossRef CAS PubMed.
- F. Maria Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustainable Chem., 2020, 21, 27–33 CrossRef.
- T. El Achkar, H. Greige-Gerge and S. Fourmentin, Environ. Chem. Lett., 2021, 19, 3397–3408 CrossRef CAS.
- D. O. Abranches and J. A. P. Coutinho, Annu. Rev. Chem. Biomol. Eng., 2023, 14, 141–163 CrossRef CAS PubMed.
- R. Nguyen, A. Auvigne, A. M. Pérez Merchán, I. Malpartida and C. Len, Org. Process Res. Dev., 2024, 28(9), 3560–3569 CrossRef CAS.
- A. F. Quivelli, F. V. Rossi, P. Vitale, J. García-Álvarez, F. M. Perna and V. Capriati, ACS Sustainable Chem. Eng., 2022, 10(13), 4065–4072 CrossRef CAS.
- N. Joshi and S. Loganathan, Catalysts, 2024, 14, 802 CrossRef CAS.
- D. Ozer, in Advances in Green Synthesis, Advances in Science, Technology & Innovation, ed. Inamuddin, R. Boddula, M. I. Ahamed and A. Khan, Springer, 2021 Search PubMed.
- G. Chatel and D. R. MacFarlane, Green Chem., 2014, 16, 271–285 RSC.
- G. Cravotto and P. Cintas, Chem. Soc. Rev., 2009, 38, 2684–2697 RSC.
- E. Kuna, R. Behling, S. Valange, G. Chatel and J. C. Colmenares, Top. Curr. Chem., 2017, 375, 41 CrossRef PubMed.
- A. A. Burgess and D. J. Brennan, Chem. Eng. Sci., 2001, 56, 2589–2604 CrossRef CAS.
- S. A. Espinosa, A. Balaa and P. Fullana-i-Palmer, Green Chem., 2022, 24, 7751–7762 RSC.
- A. A. Burgess and D. J. Brennan, Chem. Eng. Sci., 2001, 8, 2589–2604 CrossRef.
- E. Morici and N. T. Dintcheva, Polymers, 2022, 14, 4153 Search PubMed.
- J.-G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 Search PubMed.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- Chimie Verte Academy, https://www.chimieverte-academy.fr.
- Beyond Benign Green Chemistry Education, https://www.beyondbenign.org.
- R. Ratti, SN Appl. Sci., 2020, 2, 263 CrossRef.
- J. Kratzer, Curr. Opin. Green Sustainable Chem., 2020, 21, 89–92 CrossRef.
- Q. Zhang, Z. Li, Z. Liu, Y. D. Prasetyatama, W. K. Oh and I. K. M. Yu, Nat. Rev. Clean Technol., 2025, 1, 269–287 CrossRef.
- P. Guzik, P. Kulawik, M. Zając and W. Migdał, Crit. Rev. Food Sci. Nutr., 2021, 62(29), 7989–8008 CrossRef PubMed.
- E. Colacino, V. Isoni, D. Crawford and F. García, Trends Chem., 2021, 3(5), 335–339 CrossRef CAS.
- M. A. Benvenuto and H. Plaumann, Green Chemistry in Government and Industry, De Gruyter, 2020 Search PubMed.
- A. Cannon, S. Edwards, M. Jacobs, J. W. Moir, M. A. Roy and J. A. Tickner, RSC Sustainability, 2023, 1, 2092–2106 RSC.
| This journal is © The Royal Society of Chemistry 2025 |





