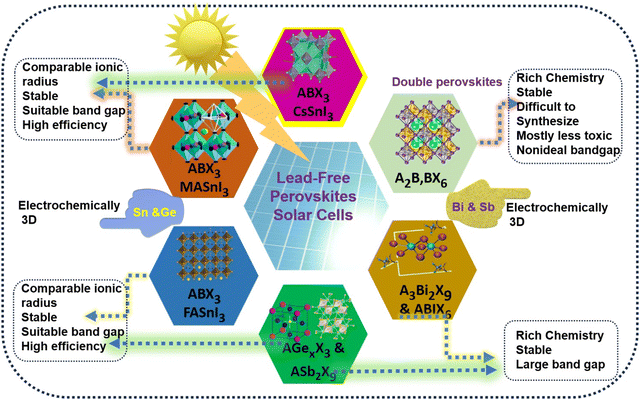
Lead-free perovskites for solar cell applications: recent progress, ongoing challenges, and strategic approaches
Imtiaz
Ahmed
 a,
Kamal
Prakash
a and
Shaikh M.
Mobin
a,
Kamal
Prakash
a and
Shaikh M.
Mobin
 *abc
*abc
aDepartment of Chemistry, Indian Institute of Technology Indore, Simrol Khandwa Road, 433552, India. E-mail: xray@iiti.ac.in
bCenter for Advanced Electronics (CAE), Indian Institute of Technology Indore, Simrol Khandwa Road, 433552, India
cCenter for Electric Vehicle and Intelligent Transport System (CEVITS), Indian Institute of Technology Indore, Khandwa Road, Simrol, Indore 453552, India
First published on 31st March 2025
Abstract
The growing perovskite solar cells (PSC) have reached a power conversion efficiency of up to 25% within a decade and demonstrated the potential to replace traditional silicon-based solar cells. However, a major issue with perovskite solar cells regarding their practical application and commercialization is their lead-based toxicity, which has harmful effects on human health and ecological systems. Thus, lead-free perovskite solar cells have emerged as one of the most promising prospects in perovskite solar cell technology due to their non-toxic nature, optimal stability, and durability. Since their discovery, lead-free perovskite solar cells have achieved a maximum power conversion efficiency of ∼15% and still require further development. In this feature article, we review the recent developments in the field of lead-free perovskite solar cells. We emphasize the advantages and limitations of Pb-free perovskites and the current state of lead-free perovskite solar cells. Furthermore, we discuss the impact of cation and anion sites on the stability and efficiency of lead-free PSCs and provide an update on the progress of lead-free perovskites for photovoltaic applications. Designing environmentally friendly lead-free perovskite devices is an imperative goal, though it comes with significant challenges. This article provides a brief analysis of the challenges and strategies required to improve the stability and efficiency of lead-free perovskites. Finally, we summarize the review to offer a better understanding of lead-free PSCs and outline the direction for further exploration.
1. Introduction
Solar energy has emerged as a powerful method of energy production that harnesses sunlight. It is expected to become a major energy source for future generations due to its unlimited and eco-friendly nature.1 Solar power will also serve as an effective sustainable energy source to reduce dependence on traditional energy sources and help combat global warming and climate change.2 In this regard, solar cells have been developed to convert solar energy into electric energy via photovoltaic (PVs) technology.3 Solar cells possess energy bandgaps ranging from 1.1 to 1.7 eV, which is close to the ideal bandgap of 1.5 eV required for optimal solar energy conversion. Several types of solar cells have been developed for photovoltaics (PVs), including inorganic solar cells such as silicon (Si), copper indium gallium selenide (CIGS), and gallium arsenide (GaAs), as well as organic solar cells like dye-sensitized solar cells (DSSCs) and organic polymer solar cells (OPVs).4–7 Crystalline silicon solar cells have demonstrated a power conversion efficiency of up to 26% and have gained wide recognition as the optimal solar cells in photovoltaics due to their superior electronic, optical, and thermal properties, as well as their high stability in various environmental conditions.8 Organic solar cells are also appealing due to their low-cost production and feasible synthesis.9 In recent years, perovskite solar cells (PSCs) have emerged as one of the most promising energy technologies, as they exhibit higher energy efficiency than other types of solar cells and offer cost-effective production with fewer and less expensive processing steps.10 Lead-free perovskites have recently received sustained attention for applications in single-crystal X-ray detectors,11–13 photodetection,14,15 water detection,16 scintillators,17,18 and potential use in solar cells,19–21 wireless light communication, and X-ray imaging,22 due to their nontoxicity, low-cost, as well as excellent stability and optoelectronic properties.Perovskite materials can be represent by the chemical formula ABX3, similar to mineral perovskite calcium titanium oxide (CaTiO3), where ‘A’ and ‘B’ are two positively charged cations with different sizes, while X represents the anion, mostly oxide, connected to both cations. Generally, perovskite materials have a cubic structure, in which B represents an atom surrounded by an anion octahedron in a 6-fold coordination, while A represents an atom with a 12-fold cuboctahedral coordination. Other forms of perovskite also exist with different arrangements of A and B atoms such as A1x−1A2x and/or B1y−1B2y configuration, in which the A and B sites change their oxidation states and coordinate with an anion (X), deviating from the ideal configuration.23 In a perovskite-based materials, alkali metals such as Cs+, Rb+, and K+ and divalent metal ions such as Pb2+, Sn2+, and Ge2+ are well-known as A and B cations having large size differences, while I, Br, or Cl are used as the X anion.24 Many organic cations such as methylammonium (CH3NH3+) and formamidinium (NH2CHNH2+) can also be utilized for the formation of perovskites. By varying the atoms//molecules in the structure of perovskites, their properties such as superconductivity, magnetism, spin-dependent transport (spintronics), and catalytic properties can be modulated for different applications. Thus, perovskites have attracted significant attention from the scientific community for material applications.23
Perovskite-based solar cells exhibit high efficiency and show promise as alternative solar cells that are low-cost, lightweight, and flexible. The crystalline layers of mixed organo-metal trihalide perovskites are capable of photo-absorption in the near IR and UV-visible regions with high molar extinction coefficients and longer exciton lifetimes, resulting in improved efficiency. In 2009, Miyaska and group employed CH3NH3PbI3 (MAPbI3) and CH3NH3PbBr3 (MAPbBr3) as photosensitizers in dye-sensitized solar cells, achieving a PCE of 3.8% and 3.1%, respectively.25 Subsequently, a lead-halide perovskite layer was coated on a TiO2 film, achieving a PCE of 9.7% under 1.5 AM sunlight illumination.26 Since then, the PCE of PSCs has increased exponentially from 3.8% to 22% within a decade, competing with the traditional Si-, CdTe-, and GaAs-based solar cells.21 Lead halide perovskite solar cells have demonstrated a high PCE of over 25%, similar to silicon-based solar cells.27–29 The electronic properties and photophysical of PSCs significantly depend on the crystal growth and morphology of perovskite structures. The high efficiencies of PSCs are attributed to the impressive optoelectrical properties of semiconducting organic–inorganic perovskite materials such as high optical absorption, tunable bandgap, effective photogenerated electron–hole pairs, and efficient charge transportation. Recently, perovskite/perovskite tandem solar cells and perovskite–silicon tandem solar cells achieved a PCE of 29.1% and 33.9%, respectively, surpassing silicon-based solar cells.30,31 Additionally, PSCs can be prepared via low-temperature solution-based processes that are low-cost and less energy-intensive, providing the advantage of cost-effectiveness and making them significantly cheaper than traditional silicon-based solar cells. Further, PSCs can be easily fabricated or deposited via solution processing methods, which are favorable in large-scale manufacturing, practical application, and subsequent commercialization.32
However, although perovskite-based solar cells have achieved unprecedented PCEs, many concerns hinder their large-scale applications. The major concerns regarding perovskite solar cells are their long-term stability and the toxicity of lead. Also, PSCs are susceptible to environmental conditions and have a short lifetime span. Thus, different strategies have been employed to extend the stability of PVSCs for longer periods with high efficiency.33 Modulating the components of perovskite solar cells such as mixed cation systems and surface passivation through two-dimensional (2D) perovskite integration have been introduced, which have significantly enhanced the stability and lifetimes of perovskite solar cells.34,35 However, the presence of lead in the perovskite structure also needs to be addressed. Pb in perovskite tends to degrade and convert into a toxic product that can cause health and environmental issues.36 Thus, lead-based PSCs are not commercially viable, motivating researchers to explore lead-free perovskites. Various metals or cations have been identified to replace Pb in perovskite solar cells based on their size, electronic configuration, and stability without compromising their impressive efficiency. These metals or cations must possess similar optoelectronic properties to perovskite solar cells such as optimal bandgap, high optical molar coefficients, longer exciton lifetimes, charge mobility, and long-term stability. Several transition metals, or lanthanides, alkaline earth metals, and p-block elements have shown the ability to substitute lead in perovskite materials.37 Some of the examples are Sn2+, Ge2+, Be2+, Sr2+, Ba2+, Ga2+, In2+, Bi3+, Sb2+ Eu2+, Tm2+, and Yb2+.38,39 Especially, Sn/Ge-based halides, Bi/Sb-based halides and some double perovskites, some with perovskite-like structures, have shown immense potential to develop lead-free PSCs.40
There has been significant advancement towards lead-free perovskites, but different challenges in realizing efficient and stable PSCs are still unresolved for their real-life application. Thus, our review mainly focuses on the recent progress and advancement in the field of lead-free PSCs. Firstly, we present the requirements of lead-free perovskite solar cells and highlight the advantages and limitations of Pb alternatives in PSCs, together with synthesis of lead-free perovskites solar cells and their essential optical characteristics. Then, the impact of different cation and anion sites on Pb-free perovskite materials for application in solar cells will be discussed. The development of Sn, Ge, Bi, Sb, and double perovskites for lead-free solar cells in recent times is comprehensively described. Finally, we describe tandem solar cells and the overall challenges regarding lead-free PSCs together with a summary and brief future perspective on developing highly efficient and environmentally friendly PSCs.
2. Advantages and limitations of Pb-free perovskites for solar cells
Lead-based perovskites, although highly efficient in converting sunlight into electricity, pose significant toxicity risks, particularly if devices degrade or are disposed of improperly. Lead contamination can harm ecosystems and accumulate in living organisms, raising environmental and regulatory concerns for the widespread, large-scale deployment of this technology.41 This has led to the emergence of alternative lead-free perovskite materials that possess low toxicity with favorable optoelectronic properties. In the search for Pb-free alternatives, materials with strong optical absorption, narrow bandgap, high charge transportation, low exciton binding energy, and robust stability are required. This can allow Pb-free materials to serve as effective absorbers in solar cells without significantly sacrificing their performance, positioning them as preferable, sustainable options for future applications. Fortunately, many low-toxicity materials with perovskite or perovskite-like structures exhibit potential for solar cell applications. Several lead-free alternatives, such as tin (Sn)-based perovskites, other structures incorporating elements such as bismuth (Bi), germanium (Ge), and antimony (Sb), defect-structured perovskites, and double perovskites, offer beneficial characteristics for photovoltaic performance and represent potential substitutes for lead in PSCs.35,37 Sn-based perovskites exhibit high charge mobilities and longer lifetimes, resulting in a high solar to power conversion.42 These materials can have immense potential to develop highly efficient PSCs, as seen in lead-based cells, with a much lower toxicity profile.Researchers are keen to develop eco-friendly perovskite solar cells that can meet industry standards for efficiency in a safer way. However, although lead-free options such as Sn-based perovskites, Bi-based compounds, and Sb-based halides are less toxic than lead, they each require unique environmental consideration.43 Tin is leading the way as one of the most commonly explored substitutes for lead-free perovskites. Sn-based perovskite structures are very similar to Pb-based perovskites and exhibit similar optoelectronic properties.44 However, the photo efficiency of Sn-based perovskite cells are relatively lower than that of Pb-based solar cells. One major issue with Sn perovskite solar cells is that Sn(II) is readily transformed into Sn(IV) via oxidation in the presence of oxygen and moisture, which reduces its stability and performance. Ge-based perovskite materials can also serve as Pb-free alternatives due to their optimal bandgaps for solar applications.45 However, germanium halide-based perovskites have been less investigated because Ge is a rare metal and very expensive, making it less suitable for a low-cost economy. Bismuth and antimony are also considered safer than lead, and their perovskite materials also demonstrate good stability for photovoltaic application in atmosphere conditions.45 However, bismuth and antimony-based perovskites exhibit a low power conversion efficiency due to their bandgap defects, insufficient charge transportation and high charge recombination rate. Additionally, the mining and refining processes for bismuth and antimony result in the release of harmful byproducts, presenting a severe concern for the ecosystem if not properly controlled. This significantly hampers their scalability and cost-effectiveness for wider applications. Another type of Pb-free perovskite family is double perovskites or ordered perovskites having a combination of monovalent and trivalent cations rather than divalent cations. Double perovskites possess a three-dimensional structure similar to ABX3 perovskites and show impressive optoelectronic properties and charge carriers with longer lifetimes.46 Moreover, many lead-free double perovskites based on cesium–silver–bismuth halides have been investigated due to their stability and relatively low toxicity.47 The key problem associated with double perovskites is their large and indirect band gap, leading to high energy loss and detrimental for solar energy harvesting. Thus, double perovskites are less efficient compared to lead-based perovskites, which may result in larger panel areas needed for equivalent power, potentially increasing the resource use and environmental footprint over time. Overall, although lead-free perovskites offer an environmentally safer profile, their stability, efficiency, and sustainable production processes need to be improved for large-scale applications. Research into encapsulation methods, material recycling, and scalable production practices will be key to minimizing the environmental impact of these promising lead-free alternatives.48,49 Finally, the move toward lead-free perovskites aligns with global goals for sustainable energy solutions, as well as regulatory trends that may eventually limit the use of hazardous materials in consumer products. Thus, lead-free options show noteworthy promise for the future of green energy technology. Fig. 1 presents a schematic illustration of Pb replacement and potential of lead-free perovskites.
3. Present status of lead-free PSCs
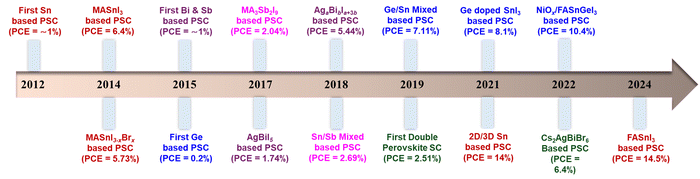
Lead-free perovskites have shown significant promise; however, their performance still lags behind their lead-based counterparts. This is primarily attributed to their susceptibility to oxidation, the formation of high-density defects, and rapid crystallization dynamics. These factors collectively contribute to their suboptimal photovoltaic performance, both in terms of efficiency and long-term stability. Thus, to address these challenges, extensive efforts have been undertaken, including advancements in solution processing techniques, compositional engineering strategies (such as tuning crystal dimensionality), and innovative device engineering approaches.50,51Bivalent Sn is a promising candidate for replacing Pb for lead-free perovskites given that both are from the same group and possess similar electron configurations. In 2012, Chen et al. first developed lead-free- PSCs using CsSnI3 perovskite. CsSnI3 perovskite served as a direct semiconductor for Schottky solar cells with a bandgap of 1.3 eV and a PCE of nearly 1%.52 Later, in 2014, Noel and team demonstrated a lead-free, CH3NH3SnI3 perovskite solar cell with a PCE efficiency of 6% and high Jsc of 22.7 mA cm−2.53 This was a significant achievement in terms of PSC efficiency and led to the rapid development of Sn-halide perovskite solar cells, achieving the highest efficiency of 15% within a few years. However, the conversion of Sn2+ to Sn4+via oxidation in Sn-halide perovskites reduces their efficiency and stability. Thus, formamidinium tin iodide (FASnI3) perovskites have been widely explored considering their ability to prevent Sn oxidation and obtain a lower hole density compared to other perovskites. In recent times, highly efficient Sn-based PSCs were prepared via composite formation, use of additives, or surface engineering to hamper the conversion of Sn2+ to Sn4+ and enhance the charge carrier mobility, leading to PSCs with superior performances and stability. The oxidation of Sn2+ to Sn4+ is one of the major challenges limiting the stability and performance of tin-based perovskite solar cells. This is due to their increased defect density, reduced charge carrier lifetime, and accelerated material degradation. Thus, various strategies are discussed in this work to mitigate these challenges. P. Wang et al. fabricated SnF2 on a methylammonium tin iodide (MASnI3) film through insertion reactions/ion exchange, achieving a PCE of 7.78%.54 The large amount of SnF2 improved the crystallinity of MASnI3 and suppressed the detrimental oxidation, which allowed it to achieve a PCE of 7.78%. Further, W. Zhang et al. utilized dimethyl ketoxime (C3H7NO, DMKO) as a multifunctional additive in CsSnI3−xBrx based organic-free and Pb-free based PSCs and obtained an enhanced PCE of over 11.2%.55 The introduction of the 2D phase can enhance the crystalline 3D growth to form a unique 2D/3D microstructure to reduce the defect density and enhance the device performance. Z. Kang and coworkers remarkably enhanced the PCE of a 2D–3D tin-based perovskite heterostructure to 12.45% by simply introducing low-dimensional phases.56 In another interesting work, the introduction of a 2D phase FASnI3 structure led to an enhancement in the PCE from 9.38% to 14.81%.57 However, although tin-based PSCs have been extensively studied, their efficiency and stability are still not comparable to that of Pb-based PSCs, and thus more efforts are required to enhance their performance. Considering elements in the same group, germanium (Ge2+) can also be promising as a divalent lead alternative. Krishnamoorthy et al. first synthesized germanium iodide perovskite solar cells using spiro-OMeTAD and TiO2 as the electron- and hole-selective layers, respectively.58 Different cations of Cs, MA, and FA were employed for the synthesis of Ge-based perovskites and investigated for their potential for photovoltaic applications. In their study, CsGeI3 and MAGeI3 displayed PCE values of 0.11% and 0.2% for solar cells with Jsc values of 5.7 and 4 mA cm−2, respectively. It was proven that the bandgap of CsGeX3 (X = I, Br, Cl) decreases with the size of the halide-ions and only the CsGeI3 perovskite has an optimal bandgap suitable for photovoltaic devices.59 Ge-based PSCs are even more susceptible to oxidation than Sn-based devices due to the high stability of the Ge4+ ion via the inert pair effect. This leads to the poor efficiency of Ge perovskites and minimizes their application as single metallic PSCs. Thus resolve this, mixed Ge/Sn-based perovskites have been developed. The amalgamation of Ge and Sn for the fabrication of perovskite solar cells represents a new approach for boosting their stability and efficiency. In 2019, the CsSnGeI3 perovskite was formed by alloying Ge(II) in the CsSnI3 perovskite composition, which showed high chemical and thermal stability and delivered a PCE of up to 7.11%.59 Recently, ozone-exposed PEDOT:PSS was applied in a Sn–Ge mixed perovskite, improving its PCE from 6.8% to 8.1%.60 The exposure of PEDOT:PSS to ozone optimized the band energy levels and enhanced the hole mobility, subsequently improving the PSC performance. Moreover, the PCE of Ge-based solar cells can be significantly improved by the selection of appropriate cation, mixed perovskite, and surface engineering. However, the commercialization of Ge-based perovskite solar cells is not economical considering the high cost of Ge.
Similar to Pb and Ge, Bi(III) and Sb(III) have also been considered possible substitutes for lead-free perovskites. As elements in group 15, Bi and Sb possess an electronic configuration similar to Pb(II) and show high stability and non-toxic nature under atmosphere conditions.61 In 2015, Park et al. initially reported Cs3Bi2I9 PSCs with a PCE of over 1%.62 Later in 2016, solution-processed AgBi2I7 thin films were developed by spin-coating silver, which formed pinhole-free surface morphologies and possessed a narrow band gap of 1.87 eV. The AgBi2I7-based devices produced a PCE of 1.22% with outstanding stability over 10 days under atmosphere conditions.63 Subsequently, various compositions of silver bismuth iodide (AgBiI) were prepared using different ratios of AgI/BiI3 and investigated for solar cells, albeit the highest efficiency of 2.31% was obtained.64 The low efficiency of Bi-based perovskites can be rationalized in terms of their large bandgap and low-dimensional structures such as 0D and 2D. Thus, to counter the dimensionality issue, three-dimensional AgBi2I7 was formed, which showed a more suitable band gap of 1.83 eV for photovoltaics compared to the 0D Cs3Bi2I9 (2.16) and 2D Rb3Bi2I9 (2.10) structures.65 Durrant and coworkers synthesized (CH3NH3)3Bi2I9 films with excellent optoelectronic properties and produced a hysteresis-free, PCE of 3.17% with good reproducibility.66 Additionally, a strategic improvement in PCE was achieved by tuning the optoelectronic properties of AgaBibIa+3bvia limited anionic sulfide substitution.67 The effect of the sulfide anion on these compositions was seen by the adjustment in valence band edge by 0.1–0.3 eV, and subsequently the corresponding PCE. Thus, a record PCE value of 5.44 ± 0.07% for Bi-based perovskites was achieved under 1 sun irradiation with a Jsc value of 14.6 mAcm−2 and excellent stability under ambient conditions for over a month. This indicated that optimizing the composition of Bi-based PSCs substantially improved their performance. Compared to other metals, Sb is a naturally occurring metal and cheaper, which is capable of achieving cost-effective PSCs by replacing Pb. Hence, many Sb-based perovskites have been investigated as capable candidates for solar cells.68 In 2015, Mitzi et al. pioneered antimony-based lead-free PSCs by developing a 2D layered perovskite derivative, Cs3Sb2I9.69 It exhibited a direct bandgap of 2.05 eV and similar photo-absorption as CH3NH3PbI3, making it very promising for optoelectronics applications. T. Kirchartz et al. prepared the (CH3NH3)3Sb2I9 perovskite material and utilized it as an absorbing layer in a heterojunction solar cell, achieving a PCE of 0.5% with low photocurrent densities.70 Further, Bhoopathi et al. and S. Chatterji lowered the optical band gap of (CH3NH3)3Sb2I9 perovskite solar cells and improved the PCE significantly.71,72 In another work, our group fabricated a smooth layer of (NH4)3Sb2I9 perovskite material in a PCS device, achieving a PCE of 0.42% with a Voc of 945 mV.72 In a recent study, theoretical calculations of the PCE of Sb-based PSCs were performed, which suggested that stable and highly efficient Sb-based PSCs may achieve a PCE of 23.10% by optimizing their perovskite layer thickness, doping concentration or density defect, etc.73 However, to replace Pb in PSCs, more work is required to make Bi and Sb compatible.
Further, double perovskites have emerged as a fascinating and alternative approach in the field of lead-free PSCs.74 In lead-free double perovskites, two Pb2+ positions are replaced with two different cations with +1 and +3 valency and form an A2B1+B3+X6 structure. Many double perovskites with the A2B1+B3+X6 structure have been developed with Cs, Cu, and MA at the A position, Ag+, In+, Na+, and K+ at B+, Bi3+, Sb3+, and In3+ at the B3+ position and X as halide ions.75 Double perovskites possess unique properties such as optimal bandgap, high thermal stability, increased charge mobility, and low exciton binding energy, making them favorable for photo applications. Fig. 2 presents the development of lead-free perovskites and their PCE values over the years.
4. Synthesis of lead-free perovskites for solar cells
Several methods are available to develop lead-free perovskite materials; the most popular are solution processing, vapor phase deposition, solid-state synthesis, and hydrothermal/solvothermal synthesis. The choice of a particular method depends on which form of perovskite material needs to be synthesized, such as nanocrystal, colloidal, or thin films. Generally, single-crystal perovskites are developed via the temperature-lowering method, traditional solvothermal method, and antisolvent crystallization. In the temperature-lowering method, single crystals of perovskite are formed at a temperature lower than typically preferred. Mitzi et al. first reported the synthesis of tin-based single crystals (C4H9NH3)2(CH3NH3)n−1SnnI3n+1 (n = 1–5) using the temperature-lowering crystallization method, keeping the temperature rate between 2 °C to 5 °C per hour.76 The double perovskite Cs2AgIBiIIIBr6 was prepared by heating the solution at 110 °C for 2 h and cooling it to room temperature. It exhibited similar photovoltaic properties to (MA)PbI3 and (MA)PbBr3, suggesting its photo-based applications without having a toxic element. Compared to the temperature-lowering method, anti-solvent crystallization can be performed under ambient conditions, which involves the addition of a second solvent to reduce the solubility of the solution and form single crystal perovskites. This method is also called precipitation, desolvation, or solvent displacement. Chen et al. utilized acetic acid (HAc) as an anti-solvent to synthesize tin-based perovskites, which increased the nucleation growth of the precursors via salting-out crystallization. Antisolvent and primary solvent DMSO form hydrogen bonds to lessen the solvation of the precursor and enhance the effective concentration to give a crystallized form from the solution.77 Chu and coworkers applied chlorobenzene, isopropyl alcohol, and anisole as antisolvents to prepare Cs3Sb2ClxI9−x perovskite films. Among them, anisole as a green antisolvent achieved a dense surface morphology for perovskite single crystals with high crystallinity and photo-absorption ability. Thus, the Sb-perovskite with anisole showed a superior photovoltaic performance with a PCE of 2.07%.Further, lead-free perovskite nanocrystals can be formed via either the hot injection method or recrystallization technique. Between them, the hot injection method is preferred because the size and morphology of perovskite colloids can be modulated by varying the reaction conditions such as precursor concentration, temperature, and time. The formation of CsSnX3 (X = Cl, Cl0.5Br0.5, Br, Br0.5I0.5, I) perovskite nanocrystals was attempted from Cs2CO3 and SnX2 unsuccessfully due to the formation of CsX precipitation. For the synthesis of CsSnX3 perovskite nanocrystals, SnX2 was dissolved in reducing tri-n-octylphosphine solvent and injected into a Cs2CO3 solution containing oleic acid and oleylamine at 170 °C, which resulted in the formation of highly stable CsSnX3 colloidal nanocrystals, as confirmed by powder X-ray diffraction (PXRD).78 Moreover, Cs2SnI6 NCs were developed via the hot injection method and could be transformed into different structural shapes, such as spherical quantum dots, nanorods, and nanowires, by changing reaction time and ligands. Oleic acid and oleylamine helped form high-quality perovskite nanocrystals.79 Likewise, colloidal Cs3Sb2I9 nanocrystals were also prepared via the hot injection method. In this context, Bi-based double perovskite nanocrystals, Cs2AgIn0.9Bi0.1Cl6, were obtained using CsCl, AgCl, InCl3, and BiCl3 solution in DMSO.80 This solution was further injected into isopropanol antisolvent, and the NCs were obtained by centrifugation. However, these two methods are the only methods available to synthesize lead-free halide perovskite colloids, and thus other techniques need to be developed.
Therefore, lead-free perovskites, particularly Sn-based perovskite thin films for PSC application, are synthesized through solution processing methods such as one- or two-step deposition and vapour deposition methods.81–83 Compared to lead-based perovskites, lead-free perovskites are mostly synthesized using organic cations such as MA and FA, inorganic cations such as Cs+, Rb+, and other alkali cations that occupy the A-position. In this regard, the solution processing and vapour deposition methods are the most compatible with forming lead-free perovskites for perovskite solar cells. The one-step solution method is widely considered for the synthesis of tin-based perovskites. SnI2 has low solubility in commonly used solvents such as N,N-dimethylformamide (DMF) and crystallizes faster that its Pb-counterparts. Thus, single solvents such as DMSO were strategically explored to develop Sn-based perovskite films through a one-step spin-coating method.84 The precursor SnI2 formed a stable intermediate with DMSO to resist the faster crystallization process and produce a high-quality perovskite thin film. An FA0.75MA0.25SnI3 perovskite thin film was fabricated using chlorobenzene antisolvent with a high film coverage and reduced pinhole generation, which achieved a PCE of 6.6%.85 Further, an FA0.75MA0.25SnI3 perovskite thin film was fabricated using various antisolvents for PSC applications. Besides the one-step solution method, the two-step solution method can also be employed to develop lead-based perovskites, but it is not suitable for Sn-based perovskites. Zhu and group first introduced a two-step process for Sn-based PSCs by using SnI2 in trimethylaluminum (TMA) solution with a lower concentration of SnF2. TMA forms an SnI2-TMA complex, which delays the fast reaction of FAI and SnI2 and enhances the perovskite crystallization by preventing SnF2 segregation. Thus, an improved SnI2 film with the maximum coverage was obtained, which achieved a superior efficiency of 7.09%.86 A bismuth-based (CH3NH3)3Bi2I9 (MBI) PSC was developed via a two-step sequential solution method using a rutile TiO2 layer and electron transporting layer (ETL). The photovoltaic result exhibited a PCE of 0.14% for this PCS with excellent stability up to 67 days under ambient conditions.87 Recently, Han and co-workers prepared Sn-based PSCs using DMSO as the solvent. The developed Sn films had a uniform morphology and showed a PCE of over 10%.88
Another strategy to achieve high-quality lead-free perovskite films is the vapor deposition method. There are several examples of the preparation of Sn-based films through the vapor-assisted method. It produces a uniform and smooth morphology thin-film without the use of any incompatible solvent. A lead-free MASnI3 film was prepared for the first time via a low-temperature vapor-assisted solution process (LT-VASP).89 The resulting high-quality Sn film-based PSC achieved a PCE value of 1.86%. This work suggested that the substrate temperature plays an important role in obtaining high surface coverage and excellent uniformity for thin films. Wang et al. utilized a sequential-vapor-deposition procedure to prepare double perovskite Cs2AgBiBr6 thin films for photovoltaic application.90 The Cs2AgBiBr6 thin films exhibited large grain sizes and uniform and smooth surface properties. Consequently, the Cs2AgBiBr6 thin film-based PSC device achieved a PCE value of 1.37% with high stability. The feasibility of the vapor deposition method to fabricate high-quality thin films leads the way for the further exploration of various PSC devices based on lead-free perovskites. Finally, the main reason behind using different methods for thin-film synthesis is to optimize the quality of thin films in terms of their crystallinity, thickness, grain size, phase purity, and crystal orientation to improve the PCE of solar cells. However, the photovoltaic efficiency of lead-free perovskite solar cells is still lower compared to that of lead-based PSCs, and thus new synthetic strategies still need to be explored.
5. Essential optical characteristics of lead-free perovskite solar cells
To replace lead perovskite solar cells with lead-free halide perovskites and achieve high PCEs, certain optoelectronic properties need to be enhanced, such as the absorption coefficient, charge mobility, carrier lifetime, small exciton binding energy of photogenerated electrons, and low trap density. The optical properties of perovskites depend on the high electronic dimensionality, metal electronic configuration, and nature of their organic cations and halides. The structural dimensionality of perovskite materials has a large impact on their photovoltaic performance. In the case of Pb-based perovskites, their bandgap increases with a reduction in structure dimensionality from 3D to 0D. It has been found that 0D and 1D perovskites possess large bandgaps and localized band edges, which are not beneficial for photovoltaic application, while 2D perovskite film growth must be aligned parallel to the layers to facilitate effective charge generation and transportation. Alternatively, 3D perovskites are more promising as absorber materials for photovoltaics due to their optimal bandgap, flat band edges, high absorption coefficient and high charge mobility. This indicates that the 3D structure of lead-free halide perovskites should be highly desirable solar cell absorber materials.Moreover, high photo absorption is very important to design efficient PSC devices. Pb-based halide perovskites display high optical absorption coefficients with a layer thickness of ≈500 nm, far ahead of traditional inorganic thin-film solar cell technologies.91,92 In the case of lead-free perovskite solar cells, Sn2+ has the same electronic configurations and structural properties as Pb2+, and thus Sn also exhibits high absorption upon solar irradiation for PSC application. MASnI3 perovskite thin films displayed a direct band gap of 1.3 eV and a PL emission band at 950 nm with high absorption.93 Replacing MA with the ratio of FA led to different emissions corresponding to their ratio in a PSC cell.94 High charge mobility of photogenerated electron and holes is essential to obtain efficient thin-film solar cells given that it increases the carrier lifetimes and reduces their recombination rate. MASnI3 and CsSnI3 exhibited high electron mobilities of 2320 and 536 cm2 V−1 s and produced power to a conversion efficiency of 6.63% and 3.79%, respectively.95,96 Similarly, PSC devices with a low trap density demonstrate high efficiency due to their facile charge carrier transportation and improved stability. The FASnI3 material exhibits lower charge-carrier mobility than CsSnI3 and MASnI3, but PSC devices based on it showed high efficiency (PCE = 12%) due to its low trap density.97 FASnI3 perovskites also show excellent thermal stability compared to others, although they have lower conductivity than MASnI3. Moreover, the doping of different ions in hybrid halide perovskites has a profound impact on the absorption and band gap of perovskite solar cells. Halide hybrid perovskites MASnI3−xBrx exhibited tuned optical properties as PL emission of MASnBr3, MASnI2Br, and MASnIBr2 appeared at 577, 795, and 708 nm with modulated band gap edges, respectively.93 This also impacted the PCE given that the MASnIBr2-based device delivered the best PCE value of 5.73% under solar illumination, while CH3NH3SnI3 showed a PCE of 5.23%. Similar to Sn(II), Ge is also a group IVA element and a potential candidate for the fabrication of perovskite solar cells. An investigation into a series of Ge-based perovskites, CsGeI3, MAGeI3, and FAGeI3, depicted that the structural dimensionality of AGeI3 is completely different from each other due to their cation size, and they exhibit direct band gaps of 1.63, 2.0, and 2.35 eV, respectively.58 The optical properties of AGeI3 are suitable for perovskites solar cell application given that they exhibit strong absorption in the range of 400–600 nm together with sharp band edges. However, the PCE of MAGeI3 and CsGeI3 were found to be 0.20% and 0.11%, which is relatively lower than that of their Sn-based counterparts, and also indicated that band gap tuning by changing the cation can improve their photovoltaic performance.58 The photovoltaic performance of tin-based PSCs is hampered by the facile oxidation of Sn(II) to Sn(IV), which degrades the absorber perovskite material significantly. In this context, a change in the dimensionality of the structure readily from 3D to 2D or mixed 2D and 3D Sn perovskites can lead to high-stability perovskites, subsequently improving the PCE.98,99
Besides Sn and Ge, Bi3+ and Sb3+ may be alternatives to lead-free perovskites due to their low toxicity and similar electron configuration. Generally, Bi and Sb form an A3B2X9 perovskite structure, where the B-positions are occupied in the perovskites. A3B2X9 perovskites mostly exhibit wider band gaps, generally larger than 1.9 eV, and large absorption coefficients in the visible region and possess excellent stability in air and moisture. A3B2X9 perovskites such as MA3Bi2I9, Cs3Bi2I9, and MA3Bi2I9, were formed via a solution processing method and exhibited weak absorption, high exciton binding energies, and low charge carrier mobilities. Their corresponding photovoltaic devices produced low efficiency, and the highest PCE of only 1.09% was obtained for Cs3Bi2I9.62 Later, ABi3I10 perovskites were also developed, and their absorption and band structure were also adjusted by varying their cations (A = MA, FA, and Cs).100 These perovskites demonstrated good stability in air and were utilized as light absorbers for solar cells devices. Among them, CsBi3I10 achieved the highest efficiency of 1.51% due to its maximum light absorption and narrow bandgap. The bismuth-based perovskite Cs3Sb2I9 was explored for photovoltaic applications due to its layered structure and high electron and hole mobility. The Cs3Sb2I9-based photovoltaic devices exhibited a PCE of less than 1%, which can be rationalized in terms of their deep level defects in a layered structure.69 Similarly, MA3Sb2I9− based thin films exhibited a PCE of 0.5% despite having a suitable band gap of 2.14 eV.70 Sb-based perovskites have poor morphology and excessive halide ions, which disturb their crystalline growth, resulting in low performance. Thus, the optimization of the contact layers and morphology of Sb-based perovskites can lead to a high power conversion efficiency.101
In the case of double perovskites, the Cs2AgBiBr6 perovskite was exploited due to its good optoelectronic properties and stability under ambient conditions. The Cs2AgBiBr6 perovskite has good crystallinity, narrow band gap, longer carrier lifetime, and high charge mobility. Thus, it showed an indirect bandgap of 1.95 eV with a photoluminescence (PL) lifetime of ca. 660 and high stability, which are beneficial for photovoltaic studies.102 Bein and coworkers synthesized a high-quality Cs2AgBiBr6 thin film using a one-step method and used it as an absorber material in PSC devices, obtaining a PCE of 2.5%.103 Shao et al. also developed a crystalline Cs2AgBiBr6 film with a smooth morphology and large grain structure via an antisolvent crystallization and annealing method, which showed an efficiency of 2.23%.104 In another work, the optical properties of the Cs2AgBiBr6 perovskite were enhanced by introducing an N719 dye interlayer. The incorporation of the dye increased the photo absorption, constructed a suitable band structure, and reduced the charge recombination rate, consequently improving its PCE up to 2.84%, which is higher than that of previously reported Cs2AgBiBr6-based PSCs.104 Thus, to achieve high efficiency for double perovskites, it is necessary to apply doping or alloying techniques effectively to improve their optoelectronic properties.
6. Importance of compositional engineering of perovskites in terms of cation and anion sites
Perovskite materials with different crystal structures are known to be fascinating materials due to their unique properties and versatile applications in various fields such as solar cells, sensing, catalysis, fuel cells, and energy storage. Generally, perovskite structures are comprised of two cation sites, A and B, while one anion site X, allowing the development of a library of perovskite materials with different compositions and tuning of their electronic and optical properties. Compositional engineering of perovskites using different types of cations and anions plays an important role in optimizing their performance, stability, and functionality for a particular application. The efficiency of perovskite solar cells can also be affected by the compositional engineering of perovskites. Herein, we delve into the importance of compositional engineering, providing examples and applications to underscore its transformative role in solar cells.6.1. Impact of the cation (A site) in Pb-free perovskites
Lead-free perovskites can assume various geometries depending on their synthesis methods and compositional variations. In terms of high carrier mobility, optimum direct bandgap, and tolerance to defects due to its higher dielectric constant, theoretical studies reveal that the corner-sharing 2D-structure of octahedron layers is more favorable compared to the face-sharing 0D-structure of octahedron dimers.69 Considering antimony (Sb) as an example, the layered phase of A3Sb2X9 is challenging to achieve through conventional solution processing. Therefore, innovative solution-processable approaches must be explored and developed to synthesize layered perovskites. The properties of lead-free based perovskites can be finely tuned by varying the concentrations of their cations. Notably, the structure of perovskites is significantly influenced by the effective ionic radius of their cation, underscoring its critical role in determining the crystallographic arrangement of these material.105 In the case of inorganic cations, density functional theory calculations revealed that the formation energies of the two structural forms of Sb-based perovskites differ by only 0.1 eV. Consequently, the specific synthesis conditions play a decisive role in determining which structural form is obtained.106 Inorganic perovskites are an emerging class of halide perovskites with enhanced stability against oxygen, moisture, and elevated temperatures. In the case of organic cations, methylammonium is a bulky group in hybrid inorganic–organic Sb perovskites (MA3Sb2X9). To get a layered structure, the stoichiometries of the halides in mixed perovskites must be varied. To adopt a well-stabilized structure in a perovskite crystal, it must possess an optimal tolerance factor.107 Accordingly, incorporating smaller cations in the structure has been shown to be an effective strategy for attaining the optimal tolerance factor.108 When comparing organic and inorganic cations, organic cations, despite being bulkier (e.g., methylammonium, formamidinium, ethylammonium, and guanidinium), demonstrate superior stability and performances compared to other monovalent inorganic cations such as Cs, Rb, K, Na.22,69,109,110 Cs as an inorganic cation provides more thermal and chemical stability to perovskite materials. Thus, Cs-based perovskite structures have been widely explored. The incorporation of the rubidium cation is beneficial for the phase stability of perovskites. The Rb+ ion effectively increases the grain boundaries of perovskite materials, which readily reduces the non-radiative recombination and enhances the charge mobility.111 Further, organic cations such as methylammonium (CH3NH3+) and formamidinium (CH(NH2)2+) were mainly utilized for the formation of hybrid perovskites. MA-based perovskite materials tend to show high excellent optoelectronic properties with excellent PCE values but are often thermally unstable. Meanwhile, FA-based perovskites offer high thermal stability but are fragile to phase instability, limiting their PCE performance. However, the optimized combination of MA and FA in perovskite solar cells has been investigated to improve their performance. Thus, the variation of the A-site cation promises significant opportunities for the development of novel materials through different combinations in double and triple-cation systems. Weak hydrogen bonding interactions and cation size contribute to the structural cohesion between the inorganic framework and the A-site cation, where dipole–dipole interactions are essential in driving the structures toward their most thermodynamically stable configurations. In this context, Ozorio et al. described the role of the A-cations in the polymorphic stability and optoelectronic properties of lead-free ASnI3 perovskites.1126.2. Impact of the cation (B site) in Pb-free perovskites
In recent years, B-site doping has emerged as the most effective and extensively adopted strategy for enhancing the performance of perovskite solar cells (PSCs). This approach has been instrumental in achieving high PCEs and improved stability. By modifying the B-site composition, the optoelectronic properties and band structure of perovskite materials can be precisely tailored, enabling optimized device performances. The limited substitution of divalent cations at the B-site with either heterovalent or homovalent ions has been proven to be an effective strategy for producing high-quality perovskite materials. As described in the previous section, this approach not only reduces the reliance on toxic elements but also induces passivation effects at the grain boundaries and surfaces. It enhances the crystallinity of perovskites by increasing their grain size, thereby reducing their trap-state density and grain boundaries. These improvements contribute to lower energy losses and reduced charge recombination at the interfaces between the perovskite layer and the corresponding charge-transport layers, ultimately enhancing the device performance.113 This strategy enables precise modulation of the bandgap energy to achieve desired values, ensuring optimal alignment with the energy levels of adjacent layers for specific applications.114 B-site dopants, including group IIIA to VA metal ions, transition metal ions, alkaline-earth metals, and lanthanides, play a noteworthy role in enhancing the stability and photovoltaic performance of perovskite-based devices. The incorporation of these dopants offers promising opportunities to improve the material properties and device efficiencies through the B-site doping strategy.6.3. Impact of the anion (X-site) in lead-free perovskites
To maintain charge neutrality between the anions and cations, halide ions play a crucial role, and due to their suitable effective ionic radii, they have been highly explored. The bandgap typically decreases as the electronegativity difference between the constituent cations and anions decreases.115 The bandgap decreases linearly from Cl to Br to I, and thus halides with lower electronegativity exhibit a bandgap more suitable for absorbing a substantial portion of the solar spectrum. Research on lead-free perovskites suggests that directly substituting lead with alternative metal cations often results in a reduced device efficiency. Sn–Pb-based perovskites show great potential for achieving relatively high efficiency however, tin (Sn) still faces significant stability challenges even it may have similar properties. The anion (X-site) in lead-free perovskites significantly influences their structural, and optical electronic properties. The anion (X-site) plays a vital role in determining the physical and functional properties of lead-free perovskites, making its selection a crucial aspect in material design. The key highlights responsible for the impact of the anion on the X-site in lead-free perovskites are as follows:(a) The nature of the anion affects the bandgap, with larger and less electronegative anions typically resulting in narrower bandgaps, enhancing light absorption in the visible spectrum. (b) Anion selection can impact the overall stability of the perovskite structure. For instance, halides such as Cl−, Br−, and I− play critical roles in stabilizing the crystal lattice, but their differing ionic radii and electronegativities influence the tolerance factors and defect formation. (c) The anion determines the defect levels and density of trap states, influencing the charge carrier recombination rates and transport properties. For example, halides with lower electronegativity such as I− are more prone to forming shallow defect levels. (d) The anion influences the phase stability of lead-free perovskites, particularly under environmental stressors such as moisture, light, and heat. Mixed halides such as I−/Br− can enhance the phase stability and mitigate degradation. (e) Anions directly impact the optical absorption, emission spectra, and device efficiency. Thus, optimizing the anion composition is critical to achieving high-performance materials for photovoltaic and optoelectronic applications.
The stability and efficiency of lead-free perovskite solar cells (PSCs) are significantly influenced by the choice of cations and anions in the perovskite structure. The A-site in perovskites (ABX3) is typically occupied by organic cations such as MA+ (methylammonium) and FA+ (formamidinium), or inorganic cations such as Cs+. In lead-free PSCs, the choice of A-site cation affects their structural stability and bandgap. Inorganic cations such as Cs+ mainly enhance the thermal stability but may increase the bandgap, reducing the light absorption, while FA+/MA+ help to increase the efficiency due to their better light absorption but may compromise the thermal and moisture stability. B-site cations of less toxic elements such as Sn2+, Ge2+, Bi2+, and Sb2+ can be used to replace the toxic Pb2+, which directly impact the electronic properties and stability. These elements may offer high efficiency, suitable bandgaps and improve the stability. Alternatively, the X-site anions are typically occupied by different halides such as I−, Br− and Cl−, which affect the bandgap, charge carrier mobility, and stability. Considering I−, it generally enables high efficiency and provides a narrow bandgap, while Br−/Cl− increase the band gap but improve the stability. We recognize that challenges related to stability and efficiency still persist. Thus, to address these issues, several strategies can be implemented, including cation and anion engineering, which involves tailoring the composition of the A/B-sites, as well as defect passivation and dimensional engineering, such as utilizing 2D/3D hybrid structures. These approaches aim to enhance the stability without compromising the efficiency, which are thoroughly discussed in the “Challenges and strategies” section.
7. Recent development of Pb-free perovskite materials for solar cells
To achieve the maximum solar conversion efficiency, the materials involved in constructing photovoltaics must have outstanding carrier transfer kinetics, tunable bandgap, appropriate band structure, and long length for the diffusion of carriers. Regarding the absorption of solar energy, the bandgap of photovoltaic-like materials is an important parameter. The PCE of lead-based PSCs is strongly influenced by the electronic structure of lead (Pb). Specifically, the 6p orbitals of the Pb atoms play a critical role in determining the band structure of perovskite materials. Consequently, alternative elements proposed to substitute for Pb should exhibit chemical compatibility with the perovskite structure to maintain the stability and optimal electronic properties of the material. Macdonald116et al. recapitulate the evolution of Sn-PSCs, including early progress to the state-of-the-art approaches benefitting the stability of devices. As is known, Pb is located in period 4, group 14 of the periodic table, and thus the most promising candidates for Pb substitution are elements from group 14, which share similar chemical properties. Additionally, elements from group15, such as bismuth (Bi) and antimony (Sb), with comparable electronic configurations and other less toxic and sustainable metals may also be considered as potential alternatives.92 To mitigate the toxicity concerns associated with lead (Pb), it is common to partially or completely replace Pb with alternative metals in the perovskite structure.117,118 Hence in this section, we explore the numerous alternatives to Pb perovskites for solar cell application. Among the commonly studied elements for the substitution of lead (Pb), including Sn, Ge, Sb, Bi, and Cu, tin has emerged as the most promising candidate in recent years.53,71,119–1227.1. Sn-based perovskite solar cells
Sn-based perovskites have the general formula of ABX3, where A represents organic/inorganic cations such as methylammonium (MA), formamidinium (FA), and cesium (Cs), B represents Sn, and X is a halide ion (i.e., Cl, Br, I). Sn-based PSCs mainly possess MA and FA cations in their structure to get highly efficient PSCs. Thus, the resulting crystal structures are primarily observed in either the orthorhombic Amm2 or cubic Pm3m phase. Sn forms a traditional perovskite because the A-cations are situated at eight corners of the cubic units to occupy the cavity and metals have 6-coordinations fold with corner-sharing [SnX6] octahedra.123 Similar to Pb, Sn perovskites display excellent optoelectronic properties compared to other perovskite-based materials. Sn-based perovskites exhibit higher charge transportation and a narrow optical band gap relative to Pb-based perovskites. According to both experimental observations and theoretical calculations, the bandgaps of Sn2+-based perovskites are in the range of 0.75–1.3 eV, positioning them as promising candidates for photovoltaic applications. In addition to their suitable bandgap, Sn-based perovskites exhibit several advantageous properties, including high absorption coefficients, direct bandgaps, low exciton binding energies, and high charge carrier mobilities.124,125 Some of the best of Sn-based perovskites are CsSnI3, MASnI3, and FASnI3, which will be discussed next.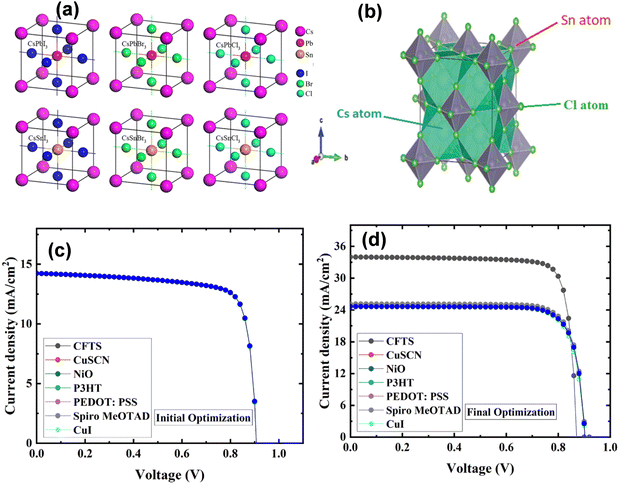 | ||
| Fig. 3 Representation of unit cells of CsBX3 (B = Pb and Sn) composed of different halide anions. Reproduced with permission from ref. 126, copyright 2017, Nature Scientific Reports. (b) Schematic of the crystal structure of defect perovskite Cs2SnCl6 (green color represents Cl atoms, while Cs atoms are positioned at the interstitial spaces). Reproduced with permission from ref. 130, copyright 2019, RSC. (c) and (d) Current density–voltage curve of the initial and final optimized structure of ITO/PCBM/CsSnI3/HTL/Se, respectively. Reproduced with permission from ref. 131, copyright 2023, RSC. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of DMSO and DMF. Further, an increase in the Br content reduced the charge recombination rate and aligned the band structure for PV application. Hence, MASn(I0.33Br0.67)3 exhibited an improved PCE by up to 3.2%.133 For the first time, Wang et al. reported a synthetic strategy for fabricating MASnI3 perovskite films using ion exchange/insertion reactions tailored for solar cells. By effectively controlling the crystallization and nucleation processes of MASnI3, they attained a highly uniform and pinhole-free perovskite structure. This advancement enabled a PCE value of 7.78%, marking the highest value for MASnI3-based solar cells to date, with excellent reproducibility and stability. The J–V curves and histograms of PCEs for 30 devices prepared using MASnI3 films are presented in Fig. 4a and b, respectively.54
1 (v/v) mixture of DMSO and DMF. Further, an increase in the Br content reduced the charge recombination rate and aligned the band structure for PV application. Hence, MASn(I0.33Br0.67)3 exhibited an improved PCE by up to 3.2%.133 For the first time, Wang et al. reported a synthetic strategy for fabricating MASnI3 perovskite films using ion exchange/insertion reactions tailored for solar cells. By effectively controlling the crystallization and nucleation processes of MASnI3, they attained a highly uniform and pinhole-free perovskite structure. This advancement enabled a PCE value of 7.78%, marking the highest value for MASnI3-based solar cells to date, with excellent reproducibility and stability. The J–V curves and histograms of PCEs for 30 devices prepared using MASnI3 films are presented in Fig. 4a and b, respectively.54
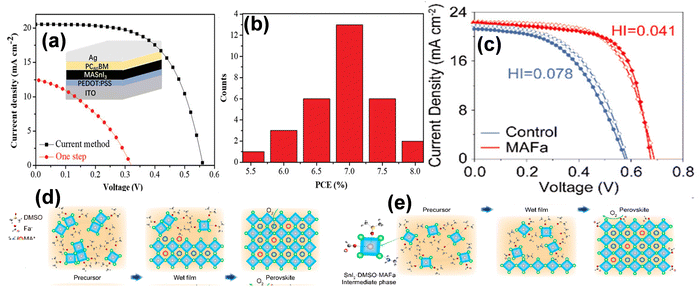 | ||
| Fig. 4 (a) and (b) J–V curves and histograms of PCEs for 30 devices of MASnI3 films, respectively. Reproduced with permission from ref. 54, copyright 2020, Wiley. (c) J–V curves of the control and MAFa-containing TPSCs. (d) and (e) Schematic of the crystallization process of the control and MAFa-derived perovskite films. Reproduced with permission from ref. 134, copyright 2023, ACS. | ||
An extremely stable lead-free PSC was developed by fabricating high-quality FA0.75MA0.25SnI3 films. This was achieved using the ionic liquid methylamine formate (MAFA), which stabilized the intermediate adduct. It effectively controlled the reaction rate of ammonium salts and SnI2, in turn modulating Sn perovskite crystallization and preventing the formation of SnI2 clusters, as shown in Fig. 4d and e. Thus, the PCE of the MAFA-derived perovskite films remarkably reached over 10% (Fig. 4c).134 In further developments, Zhao and coworkers altered the composition of a perovskite material to achieve high performance. According to the calculated tolerance factor, the organic cations (MA/FA) and halide anions (I/Br) were varied in the perovskite structure to optimize the band structure and crystallinity of the MA1−yFAySnI3−xBrx perovskite. It was observed that MA0.4–02FA0.6–0.8SnI3–xBrx exhibited a suitable bandgap, high surface, and reduced voids with an increase in the Br ratio, and MA0.3FA0.7SnI2.4Br0.6 was found to be the optimal composition for device application.135
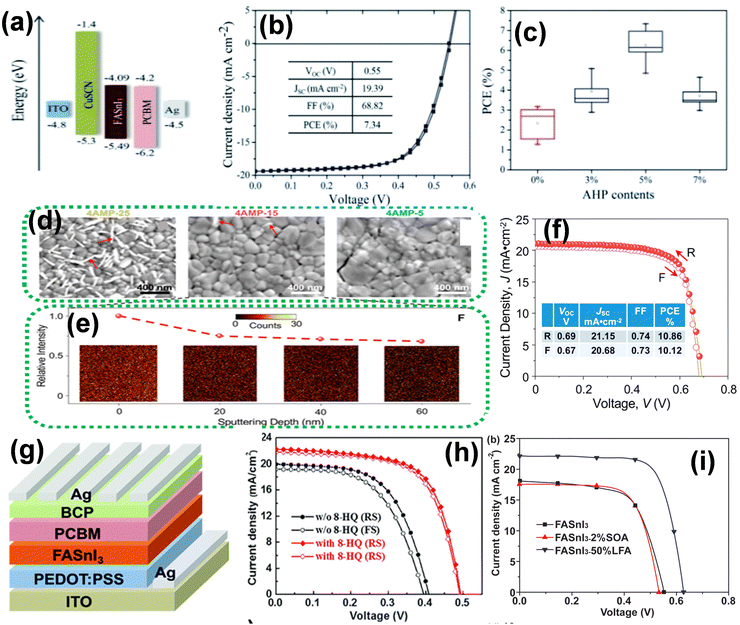 | ||
| Fig. 5 (a) Band structure, (b) J–V curve of FASnI3-based PSCs, and (c) PCE statistics for FASnI3-based PSCs with different amounts of AHP. Reproduced with permission from ref. 139, copyright 2019, RSC. (d) SEM images of the perovskite thin films of 4AMP-25, 4AMP-15, and 4AMP-5, (e) TOF-SIMS intensity as a function of sputtering depth in the 4AMP-15 thin film, and (f) J–V curve of 4AMP-15-based device. Reproduced with permission from ref. 140, copyright 2020, ACS. (g) PSC device structure based on FASnI3 and (h) J–V curves of the best devices without and with 8-HQ. Reproduced with permission from ref. 141, copyright 2020, RSC. (i) J–V curves of FASnI3-based devices. Reproduced with permission from ref. 142, copyright 2020, ACS. | ||
To date, numerous studies have been conducted on Sn-based perovskite solar cells (PSCs). Table 1 summarizes the photovoltaic parameters observed across these investigations. The favored use of the FA cation can be attributed to the higher formation energies of Sn vacancies in FASnI3, and thus a lower hole density relative to MASnI3. Lin et al. demonstrated a new strategy to restrict Sn degradation under ambient conditions by adding the bidentate ligand 8-hydroquinone in inverted FASnI3 PSCs. The 8-HQ ligand can coordinate with the Sn2+ sites via its nitrogen and oxygen atoms and inhibit the conversion of Sn2+ to Sn4+. Thus, the film quality and stability of the PSC improved effectively with reduced defect states, subsequently enhancing its photovoltaic performance. Fig. 5g and h represent the device structure based on 8-HQ coordinated FASnI3, and the corresponding devices showed an excellent PCE of 7.15% with high Jsc and Voc.141 Meng et al. reproduced an improved FASnI3 PSC by introducing liquid formic acid as a reducing solvent, resulting in the production of the FASnI3 perovskite film and leading to an efficiency of over 10.37% under AM1.5G illumination (Fig. 5i).142
| S. no. | Light absorber | Device structure | Synthetic method | Band gap (eV) | Carrier lifetime (ns) | V oc (V) | J sc (mA cm−2) | FF% | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | FASnI3 | PET/IZO/PEDOT:PSS/FASI3/C60/BCP/Ag. | One-step spin coating | — | — | 0.59 | 21.60 | 66.5 | 8.35 | 143 |
| 2. | PEA0.2FA0.8SnI3 | ITO/HTLs/PEA0.2FA0.8SnI3/ICBA/BCP/Ag | One-step spin coating | 1.42 | 10.3 | 0.60 ± 0.06 | 20.7 ± 0.4 | 51.8 ± 3.6 | 6.3 ± 0.6 | 144 |
| 3. | FASnI3 | ITO-PEDOT:PSS-perovskite/C60/BCP/Ag. | One-step spin coating | 1.43 | 21.2 | 0.84 | 23.19 | 72 | 14.5 | 145 |
| 4 | FASnI3 | ITO/PEDOT:PSS/perovskite/PCBM/BCP/Ag | Antisolvent method | 1.43 | 27 | 0.85 | 20.50 | 71 | 12.45 | 56 |
| 5. | Cs2SnI6 | FTOTiO2-Cs2SnI6/P3HT/Ag | Solution processing method | 2.14 | — | 0.25 | 7.41 | 24 | 0.47 | 130 |
| 6. | FASnI3 | ITO/PEPOT:PSS-FASnI3/PCBM/BCP/Ag | Antisolvent method | 1.4 | — | 0.31 | 17.64 | 41 | 2.28 | 146 |
| 7. | FA0.8PEA0.2SnI2.8Br0.2 | ITO/PEDOT:PSS-Sn-PVK/ICBA/BCP/Ag | One-step spin coating | 1.23 | — | 0.86 | 19.7 | 73 | 12.9 | 53 |
| 8. | FASnI3 | ITO/PEPOT:PSS-FASnI3/PCBM/PEI/Ag | — | 1.41 | — | 0.64 | 13.36 | 56 | 5.51 | 147 |
| 9. | FA0.75MA0.25SnI3 | ITO/PEDOT:PSS-FA0.75MA0.25SnI3/C60/BCP/Al | One-step spin coating | 1.34 | 5.07–6.22 | 0.69 | 22.4 | 63 | 10.1 | 134 |
| 10. | CsSnI3 | ITO/PCBM/CsSnI3/CFTS/Se | — | — | — | 0.87 | 33.99 | 83.4 | 24.73 | 131 |
| 11. | FASnI3 | FTO/WO3/FASnI3/CZGS/MoOx:Au | — | — | — | 0.75 | 32.84 | 46 | 17.10 | 148 |
| 12. | FASnI3 | ITO/CuI/CsSnI3/C60/BCP/Al | Two-step spin coating | 1.4 | 123 | 0.92 | 20.3 | 77 | 14.0 | 149 |
| 13. | FASnI3 | ITO/PEDOT:PSS//C60/BCP/Ag | One-step spin coating | 1.42 | 12.4 | 0.49 | 22.24 | 65.19 | 7.15 | 142 |
| 14. | PEA0.15FA0.85SnI3 | PEDOT:PSS | Antisolvent method | 1.39 | — | 0.47 | 22.7 | 67.8 | 7.3 | 60 |
| 15. | MASnIBr2 | FTO/cTiO2/mTiO2MASnIBr2/spiro-OMETAD/Au | One-step spin coating | 1.75 | — | 0.58 | 11.09 | 49 | 3.2 | 133 |
| 16. | PTN-Br + SnF2 | ITO/PEDOT:PSS-FASnI3/C60/BCP/Ag | Antisolvent method | 1.35 | — | 0.57 | 20.66 | 67.40 | 7.94 | 150 |
| 17. | GeI2 + SnF2 | ITO/PEDOT:PSS/PEA0.08 FA0.92Sn1−xGexI3/C60-BCP/Ag/Au | Two-step sequential coating | 1.45 | — | 0.46 | 21.92 | 73.0 | 7.45 | 151 |
| 18. | RbCl + SnF2 | ITO/PEDOT:PSS/FA1−xRbxSnI3-PCBM/BCP/Ag | Antisolvent method | — | — | 0.487 | 20.04 | 60.4 | 5.89 | 152 |
| 19. | TBAI + SnF2 | ITO/PEDOT:PSS-FASnI3/PCBM/BCP/Ag | One-step spin coating | 1.34 | — | 0.51 | 19.7 | 69.6 | 7.0 | 153 |
| 20. | PDI2 + SnF2 | ITO/PEDOT:PSS-FASnI3C60/BCP/Ag | One-step sequential coating | 1.37 | 127 ns | 0.69 | 21.85 | 75.1 | 11.39 | 154 |
| 21. | NH4SCN + SnF2 | ITO/PEDOT:PSS/PEAxFA1−xSnI3-ICBA/BCP/Ag | Antisolvent method | 1.39 | — | 0.94 | 17.4 | 75 | 12.4 | 155 |
| 22. | FASnI3 | FASnI3:PEACl | Antisolvent method | — | — | 0.59 | 22.06 | 69 | 9.1 | 156 |
| 23. | FASnI3 | ITO/PEDOT:PSSDPPAxFA1−xSnI3-C60/BCP/Ag | Antisolvent method | — | 2.16 | 0.45 | 14.47 | 63.03 | 4.12 | 157 |
| 24. | FASnI3 | glass/ITO/PEDOT:PSS/FASnI3/PCBM-BCP/Ag | One-step spin coating | — | 1.4 | 0.49 | 22.24 | 65.19 | 7.15 | 141 |
| 25. | MASnI3 | ITO/PEDOT:PSS/MASnI3/PC61BM/BCP-Ag | Multi-step sequential coating | — | — | 0.57 | 20.68 | 66 | 7.78 | 54 |
| 26. | (FA,MA)SnI3 | Au/spiroOMeTAD/FAxMA1–xSnI3/TiO2/FTO | One-step spin coating | 1.35–1.43 | 7.76–6.72 | 0.58 | 19.80 | 46.65 | 5.37 | 132 |
| 27. | FASnI3 | ITO/CuSCN/FASnI3-PCBM/Ag | Two-step sequential spin coating | 1.4 | — | 0.55 | 19.39 | 86.8 | 7.3 | 139 |
| 28. | Ag3BiI6−2xSx | FTO/TiO2/Ag3BiI6−2xSx/spiro-OMeTAD/Au | — | 1.65–1.85 | 110 | 0.57 | 14.60 | 65 | 5.44 | 158 |
| 29. | AgBi2I7 | FTO/TiO2/AgBi2I7/spiro-OMeTAD/Au | Two-step sequential coating | 1.89 | — | 0.54 | 2.81 | 46 | 0.69 | 159 |
| 30. | MA3Sb2I9 | FTO/c-TiO2/LFPSCs-6/P3HT/carbon | One-step spin coating | 2.2 | — | 0.55 | 3.70 | 48 | 0.91 | 160 |
| 31. | CsBi3I10 | ITO/NiOx/perovskite layer/PC61BM/BCP/Ag. | Ligand-assisted reprecipitation | 1.77 | — | 0.79 | 4.2 | 69 | 2.30 | 161 |
| 32. | Cs3Bi2I9 | ITO/NiOx/perovskite layer/PC61BM/BCP/Ag. | Ligand-assisted reprecipitation | 2.05 | — | 0.62 | 2.4 | 47 | 0.70 | 161 |
| 33. | Rb3Sb2I9 | FTO|c-TiO2|Rb3Sb2I9-poly-TPD|Au | Two-step deposition | 2.24 | — | 0.61 | 5.54 | 40 | 1.35 | 162 |
| 34. | (CH3NH3)3Bi2I9 | ITO/PEDOT:PSS-Sn/C60/BCP/Ag | Antisolvent method | — | — | 0.51 | 0.94 | 61 | 0.31 | 163 |
| 35. | (CH3NH3)3Bi2I9 | FTO/c-TiO2-Meso-TiO2/P3HT | One-step spin coating | 2.1 | — | 0.87 | 2.70 | 69 | 1.62 | 164 |
| 36. | AgBi2I-DMF | — | Two-step deposition | 0.54 | 46 | 0.54 | 2.81 | 46 | 0.69 | 159 |
| 37. | AgBi2I7 DMF:MeOH | — | Two-step deposition | 0.65 | 48 | 0.65 | 3.07 | 48 | 0.96 | 159 |
| 38. | AgBi2I-DMF | — | Two-step deposition | 0.54 | 46 | 0.54 | 2.81 | 46 | 0.69 | 159 |
| 39. | CsBi3I10 | ITO/PEDOT:PSS/CBI/BCP/C60/BCP/Ag. | Two-step spin coating | 1.78 | 1.92 ns | 0.67 | 2.46 | 49 | 0.80 | 165 |
| 40. | (CH3NH3)3Bi2I9 | FTO/CLTiO2/mTiO2/MBI/spiro-MEOTAD/Au | Two-step deposition | 2.2 | — | 0.87 | 1.6 | 34 | 0.41 | 166 |
| 41. | (CH3NH3)3Bi2I9 | F/SnO2/FTO/TiO2 | Chemical vapour deposition | 2.30 | — | 0.40 | 0.11 | 36 | 0.016 | 167 |
| 42. | (CH3NH3)3Bi2I9 | — | Antisolvent method | 2.0 | — | 0.59 | 0.5 | 57 | 0.17 | 168 |
| 43. | (FA)3Bi2I9 | FTO/C-TiO2-meso-TiO2/perovskite/spiro/Au | One-step spin coating | 2.19 | — | 0.48 | 0.11 | 46 | 0.022 | 169 |
| Sb-based | ||||||||||
| 44. | Cs4CuSb2Cl12 | FTO/WS2/Cs4CuSb2Cl12/CuSbS2/Ni | — | 1.6 | — | 1.17 | 23.75 | 83 | 23.10 | 73 |
| 45. | (CH3NH3)3Sb2I9 | — | Antisolvent method | 1.55 | — | 0.74 | 1.48 | 52 | 0.57 | 72 |
| 46. | Rb3Sb2I9 | ITO/cTiO2/mpTiO2/Rb3Sb2Br9–xIx/spiro-OMeTAD/Au. | Antisolvent vapor diffusion crystallization | 2.02 | — | 0.55 | 4.25 | 59.5 | 1.37 | 170 |
| 47. | Rb3Sb2I9 | — | One-step spin coating | 2.24 | — | 0.55 | 2.11 | 57 | 0.66 | 106 |
| 48. | (CH3NH3)3Sb2I9 | FTO/CL-TiO2/m-TiO2/MASbI-HTM/Au | Two-step deposition | 2.1 | — | 0.69 | 0.39 | — | 0.10 | 171 |
| 49. | (CH3NH3)3Sb2I9 | FTO/CL-TiO2/m-TiO2/MASbI/HTM/Au | Two-step deposition | 2.1 | — | 0.74 | 1.40 | — | 0.54 | 171 |
| 50. | (NH4)3Sb2I9 | FTO/CLTiO2mTiO2/(NH4)3Sb2I9-HTM/Au | Two-step deposition | 2.05 | 0.47 | 0.94 | — | — | 0.42 | 172 |
| Ge-based | ||||||||||
| 51. | NiOx/FASn0.9Ge0.1I3 | PEDOT:PSS HTL | One-step spin coating | 1.4 | — | 0.69 | 21.3 | 71 | 10.43 | 173 |
| 52. | Ge:EDA0.01FA0.98SnI3 | PEDOT:PSS | Antisolvent method | 1.39 | — | 0.51 | 21.39 | 63 | 6.8 | 60 |
| 53. | CsSnGeI3 | Au/spiro-OMeTAD/CsSn0.5Ge0.5I3/spiro-OMeTAD/Au | Solid-state synthesis | 1.5 | 10.92 | 0.63 | 18.61 | 60 | 7.11 | 174 |
| 54. | CsSnGeI3 | FTO/PCBM/(IL1)/CsSn0.5Ge0.5I3/(IL2)/spiro-OMeTAD/Au | — | 1.5 | — | 1.17 | 25.80 | 80.33 | 24.20 | 175 |
| 55. | CsSn0.5Ge0.5I3 | Au/spiro OMeTAD/PCBM/FTO | Solid-state synthesis | 1.50 | 10.92 | 0.63 | 16.61 | 60 | 7.11 | 174 |
| Double lead-free perovskites | ||||||||||
| 56. | Cs2AgxNa1−xBiyIn1−yCl6 | Cs2NaBiI6 (CNBI) | Room temperature synthesis | — | — | 0.67 | 2.38 | 75 | 23.13 | 176 |
| 57. | Cs2AgBiBr6 | FTO/bl&mpTiO2/Cs2AgBiBr6/HSL/Au | One-step spin coating | — | 10−6 s | 1.59 | 3.73 | 74 | 2.92 | 177 |
| 58. | FA4GeSbCl12 | FTO/TiO2/FA4GeSbCl12/Cu2O/Au | — | 1.3 | — | 0.76 | 34.52 | 85.1 | 22.25 | 178 |
| 59. | Cs2AgBiBr6 | SnO2, spiro-OMeTAD/ITO/Au | One-step spin coating | 1.64 | 41.86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
0.92 | 11.40 | — | 6.37 | 179 |
| 60. | Cs2AgBiBr6 | FTO/TiO2/Cs2AgBiBr6/spiro-OMeTAD/MoO3/Ag | Solution and vapour deposition | 1.99 | 39 | 1.01 | 3.82 | 65 | 2.51 | 180 |
| 61. | Cs2AgBiBr6 | FTO/CTiO2/Cs2AgBiBr6/P3HT/Cu | One-step spin coating | 2.39 | 14.44 | — | 2.58 | — | 1.91 | 181 |
| 62. | Cs2AgBiBr6 | Spiro-OMeTAD as HTL | One-step spin coating | 3.09 | — | — | 2.53 | — | 1.38 | 181 |
| 63. | Cs2AgBiBr6 | P3HT as HTL | One-step spin coating | 1.9 | — | — | — | — | 1.77 | 181 |
| 64. | Cs2BiAgI6 | ITO/TiO2/Cs2BiAgI6/CBTS/Au | — | 1.6 | — | — | 24.25 | 87.40 | 23.71 | 182 |
Li et al. developed a highly oriented 2D halide perovskite film for stable lead-free PSCs. Additive phenyl ethylammonium chloride (PEACl) was utilized on FASnI3 perovskite to grow the FASnI3:PEACl film in a highly crystalline 2D-phase, as presented in Fig. 6a. It was revealed that the annealing of Sn perovskite, facilitated by PEACl, promoted the formation of a vertically oriented, highly ordered 2D crystal structure. FASnI3:PEACl PSCs were fabricated with NiOx or PEDOT:PSS as HTLs. The PEACI additive in the PSC device functioned as a surface barrier layer, effectively protecting the Sn layer from the oxidation process at room temperature. This resulted in an improvement in device efficiency, with the PCE exceeding 9% (Fig. 6b and c).156 In a similar strategy, X. Xui and coworkers constructed a three-dimensional (3D) perovskite film by applying a mixed-cation strategy with doping of hydrophobic additive. Fig. 6d and e present the device structure of the PSCs and J–V curves of the PSCs with different amounts of mol% for DPPAI under simulation, respectively. Sn-based halide perovskites were formed using FA and 3,3-diphenylpropylammonium (DPPA) as mixed cations in different ratios. The optimal DPPA0.02FA0.98SnI3-based perovskite achieved the highest PCE of 6.75% with increased device performance across all parameters. Notably, the trace-doping of these cations enhanced the morphology and crystallinity of the perovskite film, leading to long-term stability and suppression of non-radiative recombination, resulting in a high PCE.157
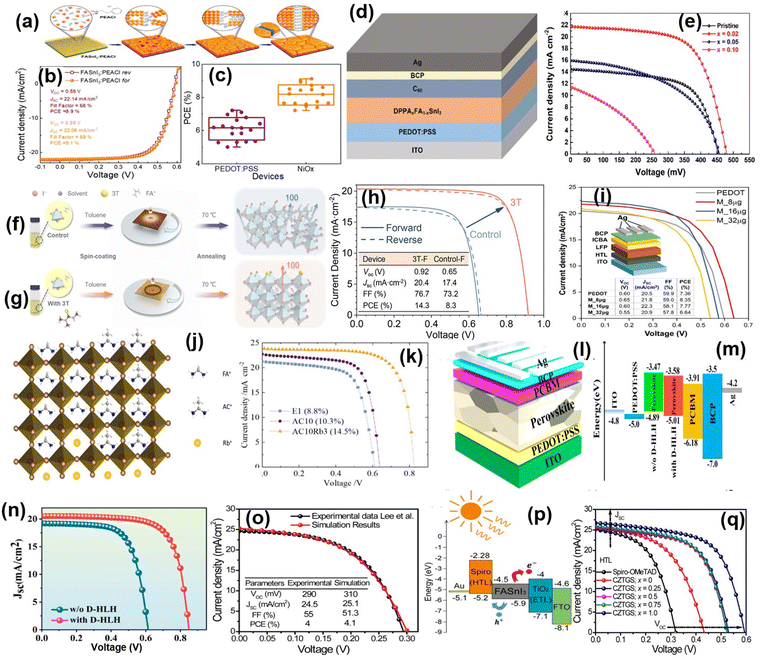 | ||
| Fig. 6 (a) Schematic of the crystallization process of FASnI3 and FASnI3:PEACl films. (b) J–V curves and (c) IPCE spectra of FASnI3:PEACl-based PSCs. Reproduced with permission from ref. 156, copyright 2020, ACS. (d) Device structure model of the PSCs. (e) J–V curves of PSCs with different DPPAI amounts at different mol%. Reproduced with permission from ref. 157, copyright 2020, ACS. (f) and (g) Schematic of the crystallization process of FASnI3 films with and without N-trimethylthiourea (3T), respectively and (h) J–V curves of devices with or without 3T. Reproduced with permission from ref. 149, copyright 2022, ACS. (i) J–V curves of MXene-embedded PEDOT:PSS-based PSC devices. Reproduced with permission from ref. 144, copyright 2024, ACS. (j) and (k) Crystalline structure and characteristic J–V curves for E1AC10Rb3 film and PSC device, respectively. Reproduced with permission from ref. 145, copyright 2024, ACS. (l) Device architecture of tin PSCs. (m) Energy level diagram of tin PSCs. (n) J–V curves of tin PSCs with and without D-HLH. Reproduced with permission from ref. 56, copyright 2024, ACS. (o) Comparison of J–V characteristics between simulation and experimental results, (p) flat band energy diagram, and (q) J–V curves of PSCs employing CZTGS with different ratios. Reproduced with permission from ref. 148, copyright 2024, ACS. | ||
FASnI3-based perovskites are similar to lead-based perovskites but have poor crystallinity. Thus, to resolve this problem, Zhu et al. developed a one-step-spin coating method to form FASnI3 films using an antisolvent. The spin coating and annealing of FASnI3 (Fig. 6f and g) were performed in the presence of N-trimethylthiourea (3T). 3T served as a Lewis base as well as an H-bond donor to provide uniform morphology and high stability to the perovskite film. The photoluminescence analysis revealed that the 3T-treated films exhibited a longer carrier lifetime of 123 ns, surpassing other reported perovskites and reducing the charge recombination effectively. Consequently, the PSC device performance was highly improved, displaying a certified PCE of 14.0% (Fig. 6h).149 Later, Zuraw et al. proposed a tin powder−carboxylic acid-assisted synthesis to prepare pure and stable SnI2 for developing lead-free perovskites. In this approach, Sn nanoparticles were inherently grafted with carboxylic acid via an in situ reaction. The utilization of carboxylic acid is not only advantageous for the formation of SnI2 but also develops a stable lead-free perovskite by mitigating Sn oxidation. It established the fabrication of a reproducible large-area (1 cm2) flexible perovskite solar cell, which exhibited a remarkable PCE of 8.35% with an improved open-circuit voltage.143 Further, Gao et al. reported an improved hybrid tin halide-based perovskite for solar cell applications by using dipropyl sulfide (DPS) as an additive. Their study demonstrated that the DPS molecules effectively coordinated with Sn molecules to increase the stability of the Sn2+ ions, facilitating the formation of an intermediate complex. This stabilization hindered the crystallization and oxidation of the prepared films. Thus, the device showed an enhanced PCE from 11.0% to 12.9%, accompanied by reduced charge recombination and a small leakage current. Additionally, the device stability was significantly enhanced. It is known that tin-based perovskites show the highest performance as lead-free PSCs. Sn-based halide perovskites, typically FASnI3, are mostly similar to their lead-based substituents in terms of optoelectronic properties but struggle with dissimilar crystallization kinetics.183
Further, Fakharuddin and coworkers proposed an interesting work with MXene-embedded PEDOT:PSS as HTL for enhancing the performance of PSCs. Due to MXene-PEDOT:PSS HTL combination, a modified perovskite film was obtained with low pinhole density and a uniform contact potential difference, leading to a high PCE. The modified PSC delivered a PCE of 8.35%, which was comparatively higher than that of its pristine counterpart (7.35%) (Fig. 6i).144 In another work, Kuan et al. explored the incorporation of acetamidinium and rubidium (Rb) as co-cations in varying proportions in the FASnI3 perovskite structure. The observation indicated that AC effectively passivated the defects on the surface of the film, at its interface with the substrate, and within the bulk material, while Rb primarily mitigated the interface defects between the PEDOT:PSS and perovskite layer, as shown in Fig. 6j. It improved phase stability, due to which non-radiative recombination was effectively inhibited and the charge mobility was drastically enhanced. The films prepared with these co-cations demonstrated the optimal performance at 10% AC (E1AC10) and 3% Rb (E1AC10Rb3), as presented in Fig. 6k. Consequently, the E1AC10Rb3 device attained a PCE of 14.5% and demonstrated exceptional stability, maintaining its performance over 3000 h.145 Recently, Hongqiang Wang and co-workers enhanced the efficiency of low-dimensional and 2D/3D Sn-PSCs from 7.97% to 12.45% by incorporating a multifunctional molecule such as D-homoserine lactone hydrochloride. The corresponding device is presented in Fig. 6l together with its band gap energy and efficiency results in Fig. 6m and n, respectively. Their study revealed that D-HLH forms H-bonds with FA+ and I− ions and binds with Sn2+ through Sn–O bonds. This improved crystallization process enhanced both the efficiency and stability of the resulting PSC device.56 Herrera et al. conducted a simulation-based (Fig. 6o) study on the photovoltaic performance of FASnI3-based PSCs with a regular device architecture (Fig. 6p). They also incorporated a typical organic HTL material, spiro-OMeTAD, to evaluate its impact on the device behaviour. The theoretical investigation suggested that the PSC showed an inspiring PCE of 17.1% by using an MoOx:Au composite in place of the Au back contact, which optimized the alignment of the energy levels at different heterojunctions and led to a high performance (Fig. 6q).148
7.2. Ge-based perovskite solar cells (AGeX3)
Ge-based perovskites exhibit low acute toxicity and share several favourable properties with lead-based perovskites, though they are not as closely matched in characteristics as their Sn analogs.184 Pb2+ and Ge2+ have almost same similar coordination types, electronic configurations, and crystal structures, and therefore Ge seems to be an alternative candidate for Pb, which can also satisfy the requirements of both charge balance and coordination. Germanium perovskites provide a safer, less toxic option and demonstrate some promising photovoltaic qualities but they typically exhibit lower stability and performance compared to lead- and tin-based alternatives. A comparison of the unit cells of cubic metal halide perovskites CsGeX3 (X = I, Br, Cl) with Pb-based complexes CsPbX3 is presented in Fig. 7a.126 This limits their current applicability in high-efficiency solar cells, although ongoing research seeks to improve their optoelectronic properties and stability to make them more competitive. The structural and electrical properties of MAGeX3 are mostly matched to MAPbX3, making it a suitable Pb substitute in halide perovskites. Current research in perovskite solar cells indicates that Ge-based perovskites exhibit lower efficiency than Sn-based perovskites. Thus, realizing high-efficiency solar cells with Ge-based perovskites remain a significant challenge. Nonetheless, theoretical investigations indicated that Ge-containing perovskites possess excellent optoelectronic properties suitable for solar applications. Researchers continue to explore ways to harness these properties, with a focus on enhancing the stability and optimizing the performance of Ge-based perovskites to make more viable in photovoltaic technologies.184–186 Qian et al. first performed DFT calculations on the CsGeI3 inorganic halide perovskite to examine its electronic structure and optical properties and compared it with the CH3NH3PbI3 perovskite. Interestingly, the bandgap of CsGeI3 and CH3NH3PbI3 was found to be 1.51 and 1.55 eV, respectively, and their charge mass transport ratio is also nearly matched, suggesting that Ge is a good replacement for the fabrication of Pb-free solar cells. This is also evident from the calculated PCE value of 27.9% and 26.7% for CsGeI3 and CH3NH3PbI3, respectively.186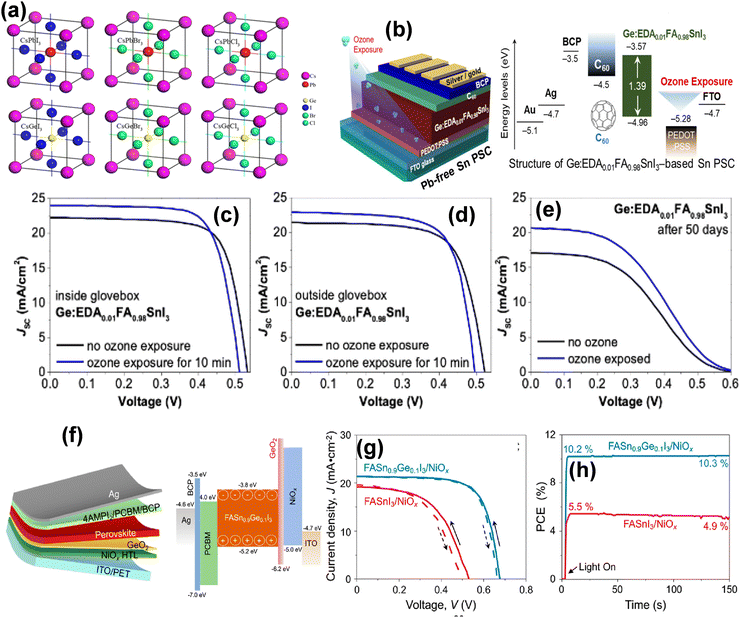 | ||
| Fig. 7 (a) Representation of unit cells of CsBX3 (B = Ge and Sn) composed of different halide anions. Reproduced with permission from ref. 126, copyright 2017, Nature Scientific Reports. (b) Ge:EDA0.01FA0.98SnI3-based PSC device and corresponding energy-level diagrams. (c)–(e) J–V curves of Ge:EDA0.01FA0.98SnI3 PSCs with and without ozone exposure, measured inside the GB, outside the GB, and after 50 days in air, respectively. Reproduced with permission from ref. 60, copyright 2020, ACS. (f) Schematic of the adopted architecture of the f-PSC and its corresponding energy-level diagram. (g) J–V responses of “champion” f-PSC devices. (h) Stable output of “champion” f-PSC devices. Reproduced with permission from ref. 173, copyright 2022, ACS. | ||
However, Ge is a rare and costly metal; thus, Ge is preferably mixed with other cations such as Sn to fabricated mixed cation-based lead-free PSCs. Considering this, Chen et al. introduced the CsSn0.5Ge0.5I3 perovskite composition by alloying Ge(II) with CsSnI3. The CsSn0.5Ge0.5I3 perovskite solid solution exhibited remarkable stability, with less than a 10% decline in efficiency over 500 h in the corresponding PSCs. It also possesses decent absorption in the visible region with an optical band gap of 1.50 eV, which lies between the bandgaps of its pure form perovskites. The Ge/Sn-mixed perovskite material was utilized as a light absorber in perovskite solar cells (PSCs) and a promising PCE of up to 7.11% was achieved.174 Ng et al. described the role of additives in new Sn/Ge-based perovskites on their photovoltaic performance. The FA0.75MA0.25Sn1−xGexI3 perovskite, depicted as FMS1−xGxI, was prepared and further coated with SnF2 and GeI2 additives. The presence of SnF2 retarded Sn2+ degradation into Sn4+ and reduce the Sn vacancies in the PSC. Alternatively, GeI2 (5 mol%) drastically reduced the trap density, resulting in a longer charge lifetime and diffusion coupled with excellent charge mobility. Thus, the Ge-doped perovskite attained a longer lifetime of excitons, higher charge mobility, and longer charge diffusion, which were significantly higher than that of the pristine perovskite, achieving a high PCE of 7.9%. This work demonstrated that modification of the optoelectronic properties via additives opens a new platform for designing Ge-based PSCs with high performance.187
Later, Nam et al. introduced a cheaper, cost-effective, and effective ozone exposure method for PEDOT:PSS to fabricate Ge-based perovskites to improve the device performance.60 They fabricated Ge:EDA0.01FA0.98SnI3-based PSCs in the configurational structure presented in the schematic illustration in Fig. 7b along with its energy level diagram. The ozone-exposed PEDOT:PSS-based devices fabricated using the synthesized perovskite demonstrated comparable power conversion efficiencies (PCEs) both outside and inside their grain boundaries, which was attributed to their enhanced stability (Fig. 7c). Specifically, the ozone-treated PEDOT:PSS utilized in the Ge:EDA0.01FA0.98SnI3-based tin perovskite solar cells (Sn-PSCs) achieved an increased PCE of 8.1%, which was higher than that of the pristine perovskite (Fig. 7d). It was revealed that the ozone exposure significantly lowered the Fermi level of PEDOT:PSS and tuned their energy levels, improving the hole mobility and reducing the trap density for the device to achieve a significantly higher performance. Furthermore, the Ge:EDA0.01FA0.98SnI3-based Sn-PSCs maintained a significant PCE performance even after 50 days of air exposure (Fig. 7e). The stability differences between the ozone-exposed and untreated PEDOT:PSS-based Sn-PSCs were negligible, which indicated that ozone exposure did not adversely impact the stability of the perovskites.
Recently, Chen et al. reported another method for the fabrication of flexible Ge-doped lead-free PSCs using an innovative compositional and interfacial engineering approach, producing the highest efficiency of 10.43% to date. Their work revealed that the in situ growth of the GeO2 layers occurred at the interface between the Sn-based perovskite film and NiOx HTL to form a new FASn0.9Ge0.1I3 layer. The role of Ge in the FASnI3 perovskite (MHP) is to stabilize the Sn2+ site in the MHP film having NiOx as the HTL. Thin films of FASnI3 and FASn0.9Ge0.1I3 were integrated into flexible perovskite solar cells (f-PSCs), and their photo-efficiency, operational stability, and mechanical strength were assessed. The adopted architecture of the f-PSC is depicted in Fig. 7f, with the corresponding energy-level diagram. In this configuration, a thin film of perovskite is positioned between two different HTL electron transport layers (ETL). This led to no apparent photocurrent leakage and restricted interfacial charge recombination, which is evident from the high Voc and FF values. Thus, the PSC device achieved an all-time record efficiency of 10.43% together with a high Voc and Jsc of 0.69 V and 21.3 mA cm−2, respectively, and a fill factor (FF) of 0.71 (Fig. 7g). This represents the highest reported PCE for a lead-free f-PSC to date. Fig. 7h further shows the stable PCE output of 10.3% at the maximum power point, consistent with the J–V-derived PCE, affirming the reduced hysteresis behavior of the device.173
7.3. Bi-based perovskite solar cells (ABiX3)
Bi and its compounds have advantages and disadvantages. Many bismuth compounds are applicable for medicinal purposes, while their chronic intake can be harmful and result in encephalopathy and renal failure. However, low concentrations of bismuth (1 to 100 mM) are non-toxic to human health. The Bi3+ ion possess an identical ionic radius and electronic configuration as Pb, and thus has potential for Pb substitution. Bi is a stable element under ambient conditions and almost non-toxic compared to the Ge and Sn elements. Bi forms trivalent ions and the perovskite structure can be obtained by replacing three Pb2+ with two Bi3+ ions. Thus, trivalent Bi3+-based perovskites have a structure of A3Bi2X9 with generally an octahedral-coordinated structure, where A is occupied by a cation such as Cs+ and MA+, while the X position is occupied by a halide ion such as Br− and I−. The first Cs3Bi2I9 PSC was reported with a PCE of over 1%. After that, several bismuth-based perovskite solar cells have been reported with an improved PCE. Jain et al. utilized a sustainable approach to fabricate lead-free, Bi-based (CH3NH3)3Bi2I9 (MBI) perovskite films by applying a non-toxic methyl acetate solution. The MBI devices were fabricated with two different HTLs, spiro-OMeTAD and P3HT, which produced an efficiency of 1.12% and 1.62%, respectively. The photovoltaic performance of the PSCs and the energy band level diagram and device architecture are displayed in Fig. 8a. Fig. 8b represents the J–V characteristics of the respective solar cells.164 Our group prepared an improved hybrid bismuth-based lead-free perovskite (AgBi2I7) through the solvent engineering technique and utilized it as the light absorber layer in PSCs. Two different solvents, DMF and MeOH, were employed in an optimum ratio to improve the photovoltaic efficiency. This work revealed that the optimal combination of the solvent system reduced the band gap to 1.89 eV, leading to a PCE value of 0.96% for the Bi-based solar cells (Fig. 8c). However, the theoretical investigation calculated a maximum efficiency of 13.26% using AgBi2I7 as a light absorber in PSCs.159 Karim et al. demonstrated stable Cs2AgBiBr6-based PSCs by optimizing the thin-film fabrication conditions and presenting a buffer interfacial layer (BIL) at the perovskite/ETL interface. The introduction of BIL as a hole leakage barrier at the interface significantly improved the electrical contact at the interface, resulting in an enhanced device performance. This interfacial engineering approach led to a PCE of 0.80% with high reproducibility, which was substantially higher than that of the control PSC (0.38%).165 In another work, our group introduced methyl acetate as an innovative solvent for preparing thin films of pristine perovskite materials. We demonstrated that the incorporation of Sn(IV) into the MA3Bi2I9 lead-free perovskite structure resulted in the formation of MA3(Bi1−xSnx)3I9. Subsequently, lead-free perovskite solar cell (LFPSC) devices were fabricated using pristine MA3Bi2I9 and Sn(IV)-doped MA3(Bi1−xSnx)3I9 (where x = 0, 0.1, 0.2, 0.3, 0.4, and 0.5) as the light-absorbing layers. Theoretical studies of the LFPSC devices demonstrated a high PCE value of 12%, highlighting the significant potential of these materials for advancing LFPSC technologies.160 For the first time, our group introduced a two-step deposition method for the fabrication of a device showing a good performance with 0.41% efficiency in comparison to the one-step deposition method for the fabrication of Pb-free PSCs.166 Ahmad et al. from our group introduced a new light Bi-based absorber for lead-free perovskites [(CH3NH3)3Bi2Cl9]n in the form of a 1D-polymeric chain.188 This perovskite exhibited non-toxic behavior and high stability with a remarkable open circuit voltage of 430 mV. However, the PCE of the device was found to be very low compared to another reported perovskite, which can be ascribed to its large bandgap of 2.85 eV. An efficient work was carried out by Vijaya et al. using the ligand-assisted re-precipitation approach (LARP) to produce two perovskites, Cs3Bi2I9 and CsBi3I10, for photovoltaic application. This is one of the best methods to prepare low-cost and highly efficient perovskites under atmospheric conditions. Interestingly, the CsBi3I10-based PSC displayed a PCE value of 2.3% with an increase in fill factor (FF) by 69% as compared to that of the Cs3Bi2I9-based device, showing a low efficiency of 0.7%. The device architecture, J–V curves and energy level diagram of the CsBi3I10-based device before and after annealing are shown in Fig. 8d–f, respectively.161 Recently, Sekar et al. conducted a theoretical simulation of the PCE efficiency of Ag3BiI6-based solar cells using CeOx as the HTL. This investigation considered various factors such as different ETLs, absorber layers, and three different HTLs to tune their thicknesses and density and calculate high-performance solar cells. Finally, the optimized planar Pb-free solar cells with Se/Te–Cu2O HTL achieved a high PCE value of 17.77%.158 This work emphasizes the fact that the efficiency of the proposed device can be calculated by theoretical investigation, which will be helpful for experimentalists to obtain highly efficient PSCs.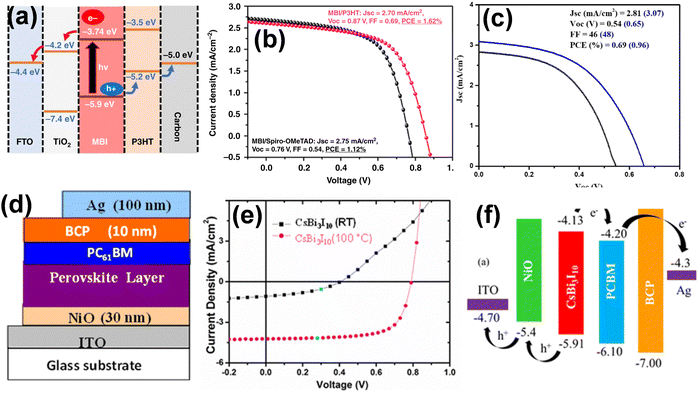 | ||
| Fig. 8 (a) Optical band gap and (b) photovoltaic performance of MBI-based PSC devices. Reproduced with permission from ref. 164, copyright 2019, Springer Nature. (c) J–V curves of SBI-D and SBI-DM under 1 sun conditions. Reproduced with permission from ref. 159, copyright 2023, RSC. (d) Device architecture, (e) J–V measurements, and (f) energy level diagram of CsBi3I10-based devices (before and after annealing). Reproduced with permission from ref. 161, copyright 2023, RSC. | ||
7.4. Sb-based perovskite solar cells
Sb-based PSCs have emerged as potential alternatives for Pb-based PSCs, offering a more cost-effective solution compared to their Sn-based counterparts. Antimony possesses a stable lone pair of 5s2 electrons, which contributes to its unique properties. This element is naturally found in environments associated with volcano and sea spray, with an average concentration in soil of approximately 0.48 ppm. Incorporating specific ions into the crystal lattice of Sb-based perovskites has been identified as a viable strategy to tune and enhance their optoelectronic properties, making them suitable for advanced photovoltaic applications.189 Most of the human population is already exposed to low levels of antimony, which shares several characteristics with arsenic due to its position in the same group of the Periodic Table. Sb-based perovskites, commonly formulated as A3Sb2X9 (where A represents MA+ or Cs+ and X represents I−, Br−, or Cl−), have shown considerable promise for these applications. These materials exhibit favorable optoelectronic properties, positioning them as strong candidates for next-generation solar cell technologies.190 Weber et al. reported the synthesis of interesting Sb-based perovskites (Rb3Sb2Br9−xIx) for solar cells, exhibiting a monoclinic structure and P21/n space group, which is consistent with the 2D crystal structure of Rb3Sb2I9 (Fig. 9a). It was observed that with an increase in the bromide content in the BX6 octahedron, the perovskite volume readily decreased from Rb3Sb2I9 to Rb3Sb2Br9. The substitution of iodide with bromide started to shrink the unit cell with a blue shift in absorption profile and a larger band gap. It disoriented the crystallinity of the perovskites and formed a layered structure, which was detrimental to the charge mobility, leading to a decrease in the photovoltaic efficiency. Thus, the highest efficiency of 1.37% was achieved for the unmasked PX-0-9 perovskite solar cells (Fig. 9b).170 Likewise, Li et al. reported the preparation of compact Rb3Sb2I9 thin films with improved microstructure for lead-free perovskite devices through the deposition strategy, as presented in Fig. 9c. The high-quality thin film was developed by controlling the high-temperature annealing process and supersaturation level. The Rb3Sb2I9 thin films attained a microstructural environment that increased the charge carrier mobility and reduce the charge recombination centers, thus boosting the photovoltaic performance. Thus, the PSC devices comprised of the Rb3Sb2I9 thin-films achieved a significantly higher PCE value of 1.35% with a maximum EQE of 65.4% (Fig. 9d).162 This indicated that thin film processing strategies can be effective in improving the microstructural and optoelectronic properties, subsequently enhancing the device performance. The resulting current density–voltage characteristics are presented in Fig. 9e.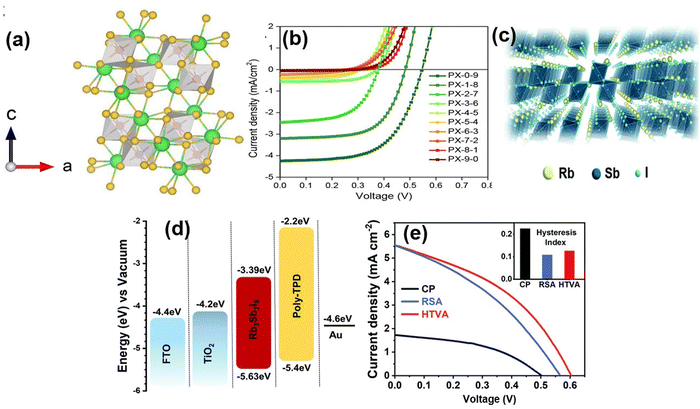 | ||
| Fig. 9 (a) 2D crystal structure of Rb3Sb2I9 (red, Sb; green, Rb; and yellow, I) and (b) J–V curves of Rb3Sb2Br9–xIx-based solar cells. Reproduced with permission from ref. 170, copyright 2019, ACS. (c) Compact 2D structure of Rb3Sb2I9, (d) energy band structure of Rb3Sb2I9-based devices, and (e) J–V characteristics of different Rb3Sb2I9 devices. Reproduced with permission from ref. 162, copyright 2020, RSC. | ||
Furthermore, our group developed a PSC device by fabricating MASbI perovskite as a light absorber.171 The thin film was prepared by employing a two-step modified sequential deposition method. The MASbI-based PSC device exhibited a maximum efficiency of 0.54%, which is low compared to other reported Sb-based perovskites. However, the device exhibited an impressive Voc of 740 mV, demonstrating the potential of MASbI-based materials for future improvements in PSC performance.171,172
8. Double perovskites
To replace Pb-halide perovskites, another approach is the use of halide double perovskites. Double perovskites with the general formula of A2B′B′′X6 were developed to address the complexity associated with lead substitution, where the A position is a large cation such as Cs+, while the B′ and B′′ positions are either trivalent or monovalent cations, and the X component is a halide.191 Due to their high stability and non-toxic nature, halide-based double perovskites have attracted significant attention for application in PSCs. Six adjacent octahedral derivatives of BIX6 or BIIIX6 units coordinate at intersections with different ions to develop a 3D perovskite structure. Examining various combinations of B′ and B′′ positions in A2B′B′′X6 is anticipated to facilitate the development of efficient alternatives to lead-based halide perovskites.192Since their initial synthesis in the 1970s, only a limited selection of double perovskites such as Cs2AgBiBr6 and Cs2NaBiI6 has been utilized in solar cell applications. When double perovskite materials are confined to single monolayers at the nanoscale, their stability and quantum size effects improve. This behavior is due to the elevated nanocrystal surface energy in their metastable phases. Firstly, Zhao et al. led a study to understand the optoelectronic properties of a series of double perovskites, Cs2NaBX6 (B = Sb, Bi; X = Cl, Br, I), by applying first-principles calculations, aiming to evaluate their potential for solar cell applications. Their studies predicted that these perovskites exhibit large indirect band gaps, limiting their light absorption capabilities. However, the perovskite with iodide as the halide ion displayed a lower band gap than that with chloride and bromide halide. It was observed that bromide and iodide perovskites have positive formation enthalpies and can be stable under certain conditions. It was also revealed that the charge mobility of these perovskites is lower than that of lead-based perovskites and needs to be tuned to achieve high efficiency.193 Later, G. Volonakis performed simulation studies of many double perovskites of the Cs2BiB′′X6 and CsSbB′′X6 family, where X represents Cl, Br, and I.194 Based on their results, Cs2BiAgCl6 was successfully synthesized, which consists of a face-centered cubic double perovskite. Theoretical calculations indicated that these double perovskites can serve as indirect bandgap semiconductors and possess tunable carrier effective masses in the visible absorption range, which was also confirmed through optical characterization of the synthesized Cs2BiAgCl6. An interesting comparison was executed by Yang et al. to prepare the Cs2AgBiBr6 double perovskite via solution-processing and vacuum sublimation for highly efficient lead-free PSCs. The solution-processed films demonstrated superior properties, including higher crystallinity, reduced bandgap, longer lifetimes, and enhanced charge carrier mobility, compared to the vacuum-sublimated films. Consequently, the optimized power conversion efficiency was 2.51% for the solution-processed films, which is comparatively higher than that of 1.41% for their vacuum-processed counterparts (Fig. 10a and b), respectively. This signified that the synthetic strategy can impact the precise composition stoichiometry and lead to a high-quality perovskite film, beneficial for photovoltaic performance.180
 | ||
Fig. 10 (a) J–V curves of PSC devices fabricated and (b) ambient stability characteristics of the perovskite solar cells fabricated with optimized solution- and vacuum-processed Cs2AgBiBr6 thin films. Reproduced with permission from ref. 180, copyright 2019, ACS. (c) Statistics of device efficiency of 35 nm-thick P3HT films stored under atmosphere conditions, (d) device configuration of the Cs2AgBiBr6 solar cell, and (e) schematic of dual-ion-diffusion-induced degradation of Cs2AgBiBr6 in a PSC device. Reproduced with permission from ref. 181, copyright 2020, John Wiley and Sons. (f) J–V curve of Cs2AgBiBr6 PSCs with different hydrogenation times (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s, 600 s, 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s and 1200 s and 1200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s). (g) Average photoelectric conversion efficiency (PCE) distribution of Cs2AgBiBr6 PSCs at different hydrogenation times, along with a layered PSC in the inset picture. Reproduced with permission from ref. 179, copyright 2022, Nature Commun. (h) J–V curves of Cs2AgBiBr6 solar cells with different HSLs. (i) Stabilized power output of PyDAnCBZ HSL. Reproduced with permission from ref. 177, copyright 2023, ACS. s). (g) Average photoelectric conversion efficiency (PCE) distribution of Cs2AgBiBr6 PSCs at different hydrogenation times, along with a layered PSC in the inset picture. Reproduced with permission from ref. 179, copyright 2022, Nature Commun. (h) J–V curves of Cs2AgBiBr6 solar cells with different HSLs. (i) Stabilized power output of PyDAnCBZ HSL. Reproduced with permission from ref. 177, copyright 2023, ACS. | ||
Since its discovery, Cs2AgBiBr6 has emerged as an important lead-free double perovskite material to address the stability and toxicity issues of lead halide perovskites. Ghasemi et al. developed a high-quality Cs2AgBiBr6 thin film for the fabrication of a lead-free double PSC, which exhibited an efficiency of 1.91% (Fig. 10c and d). However, the long-term stability of the PSC devices was affected by the dual-ion-diffusion-induced degradation of Cs2AgBiBr6, in which Ag and Br readily diffused through the HTL layer (Fig. 10e). Nevertheless, this phenomenon can be restricted by using a thick layer of hole-transporting material in the devices. This work emphasized the fundamental understanding of ion behavior to achieve high stability. Similarly, our group further explored (CH3NH3)3Sb2I9 and (NH4)3Sb2I9 perovskites as light-absorbing materials to fabricate lead-free PSCs. Between them, the (NH4)3Sb2I9-based PSC devices demonstrated an improved PCE of 0.42% and a remarkable open-circuit voltage (Voc) of 0.94 mV, highlighting the potential of (NH4)3Sb2I9 as an alternative for Pb-free PSCs.181 The efficiency of Cs2AgBiBr6-based PSCs is normally lower because of its wide bandgap and limited light absorption area. Notably, Zhang et al. achieved a breakthrough in this regard and reported a stable hydrogenated Cs2AgBiBr6 lead-free double perovskite solar cell with a power conversion efficiency of 6.37% (Fig. 10f and g). Cs2AgBiBr6 films were successfully doped with atomic hydrogen via a hydrogenation method to lower the bandgap of the double perovskite from 2.18 to 1.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV. First-principles calculations suggested that H-atoms are integrated into the interstitial sites of the perovskite crystal and significantly enhance its optical properties, charge carrier mobility, and lifetime compared to the pristine perovskite structure. This demonstrated that an effective lattice engineering strategy for double perovskites can be beneficial to form highly stable and environment-friendly efficient PSCs with tunable optoelectronic properties.179 Recently, Huang et al. fabricated modified Cs2AgBiBr6 PSC devices using different hole transporting layers. Four pyridine-based organic moieties with different functionalization at the 2,6-position of pyridine were investigated as the HTL for PSC application. Among them, PyDAnCBZ with carbazole functional moieties significantly improved the electro-optical properties and facilitated charge carrier transport in the PSC (Fig. 10h and i). Thus, the PyDAnCBZ-incorporated PSC device exhibited an improved power conversion efficiency of 4.9% with high thermal and moisture stability.177
eV. First-principles calculations suggested that H-atoms are integrated into the interstitial sites of the perovskite crystal and significantly enhance its optical properties, charge carrier mobility, and lifetime compared to the pristine perovskite structure. This demonstrated that an effective lattice engineering strategy for double perovskites can be beneficial to form highly stable and environment-friendly efficient PSCs with tunable optoelectronic properties.179 Recently, Huang et al. fabricated modified Cs2AgBiBr6 PSC devices using different hole transporting layers. Four pyridine-based organic moieties with different functionalization at the 2,6-position of pyridine were investigated as the HTL for PSC application. Among them, PyDAnCBZ with carbazole functional moieties significantly improved the electro-optical properties and facilitated charge carrier transport in the PSC (Fig. 10h and i). Thus, the PyDAnCBZ-incorporated PSC device exhibited an improved power conversion efficiency of 4.9% with high thermal and moisture stability.177
Recently, Sekar et al. investigated the performance of a new FA4GeSbCl12 double perovskite solar cell using drift-diffusion SCAPS-1D simulation and achieved an efficiency of 22.5%. Their studies displayed that the device performance can be boosted by optimizing the band structure of the ETL and HTL layers and varying their thickness and defect density.178 In a similar approach, Karmaker et al. introduced a novel Cs4CuSb2Cl12 perovskite nanocrystal cell, a promising material for PSCs. Various numerical simulations were carried out to optimize the Cs4CuSb2Cl12 nanocrystal-based solar cells, addressing the challenging task of balancing the device parameters. Their findings revealed that while thicker perovskite layers enhanced the light absorption, they also impeded the charge transport efficiency. Through computational modeling, this study optimized the key parameters, including perovskite layer thickness, doping material concentration, and defect density. Further, the proposed PSC exhibited a PCE of 23.10% with a Voc of 1.1675 V and fill factor (FF) of 83.33%.73 Recently, Kong et al. reported a luminescence down-shifting lead-free Cs2AgxNa1−xBiyIn1−yCl6 double perovskite phosphor (CANBIC) for silicon solar cells. The phosphor was incorporated into a polydimethylsiloxane (PDMS) film to improve the energy conversion in the ultraviolet (UV) spectrum. The CANBIC microcrystal served as a bifunctional fluorescent compound that absorbs light from the full solar spectrum through light scattering for silicon solar cells. By optimizing the CANBIC content in the textured PDMS film, an optical efficiency enhancement of 5.40% was obtained and the LSC-PV system showed a significant improvement in PCE, reaching up to 0.463% with a Jsc of up to 1.232 mA cm-2. By using an Al foil, the LSC-4PV system further reached a champion efficiency of 3.14% with high photostability and air stability.176
9. Other lead-free perovskites
Despite the significant advancements in rare-earth-based lead-free perovskites, maintaining high luminescence stability remains a considerable challenge. Considering this challenge, Zhao and coworkers performed first principles calculations for Cs2NaBX6, where B represents Sb and Bi and X represents Cl, Br, and I to understand their electronic properties for the development of lead-free double perovskites. It was found that Cs2NaSbI6 and Cs2NaBiI6 perovskite have an indirect band gap of 1.65 and 1.68 eV, respectively, similar to the Cs2AgBiBr6 double perovskite. Furthermore, DFT calculations indicated that the mobility of holes in these double perovskite is lower than the corresponding photogenerated electrons, suggesting that their band structure and charge carrier mobility need to be optimized in terms of potential photovoltaic application.193 In 2015, DFT studies were performed for AMX3 using K, Rb, and Cs as the A cations and Cl, Br, and I as the halide anions to investigate their bandgap and stability. The calculations indicated that RbSnBr3, CsSnBr3, and CsGeI3 are promising for solar cells. Among them, CsGeI3 possesses the optimal bandgap but displays a low PCE value. Many alkaline metals such as Ca, Sr, and Ba can be interesting elements for the synthesis of lead-free perovskite materials. They are usually low-cost and beneficial for large-scale applications. The Sr2+ and Ca2+ cations have the capability to form perovskites and are more stable compared to Sn and Ge. T. Edvinsson et al. investigated the simulation calculation of the structural and optoelectronic properties of CH3NH3SrI3 and CH3NH3PbI3. The DFT analysis revealed that the bandgap of CH3NH3SrI3 is large given that Sr2+ is less electronegative and has an empty d-orbital. Moreover, the formation of Sr-based perovskites was unsuccessful given that metal iodine bonding restricts the interaction of MA with strontium.195Transition metals were predominantly investigated for Pb replacement in lead-free perovskite due to their tendency to form complex through vacant d-orbitals. Cui et al. applied a low-temperature solution-based technique to synthesize copper-based perovskites, (p-F-C6H5–C2H4NH3)2CuBr4 and (CH3(CH2)3NH3)2CuBr4, which possessed an optimal band gap of 1.75 for photovoltaic application. However, the PSC devices fabricated using these perovskites exhibited low PCE values of 0.51% and 0.63%, respectively.196 Later, N. Mathew reported the synthesis of a series of copper-based layered 2D perovskites, (CH3NH3)2CuClxBr4−x, using different ratios of halide ions for bandgap optimization and tuning their optoelectronic properties. These perovskites were easily deposited as highly oriented films via the spin coating method. Cu2+ was converted to Cu+ during the annealing process due to the presence of the bromide ion, which increased the green photoluminescence properties. Among them, (CH3NH3)2CuCl2Br2-based PSC device exhibited a power conversion efficiency of 0.017%.197 Our group also reported the synthesis of organic–inorganic copper(II)-based perovskites, C6H4NH2CuBr2I and C6H4NH2CuCuCl2, for the fabrication of perovskite solar cells. Both perovskites showed absorption in the region of 400–700 nm with band gaps of 1.65 eV and 2.01 eV, respectively. These perovskites were utilized as light absorbers in PSC devices with ZnO as the HTL layer, exhibiting a PCE value of 0.42% and 0.61%, respectively, which was comparatively higher than that of TiO2-based devices.198 Further, Wilett et al. studied the magnetic properties of two iron-based 2D perovskites but their material application remained unexplored.199 Mn-based perovskites can also be good photovoltaics considering their absorption region in the visible and UV regions. Considering this, Zhou et al. prepared a CH3NH3MnI3 (a-MAMnI3) thin film via the spin coating technique and developed a photovoltaic device. This device showed a PCE of 0.02% and Voc of 0.33 V, indicating that Mn-based perovskites may have potential for application in solar cells.200 The Zhigang Zang group propose an alternative saturated precursor cyclic crystallization method to prepare centimeter-sized CsCu2I3 single crystals (SCs) with a one-dimensional (1D) structure. Their report revealed unidirectional and efficient charge carrier transport in the 1D CsCu2I3 SCs, and consequently the CsCu2I3 SC-based detector exhibited an excellent X-ray detection performance.12
Palladium and titanium are also viable options for the fabrication of lead-free PSCs. Ju and coworkers synthesized several Ti4+-based perovskites, Cs2TiIxBr6−x (x = 0, 2, 4, 6) via a melt-crystallization method and studied their potential for photovoltaic application. Among them, the Cs2TiI2Br4 perovskite attained a bandgap of 1.38 eV, making it suitable for use in single-junction solar cells. Ti-based perovskites displayed excellent light absorption capability, absorption behavior, defect density, and high carrier mobilities comparable to lead-based perovskites and can serve as light absorber layers in PSC devices considering their carrier mobilities.201 Furthermore, Huang et al. synthesized the (CH3NH3)2PdCl3 perovskite via a low-temperature solution method. It formed a 2D layered phase with a high absorption coefficient and was stable in air, making it advantageous to several existing perovskites. However, it possessed a direct bandgap of ∼2.2 eV, limiting its potential for solar applications but this can be tuned by replacing its halide ion.
Recent studies have shown that the performance of PVSCs is highly related to the existence of Pb2+ ions. Simply substituting lead with other metal cations typically results in a significant deterioration in device efficiency. However, mixed Pb–Sn perovskites can maintain relatively high efficiency. Mixed metal cations and halide double perovskites featuring a 3D structure should exhibit a good charge transport performance, although more research is needed. Overall, although many challenges have been identified, many more remain unaddressed, and thus the further development of lead-free materials with comparable performances to Pb-based perovskites is required.
10. Defect ordered perovskites
The defect properties in lead-free perovskite materials have been actively investigated because their unavoidable presence in the active layer highly impacts the performance of PSCs. A change in the 0D configuration to 2D layered structure in A3B2X9 defected perovskites, along with enhanced electronic dimensionality, markedly improves the performance of the corresponding PSCs.202 In PSCs, deep-level defects primarily serve as recombination centers, reducing the carrier lifetime and limiting the device performance. In contrast, shallow-level defects function as donors or acceptors and contribute to the generation and transportation of charge carriers. Bismuth exhibits defects due to its oxidation state and presence of lone pairs and has possibly benign properties, demonstrating less toxicity, and thus making it as effective as a heavy metal. Excessive exposure to MAI vapor led to a reduction in Bi-based metal defect sites, thereby decreasing the recombination losses. Defects in Cs3Bi2I9 exhibit low formation energies and a propensity to introduce deep-level states within the bandgap, leading to significant carrier recombination. Scanning tunneling spectroscopy revealed that the band edges of the Cs3Bi2I9 perovskite exhibit p-type characteristics, which is attributed to the electron-accepting nature of the defects located above the valence band. However, when methylammonium (MA) occupies the A positions, defect formation becomes less favorable because of the defect-tolerant properties of MA-based perovskites.203 As a result, the defect-related challenges are effectively mitigated by transitioning A3B2X9 from a zero-dimensional dimer configuration to a 2D structure. Furthermore, the improved phase purity of the 2D layered configuration contributes to a decrease in defect density.204In addition to point defects, interfacial defects play a critical role in device performance, predominantly occurring at the grain boundaries where their density is significantly higher than within the grains. As the grain size increases and the number of grain boundaries decreases, perovskite films contain a lower defect density, thereby minimizing the recombination rate and improving the PCE. To reduce the deep-energy-related defects or interfacial defects, several methods have been employed, thus enhancing the optoelectronic properties. Materials engineering, i.e., structural engineering by varying the cations (MA, FA, and Cs) and anions (Cl, Br, I) in perovskites, has been proven to be an effective approach for enhancing the performance and stability of PSCs. High-efficient solar cells incorporating these mixed perovskites demonstrate a reduction in defects and enhanced defect tolerance.205,206
11. Tandem solar cells
Additionally, multi-junction devices, such as tandem solar cells, are widely regarded as a promising approach to achieve higher power conversion efficiencies and surpass the Shockley–Queisser efficiency limit inherent to single-junction devices.207 In tandem solar cell configurations, high-energy and low-energy photons can be efficiently captured by the front and rear subcells, respectively, which are designed with complementary bandgaps. Subsequently, these photons are converted into electrical energy and collected through a transparent interconnecting layer or internal electrode, enabling enhanced photon utilization and improved overall device performance.208 For constructing tandem devices by themselves or interfacing with a variety of solar cells, tunable bandgaps in the range of 1.17 to 2.3 eV are required. The bandgap can also be tuned via compositional engineering, which make perovskites highly desirable for tandem cell applications. Some reports on copper indium gallium selenide (CIGS) perovskites,209,210 Si- based perovskites,211,212 and perovskite–organic-photovoltaic (OPV) tandems213,214 have demonstrated promising energy conversion efficiencies. Two different types of configurations are used to fabricate these perovskite-containing tandem solar cells, i.e. monolithic 2-terminal tandems, which electrically connect two subcells through a transparent internal electrode, and mechanically stacked 4-terminal tandems, where the two cells are fabricated independently, and then mechanically stacked on top of each other. The incorporation of an additional transparent electrode in four-terminal devices introduces increases the optical absorption losses and elevates the overall fabrication costs, posing challenges for their practical implementation and scalability.215 In contrast, the challenges inherent to two-terminal perovskite tandem solar cells encompass the design and integration of an efficient charge recombination interlayer, the intricate fabrication process involving multiple layers, and the precise optimization of the optical and electrical properties of the thick subcells to achieve the optimal performance. Multi-junction tandem solar cells utilize a stacked configuration of photovoltaic subcells with varying bandgap energies, enabling each subcell to selectively absorb distinct spectral regions of sunlight. This design optimizes the photon utilization by minimizing the sub-bandgap transmission losses and reducing thermalization losses, thereby enhancing the overall energy conversion efficiency.207Fig. 11a and b illustrate two configurations for double-junction tandem cells. A double-junction tandem solar cell represents the most fundamental configuration within the multi-junction architecture, which can be fabricated by mechanically stacking the high-bandgap cell on top of the low-bandgap cell. A monolithically integrated tandem solar cell represents a more sophisticated and streamlined approach, which is typically achieved by directly fabricating a high-bandgap subcell on a low-bandgap subcell. This integration facilitates efficient charge carrier transport and minimizes the optical losses, thereby enhancing the overall performance and scalability of the tandem architecture.216 Hybrid metal halide perovskite solar cells have recently emerged as highly promising candidates for multi-junction tandem applications, driven by their rapid advancements in power conversion efficiency (PCE), which has reached 26%.215 To enhance the performance of perovskite solar cells (PSCs), the development of tandem solar cell architectures represents a promising strategy for augmenting the power conversion efficiency (PCE) of existing photovoltaic technologies, while maintaining cost-effectiveness.217,218 | ||
| Fig. 11 Schematic of two types of two-junction tandem configurations: (a) four-terminal (4T) mechanically stacked tandem and (b) two-terminal (2T) monolithically integrated tandem. Reproduced with permission from ref. 207, copyright 2021, AIP. | ||
12. Challenges and strategies
Lead-free halide perovskites have emerged as ideal options to replace lead-based perovskites and have developed tremendously in the last decade. However, despite the recent advances in lead-free perovskites, they still lag behind lead-based perovskites in terms of efficiency and stability. There are many challenges in lead-free perovskite regarding their synthetic strategies, poor morphology, weak optoelectronic properties, stability under environmental conditions, cost-effectiveness, and scalability for practical application. The following points explain the pressing challenges in the field of lead-free perovskite solar cells, and also provide strategies to tackle them from distinct perspectives.12.1. Stability issue
Despite attaining high PCEs, the stability of lead-free perovskites under atmospheric conditions poses a major issue, preventing the large-scale application of PSCs and their commercialization. Sn2+ and Ge2+ in perovskite solar cells easily oxidize into Sn4+ and Ge4+, respectively, in the presence of air and under degradation at room temperature, making the PSC devices highly unstable.219,220 Lead-free PSCs must be operational for longer times, such as days or years, to replace the traditional solar cells. Thus, to counter this problem, doping or alloying of Sn-based perovskites can be performed to effectively suppress the oxidation process.221 Further, utilizing organic ligands or self-assembled monolayers can also reduce defect formation and suppress oxidation to stabilized these materials.222 In the case of doping or incorporation of additives, bipyridine-based organic/inorganic complexes can play an important role in perovskites because bipyridine can coordinate with Sn2+ and inhibit its conversion to Sn4+, enhancing the stability of Sn-based perovskites.223 Additionally, it also serves to optimize the band gap, lower the binding energy, and enhance the transportation and lifetime of charge carriers, resulting in high photovoltaic performances. Additives such as SnF2 were mostly added in low amounts for stabilizing tin-based perovskites. Incorporating excessive halides during the fabrication process can mitigate the performance issue because they suppress the oxidation, and thereby enhance the stability of the perovskite structure, improving the overall device performance. Some studies have shown that the addition of halides, particularly “fluoride”, significantly reduced the rate of degradation under conditions of high humidity and elevated temperatures.224 Using excess of halide ions such as Cl−/Br− in the perovskite precursor can also produce the same effect. Further, halides can also improve the morphology, passivate defects, and influence the crystallization process, leading to better charge transport properties and enhancing the structural integrity to increase the efficiency.225 Further, Bi and Sb-based perovskites do not undergo degradation via the oxidation process and exhibit great stability in air and moisture, and thus PSC devices with Bi and Sb perovskites need to be further explored. Also, Sb is considered an auspicious substitute for lead-based perovskites with enhanced performances226 but there are still challenges that need to be addressed to produce efficient perovskite solar cells. For example, Sb devices are solution-processed, favouring the dimer phase, which exhibits poor photovoltaic properties.227 It has also been found that inorganic cations possess better stability compared to organic cations, especially high thermally stable. Therefore, more perovskite cells with cesium or Rb cations rather than organic cations need to be developed for PSC application. Likewise, inorganic halide perovskites show excellent stability compared to organic–inorganic hybrid halide perovskites.12.2. Synthetic strategy modification
To achieve a high power conversion efficiency (PCE), the formation of high-quality thin films with high crystallinity and minimum defect density is a necessary but challenging target. The synthetic methodology plays a vital role in producing high-quality perovskite thin films with zero defects, uniform morphology, and high stability.228,229 Simple or inexpensive methods such as the solvothermal and hydrothermal methods are not beneficial for developing lead-free perovskite solar cells. Solution processing, spin coating, and vapour deposition are widely known techniques for the fabrication of high-quality perovskite thin films. For example, Ran et al. developed a two-step evaporation-spin-coating film fabrication technique, where they observed a low FF, which is still a challenge. This might be because of the high trap-state density, short carrier diffusion length, and low charge mobility in the thin film.230 However, Sn-based thin films undergo faster crystallization during their synthesis, which often results in low stability and poor photovoltaic performances. The vapour deposition method forms a uniform morphology of perovskite thin films with different scale lengths and minimum pinhole generation, subsequently obtaining better photovoltaic performances.231 Interestingly, one of the effective strategies is to combine the solution and vapor-deposition methods, which enables slow film growth to acquire high-quality thin films. Besides, significant efforts have been dedicated to forming thin films free of pinholes with the minimum defect density, while controlling their grain size and structure dimensionality, which results in high performances in lead-free perovskite-based PSC devices. Furthermore, it was discussed in the previous section that the incorporation of additives, doping or alloying was implicated in the production of smooth and crystalline thin films with a large coverage area, optimal band structure, high charge mobility and improved stability under ambient conditions, developing high-efficient PSC devices. Owing to these developed fabrication methods, there has been significant advancement in lead-free perovskite solar cells in terms of quality and device with a small area, but scalability remains the main issue for their large-scale application. For progress towards real applications, continuous efforts are necessary to develop crystalline lead-free perovskite films in large areas with good reproducibility.12.3. Improvement in optical properties
Fabrication strategies for perovskite materials should yield high-quality films with excellent optoelectronic properties by controlled nucleation and crystal growth. One of the major challenges is to tune the optoelectronic properties of perovskite materials in terms of their absorption coefficient, band structure, charge mobility, carrier lifetimes, etc.232,233 In the aspects of absorption coefficient and band gap tuning, different strategies have been employed, such as modulation of the cation and anion sites and high-quality thin film formation. Addition of reducing or additives with perovskite material might be very effective to increase absorption in the solar spectrum. (example needed) Regarding the charge mobility and carrier lifetimes, perovskites exhibit a high excitation binding energy, which restricts the free movement of photogenerated electrons and holes and retards the charge mobility. This limits the photovoltaic performance of lead-free halide perovskites compared to lead-based perovskites. High-quality thin films with crystalline growth and uniform grain size possess greatly a minimized defect density, which significantly reduces the recombination of excitons and exciton carrier lifetimes and increases the charge mobility, thus enhancing the device performance. Alternatively, the choice of appropriate hole transporting materials (HTMs), surface passivation, and additive engineering are also important to realize efficient charge transportation. Notably, the incorporation of photosensitizers and dye in perovskite solar cells can be effective to tune their optical properties, which can lead to broader absorption in the UV-visible region, efficient charge migration, longer lifetimes and stability in PSC devices, drastically improving the cell performance.12.4. Theoretical aspect
The formation of perovskite materials is dependent on trial-based methods, and sometimes, expected results are not produced from newly developed PSC devices. In this direction, theoretical calculations and new methodologies such as DFT studies, artificial intelligence, and machine learning can be helpful to assist in designing perovskite materials with desirable properties. A data-driven screening of possible perovskites from simulation studies based on a preliminary perovskite design or literature reports might open new avenues and encourage the development of highly efficient perovskite solar cells. Also, it might be helpful to gain information about structural and electronic properties, such as how organic and inorganic cations impact the optical or electronic properties of perovskites. Ge-based inorganic perovskites, AGeX3 (A = Cs or Rb; X = I, Br, or Cl), were investigated by theoretical simulation to calculate their absorption coefficients, exciton binding energies, and band gaps, which predicted their behaviour as light absorber materials. Recently, theoretical investigations have attracted significant attention in synthetic chemistry, which holds promise to revolutionize this field.12.5. Scalability
There is growing interest in the synthesis of perovskites for solar cells, but most synthetic strategies cannot facilitate large-scale production and are only limited to the milligram level, thereby restricting their scalability and practical applications. One of the serious issues with PSCs are large-area processing and scaling-up, which restrict their practical applications. Thus, to mitigate these challenges, potential strategies need to be employed such as doping, which stabilizes the perovskite lattice and reduces oxidation, and use of reducing agents that may prevent oxidation in lead-free PSCs.234 Spin-coating is a popular method for synthesizing lead-free halide perovskite solar cells, but it is not suitable at the large-scale level due to its high tangential velocity. In the context of large-area processing and scaled-up deposition, techniques such as blade coating are employed to fabricate large-area devices, which can help stabilize flexible perovskite solar modules. Growth control and composition and inferential engineering can improve the large-area processing of lead-free PSCs.235 To overcome the issue of scaling-up, work on surface passivation techniques, reducing agents, and chemical modifications can help to enhance the stability and efficiency.236 Modifying the device architecture and incorporating additives into the perovskite precursor solution can help scale-up the process.237 Synthetic methodologies such as spray coating, slot-die coating, inkjet printing, and soft film coating have also been employed for the realization of large-scale perovskite film development. These strategies for fabricating perovskite films are cost-effective, efficient, and straightforward and mostly explored for lead-based perovskites. However, significant progress has been made for lead-free perovskites with better device performances, but the above-mentioned methods also need to be explored.To summarize these challenges and strategies, lead-free PSCs exhibit lower PCEs compared to lead-based halide perovskites because they possesses an indirect band gap, very low carrier mobility and short charge carrier diffusion length, which further affect the device performance. Thus, many other strategies have been applied to overcome these barriers including the use of a reducing agent, introduction of additives, solvent and device structure engineering, and use of 2D structural components.237,238 Numerous fabrication techniques can help boost the PCEs. Sn-based perovskites have now achieved an efficiency of more than 15%, while other lead-free Bi, Sb, and Ge-based perovskites is approaching 5% due to the modifications of (a) the addition of an anti-solvent, which accelerates heterogeneous supersaturation, nucleation, and precipitation processes, leading to a more uniform, pinhole-free crystal morphology with enhanced PCE, (b) uniform morphology of compact films and well-defined grain boundaries and (c) modification of A–and X-site mixing.
13. Summary and future outlook
Owing to the toxic nature of lead-based perovskites for PSCs, this feature article attempted to provide a concise overview of the applications of lead-free PSCs to significantly expand the panorama of possible metals such as Sn, Bi, Sb, and Ge, among which Sn is the major player to achieve a sustainable future for PSCs. These lead-free PSC materials are attracting increasing attention because of their nontoxicity and outstanding performances. Some significant parameters for the champion device require a low background density charge carrier, appropriate band gap, high absorption coefficient, effective carrier transport, and stronger stability in the ambient atmosphere. This review presented bright insight into the advantages and limitations of Pb-free perovskites, the present status, impact of the cations at the A, B, and X sites, up-to-date progress, challenges, strategies, and outlook in lead-free perovskite materials for solar cell applications. In 2009, the birth of PSCs certainly prefigured a new edge for next-generation photovoltaics. In the past one and half decades, the PCE of PSCs increased from the initial 3.8% to 26% with a great change from 2-dimensional to 3-dimensional structures along with liquid to solid junctions. Lead-free PSCs are a promising candidates because of their more stable phase and highly attractive based on existing technology. Large-area processing and scale-up technology has become a tricky problem that needs to be addressed before commercialization. To date, researchers have been focused on devices having large areas and uniform films because their efficiency is still poor. However, many of the film-making techniques including drop-casting and spin-coating used in different laboratories do not suit large-scale production. The printing and spraying techniques are considered promising methods for scale-up but scaling up the size of PSCs without the significant loss of efficiency is a challenge needs to be resolved. All perovskites must be environment-friendly and not harm the human body like Pb. Enhancing the stability of manufacturing long-lasting PSC devices with a high PCE is still an urgent issue to be implemented. To advance the development of integrated photovoltaics, numerous studies have focused on all-perovskite solar cells; however, research on inorganic PSCs remains relatively limited. Excellent PCEs have been achieved thus far for lead-free perovskites. However, they exhibit poor stability, and thus researchers are devoting great efforts to overcome this problem, such as using reducing agents to inhibit oxidation and excess amounts of halide to produce a rich environment. To improve the film quality of tin-based PSCs, additives can be introduced, significantly enhancing the device performance. At the beginning of this review, we discussed the synthesis of lead-free perovskite solar cells and their essential optical characteristics, together with the impact of the A-site cations on their chemical modification, which can not only improve the perovskite film morphology and device performance but also increase its long-term stability. Epitaxial growth-like techniques can play a pivotal role in improving the perovskite crystallinity, which is effective in improving the device performance. A few challenges exist and researchers have devoted great efforts to settle them to date. Moreover, to improve the performance of lead-free-based PSCs, we need to consider more effective approaches to overcome their limitations. In the last few years, the Jsc, FF (fill factor), PCE, Voc and device stability of solar cells have been enhanced by utilizing different approaches. Interfacial defects play a crucial role in improving the power conversion efficiency (PCE) of these devices. For commercialization, all the discussed lead-free perovskite materials seem to be promising alternatives because of their less toxicity, good stability, and reasonable optoelectronic properties. Preparing these types of lead-free perovskites will be a significant step toward developing high-efficiency lead-free perovskite cells.Data availability
The original data are available upon request and can be obtained by contacting the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. M. M. acknowledges SERB-DST, New Delhi, India (Project CRG/2020/001769), BRNS, Mumbai, India (Project 58/14/17/2020-BRNS/37215), and expresses deep gratitude to the Ministry of Textiles, India. I. A is thankful to CSIR for awarding the Postdoctoral Research Associate fellowship (09/1022(19289)/2024-EMR-I). K. P. thanks the Science and Engineering Research Board (SERB), India, for the National Postdoctoral Fellowship (PDF/2022/000110).References
- N. Kannan and D. Vakeesan, Renewable Sustainable Energy Rev., 2016, 62, 1092–1105 Search PubMed.
- A. Striolo and S. Huang, J. Phys. Chem. C, 2022, 126, 21527–21541 Search PubMed.
- M. A. Green, Nat. Energy, 2016, 1, 1–4 Search PubMed.
- C. Ballif, F.-J. Haug, M. Boccard, P. J. Verlinden and G. Hahn, Nat. Rev. Mater., 2022, 7, 597–616 Search PubMed.
- C. Isabela, R. A. M. Lameirinhas, J. P. N. Torres and C. A. Fernandes, Sustainable Energy Fuels, 2021, 5, 2273–2283 Search PubMed.
- N. Papež, R. Dallaev, Ş. Ţălu and J. Kaštyl, Mater, 2021, 14, 3075 Search PubMed.
- A. B. Muñoz-García, I. Benesperi, G. Boschloo, J. J. Concepcion, J. H. Delcamp, E. A. Gibson, G. J. Meyer, M. Pavone, H. Pettersson and A. Hagfeldt, Chem. Soc. Rev., 2021, 50, 12450–12550 Search PubMed.
- K. Yoshikawa, H. Kawasaki, W. Yoshida, T. Irie, K. Konishi, K. Nakano, T. Uto, D. Adachi, M. Kanematsu and H. Uzu, Nat. Energy, 2017, 2, 1–8 Search PubMed.
- L. X. Chen, ACS Energy Lett., 2019, 4, 2537–2539 Search PubMed.
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, Chem. Rev., 2020, 120, 7867–7918 Search PubMed.
- K. Galkowski, A. Surrente, M. Baranowski, B. Zhao, Z. Yang, A. Sadhanala, S. Mackowski, S. D. Stranks and P. Plochocka, ACS Energy Lett., 2019, 4, 615–621 Search PubMed.
- B. Wang, X. Yang, R. Li, S. M. Qaid, W. Cai, H. Xiao and Z. Zang, ACS Energy Lett., 2023, 8, 4406–4413 Search PubMed.
- T.-B. Song, T. Yokoyama, C. C. Stoumpos, J. Logsdon, D. H. Cao, M. R. Wasielewski, S. Aramaki and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 836–842 Search PubMed.
- Y. Yang, H. Dai, F. Yang, Y. Zhang, D. Luo, X. Zhang, K. Wang, X. W. Sun and J. Yao, Nanoscal. Res. Lett., 2019, 14, 1–8 Search PubMed.
- W. Zhang, Y. Sui, B. Kou, Y. Peng, Z. Wu and J. Luo, ACS Appl. Mater. Interfaces, 2019, 12, 9141–9149 Search PubMed.
- L. Zhou, J. F. Liao, Z. G. Huang, J. H. Wei, X. D. Wang, W. G. Li, H. Y. Chen, D. B. Kuang and C. Y. Su, Angew. Chem., 2019, 131, 5331–5335 Search PubMed.
- S. Zhao, W. Cai, H. Wang, Z. Zang and J. Chen, Small Methods, 2021, 5, 2001308 Search PubMed.
- Y. Zhou, J. Chen, O. M. Bakr and O. F. Mohammed, ACS Energy Lett., 2021, 6, 739–768 Search PubMed.
- J. Petrulevicius, Y. Yang, C. Liu, M. Steponaitis, E. Kamarauskas, M. Daskeviciene, A. S. Bati, T. Malinauskas, V. Jankauskas and K. Rakstys, ACS Appl. Mater. Interfaces, 2024, 16, 7310–7316 Search PubMed.
- J. Xu, H. Chen, L. Grater, C. Liu, Y. Yang, S. Teale, A. Maxwell, S. Mahesh, H. Wan and Y. Chang, Nat. Mater., 2023, 22, 1507–1514 Search PubMed.
- W. Ke and M. G. Kanatzidis, Nat. Commun., 2019, 10, 965 Search PubMed.
- S. Zhao, Z. Jia, Y. Huang, Q. Qian, Q. Lin and Z. Zang, Adv. Funct. Mater., 2023, 33, 2305858 Search PubMed.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 Search PubMed.
- S. Ahmed, M. Gondal, A. Alzahrani, M. Parvaz, A. Ahmed and S. Hussain, ACS Appl. Energy Mater., 2024, 7, 1382–1397 Search PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 Search PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 Search PubMed.
- Q. Jiang, J. Tong, Y. Xian, R. A. Kerner, S. P. Dunfield, C. Xiao, R. A. Scheidt, D. Kuciauskas, X. Wang and M. P. Hautzinger, Nature, 2022, 611, 278–283 Search PubMed.
- Z. Xu, Z. Guo, H. Li, Y. Zhou, Z. Liu, K. Wang, Z. Li, H. Wang, S. M. Qaid and O. F. Mohammed, Energy Environ. Sci., 2025, 18, 1354–1365 Search PubMed.
- H. Yang, Z. Xu, H. Wang, S. M. Qaid, O. F. Mohammed and Z. Zang, Adv. Mater., 2024, 36, 2411721 Search PubMed.
- M. Green, E. Dunlop, J. Hohl-Ebinger, M. Yoshita, N. Kopidakis and X. Hao, Prog. Photovoltaics, 2021, 29, 3–15 Search PubMed.
- C. Deline, D. Jordan, B. Sekulic, J. Parker, B. McDanold and A. Anderberg, PV Lifetime Project-2024 NREL Annual Rep, National Renewable Energy Laboratory (NREL), Golden, CO (United States), 2024 Search PubMed.
- Y. Li, L. Ji, R. Liu, C. Zhang, C. H. Mak, X. Zou, H.-H. Shen, S.-Y. Leu and H.-Y. Hsu, J. Mater. Chem. A, 2018, 6, 12842–12875 Search PubMed.
- S. Yang, W. Fu, Z. Zhang, H. Chen and C.-Z. Li, J. Mater. Chem. A, 2017, 5, 11462–11482 Search PubMed.
- M. M. Byranvand, C. Otero-Martínez, J. Ye, W. Zuo, L. Manna, M. Saliba, R. L. Hoye and L. Polavarapu, Adv. Opt. Mater., 2022, 10, 2200423 Search PubMed.
- L. Mao, C. C. Stoumpos and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 141, 1171–1190 Search PubMed.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nat. Commun., 2020, 11, 310 Search PubMed.
- F. Giustino and H. J. Snaith, ACS Energy Lett., 2016, 1, 1233–1240 Search PubMed.
- A. E. Magdalin, P. D. Nixon, E. Jayaseelan, M. Sivakumar, S. K. N. Devi, M. Subathra, N. M. Kumar and N. Ananthi, Results Eng., 2023, 101438 Search PubMed.
- C. Zhang, L. Gao, S. Hayase and T. Ma, Chem. Lett., 2017, 46, 1276–1284 Search PubMed.
- R. Nie, R. R. Sumukam, S. H. Reddy, M. Banavoth and S. I. Seok, Energy Environ. Sci., 2020, 13, 2363–2385 Search PubMed.
- C. H. Chen, S. N. Cheng, L. Cheng, Z. K. Wang and L. S. Liao, Adv. Energy Mater., 2023, 13, 2204144 Search PubMed.
- M. M. Byranvand, W. Zuo, R. Imani, M. Pazoki and M. Saliba, Chem. Sci., 2022, 13, 6766–6781 Search PubMed.
- M. Wang, W. Wang, B. Ma, W. Shen, L. Liu, K. Cao, S. Chen and W. Huang, Nano-Micro Lett., 2021, 13, 1–36 Search PubMed.
- X. Jiang, Z. Zang, Y. Zhou, H. Li, Q. Wei and Z. Ning, Acc. Mater. Res., 2021, 2, 210–219 Search PubMed.
- M. S. A. Azizman, A. W. Azhari, D. S. C. Halin, N. Ibrahim, S. Sepeai, N. A. Ludin, M. N. M. Nor and L. N. Ho, Synth. Met., 2023, 299, 117475 Search PubMed.
- P.-K. Kung, M.-H. Li, P.-Y. Lin, J.-Y. Jhang, M. Pantaler, D. C. Lupascu, G. Grancini and P. Chen, Sol. RRL, 2020, 4, 1900306 Search PubMed.
- X. Yang, W. Wang, R. Ran, W. Zhou and Z. Shao, Energy Fuels, 2020, 34, 10513–10528 Search PubMed.
- L. Chouhan, S. Ghimire, C. Subrahmanyam, T. Miyasaka and V. Biju, Chem. Soc. Rev., 2020, 49, 2869–2885 Search PubMed.
- D. Kumar, R. S. Yadav, A. K. Singh and S. B. Rai, Perovskite Mater., Devices Integr., 2020,(4), 190 Search PubMed.
- G. Nasti and A. Abate, Adv. Energy Mater., 2020, 10, 1902467 Search PubMed.
- S. Hu, K. Otsuka, R. Murdey, T. Nakamura, M. A. Truong, T. Yamada, T. Handa, K. Matsuda, K. Nakano and A. Sato, Energy Environ. Sci., 2022, 15, 2096–2107 Search PubMed.
- Z. Chen, J. J. Wang, Y. Ren, C. Yu and K. Shum, Appl. Phys. Lett., 2012, 101, 093901 Search PubMed.
- N. K. Noel, S. D. Stranks, A. Abate, C. Wehrenfennig, S. Guarnera, A.-A. Haghighirad, A. Sadhanala, G. E. Eperon, S. K. Pathak and M. B. Johnston, Energy Environ. Sci., 2014, 7, 3061–3068 Search PubMed.
- P. Wang, F. Li, K. J. Jiang, Y. Zhang, H. Fan, Y. Zhang, Y. Miao, J. H. Huang, C. Gao and X. Zhou, Adv. Sci., 2020, 7, 1903047 Search PubMed.
- W. Zhang, Y. Cai, H. Liu, Y. Xia, J. Cui, Y. Shi, R. Chen, T. Shi and H. L. Wang, Adv. Energy Mater., 2022, 12, 2202491 Search PubMed.
- Z. Kang, Y. Tong, K. Wang, Y. Chen, P. Yan, G. Pan, P. Müller-Buschbaum, L. Zhang, Y. Yang and J. Wu, ACS Mater. Lett., 2023, 6, 1–9 Search PubMed.
- B. B. Yu, Z. Chen, Y. Zhu, Y. Wang, B. Han, G. Chen, X. Zhang, Z. Du and Z. He, Adv. Mater., 2021, 33, 2102055 Search PubMed.
- T. Krishnamoorthy, H. Ding, C. Yan, W. L. Leong, T. Baikie, Z. Zhang, M. Sherburne, S. Li, M. Asta and N. Mathews, J. Mater. Chem. A, 2015, 3, 23829–23832 Search PubMed.
- W. Ming, H. Shi and M.-H. Du, J. Mater. Chem. A, 2016, 4, 13852–13858 Search PubMed.
- J.-S. Nam, K. Kim, J. Han, D. Kim, I. Chung, D. H. Wang and I. Jeon, ACS Appl. Mater. Interfaces, 2021, 13, 42935–42943 Search PubMed.
- Y. Cui, L. Yang, X. Wu, J. Deng, X. Zhang and J. Zhang, J. Mater. Chem. C, 2022, 10, 16629–16656 Search PubMed.
- B.-W. Park, B. Philippe, X. Zhang, H. Rensmo, G. Boschloo and E. Johansson, Adv. Mater., 2015, 27, 6806–6813 Search PubMed.
- Y. Kim, Z. Yang, A. Jain, O. Voznyy, G. H. Kim, M. Liu, L. N. Quan, F. P. García de Arquer, R. Comin and J. Z. Fan, Angew. Chem., Int. Ed., 2016, 55, 9586–9590 Search PubMed.
- K. W. Jung, M. R. Sohn, H. M. Lee, I. S. Yang, S. Do Sung, J. Kim, E. W.-G. Diau and W. I. Lee, Sustainable Energy Fuels, 2018, 2, 294–302 Search PubMed.
- M. Khazaee, K. Sardashti, J.-P. Sun, H. Zhou, C. Clegg, I. G. Hill, J. L. Jones, D. C. Lupascu and D. B. Mitzi, Chem. Mater., 2018, 30, 3538–3544 Search PubMed.
- S. M. Jain, D. Phuyal, M. L. Davies, M. Li, B. Philippe, C. De Castro, Z. Qiu, J. Kim, T. Watson and W. C. Tsoi, Nano Energy, 2018, 49, 614–624 Search PubMed.
- N. Pai, J. Lu, T. R. Gengenbach, A. Seeber, A. S. Chesman, L. Jiang, D. C. Senevirathna, P. C. Andrews, U. Bach and Y. B. Cheng, Adv. Energy Mater., 2019, 9, 1803396 Search PubMed.
- N. A. Raval and V. Kheraj, Sol. Energy, 2025, 286, 113128 Search PubMed.
- B. Saparov, F. Hong, J.-P. Sun, H.-S. Duan, W. Meng, S. Cameron, I. G. Hill, Y. Yan and D. B. Mitzi, Chem. Mater., 2015, 27, 5622–5632 Search PubMed.
- J.-C. Hebig, I. Kuhn, J. Flohre and T. Kirchartz, ACS Energy Lett., 2016, 1, 309–314 Search PubMed.
- K. M. Boopathi, P. Karuppuswamy, A. Singh, C. Hanmandlu, L. Lin, S. A. Abbas, C. C. Chang, P. C. Wang, G. Li and C. W. Chu, J. Mater. Chem. A, 2017, 5, 20843–20850 Search PubMed.
- S. Chatterjee and A. J. Pal, ACS Appl. Mater. Interfaces, 2018, 10, 35194–35205 Search PubMed.
- H. Karmaker, A. Siddique, B. K. Das and M. N. Islam, Results Eng., 2024, 22, 102106 Search PubMed.
- F. Ji, G. Boschloo, F. Wang and F. Gao, Sol. RRL, 2023, 7, 2201112 Search PubMed.
- J. Kangsabanik, V. Sugathan, A. Yadav, A. Yella and A. Alam, Phys. Rev. Mater., 2018, 2, 055401 Search PubMed.
- D. B. Mitzi, C. Feild, W. Harrison and A. Guloy, Nature, 1994, 369, 467–469 Search PubMed.
- Y. Su, J. Yang, H. Rao, Y. Zhong, W. Sheng, L. Tan and Y. Chen, Energy Environ. Sci., 2023, 16, 2177–2186 Search PubMed.
- T. C. Jellicoe, J. M. Richter, H. F. Glass, M. Tabachnyk, R. Brady, S. E. Dutton, A. Rao, R. H. Friend, D. Credgington and N. C. Greenham, J. Am. Chem. Soc., 2016, 138, 2941–2944 Search PubMed.
- A. Wang, X. Yan, M. Zhang, S. Sun, M. Yang, W. Shen, X. Pan, P. Wang and Z. Deng, Chem. Mater., 2016, 28, 8132–8140 Search PubMed.
- B. Yang, X. Mao, F. Hong, W. Meng, Y. Tang, X. Xia, S. Yang, W. Deng and K. Han, J. Am. Chem. Soc., 2018, 140, 17001–17006 Search PubMed.
- M. Xiao, F. Huang, W. Huang, Y. Dkhissi, Y. Zhu, J. Etheridge, A. Gray-Weale, U. Bach, Y. B. Cheng and L. Spiccia, Angew. Chem., Int. Ed., 2014, 53, 9898–9903 Search PubMed.
- C.-H. Chiang and C.-G. Wu, Nat. Photonics, 2016, 10, 196–200 Search PubMed.
- E. Jokar, P.-Y. Cheng, C.-Y. Lin, S. Narra, S. Shahbazi and E. Wei-Guang Diau, ACS Energy Lett., 2021, 6, 485–492 Search PubMed.
- F. Hao, C. C. Stoumpos, P. Guo, N. Zhou, T. J. Marks, R. P. Chang and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 11445–11452 Search PubMed.
- J. Liu, M. Ozaki, S. Yakumaru, T. Handa, R. Nishikubo, Y. Kanemitsu, A. Saeki, Y. Murata, R. Murdey and A. Wakamiya, Angew. Chem., 2018, 130, 13405–13409 Search PubMed.
- Z. Zhu, C. C. Chueh, N. Li, C. Mao and A. K. Y. Jen, Adv. Mater., 2018, 30, 1703800 Search PubMed.
- M. Chen, L. Wan, M. Kong, H. Hu, Y. Gan, J. Wang, F. Chen, Z. Guo, D. Eder and S. Wang, J. Alloys Compd., 2018, 738, 422–431 Search PubMed.
- X. Liu, T. Wu, X. Luo, H. Wang, M. Furue, T. Bessho, Y. Zhang, J. Nakazaki, H. Segawa and L. Han, ACS Energy Lett., 2021, 7, 425–431 Search PubMed.
- T. Yokoyama, D. H. Cao, C. C. Stoumpos, T.-B. Song, Y. Sato, S. Aramaki and M. G. Kanatzidis, J. Phys. Chem. Lett., 2016, 7, 776–782 Search PubMed.
- M. Wang, P. Zeng, S. Bai, J. Gu, F. Li, Z. Yang and M. Liu, Sol. RRL, 2018, 2, 1800217 Search PubMed.
- J. Chen, L. Zuo, Y. Zhang, X. Lian, W. Fu, J. Yan, J. Li, G. Wu, C. Z. Li and H. Chen, Adv. Energy Mater., 2018, 8, 1800438 Search PubMed.
- G. Schileo and G. Grancini, J. Mater. Chem. C, 2021, 9, 67–76 Search PubMed.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. Chang and M. G. Kanatzidis, Nat. Photonics, 2014, 8, 489–494 Search PubMed.
- W. Ke, C. C. Stoumpos, M. Zhu, L. Mao, I. Spanopoulos, J. Liu, O. Y. Kontsevoi, M. Chen, D. Sarma and Y. Zhang, Sci. Adv., 2017, 3, e1701293 Search PubMed.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 Search PubMed.
- W. Ke, C. C. Stoumpos, I. Spanopoulos, L. Mao, M. Chen, M. R. Wasielewski and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 14800–14806 Search PubMed.
- C. Wang, Y. Zhang, F. Gu, Z. Zhao, H. Li, H. Jiang, Z. Bian and Z. Liu, Matter, 2021, 4, 709–721 Search PubMed.
- Y. Liao, H. Liu, W. Zhou, D. Yang, Y. Shang, Z. Shi, B. Li, X. Jiang, L. Zhang and L. N. Quan, J. Am. Chem. Soc., 2017, 139, 6693–6699 Search PubMed.
- M.-G. Ju, J. Dai, L. Ma, Y. Zhou, W. Liang and X. C. Zeng, J. Mater. Chem. A, 2019, 7, 16742–16747 Search PubMed.
- J. Shin, M. Kim, S. Jung, C. S. Kim, J. Park, A. Song, K.-B. Chung, S.-H. Jin, J. H. Lee and M. Song, Nano Res., 2018, 11, 6283–6293 Search PubMed.
- Y. Yang, C. Liu, M. Cai, Y. Liao, Y. Ding, S. Ma, X. Liu, M. Guli, S. Dai and M. K. Nazeeruddin, ACS Appl. Mater. Interfaces, 2020, 12, 17062–17069 Search PubMed.
- A. H. Slavney, T. Hu, A. M. Lindenberg and H. I. Karunadasa, J. Am. Chem. Soc., 2016, 138, 2138–2141 Search PubMed.
- E. Greul, M. L. Petrus, A. Binek, P. Docampo and T. Bein, J. Mater. Chem. A, 2017, 5, 19972–19981 Search PubMed.
- W. Gao, C. Ran, J. Xi, B. Jiao, W. Zhang, M. Wu, X. Hou and Z. Wu, ChemPhysChem, 2018, 19, 1696–1700 Search PubMed.
- C. J. Bartel, C. Sutton, B. R. Goldsmith, R. Ouyang, C. B. Musgrave, L. M. Ghiringhelli and M. Scheffler, Sci. Adv., 2019, 5, eaav0693 Search PubMed.
- P. C. Harikesh, H. K. Mulmudi, B. Ghosh, T. W. Goh, Y. T. Teng, K. Thirumal, M. Lockrey, K. Weber, T. M. Koh and S. Li, Chem. Mater., 2016, 28, 7496–7504 Search PubMed.
- J. W. Lee, D. H. Kim, H. S. Kim, S. W. Seo, S. M. Cho and N. G. Park, Adv. Energy Mater., 2015, 5, 1501310 Search PubMed.
- N. Pellet, P. Gao, G. Gregori, T. Y. Yang, M. K. Nazeeruddin, J. Maier and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 3151–3157 Search PubMed.
- Y. Peng, F. Li, Y. Wang, Y. Li, R. L. Hoye, L. Feng, K. Xia and V. Pecunia, Appl. Mater. Today, 2020, 19, 100637 Search PubMed.
- L. Liang and P. Gao, Adv. Sci., 2018, 5, 1700331 Search PubMed.
- F. Yang, R. W. MacQueen, D. Menzel, A. Musiienko, A. Al-Ashouri, J. Thiesbrummel, S. Shah, K. Prashanthan, D. Abou-Ras and L. Korte, Adv. Energy Mater., 2023, 13, 2204339 Search PubMed.
- M. S. Ozório, M. Srikanth, R. Besse and J. L. Da Silva, Phys. Chem. Chem. Phys., 2021, 23, 2286–2297 Search PubMed.
- T. Ozturk, E. Akman, A. E. Shalan and S. Akin, Nano Energy, 2021, 87, 106157 Search PubMed.
- Z. Fang, M. Shang, X. Hou, Y. Zheng, Z. Du, Z. Yang, K.-C. Chou, W. Yang, Z. L. Wang and Y. Yang, Nano Energy, 2019, 61, 389–396 Search PubMed.
- A. Singh, N.-C. Chiu, K. M. Boopathi, Y.-J. Lu, A. Mohapatra, G. Li, Y.-F. Chen, T.-F. Guo and C.-W. Chu, ACS Appl. Mater. Interfaces, 2019, 11, 35088–35094 Search PubMed.
- T. J. Macdonald, L. Lanzetta, X. Liang, D. Ding and S. A. Haque, Adv. Mater., 2023, 35, 2206684 Search PubMed.
- N. Ghimire, R. S. Bobba, A. Gurung, K. M. Reza, M. A. R. Laskar, B. S. Lamsal, K. Emshadi, R. Pathak, M. A. Afroz and A. H. Chowdhury, ACS Appl. Energy Mater., 2021, 4, 1731–1742 Search PubMed.
- J. Zhou, F. Zhao, J. Shen, Y. Zhou, Y. Wu, Y. Guo, J. Jiang and J. Chu, J. Mater. Chem. C, 2021, 9, 15301–15308 Search PubMed.
- Z. Jin, Z. Zhang, J. Xiu, H. Song, T. Gatti and Z. He, J. Mater. Chem. A, 2020, 8, 16166–16188 Search PubMed.
- R. Chiara, M. Morana and L. Malavasi, ChemPlusChem, 2021, 86, 879–888 Search PubMed.
- K. Ahmad, P. Kumar and S. M. Mobin, Chem. – Asian J., 2020, 15, 2859–2863 Search PubMed.
- K. Ahmad and S. M. Mobin, ACS Omega, 2020, 5, 28404–28412 Search PubMed.
- H. Yao, F. Zhou, Z. Li, Z. Ci, L. Ding and Z. Jin, Adv. Sci., 2020, 7, 1903540 Search PubMed.
- A. K. Deb and V. Kumar, AIP Adv., 2015, 5, 077158 Search PubMed.
- M. Konstantakou and T. Stergiopoulos, J. Mater. Chem. A, 2017, 5, 11518–11549 Search PubMed.
- M. Roknuzzaman, K. Ostrikov, H. Wang, A. Du and T. Tesfamichael, Sci. Rep., 2017, 7, 14025 Search PubMed.
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Science, 2017, 358, 745–750 Search PubMed.
- L.-J. Chen, C.-R. Lee, Y.-J. Chuang, Z.-H. Wu and C. Chen, J. Phys. Chem. Lett., 2016, 7, 5028–5035 Search PubMed.
- G. Wang, J. Chang, J. Bi, M. Lei, C. Wang and Q. Qiao, Sol. RRL, 2022, 6, 2100841 Search PubMed.
- R. Ganesan, S. Vinodhini, V. Balasubramani, G. Parthipan, T. Sridhar, R. Arulmozhi and R. Muralidharan, New J. Chem., 2019, 43, 15258–15266 Search PubMed.
- M. K. Hossain, M. S. Uddin, G. I. Toki, M. K. Mohammed, R. Pandey, J. Madan, M. F. Rahman, M. R. Islam, S. Bhattarai and H. Bencherif, RSC Adv., 2023, 13, 23514–23537 Search PubMed.
- B. P. Nguyen, H. R. Jung, K. Y. Ryu, K. Kim and W. Jo, J. Phys. Chem. C, 2019, 123, 30833–30841 Search PubMed.
- B. P. Nguyen, D. Shin, H. R. Jung, J. Kim, T. T. T. Nguyen, S. Yoon, Y. Yi and W. Jo, Sol. Energy, 2019, 186, 136–144 Search PubMed.
- Y. Zhou, D. Yan, H. Zhang, Y. Jing, L. Chao, M. Li, M. Li, Y. Chen, R. Chen and L. Xu, ACS Mater. Lett., 2023, 5, 2096–2103 Search PubMed.
- Y. Sasaki, M. Murayama and X. Zhao, ACS Omega, 2024, 9, 34339–34344 Search PubMed.
- C. Ferrara, M. Patrini, A. Pisanu, P. Quadrelli, C. Milanese, C. Tealdi and L. Malavasi, J. Mater. Chem. A, 2017, 5, 9391–9395 Search PubMed.
- Z. Zhao, F. Gu, Y. Li, W. Sun, S. Ye, H. Rao, Z. Liu, Z. Bian and C. Huang, Adv. Sci., 2017, 4, 1700204 Search PubMed.
- T. M. Koh, T. Krishnamoorthy, N. Yantara, C. Shi, W. L. Leong, P. P. Boix, A. C. Grimsdale, S. G. Mhaisalkar and N. Mathews, J. Mater. Chem. A, 2015, 3, 14996–15000 Search PubMed.
- J. Cao, Q. Tai, P. You, G. Tang, T. Wang, N. Wang and F. Yan, J. Mater. Chem. A, 2019, 7, 26580–26585 Search PubMed.
- M. Chen, Q. Dong, F. T. Eickemeyer, Y. Liu, Z. Dai, A. D. Carl, B. Bahrami, A. H. Chowdhury, R. L. Grimm and Y. Shi, ACS Energy Lett., 2020, 5, 2223–2230 Search PubMed.
- Z. Lin, C. Liu, G. Liu, J. Yang, X. Duan, L. Tan and Y. Chen, Chem. Commun., 2020, 56, 4007–4010 Search PubMed.
- X. Meng, T. Wu, X. Liu, X. He, T. Noda, Y. Wang, H. Segawa and L. Han, J. Phys. Chem. Lett., 2020, 11, 2965–2971 Search PubMed.
- W. Żuraw, D. Kubicki, R. Kudrawiec and Ł. Przypis, ACS Energy Lett., 2024, 9, 4509–4515 Search PubMed.
- J. Ling, D. T. Cuzzupè, M. F. U. Din, A. Stepura, T. Burgard, Y. A. Temitmie, E. Majkova, M. Omastova, R. Jose and L. Schmidt-Mende, ACS Appl. Energy Mater., 2024, 7, 7152–7158 Search PubMed.
- C.-H. Kuan, T.-S. Liao, S. Narra, Y.-W. Tsai, J.-M. Lin, G.-R. Chen and E. W.-G. Diau, J. Phys. Chem. Lett., 2024, 15, 7763–7769 Search PubMed.
- W. Zeng, D. Cui, Z. Li, Y. Tang, X. Yu, Y. Li, Y. Deng, R. Ye, Q. Niu and R. Xia, Sol. Energy, 2019, 194, 272–278 Search PubMed.
- L. Peng and W. Xie, RSC Adv., 2020, 10, 14679–14688 Search PubMed.
- D. Mora-Herrera and M. Pal, J. Phys. Chem. C, 2022, 126, 5847–5862 Search PubMed.
- Z. Zhu, X. Jiang, D. Yu, N. Yu, Z. Ning and Q. Mi, ACS Energy Lett., 2022, 7, 2079–2083 Search PubMed.
- C. Liu, J. Tu, X. Hu, Z. Huang, X. Meng, J. Yang, X. Duan, L. Tan, Z. Li and Y. Chen, Adv. Funct. Mater., 2019, 29, 1808059 Search PubMed.
- C. H. Ng, K. Hamada, G. Kapil, M. A. Kamarudin, Z. Wang, Q. Shen, K. Yoshino, T. Minemoto and S. Hayase, J. Mater. Chem. A, 2020, 8, 2962–2968 Search PubMed.
- D. B. Khadka, Y. Shirai, M. Yanagida and K. Miyano, J. Mater. Chem. C, 2020, 8, 2307–2313 Search PubMed.
- J. Li, P. Hu, Y. Chen, Y. Li and M. Wei, ACS Sustainable Chem. Eng., 2020, 8, 8624–8628 Search PubMed.
- X. Meng, Y. Li, Y. Qu, H. Chen, N. Jiang, M. Li, D. J. Xue, J. S. Hu, H. Huang and S. Yang, Angew. Chem., 2021, 133, 3737–3742 Search PubMed.
- X. Jiang, F. Wang, Q. Wei, H. Li, Y. Shang, W. Zhou, C. Wang, P. Cheng, Q. Chen and L. Chen, Nat. Commun., 2020, 11, 1245 Search PubMed.
- M. Li, W.-W. Zuo, Y.-G. Yang, M. Aldamasy, Q. Wang, S. H. T. Cruz, S.-L. Feng, M. Saliba, Z.-K. Wang and A. Abate, ACS Energy Lett., 2020, 5, 1923–1929 Search PubMed.
- X. Xu, K. Cao, W. Zhu, W. Gu, B. Ma, M. Qin, J. Qian, Y. Lu, Z. Liu and S. Chen, ACS Appl. Energy Mater., 2020, 3, 5415–5426 Search PubMed.
- K. Sekar, L. Marasamy, S. Mayarambakam, P. Selvarajan and J. Bouclé, Mater. Today Commun., 2024, 38, 108347 Search PubMed.
- P. Kumar, K. Ahmad and S. M. Mobin, Nano. Adv., 2023, 5, 1624–1630 Search PubMed.
- K. Ahmad, P. Kumar, P. Shrivastava and S. M. Mobin, Energy Technol., 2022, 10, 2100717 Search PubMed.
- S. Vijaya, J. Subbiah, D. J. Jones and S. Anandan, RSC Adv., 2023, 13, 9978–9982 Search PubMed.
- F. Li, Y. Wang, K. Xia, R. L. Hoye and V. Pecunia, J. Mater. Chem. A, 2020, 8, 4396–4406 Search PubMed.
- X. Meng, Y. Wang, J. Lin, X. Liu, X. He, J. Barbaud, T. Wu, T. Noda, X. Yang and L. Han, Joule, 2020, 4, 902–912 Search PubMed.
- S. M. Jain, T. Edvinsson and J. R. Durrant, Commun. Chem., 2019, 2, 91 Search PubMed.
- M. A. Karim, K. Matsuishi, T. H. Chowdhury, W. I. Chowdhury, M. Abdel-shakour and A. Islam, J. Mater. Sci.: Mater. Electron., 2022, 33, 8114–8126 Search PubMed.
- K. Ahmad, S. N. Ansari, K. Natarajan and S. M. Mobin, ChemElectroChem, 2019, 6, 1192–1198 Search PubMed.
- S. Sanders, D. Stümmler, P. Pfeiffer, N. Ackermann, G. Simkus, M. Heuken, P. Baumann, A. Vescan and H. Kalisch, Sci. Rep., 2019, 9, 9774 Search PubMed.
- M.-C. Tang, D. Barrit, R. Munir, R. Li, J. M. Barbé, D.-M. Smilgies, S. Del Gobbo, T. D. Anthopoulos and A. Amassian, Sol. RRL, 2019, 3, 1800305 Search PubMed.
- C. Lan, G. Liang, S. Zhao, H. Lan, H. Peng, D. Zhang, H. Sun, J. Luo and P. Fan, Sol. Energy, 2019, 177, 501–507 Search PubMed.
- S. Weber, T. Rath, K. Fellner, R. Fischer, R. Resel, B. Kunert, T. Dimopoulos, A. Steinegger and G. Trimmel, ACS Appl. Energy Mater., 2018, 2, 539–547 Search PubMed.
- K. Ahmad, P. Kumar and S. M. Mobin, ChemElectroChem, 2020, 7, 946–950 Search PubMed.
- P. Kumar, K. Ahmad, J. Dagar, E. Unger and S. M. Mobin, ChemElectroChem, 2021, 8, 3150–3154 Search PubMed.
- M. Chen, Q. Dong, C. Xiao, X. Zheng, Z. Dai, Y. Shi, J. M. Luther and N. P. Padture, ACS Energy Lett., 2022, 7, 2256–2264 Search PubMed.
- M. Chen, M.-G. Ju, H. F. Garces, A. D. Carl, L. K. Ono, Z. Hawash, Y. Zhang, T. Shen, Y. Qi and R. L. Grimm, Nat. Commun., 2019, 10, 16 Search PubMed.
- Y. Raoui, S. Kazim, Y. Galagan, H. Ez-Zahraouy and S. Ahmad, Sustainable Energy Fuels, 2021, 5, 4661–4667 Search PubMed.
- M. Kong, O. Raievska, O. Stroyuk, A. Barabash, Z. Wu, J. Tian, C. Erban, A. Osvet, M. Batentschuk and C. J. Brabec, ACS Appl. Mater. Interfaces, 2024, 16, 43713–43723 Search PubMed.
- P. Huang, M. Sheokand, D. Payno Zarceño, S. Kazim, L. Lezama, M. K. Nazeeruddin, R. Misra and S. Ahmad, ACS Appl. Energy Mater., 2023, 6, 7955–7964 Search PubMed.
- K. Sekar, L. Marasamy, S. Mayarambakam, H. Hawashin, M. Nour and J. Bouclé, RSC Adv., 2023, 13, 25483–25496 Search PubMed.
- Z. Zhang, Q. Sun, Y. Lu, F. Lu, X. Mu, S.-H. Wei and M. Sui, Nat. Commun., 2022, 13, 3397 Search PubMed.
- F. Igbari, R. Wang, Z.-K. Wang, X.-J. Ma, Q. Wang, K.-L. Wang, Y. Zhang, L.-S. Liao and Y. Yang, Nano Lett., 2019, 19, 2066–2073 Search PubMed.
- M. Ghasemi, L. Zhang, J. H. Yun, M. Hao, D. He, P. Chen, Y. Bai, T. Lin, M. Xiao and A. Du, Adv. Funct. Mater., 2020, 30, 2002342 Search PubMed.
- M. K. Hossain, D. Samajdar, R. C. Das, A. Arnab, M. F. Rahman, M. Rubel, M. R. Islam, H. Bencherif, R. Pandey and J. Madan, Energy Fuels, 2023, 37, 3957–3979 Search PubMed.
- C. Gao, X. Wang, Q. Yang, C. Gao, X. Wang and X. Liu, J. Phys. Chem. Lett., 2024, 15, 8896–8902 Search PubMed.
- C. C. Stoumpos, L. Frazer, D. J. Clark, Y. S. Kim, S. H. Rhim, A. J. Freeman, J. B. Ketterson, J. I. Jang and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 6804–6819 Search PubMed.
- R. Chen, C. Liu, Y. Chen, C. Ye, S. Chen, J. Cheng, S. Cao, S. Wang, A. Cui and Z. Hu, J. Phys. Chem. C, 2022, 127, 635–641 Search PubMed.
- J. Qian, B. Xu and W. Tian, Org. Electro, 2016, 37, 61–73 Search PubMed.
- C. H. Ng, K. Nishimura, N. Ito, K. Hamada, D. Hirotani, Z. Wang, F. Yang, Q. Shen, K. Yoshino and T. Minemoto, Nano Energy, 2019, 58, 130–137 Search PubMed.
- K. Ahmad, S. N. Ansari, K. Natarajan and S. M. Mobin, ACS Appl. Energy Mater., 2018, 1, 2405–2409 Search PubMed.
- N. Pradhan, S. Das Adhikari, A. Nag and D. Sarma, Angew. Chem., Int. Ed., 2017, 56, 7038–7054 Search PubMed.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Chem. Rev., 2019, 119, 3036–3103 Search PubMed.
- H. Lei, D. Hardy and F. Gao, Adv. Funct. Mater., 2021, 31, 2105898 Search PubMed.
- P. Zhang, J. Yang and S.-H. Wei, J. Mater. Chem. A, 2018, 6, 1809–1815 Search PubMed.
- S. Zhao, K. Yamamoto, S. Iikubo, S. Hayase and T. Ma, J. Phys. Chem. Solids, 2018, 117, 117–121 Search PubMed.
- G. Volonakis, M. R. Filip, A. A. Haghighirad, N. Sakai, B. Wenger, H. J. Snaith and F. Giustino, J. Phys. Chem. Lett., 2016, 7, 1254–1259 Search PubMed.
- T. J. Jacobsson, M. Pazoki, A. Hagfeldt and T. Edvinsson, J. Phys. Chem. C, 2015, 119, 25673–25683 Search PubMed.
- X.-P. Cui, K.-J. Jiang, J.-H. Huang, Q.-Q. Zhang, M.-J. Su, L.-M. Yang, Y.-L. Song and X.-Q. Zhou, Synth. Met., 2015, 209, 247–250 Search PubMed.
- D. Cortecchia, H. A. Dewi, J. Yin, A. Bruno, S. Chen, T. Baikie, P. P. Boix, M. Grätzel, S. Mhaisalkar and C. Soci, Inorg. Chem., 2016, 55, 1044–1052 Search PubMed.
- K. Ahmad and S. M. Mobin, Energy Technol., 2020, 8, 1901185 Search PubMed.
- M. F. Mostafa and R. D. Willett, Phys. Rev. B, 1971, 4, 2213 Search PubMed.
- X. Zhang, J. Yin, Z. Nie, Q. Zhang, N. Sui, B. Chen, Y. Zhang, K. Qu, J. Zhao and H. Zhou, RSC Adv., 2017, 7, 37419–37425 Search PubMed.
- M.-G. Ju, M. Chen, Y. Zhou, H. F. Garces, J. Dai, L. Ma, N. P. Padture and X. C. Zeng, ACS Energy Lett., 2018, 3, 297–304 Search PubMed.
- Z. Xiao, Z. Song and Y. Yan, Adv. Mater., 2019, 31, 1803792 Search PubMed.
- B. Ghosh, B. Wu, H. K. Mulmudi, C. Guet, K. Weber, T. C. Sum, S. Mhaisalkar and N. Mathews, ACS Appl. Mater. Interfaces, 2018, 10, 35000–35007 Search PubMed.
- F. Jiang, D. Yang, Y. Jiang, T. Liu, X. Zhao, Y. Ming, B. Luo, F. Qin, J. Fan and H. Han, J. Am. Chem. Soc., 2018, 140, 1019–1027 Search PubMed.
- H. Cho, S.-H. Jeong, M.-H. Park, Y.-H. Kim, C. Wolf, C.-L. Lee, J. H. Heo, A. Sadhanala, N. Myoung and S. Yoo, Science, 2015, 350, 1222–1225 Search PubMed.
- H. Wang, C. Zhang, W. Huang, X. Zou, Z. Chen, S. Sun, L. Zhang, J. Li, J. Cheng and S. Huang, Phys. Chem. Chem. Phys., 2022, 24, 27585–27605 Search PubMed.
- A. W. Ho-Baillie, J. Zheng, M. A. Mahmud, F.-J. Ma, D. R. McKenzie and M. A. Green, Appl. Phys. Rev., 2021, 8, 041307 Search PubMed.
- K. O. Brinkmann, P. Wang, F. Lang, W. Li, X. Guo, F. Zimmermann, S. Olthof, D. Neher, Y. Hou and M. Stolterfoht, Nat. Rev. Mater., 2024, 9, 202–217 Search PubMed.
- N. Shrivastav, J. Madan and R. Pandey, Ind. J. Phys, 2024, 1–9 Search PubMed.
- Q. Han, Y.-T. Hsieh, L. Meng, J.-L. Wu, P. Sun, E.-P. Yao, S.-Y. Chang, S.-H. Bae, T. Kato and V. Bermudez, Science, 2018, 361, 904–908 Search PubMed.
- K. Amri, R. Belghouthi, M. Aillerie and R. Gharbi, Energies, 2021, 14, 3383 Search PubMed.
- A. Al-Ashouri, E. Köhnen, B. Li, A. Magomedov, H. Hempel, P. Caprioglio, J. A. Márquez, A. B. Morales Vilches, E. Kasparavicius and J. A. Smith, Science, 2020, 370, 1300–1309 Search PubMed.
- T. Serikova, G. Bizhanova, H. Hu and A. Ng, Bullet. Karaganda Univ. Phys. Series, 2023, 111, 6–15 Search PubMed.
- X. Chen, Z. Jia, Z. Chen, T. Jiang, L. Bai, F. Tao, J. Chen, X. Chen, T. Liu and X. Xu, Joule, 2020, 4, 1594–1606 Search PubMed.
- T. Duong, Y. Wu, H. Shen, J. Peng, X. Fu, D. Jacobs, E. C. Wang, T. C. Kho, K. C. Fong and M. Stocks, Adv. Energy Mater., 2017, 7, 1700228 Search PubMed.
- K. A. Bush, A. F. Palmstrom, Z. J. Yu, M. Boccard, R. Cheacharoen, J. P. Mailoa, D. P. McMeekin, R. L. Hoye, C. D. Bailie and T. Leijtens, Nat. Energy, 2017, 2, 1–7 Search PubMed.
- Z. Zhu, K. Mao and J. Xu, J. Chem., 2021, 58, 219–232 Search PubMed.
- T. Leijtens, R. Prasanna, K. A. Bush, G. E. Eperon, J. A. Raiford, A. Gold-Parker, E. J. Wolf, S. A. Swifter, C. C. Boyd and H.-P. Wang, Sustainable Energy Fuels, 2018, 2, 2450–2459 Search PubMed.
- I. Kopacic, B. Friesenbichler, S. F. Hoefler, B. Kunert, H. Plank, T. Rath and G. Trimmel, ACS Appl. Energy Mater., 2018, 1, 343–347 Search PubMed.
- X. Wang, T. Zhang, Y. Lou and Y. Zhao, Mater. Chem. Front., 2019, 3, 365–375 Search PubMed.
- S. Gupta, T. Bendikov, G. Hodes and D. Cahen, ACS Energy Lett., 2016, 1, 1028–1033 Search PubMed.
- C. Gong, H. Li, Z. Xu, Y. Li, H. Wang, Q. Zhuang, A. Wang, Z. Li, Z. Guo and C. Zhang, Nat. Commun., 2024, 15, 9154 Search PubMed.
- S. Joy, H. R. Atapattu, S. Sorensen, H. Pruett, A. B. Olivelli, A. J. Huckaba, A.-F. Miller and K. R. Graham, J. Mater. Chem. A, 2022, 10, 13278–13285 Search PubMed.
- D. Nematov, I. Raufov, A. Ashurov, S. Sattorzoda, T. Najmiddinov, S. Nazriddinzoda, M. Umarzoda and K. Yusupova, International Journal of Research and Innovation in Applied Science, 2025, 353–377 Search PubMed.
- S. Sajid, S. Alzahmi, I. B. Salem, N. Tabet, Y. Haik and I. M. Obaidat, Mater. Renewable Sustainable Energy, 2024, 13, 133–153 Search PubMed.
- Y.-L. Liu, C.-L. Yang, M.-S. Wang, X.-G. Ma and Y.-G. Yi, J. Mater. Sci., 2019, 54, 4732–4741 Search PubMed.
- A. Singh, K. M. Boopathi, A. Mohapatra, Y. F. Chen, G. Li and C. W. Chu, ACS Appl. Mater. Interfaces, 2018, 10, 2566–2573 Search PubMed.
- Z. Zhang, X. Li, X. Xia, Z. Wang, Z. Huang, B. Lei and Y. Gao, J. Phys. Chem. Lett., 2017, 8, 4300–4307 Search PubMed.
- P. Karuppuswamy, K. M. Boopathi, A. Mohapatra, H.-C. Chen, K.-T. Wong, P.-C. Wang and C.-W. Chu, Nano Energy, 2018, 45, 330–336 Search PubMed.
- C. Ran, Z. Wu, J. Xi, F. Yuan, H. Dong, T. Lei, X. He and X. Hou, J. Phys. Chem. Lett., 2017, 8, 394–400 Search PubMed.
- Z. Liu, M. Chen, L. Wan, Y. Liu, Y. Wang, Y. Gan, Z. Guo, D. Eder and S. Wang, SN Appl. Sci., 2019, 1, 1–11 Search PubMed.
- M. H. Miah, M. U. Khandaker, M. B. Rahman, M. Nur-E-Alam and M. A. Islam, RSC Adv., 2024, 14, 15876–15906 Search PubMed.
- P. Baraneedharan, S. Sekar, S. Murugesan, D. Ahamada, S. A. B. Mohamed, Y. Lee and S. Lee, Nanomaterials, 2024, 14, 1867 Search PubMed.
- T. Cai, W. Shi, R. Wu, C. Chu, N. Jin, J. Wang, W. Zheng, X. Wang and O. Chen, J. Am. Chem. Soc., 2024, 146, 3200–3209 Search PubMed.
- W. Żuraw, F. A. Vinocour Pacheco, J. Sánchez-Diaz, Ł. Przypis, M. A. Mejia Escobar, S. Almosni, G. Vescio, J. P. Martínez-Pastor, B. Garrido and R. Kudrawiec, ACS Energy Lett., 2023, 8, 4885–4887 Search PubMed.
- E. Aktas, N. Rajamanickam, J. Pascual, S. Hu, M. H. Aldamasy, D. Di Girolamo, W. Li, G. Nasti, E. Martínez-Ferrero and A. Wakamiya, Commun. Mater., 2022, 3, 104 Search PubMed.
- T. Dai, Q. Cao, L. Yang, M. H. Aldamasy, M. Li, Q. Liang, H. Lu, Y. Dong and Y. Yang, Crystals, 2021, 11, 295 Search PubMed.
- A. D. Khan, M. Mustajab, S. Moeen, M. Imran, M. Ikram, Q. Khan and M. Khan, Environ. Sci. Adv., 2024, 3, 1004–1029 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |