The nucleation and growth mechanism of spherical Li for advanced Li metal anodes – a review†
Mengting
Wang‡
a,
Xingtong
Guo‡
a,
Rui
Luo
a,
Xiaonuo
Jiang
a,
Yongfu
Tang
 *b and
Tao
Wei
*b and
Tao
Wei
 *a
*a
aSchool of Energy and Power, Jiangsu University of Science and Technology, Zhenjiang 212003, China. E-mail: wt863@126.com
bState Key Laboratory of Metastable Materials Science and Technology (MMST), Hebei Key Laboratory of Applied Chemistry, Yanshan University, Qinhuangdao 066004, P. R. China. E-mail: tangyongfu@ysu.edu.cn
First published on 30th January 2025
Abstract
Metallic lithium (Li) is known as the “Holy Grail” of anode materials in the research area of Li-based batteries. However, Li metal anodes (LMAs) are plagued by infinite volume changes and dendrite formation during operation. Spherical Li exhibits rounded surfaces, which effectively mitigates the short circuit risks associated with dendritic Li, and has the smallest specific surface area compared to other deposit morphologies, thus enabling less electrolyte consumption and higher Coulombic efficiency (CE). What's more, three-dimensional (3D) conductive frameworks have good mechanical robustness and flexibility to withstand the volume changes that occur during cycling. This review systematically depicts the theoretical models for Li deposition, the mechanisms and formation conditions of spherical Li, and the benefits of the Li deposition model as well as the advantages of combining Li spheres with 3D conductive frameworks based on our previous works. We hope that this review can inspire researchers in this filed to pave the way for advanced LMAs.
1. Introduction
The escalating consumption of fossil fuels and global environmental degradation problems are propelling the surging demand for renewable, eco-friendly, and cost-effective energy conversion or storage technologies.1–4 Among different kinds of new energy technologies, batteries have achieved significant success due to their user-friendly nature and cost-effectiveness.5–7 After the invention of the first battery by Volta in the 19th century, a variety of electrochemical batteries have gradually stepped into the commercial market, including lead–acid, nickel–cadmium, and lithium-ion batteries (LIBs).8–10 Among them, the LIB with Li cobaltate as the positive electrode and carbon material as the negative electrode, which was successfully launched by Sony Corporation of Japan in 1991, occupies an important position in the commercial market; this kind of battery overcomes the memory effect of the traditional batteries, and has the advantages of high energy density and long cycle life, and hence is rapidly occupying the consumer electronics market.11–16 At the beginning of the 21st century, LIBs continued to expand in the field of consumer electronics, revolutionising the way modern life is communicated and transported, enabling the rise of camcorders, mobile phones, laptop computers and more recently electric vehicles. However, the current graphite anode (370 mA h g−1) is not suitable for powering the booming development of new and advanced electronics.17–20 Fortunately, owing to the remarkable theoretical capacity of Li metal (3860 mA h g−1) and the exceptionally low electrochemical potential (−3.04 V vs. H+/H2), the early lapsed Li-metal batteries (LMBs) have returned to the public eyes.21–24 This is also an opportune moment to reassess the two primary challenges inherent in LMBs.(1) The Li dendrite problem
The disordered characteristics due to the uneven deposition of Li will trigger dendritic Li growth, cause repeated elongation, and even puncture the separator so that the batteries short-circuit, resulting in safety accidents.25–29(2) The infinite volume expansion problem
Continuous relative volume expansion will make the solid electrolyte interface (SEI) film fracture, exposing the surface of fresh Li in contact with the electrolyte, resulting in endless consumption of the electrolyte and Li, reducing the Coulombic efficiency (CE) and producing “dead Li”.30–35LMBs are plagued by these two major issues, which have hindered their commercial success. Consequently, strategies aimed at inhibiting dendrite growth and moderating volume changes have sparked intense discussions over the past two decades. These solutions primarily focus on the implementation of Li alloy electrodes to mitigate dendrite formation,36–39 the improvement of organic electrolytes and the establishment of artificial passivation films for stabilizing the interface of Li metal/electrolyte,40–43 the realization of solid-state electrolytes to improve shear modulus and safety,44–47 and developing a structured anode design by taking advantage of the revolution in nanotechnology and nanomaterials.48–51
The development of LMBs has been propelled by these proposed strategies; however the behavior of Li deposition is one of the most critical issues which is still waiting to be explored. In general terms, Li metal deposits exhibit various shapes, such as mossy,52,53 fibrous,54,55 whisker-like,56 planar nodular,2,57 and spherical forms.57,58 Randomly distributed and disorganized Li deposits with needle-like or branching morphology can give rise to significant safety concerns, whereas ordered and uniform Li deposits with smooth surfaces pose challenges in terms of separator penetration.52,59 Furthermore, among the various depositional morphologies, spherical Li metal deposits exhibit rounded surfaces and a lack of protruding tips, effectively mitigating the short circuit risks associated with dendritic Li deposits while also contributing to volume change mitigation.60 Additionally, spherical Li has the smallest specific surface area compared to other deposit situations, which reduces the contact area between the Li anode and the electrolyte, resulting in better electrochemical performance, such as higher CE and longer cycling life.61 Considering the aforementioned factors, spherical Li deposition holds immense potential for advancing highly energy-efficient Li metal anodes (LMAs). Currently, numerous studies have documented the emergence of spherical Li deposits with promising results.62–64 However, there remains a notable gap in comprehensive and systematic elucidation regarding the formation mechanisms and influencing conditions of these spherical Li deposits. Consequently, this work is aiming to deliver a systematic overview of spherical Li deposition to facilitate advancements in the field and inspire future innovations in LMA design.
In this study, firstly, some theoretical models that have been developed to interpret the mathematical relationships between different Li metal structures and their main influencing issues are summarized. Secondly, the distinctive properties and electrochemical behavior of polycrystalline Li are comprehensively elucidated, thereby facilitating the development of targeted inhibition strategies. Subsequently, a summarized description of the formation advantages, formation conditions, and formation mechanisms of spherical Li deposits is given. Finally, the benefits of the Li deposition model are analyzed and the advantages of combining Li spheres with three-dimensional (3D) conductive frameworks are considered based on our previous works.
2. General mechanism and growth morphology of Li
Li stands out among the alkali metals due to its distinct properties, such as being the lightest and having the smallest atomic diameter. These intrinsic features endow Li with a remarkable high capacity and enable rapid transfer.61 Therefore, the investigation of using Li metal as the anode for applications has been a hot research topic. Nevertheless, there are still many challenges for the wide commercialization of LMAs. The high reactivity and low potential of Li metal make Li unstable when it encounters electrolytes in rechargeable batteries, resulting in a potential crisis. In brief, upon contact with the electrolyte, Li undergoes corrosion and experiences a decrease in its utilization. Simultaneously, during the electrodeposition of Li, Li+ lacks a definitive position and exhibits non-homogeneous initial nucleation of Li.65 It is widely recognized that the formation of dendritic Li significantly hinders the advancement of LMBs. The prevention of Li dendrite growth has been a major focus and challenge in this area. Despite the emergence of numerous strategies to inhibit dendrite growth, there is a lack of comprehensive exploration into Li deposition behavior, which directly influences dendrite formation. Therefore, this summary will briefly introduce the Li deposition behavior from the perspectives of multi-physical fields (ion concentration field model, electric field model) and Li nucleation. It aims to reveal the formation mechanism of different Li morphologies and make contributions to correctly guiding the smooth plating of Li.2.1. Modeling Li deposition behavior with multifield and nucleation models
The Li deposition process follows the basic electrochemical principles, including four steps: (1) ion diffusion, (2) ion adsorption, (3) ion reduction nucleation, and (4) Li metal growth. According to the simplified electrodeposition behavior, solvated Li+ migrates to the anode side as a result of electric fields, adsorbs on the surface of the electrode through the double electric layer effect, undergoes the desolvation process, and finally produces Li metal on the anode through continuous reduction reactions. Among them, nucleation is widely acknowledged as a pivotal process in dictating the final form of deposited Li and ensuring anode surface stability during subsequent plating/stripping cycles. The Li+ diffusion and adsorption process as a precursor strictly controls Li nucleation behavior and provides balanced “power” and “escort” for Li nucleation.66,67 Consequently, when LMBs are operated under practical conditions – such as electrolyte limitations and elevated operating current densities – the ion adsorption and diffusion processes exert a more pronounced influence on Li deposition compared to the nucleation and growth phases.63 Under these circumstances, parameters including current distribution, Li-ion homogeneity, and salt concentration at the interface of the anode side critically determine the final morphologies and cycle stability of the LMA.68,69 Therefore, a better understanding of the close connection between the ion concentration field and the electric field distribution and the morphological evolution of Li is needed to develop precise strategies to regulate diffusion and adsorption.(1) The well-known Sand's time (τSand) model is based on the ion concentration field. In 1901, Sand et al.70 discovered the formation of branching structures in the electrodeposition process of a mixture of CuSO4 and H2SO4, which was controlled by the salt concentration close to the electrode surface. In the process of deposition, positively charged ions and negatively charged ions move in opposite directions. Cations gather close to the anode, while negatively charged ions go opposite to the cathode side. Concurrently, positively charged ions are used up during the process of depositing the metal, resulting in a gradual reduction of the amount of salt on the surface of the positive electrode. When the salt concentration on the anode surface becomes 0, the stable deposition will change to a self-amplifying dendritic growth mode. The onset of dendrite growth is represented by τSand (eqn (1)):
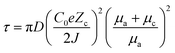 | (1) |
Here, D represents the binary diffusion coefficient, e denotes the elementary charge, C0 stands for the initial concentration of cations (Li salt), J signifies the effective current density, Zc indicates the cation charge number, and μc and μa represent the migration numbers of cations and anions respectively. Sand's model provides a quantitative understanding of the development of Li dendrites. It suggests that by reducing the effective current density or increasing the speed of ion movement, the lifespan of τSand could be prolonged. Furthermore, in 1999, Brissot et al.71 proposed the utilization of a bipolar diffusion equation at the electrode for the detection of changes in ion concentration, as represented by eqn (2):
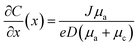 | (2) |
The symbolic meaning here is equivalent to the equation (eqn (1)) above. If dc/dx < 2C0/L (where L is the inner electrode distance), the concentration of the ion at the negative electrode remains stable, and both the electrostatic potential value and the concentration gradient remain unchanged, indicating uniform Li+ deposition. However, when dc/dx < 2C0/L, as depicted in Fig. 1(a), the ion concentration at the electrode drops to 0, and there is a spike in the potential value. The critical diffusion time derived at the inflection point corresponds to the Sand time. In addition, to simplify the identification of crucial running conditions for the Li negative electrode in actual scenarios, by utilizing the inverse correlation between dendrite nucleation time and total deposition capacity, τSand is changed into Sand's capacity (CSand = JτSand).52
 | ||
| Fig. 1 (a) At J > Jlimit (semi-infinite approximation), the ionic concentration drops to zero and the cell potential diverges at Sand's time.71 (b) Illustration of the simulation-based symmetrical cell setup. (c) The distribution of cation/anion concentration. (d) The voltage distribution along the battery.72 (e) Illustration of the nucleation processes of Li on conductive carbon frameworks.73 | ||
While Sand's time model holds certain reference value for predicting battery performance, its accuracy is limited by multiple factors. The intricate internal microstructure of batteries, exemplified by the irregular size and distribution of electrode material particles, results in variations in ion diffusion paths and velocities, thereby impacting model predictions. Moreover, environmental factors such as temperature fluctuations significantly influence chemical reaction rates and ionic conductivity. Particularly at low temperatures, the actual discharge duration of batteries markedly deviates from model predictions. Additionally, polarization during high-rate charging and discharging further contributes to discrepancies in capacity and timing. Considering these existing limitations, by setting some specific conditions, Sand's time model can still provide more accurate prediction results. When the battery operates in a relatively stable ambient temperature environment with low charge/discharge multipliers, the chemical reactions within the battery are closer to the ideal state assumed by the model. At this time, the ion diffusion rate is relatively stable, the polarization phenomenon is not obvious, and the microscopic difference of electrode materials has a relatively small impact on the overall performance, so Sand's time model can predict the charging and discharging time of the battery more accurately.74
(2) The space charge theory is a conceptual framework that relies on the presence of an electric field. The space charge theory which was first introduced by Chazalviel et al. in 199075 seeks to explain the formation of dendrites in terms of the unequal migration of positively and negatively charged particles. This model is suitable for situations involving weak electrolytes, where the movement of ions is only influenced by their mobility and diffusion coefficient and not by the convection of the electrolyte.76 It highlights the significance of anions in the process of charging. Specifically, during rapid Li plating, while Li+ can be continuously replenished, anions cannot; thus, they are more significantly influenced by the electric field in terms of concentration. As a result, depletion zones form quickly at the Li surface during the process of charging, causing a reduction in potential. With the preferential depletion of anions, the accelerated movement of cations is also rapidly depleted at the interface, causing the priority deposition of new cations at the discharge tip. A concentrated space charge and a strong electric field will form due to the depletion of anions and accumulation of Li close to the anode, which promotes dendrite growth. It is noteworthy that ion concentration and electrostatic potential are also calculated by Chazalviel et al. in thin rectangular symmetric cells to validate this model.72 As can be seen in Fig. 1(b–d), region I represents the bulk electrolyte, while region II corresponds to the small domain (space charge) on and around the surface of the electrode. When the supply rate of Li+ (Li+ mobility) in the ion concentration distribution diagram shown in Fig. 1(c) is lower than the consumption rate of Li+ (reaction rate), dendrite growth will be initiated. Simultaneously, in the voltage curve distribution diagram shown in Fig. 1(d), a space charge region still exists, generating a significant local electric field and inducing dendrite formation.
Similarly, the prediction of the space charge model in real battery systems can be affected by a number of factors. Space charge models typically rely on simplifying assumptions, such as the assumption of a homogeneous and ideal electrode/electrolyte interface. However, real battery interfaces exhibit microscopic roughness, impurities, and crystal defects, making it challenging to accurately characterize these complex interfacial properties. Furthermore, space charge models often concentrate solely on charge-related factors, neglecting the intricate effects of multi-physical field interactions, including thermal effects, stress–strain, and material diffusion. However, when the battery is in a relatively stable state of low-rate charge and discharge, the temperature inside the battery changes little, the multi-physical field coupling effect is relatively weak, and the electrode/electrolyte interface changes slowly. In this case, the space charge model based on the theory of static charge distribution and simple electric field hypothesis can better describe the charge transfer and ion transport process inside the battery. The prediction of the open circuit voltage and the ion concentration distribution in equilibrium state has high accuracy.
The relationship between the formation of dendrites and the controlled salt consumption on the electrode surface has been established in the aforementioned models, and the estimated critical growth conditions of dendrites were relatively consistent. Therefore, in order to control early Li plating behavior, it is essential to consider the mobility of cations and anions, as well as the initial concentration of electrolytes, salts, and current density.59,77–79 The following reduction of Li+ to metallic Li participates the nucleation and growth of crystals, and it is vital that the nucleation site plays here in the Li deposition process, it will further affect the final deposition morphology greatly.80,81 Currently, several nucleation models are described in the literature to explore the behavior of Li deposition, which are summarized below.
Ely et al.82 conducted a study on heterogeneous nucleation using mathematical simulation. Five distinct states of heterogeneous nucleation and growth were classified: nucleation suppression, long incubation time, short incubation time, early growth and late growth. Thermodynamically unstable nucleating embryos have a tendency to dissolve into electrolytes when they are in a condition of nucleation inhibition. Afterward, the embryos experienced coarsening due to Gibbs–Thomson interaction during an extended incubation period. Beyond the critical overpotential, the reduced incubation time plays a role in creating a more limited range of embryo sizes. Ultimately, Li initiates nucleation at a critical kinetic radius and experiences rapid growth as the overpotential increases. In conclusion, the inhibition of dendrite formation can be achieved through the reduction of surface roughness on the fluid collector, designing smaller particle size of the anode which is below the critical radius, ensuring that the coating overpotential is smaller than the critical value, and optimizing Li deposit infiltration. As depicted in Fig. 1(e), Chen et al.70 drew inspiration from this model and utilized heteroatom-doped carbon to enhance the Li affinity of the collector fluid, thereby achieving a relatively stable nucleation state.
2.2. Classification and electrochemical behavior of Li crystals
In practical applications, the plating of Li atoms often shows an irregular porous pattern, which can be broadly classified as dendritic Li, whisker-like Li, mossy Li, and smooth Li.85 Among these structures, smooth Li represents the desired morphology for efficient Li metal plating. However, the most commonly observed irregular deposition structures are whisker-like Li, mossy Li, and dendritic Li. This concise summary aims to discuss and compare the morphology as well as electrochemical behavior of these three distinct forms of Li deposits.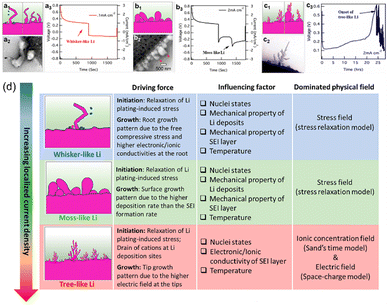 | ||
| Fig. 2 Comparison of different morphologies leaded by different pathways of Li.88 | ||
Apart from their distinctions, under specific circumstances, the morphologies of these Li coatings can also interchange.75 For instance, the dendritic Li deposit will progressively take on the characteristics of moss if the electric field changes and the mean dendritic spacing varies.52,93 The mossy Li that was initially growing at the root will transform into acicular Li growing at the tip if electrolyte diffusion is restricted.71,94 As a result, it is challenging to distinguish these three modes because they always coexist in Li deposits. For the purpose of efficiently tailoring electrode surface topography and constructing safe batteries with dendritic-free deposits, it is necessary to simplify the shape of Li deposits readily.
3. Spherical Li
Although the metallic Li is regarded as the ultimate anode for the future advanced Li batteries, low CE and dendritic Li deposition pose serious risks to public safety since there will inevitably be a Li deposition issue due to the ongoing interconversion between Li ions and Li atoms throughout the process of Li plating. As a result, controlling Li deposition is a common strategy used to prevent dendritic development. The emergence of spherical Li deposits in recent years may inhibit the establishment of Li dendrites effectively.3.1. Advantages of spherical Li
With the study of the mechanism of Li nucleation and growth as well as the morphology and internal structure of Li deposits, spherical Li has attracted more and more attention. Compared with existing Li metal deposits, spherical Li deposits have two important advantages due to their unique morphology. Firstly, spherical Li exhibits distinct morphological characteristics with a smooth surface and the absence of sharp protrusions, thereby effectively mitigating the severe safety risks associated with dendritic Li deposition.95 Back in 2017, Ye et al.96 capitalized on these spherical attributes to achieve stable electrochemical behavior (Fig. 3(a)). Initially, spherical carbon particles were prepared via chemical vapor deposition (CVD) onto a 3D nickel skeleton, followed by gradual coating of these particles with Li growth. Although the direct formation of Li spheres was not achieved in this study, the intermittent coating of spherical carbon particles by Li resulted in an electrode that demonstrated remarkable inhibition of dendrites and high utilization efficiency for Li (Fig. 3(b)), which can be attributed to the unique morphology. At this time, spherical Li deposits had not received widespread attention. More notably, in 2019, Li et al.97 successfully induced the formation of spherical Li deposits by constructing an artificially rich inorganic SEI film which was obtained by using reducing salt anions on a Cu substrate through electroreduction treatment in LiTFSI's water-salt electrolyte (Fig. 3(c)). In the first nuclear topography of Li on different substrates (with and without SEI films), dendritic growth was found on a Cu substrate without SEI films under the current densities of 1 and 2 mA cm−2 (Fig. 3(d1 and d2)). However, more uniform growth of Li balls was achieved on Cu substrates pretreated with electroreduction (Fig. 3(d3 and d4)). More pronounced differences can be found by observing the SEM images of the Li||Cu battery which was deposited at an areal capacity of 1 mA h cm−2 (Fig. 3(d5 and d6)). This is due to the induction of uniform spherical Li, which resulted in a lower crossover point and higher CE (98.2% on average). Furthermore, this improvement in battery performance is also evident in assembled Li–S full batteries (Fig. 3(e–g)), where high CE and cycle stability are observed. The discovery also highlights another advantage of spherical Li over other Li deposits, namely, its minimal surface area per unit volume. This characteristic leads to a reduced area of contact between the electrolyte and fresh Li, resulting in a decreased formation of the SEI interface. Consequently, this phenomenon contributes to higher CE and an extended cycle life. | ||
| Fig. 3 (a) Illustration of the deposition of Li. (b) Long-term performance of the full cell.96 (c) Illustrations of the Li deposition behavior on two substrates. (d) SEM images of the 1st Li nuclei on a Cu substrate without or with a salt-derived SEI (donated as SDSEI) film at different areal capacities. (e) Comparison of different substrates at 0.5 mA cm−2 after the 1st Li nucleation. (f) CE at 0.5 mA cm−2 for Li deposited on a bare Cu substrate and on the Cu substrate with the SDSEI film. (g) Electrochemical performance of different batteries.97 | ||
With the advancement and diversification of technology, spherical Li deposition has gradually appeared in the public's vision and shown better development. It is designed to promote the formation of smooth and uniform sediment layers with minimal area to improve circulation performance and ensure safety issues. For instance, in 2020, Zhu et al.98 employed Ag as a modifier on Cu foil to create a spherical island model for enhancing the deposition of Li with smooth surface (Fig. 4a). Initially, the globular Ag nuclei facilitate the reduced nucleation potential of deposited Li atoms. Subsequently, Li uniformly grows around these spherical islands to form a flat surface layer, which is opposite to the irregular growth observed on pure Cu foil. The SEM comparison image after 120 cycles (Fig. 4b) further indicates that the electrode formed using Ag nanoparticles wrapped around 3D copper foam combined with molten Li (Li-ANCF) exhibits smoother surfaces with no dendrite formation. Undoubtedly, the rate performance when using the Li-ANCF electrode surpasses that of the electrode produced by melting Li on bare copper foil (Li-BCF) (Fig. 4c). In the same year, Wang et al.99 successfully achieved uniform dispersion of carbonized metal–organic frameworks (MOFs) of nanosized Co coated by N-doped graphene (Co@N-G). This material effectively enhances graphene's affinity for Li, while the unique cubic hexahedral structure facilitates the formation of spherical Li during Li deposition, thereby promoting a more stable deposition/stripping process. The remarkable advantage of the Co@N-G electrode can be observed in both the CE image (Fig. 4e) and the Li magnification test (Fig. 4f), which is closely associated with its ability to facilitate spherical Li deposition. To summarize, the presence of spherical Li deposition effectively inhibits adverse effects caused by irregular dendrites and enables a stable long-cycle performance with high efficiency.
 | ||
| Fig. 4 (a) Deposition of Li on the surface of Cu with Ag nanoparticle coating (named ANC) and bare Cu. (b) Comparison of Li-ANCF and Li-BCF electrodes after 120 cycles. (c) Rate performances of two kinds of batteries.98 (d) Schematic illustrations of the Li deposition process on Co@N-G. (e) CE of different electrodes with an areal capacity of 1 mA h cm−2 at various current densities. (f) Rate performance of various symmetrical cells.99 | ||
3.2. Acquisition of spherical Li
The distinct characteristics of spherical Li, such as its non-tip shape and the promotion of SEI formation to minimize the low barrier, have significantly propelled LMBs toward a new direction.100,101 Currently, despite numerous reports on inducing spherical Li deposition and achieving efficient and stable circulation,102,103 there is a comprehensive interpretation of obtaining spherical Li deposition. It is well known that a number of variables, including areal capacity, current density, and electrolyte, lead to different Li deposition morphologies.104,105 Thus, it is possible to effectively regulate spherical Li deposition by taking these important aspects into account. Furthermore, this part provides a systematic overview of the existing literature on the formation of spherical Li deposits and elucidates the underlying factors contributing to diverse morphologies of Li deposition, thereby facilitating enhanced control over Li deposition morphology.3.3. Factors affecting the formation of spherical Li
In the research on electrolytes for Li batteries, different types of electrolyte additives have been employed for the purpose of improving different properties of various kinds of LMAs. Among them, film-forming additives are one of the most widely studied and representative strategies for stabilizing SEI films. For example, 1,3-propane sultone (1,3-PS), fluorinated ethylene carbonate (FEC), and vinylene carbonate (VC) have been added to Li batteries, which all contribute to the formation of a robust SEI film on the surface of Li anodes. Undoubtedly, these additives have contributed to the promotion of Li batteries but have played little role in obtaining spherical Li deposits (Fig. 5(a)).109,110 With the development of this area, LiNO3 is widely recognized as a “prominent” film-forming additive to control the dissolution sheath of electrolytes, forming nitrogen-containing SEIs, which can change the morphology of deposited Li and thereby improve the cycle stability of LMAs.111 Importantly, the introduction of LiNO3 can cause Li deposition to grow in the form of spherical particles. To be honest, this feature is effective for LMBs; however, for Li–sulfur batteries, the deposition of spherical Li cannot be fully achieved through the sole use of LiNO3, which requires the additional introduction of polysulfides to play a synergistic role. For example, the combined effect of LiNO3 and Li polysulfide in inhibiting dendrite development, explored by Li et al. in 2015,2 demonstrated that only using LiNO3 as the additive to the electrolyte was not sufficient to inhibit dendrite growth effectively. As can be seen in Fig. 5(b), in the case where LiNO3 alone is incorporated, the SEI film formed by the electrolyte without polysulfide is of insufficient mechanical strength, resulting in cracks and pits where Li filaments grow. In contrast, supplementation of electrolytes with polysulfide (Li2S8) diminishes Li nucleation sites within the electrolyte through the reaction between metallic Li anode and Li2S8, thereby facilitating flattened deposition of Li. Based on the SEM pictures (Fig. 5(c)), the current density is 2 mA cm−2, and the Li deposition capacity is 0.2, 2, and 6 mA h cm−2, respectively. When only LiNO3 is introduced into the electrolyte, Li filaments can be seen to varying degrees (Fig. 5(c1, c3 and c5)). As shown in Fig. 5(a), filaments gradually grow out of the spherical particles. As Li deposition continues, large areas of filamentary Li spread over the surface (Fig. 5(c)). These structures are not only a threat to the safety of the battery but also lead to a higher dead Li stripping rate. In contrast, due to the combined effect of LiNO3 and polysulfides, the substrate surface is gradually covered by flat circular Li deposits with uniform distribution and no obvious Li-shaped stacking (Fig. 5(c2 and c4)). Fig. 5(c6) shows the appearance of long filamentary dendrites that could be connected to the cyclic areal capacity and current density. In addition, the introduced O2 at the cathode side also favors the formation of spherical Li, according to the literature published by Shi et al. in 2017.112 As illustrated in Fig. 5(d), other conventional Li batteries produce disorganized whisker-like Li at different current densities (0.1 and 1 mA cm−2) with the same areal capacity of 1 mA h cm−2 (Fig. 5(d1–d4)). In contrast, SEM images of the LMA with the same electrolyte composition in Li–O2 batteries depicted in Fig. 5(d5 and d6) reveal a circular morphology and a uniform distribution of Li deposits, which confirm the important role of introduced O2.
 | ||
| Fig. 5 (a) Li deposition morphology after adding mold-forming additives (a1) VC109 and (a2) FEC110 to different types of electrolytes. (b) Illustration of Li deposited morphologies in various electrolytes. (c) SEM images of Li deposition on a stainless-steel substrate.2 (d) SEM images of deposited Li at different current densities and Li–O2 battery. (Scale bars: d1, d3 and d5, 5 μm; d2 and d4, 2 μm; d6, 1 μm.)112 | ||
To sum up, the incorporation of strong inhibitors such as LiNO3 and Li polysulfide promotes the formation of dense spherical Li morphology while minimizing the surface area, thereby mitigating electrolyte consumption and dead Li accumulation, ultimately leading to improved cycling efficiency. Furthermore, other components (O2 and polysulfides) also facilitate the adhesion of these spherical Li deposits.113
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), which is a typical ether-based electrolyte, and the stacking of clumped Li deposits was found ((as shown in the zoomed-in view) which might be due to the outstanding anode stability of the ether-based electrolyte itself.114 Fortunately, spherical Li deposits can generally be achieved through the addition of specific additives, such as LiNO3, to ether-based electrolytes, as described in the study of Li deposition morphology in different electrolytes by Shi et al. in 2017.112 Specifically, they demonstrated that incorporating 1% LiNO3 into an electrolyte comprising DOL/DME (in the ratio of 1
1 v/v), which is a typical ether-based electrolyte, and the stacking of clumped Li deposits was found ((as shown in the zoomed-in view) which might be due to the outstanding anode stability of the ether-based electrolyte itself.114 Fortunately, spherical Li deposits can generally be achieved through the addition of specific additives, such as LiNO3, to ether-based electrolytes, as described in the study of Li deposition morphology in different electrolytes by Shi et al. in 2017.112 Specifically, they demonstrated that incorporating 1% LiNO3 into an electrolyte comprising DOL/DME (in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) within a 1 M LiTFSI solution successfully transformed Li deposition morphology into a spherical shape (Fig. 6(a3 and a4)). In addition, uniform round Li deposits were also obtained at higher current densities when additional polysulfides at a concentration of 5 M were added to the Li–S full cell to form the cathode solution for electroplating, as shown in Fig. 6(a5 and a6). This finding is in agreement with the previous report by Li et al.2 Unfortunately, despite their advantages, the poor oxidative stability of ether-based electrolytes significantly restricts their applicability in batteries with high-voltage cathodes (≥4.3 V).115–117 In contrast, carbonate-based electrolytes possess higher anodic potentials and lower flammability profiles, which make them much more compatible than ether-based electrolytes for high-voltage cathodes. Similarly, as shown in Fig. 6(a7 and a8), significant dendrites appeared when the electrolyte was 1 M LiPF6 containing 1
1 v/v) within a 1 M LiTFSI solution successfully transformed Li deposition morphology into a spherical shape (Fig. 6(a3 and a4)). In addition, uniform round Li deposits were also obtained at higher current densities when additional polysulfides at a concentration of 5 M were added to the Li–S full cell to form the cathode solution for electroplating, as shown in Fig. 6(a5 and a6). This finding is in agreement with the previous report by Li et al.2 Unfortunately, despite their advantages, the poor oxidative stability of ether-based electrolytes significantly restricts their applicability in batteries with high-voltage cathodes (≥4.3 V).115–117 In contrast, carbonate-based electrolytes possess higher anodic potentials and lower flammability profiles, which make them much more compatible than ether-based electrolytes for high-voltage cathodes. Similarly, as shown in Fig. 6(a7 and a8), significant dendrites appeared when the electrolyte was 1 M LiPF6 containing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v EC/DEC, without additional additives. According to Liu et al.'s research,60 introducing certain amounts of LiNO3 into this carbonate matrix resulted in well-defined spherical Li deposits (Fig. 6(b)). However, the solubility of LiNO3 is limited (<10−5 g mL−1) in carbonate-based electrolytes, which has greatly hindered its application in traditional carbonate electrolytes.118–120 In order to solve this problem, Liu et al.60 designed a solubility-mediated slow-release mechanism wherein nanoparticles of LiNO3 are embedded within a porous polymer framework on the anode surface and gradually dissolve when running the battery as soluble forms are consumed as shown in Fig. 6(c). Fig. 6(d) illustrates unique morphological evolution characterized by spherical growth patterns distinct from those associated with dendritic formation typically seen under nitrogen oxide-saturated conditions in carbonate environments. In addition, the performance of the electrolyte employing dissolved LiNO3 was significantly improved compared to the other two electrolytes (Fig. 6(e)). Other methodologies aimed at enhancing solubility levels concerning LiNO3 have been documented elsewhere but will not be reiterated here.121–124
1 v/v EC/DEC, without additional additives. According to Liu et al.'s research,60 introducing certain amounts of LiNO3 into this carbonate matrix resulted in well-defined spherical Li deposits (Fig. 6(b)). However, the solubility of LiNO3 is limited (<10−5 g mL−1) in carbonate-based electrolytes, which has greatly hindered its application in traditional carbonate electrolytes.118–120 In order to solve this problem, Liu et al.60 designed a solubility-mediated slow-release mechanism wherein nanoparticles of LiNO3 are embedded within a porous polymer framework on the anode surface and gradually dissolve when running the battery as soluble forms are consumed as shown in Fig. 6(c). Fig. 6(d) illustrates unique morphological evolution characterized by spherical growth patterns distinct from those associated with dendritic formation typically seen under nitrogen oxide-saturated conditions in carbonate environments. In addition, the performance of the electrolyte employing dissolved LiNO3 was significantly improved compared to the other two electrolytes (Fig. 6(e)). Other methodologies aimed at enhancing solubility levels concerning LiNO3 have been documented elsewhere but will not be reiterated here.121–124
 | ||
| Fig. 6 (a) The morphologies of deposited Li in different electrolytes at 0.1 mA cm−2 and 1 mA h cm−2.112,114 (b) The morphologies of deposited Li in a 1.0 M LiPF6 EC/DEC electrolyte with saturated LiNO3. (c) Illustration of the fabrication and working process of a freestanding membrane consisting of LiNO3 dissolved in a polymeric matrix (termed LiNO3 sustained-release film, LNO-SRF). (d) The morphologies of deposited Li on a Cu substrate covered by LNO-SRF. (e) CE at 1 mA cm−2 and 1 mA h cm−2 in different electrolytes.60 | ||
It is noteworthy that Luo et al.125 have developed a composite electrolyte (nitrocellulose (NC) combined with Li bis(fluorosulfonyl)imide (LiFSI)) which showed excellent reversible properties in lean electrolyte cells under the conditions of high current density. Fig. 7(a) demonstrates that an inorganic LiF-rich layer was formed promoted by LiFSI, and NC played the role of a passivated organic layer which encapsulated the above inorganic layer. This joint effect of the nitro group in NC and the LiF-rich interface facilitates even plating/stripping of Li, leading to the formation of spherical nuclei of Li even at elevated current densities. With this advantage, LMBs showed excellent electrochemical performance (Fig. 7(b)). Furthermore, Fig. 7(c) illustrates the SEM images of the Li crystal nucleus under different current densities between 0.5 and 10 mA cm−2 at a fixed areal capacity of 0.1 mA h cm−2. Interestingly, when 2% NC is added, the Li nucleus changes from rod to ball with the increase of current density. In the morphological evolution diagram of the electroplated/stripped Li electrodeposited layer observed through in situ/ex situ techniques at various current densities, it is observed that Li metal assumes a spherical morphology when applied at a high current density (>1 mA cm−2) in a 2 M LiFSI/DME(FM) + 2% NC electrolyte.
 | ||
| Fig. 7 (a) Schematic diagram of dendritic/spherical Li deposition. (b) The CE of Li||Cu and Li||Li cells and the cyclic performance of Li||Li cells at 1 mA cm−2 and 1 mA h cm−2. (c) Deposited Li in different electrolytes.125 | ||
 | ||
| Fig. 8 (a) Illustration of the density and size of Li nuclei at different overpotentials. (b) Morphologies of deposited Li at various current densities between 0.025 and 10 mA cm−2 at an areal capacity of 0.1 mA h cm−2. (c) Morphologies of deposited Li at different current densities and areal capacities.93 (d) Morphologies of deposited Li at the areal capacity of 0.1 and 0.2 mA h cm−2 at various current densities. (e) Cross-sectional images of deposited Li at an areal capacity of 1 mA h cm−2 with different current densities. (f) Illustration of the evolution of morphology.126 | ||
Additionally, in 2021, under identical experimental conditions (DOL/DME with 1 wt% LiNO3, deposited on copper foil), Lee et al.126 not only investigated the disparity of Li deposition behavior with the alteration of current density and areal capacity but also indicated that the dendrite Li phenomenon would arise with the increment of deposition amount. As depicted in Fig. 8(d), at the beginning with a current of 0.1 mA h cm−2, the size of Li nuclei reduces, and the size increases with increasing current density, which is in agreement with the conclusion of Pei's results. Nevertheless, with the augmentation of the amount of electrodeposition, dendrite Li emerges at lower current densities (Fig. 8(d5 and d6)), while at 1.0 and 1.8 mA cm−2, multiple layers of spherical Li are covered on the Cu substrate compactly, as well as the particle size of Li increases vertically from bottom to top. When the areal capacity increased to 1 mA h cm−2 (Fig. 8(e)), only filamentous deposition of Li existed at 0.2 mA cm−2, and when the current density gradually increased from 0.5 mA cm−2 to 1.8 mA cm−2, there were spherical Li particles grown on the copper surface, combined with sparse Li spheres and multi-layer Li spheres. However, when the spherical Li particles grew to a certain extent, that is, when they reached a certain size, the Li particles transformed into fibrous growth, which is decided by the electrochemical environment of the cell. As illustrated in Fig. 8(f), since the stress was released when spherical Li was converted into columnar deposition, Li would be preferentially deposited onto the formed fibrous Li, while for the smaller Li balls, they remain unchanged. Consequently, only filamentous Li deposits exist at a low current density, and their diameters and lengths are large. When operated using a preset high current density, the Li particles grow spherically during the initial nucleation on the surface of the substrate. And the Li nuclei can grow without becoming coarse under the dynamic boundary conditions. However, with the growing of the particle, the base-controlled growth mechanism can be used to explain the electrodeposition conditions, which causes the Li deposits to deform and cause columnar growth plastically. In view of this phenomenon, Lee's research group made improvements on this basis to induce uniform and compact columnar Li deposition, which will be elaborated in the next section.
Nevertheless, in traditional carbonate electrolytes, the deposition of Li might differ from the aforementioned research findings. For instance, in 2021, Dong et al.127 disclosed that the low local current distribution during the deposition of Li is more beneficial for the formation of compact spherical Li. Specifically, by observing the SEM image depicted in Fig. 9(a), the spherical Li (approximately 500 nm) is identified when the deposition amount is 1 mA h cm−2 at 0.2 mA cm−2 (Fig. 9(a1 and a2)). Some of the smaller spheres found in the enlarged image might be newly formed Li electrodeposits, which would continue to expand if the electrodeposition proceeds. Subsequently, with the increase of current density, the Li deposition becomes increasingly uneven, and spherical Li and columnar Li coexist.
 | ||
| Fig. 9 (a) Morphologies of deposited Li at various current densities ranging from 0.2 to 10.0 mA cm−2 at an areal capacity of 1.0 mA h cm−2. (b) Internal view of the granular and bush-shaped Li obtained by FIB/SEM. (c) Illustration of the deposited Li at different currents.127 | ||
Spherical and dendritic structures are more pronounced at higher current densities (5 and 10 mA cm−2). This phenomenon can be accounted for by the space charge theory, namely, current densities below Jlim (2.1 mA cm−2) can give rise to relatively flat deposits, while those above Jlim lead to dendritic deposits. By inspecting their internal structure, it can also be discovered (Fig. 9(b)) that spherically stacked uniform and compact structures emerge at 0.2 mA cm−2 (Fig. 9(b1 and b3)), in contrast with the shrubby electrosedimentary layer at 5 mA cm−2 (Fig. 9(b2 and b4)). Based on these outcomes, the Li electrodeposition and growth processes in the carbonate-based electrolyte should comply with the mechanism diagram in Fig. 9(c). That is, at a low deposition rate, Li+ is distributed relatively evenly, and Li spheres can arise adjacent to the electrode surface and accumulate with the deposition. Conversely, once the critical rate is surpassed, the Li deposition occurs in a disordered manner, and the coexistence of spherical Li and shrub-like Li is found.
In conclusion, the role of electrolyte additives, electrolyte components, and current density/areal capacity in Li deposition morphology was analyzed, and the operating conditions conducive to the formation of spherical Li morphology were summarized. This will also effectively facilitate the subsequent obtaining of Li spheres.
3.4. Nucleation and growth mechanisms of spherical Li
Spherical Li deposition can effectively prevent the severe safety risks caused by dendritic Li formation. The previous section elaborates on the strategies for regulating the formation of spherical Li deposits; however, the formation mechanisms of spherical Li are still not well comprehended. The elucidation of the formation mechanism of Li balls can further guarantee the realization of safe and stable LMBs. In this respect, Zhang et al. developed a mechanism of diffusion–reaction competition for Li+, which achieves the deposition of Li in different scenarios by dividing the controlling step of Li deposition into two categories, namely diffusion and reaction.128 Typically, the deposition of Li occurs below the SEI coating. This involves Li+ passing through the SEI film and gaining electrons to convert into Li atoms. However, as shown in Fig. 10(a), when the diffusion rate decreases throughout the Li deposition process with the same electrode reaction rate, it becomes difficult for Li+ to pass through the SEI film, which is referred to as a sluggish SEI. Concurrently, the reduction of ions will lead to a shortage of Li+ below the SEI, which is referred to as the diffusion-controlled mechanism. | ||
| Fig. 10 (a) Illustration of dendritic/spherical deposition of Li under slow and fast SEI. (b) Li deposition morphology and local current density distribution from phase field modeling. (c) SEM images of dendritic/spherical Li deposition at a current density of 0.50 mA cm−2 and an areal capacity of 0.125 mA h cm−2 in different electrolytes.128 (d) Illustration of the reaction rates at different electroplating steps. (e) Morphologies of deposited Li in the electrolyte of LiPF6 dissolved in EC/DEC at a current density of 0.1 mA cm−2 under different capacities. (f) Top-view of columnar metallic Li after stripping under different areal capacities.129 | ||
As a result, there is a lack of Li+, and they tend to gather at the tips, leading to the formation of dendritic Li deposits. In the opposite, in the case of fast SEI, the nucleation of Li will occur under the SEI and spherical Li will be achieved. In order to validate this diffusion–reaction mechanism, Chen et al.128 employed a phase field model to replicate the spherical/dendritic formation of Li during rapid/slow SEI deposition.
As shown in Fig. 10(b), it is evident that in situations where diffusion is the controlling factor, Li+ faces obstacles in diffusing through the SEI layer. This leads to the accumulation of local current density at the tip, indicating the formation of dendritic structures. In contrast, when the reaction control dominates, spherical Li deposits are created successfully accompanied by even local current densities around the spherical Li. This characterization technique can be widely employed in the subsequent investigation of spherical Li and can clearly emphasize the production of spherical Li. In addition to the physical field model, Zhang et al. also employed a series of electrolytes in DOL/DME containing 1.0 M LiNO3 and LiTFSI as a model system for constructing SEI with a diffusion kinetic gradient. Conventional TFSI− partially substitutes NO3− to generate Li3N and LiNxOy in the SEI film, and thereby increases the Li+ conductivity of the SEI film. Specifically, the electrolytes with different molar ratios of LiNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiTFSI at 0
LiTFSI at 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 2
10, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 4
8, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 6
6, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 8
4, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were utilized to form SEI with enhanced ionic diffusion coefficients. Fig. 10(c1–c5) presents the SEM images of deposited Li on Cu foil at 0.5 mA cm−2 and 0.125 mA h cm−2. With the escalation of the molar ratio of LiNO3 and LiTFSI, spherical Li deposits gradually emerge. When the N8F2 (LiNO3
2 were utilized to form SEI with enhanced ionic diffusion coefficients. Fig. 10(c1–c5) presents the SEM images of deposited Li on Cu foil at 0.5 mA cm−2 and 0.125 mA h cm−2. With the escalation of the molar ratio of LiNO3 and LiTFSI, spherical Li deposits gradually emerge. When the N8F2 (LiNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiTFSI = 8
LiTFSI = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) electrolyte is utilized, all Li atoms have the tendency of depositing in the form of spheres. The gradual spherical Li deposition here is for the reason that with the increase of the diffusion rate of Li+, the ion concentration under the SEI film with the same electrode reaction rate increases. Additionally, the morphology of Li deposition in the N8F2 electrolyte after two plating/stripping processes (Fig. 10(c6)) is also observed to maintain a spherical structure, indicating that the deposited Li morphology is constantly controlled by adjusting the competition between diffusion and reaction processes.
2) electrolyte is utilized, all Li atoms have the tendency of depositing in the form of spheres. The gradual spherical Li deposition here is for the reason that with the increase of the diffusion rate of Li+, the ion concentration under the SEI film with the same electrode reaction rate increases. Additionally, the morphology of Li deposition in the N8F2 electrolyte after two plating/stripping processes (Fig. 10(c6)) is also observed to maintain a spherical structure, indicating that the deposited Li morphology is constantly controlled by adjusting the competition between diffusion and reaction processes.
In addition to the aforementioned diffusion–reaction mechanism, there is another fascinating query. Lee129 also elucidated the formation mechanisms of columnar Li metal, multilayered spherical Li accumulation, and dendrite Li based on the rates of Li nucleation on the substrate, Li growth and Li nucleation on Li (Fig. 10(d)). Among them, ordered columnar Li deposition represents the optimal state for forming a dendrite-free anode, which can effectively avert the safety hazards caused by dendrite Li. Specifically, when the rate of Li nucleation on the substrate (Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sub) exceeds the rate of Li growth (Vgrowth) and Li nucleation on Li deposits (Vnuc
sub) exceeds the rate of Li growth (Vgrowth) and Li nucleation on Li deposits (Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li), the Li nuclei will first grow on the substrate rather than form metallic Li. Thus, with the increase of areal capacity, one-dimensional growth of Li occurs, leading to the formation of columnar Li metal. However, if Vgrowth surpasses Vnuc
Li), the Li nuclei will first grow on the substrate rather than form metallic Li. Thus, with the increase of areal capacity, one-dimensional growth of Li occurs, leading to the formation of columnar Li metal. However, if Vgrowth surpasses Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sub and Vnuc
sub and Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li, the deposition of Li nuclei will be hard to see, and the metallic Li grows in every direction as the continuous plating of Li, resulting in the formation of multiple layers of spherical Li. Eventually, when Vnuc
Li, the deposition of Li nuclei will be hard to see, and the metallic Li grows in every direction as the continuous plating of Li, resulting in the formation of multiple layers of spherical Li. Eventually, when Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li is greater than Vnuc
Li is greater than Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sub and Vgrowth, the Li metal is more prone to deposit at the surface of formed Li instead of depositing at the surface of the substrate. Therefore, during operating, the Li metal grows randomly in the form of dendrites. Simultaneously, the SEM images of the surface/cross-section of various substrates, namely the Cu foil (Fig. 10(e1–e3)) and the Ti foil (Fig. 10(e4–e6)), at 0.2, 4, and 6 mA h cm−2 were characterized. At 0.2 mA h cm−2, the Li nucleus on Cu foil is denser and smaller than that on the Ti foil, and the disparity becomes more pronounced with the augmented deposition amount. This reveals that a hetero-nucleation rate is associated with the different substrates, namely, a faster hetero-nucleation rate is associated with the deposition of regular columnar Li metal. Finally, the role of columnar Li metal in enhancing electrochemical performance compared with traditional Li metal was elucidated by comparing the morphological alterations of the Li metal cylinder with traditional metallic Li electrodes in terms of the exfoliation process of Li. Fig. 10(f1–f3) and (f4–f6) present the different views (top and cross-section) of the Li cylinder electrode when stripping under 0.1 mA cm−2. With the increase in the stripping amount, each Li metal cylinder shrinks uniformly, indicating that the exfoliation process of Li metal across the whole electrode surface occurs uniformly. On the other hand, non-uniform stripping phenomena were witnessed when using the traditional Li metal electrode, and the area of the pores enlarged gradually following the increase in the stripping amount, as depicted in Fig. 10(f7–f9).
sub and Vgrowth, the Li metal is more prone to deposit at the surface of formed Li instead of depositing at the surface of the substrate. Therefore, during operating, the Li metal grows randomly in the form of dendrites. Simultaneously, the SEM images of the surface/cross-section of various substrates, namely the Cu foil (Fig. 10(e1–e3)) and the Ti foil (Fig. 10(e4–e6)), at 0.2, 4, and 6 mA h cm−2 were characterized. At 0.2 mA h cm−2, the Li nucleus on Cu foil is denser and smaller than that on the Ti foil, and the disparity becomes more pronounced with the augmented deposition amount. This reveals that a hetero-nucleation rate is associated with the different substrates, namely, a faster hetero-nucleation rate is associated with the deposition of regular columnar Li metal. Finally, the role of columnar Li metal in enhancing electrochemical performance compared with traditional Li metal was elucidated by comparing the morphological alterations of the Li metal cylinder with traditional metallic Li electrodes in terms of the exfoliation process of Li. Fig. 10(f1–f3) and (f4–f6) present the different views (top and cross-section) of the Li cylinder electrode when stripping under 0.1 mA cm−2. With the increase in the stripping amount, each Li metal cylinder shrinks uniformly, indicating that the exfoliation process of Li metal across the whole electrode surface occurs uniformly. On the other hand, non-uniform stripping phenomena were witnessed when using the traditional Li metal electrode, and the area of the pores enlarged gradually following the increase in the stripping amount, as depicted in Fig. 10(f7–f9).
4. Combining spherical Li with conductive 3D frameworks
As discussed above, the main challenges of LMAs are Li dendrite growth and infinite volume expansion. Although spherical Li can effectively address the dendritic Li formation, the volume expansion problem still needs to be solved.130–134 Fortunately, the 3D conductive frameworks have high mechanical robustness and flexibility to withstand the volume changes that occur during charging/discharging.135–140 The combination of the 3D conductive frameworks and spherical Li may have a profound influence on improving the inhibition of the volume change and Li dendrite growth of the LMA.However, frequently employed 3D skeletons (Cu foam, Ni foam (NF), carbon cloth (CC), etc.) exhibit lithiophobic characteristics.141–143 Therefore, there is a necessity of depositing lithiophilic materials on the surface of these 3D skeletons. Various methods have been developed to uniformly coat these materials on the skeleton, such as magnetron sputtering,144 vapor phase deposition,145 aqueous metal salt treatment,146 atomic layer deposition,147 and the use of metal–organic framework (MOF) derivatives.148 Among them, using MOF derivatives to fabricate the lithiophilic 3D frameworks shows significant advantages in the above-mentioned techniques, which can be referred to in our previous review.149
Unfortunately, it seems still difficult to acquire spherical Li in these monometallic MOF derivative modified 3D frameworks. For example, in the previous study of our group, a MOF-derived (ZIF-67) Co3O4 layer was uniformly coated on the NF framework and injected with molten Li to obtain a Li–Co3O4-NF composite anode (Fig. 11(a)).150 In ether electrolytes, the anode demonstrated relatively uniform Li deposition behavior. Nevertheless, the growth of Li dendrites was still found by using in situ optical microscopy (OM) (Fig. 11(b)). Zhuang et al. synthesized c-ZNCC (ZnO, N binary doped nanocages) as the main body of the LMA using ZIF-8 (zeolite imidazolate framework-8) (Fig. 11(c)).151 The anode has excellent cycling stability and efficient charge/discharge capability. However, regarding the morphology of the surface of this anode after cycling, the formation of dendritic Li cannot be avoided, although the thickness of c-ZNCC-Li had been significantly reduced (Fig. 11(d)).
 | ||
| Fig. 11 (a) Synthesis procedure of the Co3O4-NF electrode.150 (b) In situ OM tests of the Li–Co3O4-NF symmetric cells. (c) Illustration of the preparation of c-ZNCC.151 (d) SEM images of c-ZNCC-Li and bare Li anodes after cycling for 500 cycles. | ||
Interestingly, while utilizing bimetallic MOF derivatives to fabricate lithiophilic 3D frameworks, spherical Li deposition behavior can be clearly seen without changes of the electrolyte components or any electrolyte additives. For instance, based on our previous study, the formation of spherical Li was clearly found on the surface of a lithiophilic framework constructed based on ZnCo-MOF derivatives (ZnCo2O4 and ZnO) (CC@ZZCO).152 This phenomenon was first theoretically supported by DFT calculations, which showed that ZnO has a stronger affinity for Li adsorption and nucleation (Fig. 12(a and b)). It is demonstrated that Li prefers preferential nucleation and growth on ZnO. To investigate the Li deposition behavior further, we conducted Li deposition experiments at 1 mA cm−2 under various areal capacities (Fig. 12(c1–c4)). When operating under 5 mA h cm−2, Li deposited on the CC formed small protrusions aligned along the fiber direction and presented a spherical Li morphology (Fig. 12(c1 and c2)). The spherical Li was densely arranged on the CC surface, which inhibited the growth of Li dendrites effectively. As the deposition time increases (under 10 mA h cm−2), Li deposition begins to abandon the protruding regions and instead fills the surface (Li deposition on ZnCo2O4), eventually forming a smooth surface (Fig. 12(c3 and c4)). Furthermore, the diameter was 8.69 μm for the original CC, which increased to 9.81 μm after deposition at 1 mA cm−2 with the capacity of 5 mA h cm−2 for CC@ZZCO; with more Li deposition, the diameter increased to about 11.00 μm. This indicates that most of the deposited Li are formed on the CC instead of forming large clumps or dendrites at the top of the framework. Nevertheless, when deposited on pure CC under the same current deposition conditions, Li exhibited a disordered stacking morphology (Fig. 12(c5 and c6)). This phenomenon further proves that CC@ZZCO can induce the uniform nucleation of Li effectively. The specific mechanism is shown in Fig. 12(d), where ZnCo2O4 and ZnO are intermittently aligned on the CC, which contributes to guiding the uniform deposition of Li.
 | ||
| Fig. 12 (a) and (b) DFT calculation of binding energies of Li atoms with ZnO and ZnCo2O4. (c) SEM images of plated Li on CC@ZZCO under different capacities. (d) Schematic of Li interstitial deposition consisting of ZnO and ZnCo2O4.152 (e) and (f) DFT calculation of binding energies of Li atoms with Fe2O3 and NiFe2O4. (g) Li encapsulation process of the CC@NFFO skeleton under different capacities. (h) Illustration of Li interstitial deposition consisting of Fe2O3 and NiFe2O4.153 (i) Illustration of Li interstitial deposition consisting of MnO and Co. (j1)–(j6) In situ OM of Li deposition on CC@MnO/Co/C. (j7)–(j9) SEM images of plated Li on CC@MnO/Co/C under different capacities.154 | ||
Similarly, in another study also conducted by our group, a lithiophilic framework (CC@NFFO) was constructed based on bimetallic NiFe-MOF derivatives (Fe2O3 and NiFe2O4).153 The binding energies of both Fe2O3 and NiFe2O4 were demonstrated to be lower than that of graphitic carbon (−1.63 eV) using DFT calculations (Fig. 12(e and f)), which illustrated the strong lithiophilicity of both of them. Furthermore, since the binding energy of NiFe2O4 (−2.71 eV) is higher than that of Fe2O3 (−3.64 eV), the Li atoms are adsorbed on Fe2O3 prior to the adsorption onto NiFe2O4. Equally, we electrodeposited different amounts of Li at 1 mA cm−2 (Fig. 12(g)) and at an areal capacity of 1 mA h cm−2; as shown in Fig. 12(g1 and g2), there is a dense granular arrangement, demonstrating that Li atoms are preferentially deposited in the Fe2O3 region. Subsequently, Li atoms were deposited in the NiFe2O4 residual zone (Fig. 12(g3 and g4)). It culminated in the uniform Li deposition on the smooth skeleton surface at 8 mA h cm−2 (Fig. 12(g5 and g6)). However, the bare Li electrode morphology (Fig. 12(g7 and g8)) revealed that the disordered nature of Li led to its random deposition on the CC and aggregation. The Ni and Fe metal atoms from NiFe-MOFs are separated by organic ligands, while their derivatives could maintain the structure of the MOFs, and the intermittent distribution of Fe2O3 and NiFe2O4 on the CC facilitates homogeneous deposition (Fig. 12(h)). In contrast, the deposition of Li on the bare CC surface resulted in the growth of Li dendrites. Therefore, the model not only avoids the Li ion accumulation issue, but also maximizes the role of the 3D skeleton and achieves high-capacity maintenance.
What's more, our recent work also found the presence of spherical Li deposition, where the 3D framework consisted of intermittent arrangements of Co and MnO derived from Mn-Co bimetallic MOFs.154 Since the conductivity of Co is higher than that of MnO, it can be hypothesized that electrons conduct faster in Co, and therefore Li atoms preferentially nucleate on the surface of Co, followed by nucleation on MnO. Based on this theory, we mapped the Li deposition mechanism (Fig. 12(i)). In the present study, the nucleation process of Li was observed by in situ OM (Fig. 12(j1–j6)). It was observed that the electrode surface was initially flat; when little metallic Li was deposited, Li ions readily nucleated on the surface of Co due to its good electronic conductivity (10 min, tiny lumps). Subsequently, Li intermittently deposited on adjacent small protruding sites (such as MnO), and then the surface of the electrode became flatter (30 min). Consequently, observation of the Li nucleation process by in situ OM revealed that Li still tends to form smooth spherical circular shapes on the surface. After the final nucleation, a dense and flat Li film was formed on the surface of the electrode, which promoted the Li deposition stably and effectively avoided the growth of dendritic Li. To analyze this behavior further, we performed Li deposition on an electrode at 1 mA cm−2 under various areal capacities and observed the changes in the deposition behavior by SEM. Li was preferentially deposited in a number of rounded blocky areas (Fig. 12(j7)). As the areal capacity increased (Fig. 12(j8)), multiple banded protrusions appeared. Under the situation of 20 mA h cm−2, the electrode surface became smoother without dendrites or interfacial collapse, and the above characterization was in good agreement with the results observed by in situ OM (Fig. 12(j9)).
Hence, these three examples of bimetallic MOF derivatives combined with the 3D conductive frameworks (CC@ZZCO, CC@NFFO and CC@MnO/Co/C) confirmed that obtaining spherical Li on these kinds of substrates is not occasional. And the construction of a lithiophilic 3D framework by introducing bimetallic MOF derivatives not only significantly improves the morphology of deposited Li, but also promotes the homogeneous nucleation of Li, which optimizes the long-term stability and cycling performance of the cell and further improves the overall electrochemical performance.
As known to all, in the history of the development of conductive 3D frameworks for LMAs, primarily two models have been proposed, namely the top growth model with homogeneous lithiophilicity (Fig. 13(a))143,155–157 and the bottom-up growth model with gradient lithiophilicity (Fig. 13(b)).158–162 However, for the top-growth model, Li prefers to deposit on the surface of the frameworks due to the homogeneous lithophilicity, thus predisposing to the growth of dendritic Li (Fig. 11(a)). To overcome this issue, the bottom-up growth model was developed. For this model, the majority of Li nucleation sites were missed from the top of these frameworks, thus possibly preventing the maintenance of high areal capacity (Fig. 13(b)). Based on the above discussions, we proposed a new model to explain the Li deposition behavior, namely the intermittent lithiophilic model (Fig. 13(c)).163 This model is consisting of lithiophilic material A and lithiophilic material B which intermittently located on the surface of skeletons in the nano or micro scale where the lithiophilicity of A is stronger than that of B.152,154,163,164 Since the lithiophilicity of A is superior to B, Li+ preferentially nucleates and grows on A. Meanwhile, the Li+ concentration in the electrolyte near A is reduced, combined with the lithiophilicity of B, which will lead to the following nucleation and growth of Li on B. This approach effectively avoids Li accumulation in localized regions while fully utilizing the 3D conducting framework as a nucleation site for Li.
 | ||
| Fig. 13 Schematic diagram of Li deposition behavior on three different conductive 3D frameworks: (a) homogeneous lithiophilic model; (b) gradient lithiophilic model; and (c) intermittent lithiophilic model.163 | ||
5. Conclusions and outlook
In general, a systematic exploration of the Li deposition process has significantly advanced the development of LMBs. However, to achieve the actual commercialization of LMBs, it is essential to consider the dendrite problem. Spherical Li holds immense potential for advancing highly energy-efficient LMAs. Since neither electrolyte modification nor hosting strategies can completely eliminate dendrites during large-scale practical applications, it is essential to investigate the mechanism for spherical Li deposition so as to seek a new development path to promote LMBs into a higher stage of development. Here, we summarise the key factors affecting spherical Li deposition and the formation mechanism of spherical Li deposition, and conclude by combining the advantages of 3D skeletons to provide inspiration for the future research and development of Li metal batteries.(1) Influencing factors of spherical Li deposition
Electrolyte additives. Electrolyte additives exert distinct influences on the morphology of Li deposition. Specifically, LiNO3 functions as an effective film-forming additive that modifies the structure of deposited Li, promoting its growth into spherical particles. This modification significantly enhances the cycling stability of LMAs. However, in Li-sulfur batteries, LiNO3 alone is insufficient to achieve fully spherical Li deposition; the presence of additional polysulfides, such as Li2S3, is necessary to synergistically facilitate this process. Moreover, introducing oxygen (O2) on the cathode side further promotes the formation of spherical Li.(2) Mechanism of spherical Li formation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sub), the rate of growth (Vgrowth), and the rate of Li nucleation on the Li deposit (Vnuc
sub), the rate of growth (Vgrowth), and the rate of Li nucleation on the Li deposit (Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li). In this case, if Vgrowth exceeds Vnuc
Li). In this case, if Vgrowth exceeds Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sub and Vnuc
sub and Vnuc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li, the deposition of Li nuclei is difficult to observe, and as Li continues to be plated, Li metal grows in all directions, leading to the acquisition of multilayered spherical Li.
Li, the deposition of Li nuclei is difficult to observe, and as Li continues to be plated, Li metal grows in all directions, leading to the acquisition of multilayered spherical Li.
(3) Combining the 3D conductive frameworks and spherical Li
As discussed above, although spherical Li can address the problems of Li dendrites effectively, the volume expansion still needs to be taken into account for host-less Li. Fortunately, the 3D conductive frameworks are used as supplementary materials to prevent the volume expansion. Thus, the combination of the spherical Li and 3D conductive frameworks may be an effective strategy for obtaining LMAs with excellent electrochemical performances.In addition, this work summarises several methods to obtain spherical Li, including the addition of electrolyte additives, adjusting the electrolyte composition, varying the current density or surface capacity (Section 3.2.1) and using bimetallic MOF derivatives (Section 4). However, the formation mechanism of spherical Li is far from being well investigated. Therefore, apart from the diffusion–reaction competition mechanism and the rate of Li nucleation, growth and nucleation on Li deposits (Section 3.2.2), other mechanisms which can be used under multi-factors concomitant conditions should also be studied. On the other hand, understanding of the mechanisms of spherical Li deposition can be supported by more advanced in situ characterisation. Most of the characterisation techniques carried out to explore the LMA fall into two main categories, in situ and ex situ techniques. To date, most characterisation studies of the LMA have been performed by non-in situ techniques or under static conditions. The development of in situ characterisation techniques in real cells under different modes of operation is of great interest and urgency. We fully believe that the combination of these two types of characterisation techniques can facilitate the understanding of the behaviour of spherical Li. On the whole, more efforts should be made to pave the way to the commercialization of LMBs with longer lifespan, higher energy density and safety.
Author contributions
M. T. Wang, X. T. Guo, R. Luo, and X. N. Jiang collected the data, M. T. Wang and X. T. Guo wrote the original manuscript, and Y. F. Tang and T. Wei conceived the idea and revised the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21701083, 22179054) and the Key Technologies R & D Program of Jiangsu Province (BZ2023010).Notes and references
- M. R. Palacín and A. de Guibert, Science, 2016, 351, 1253292 CrossRef PubMed.
- W. Li, H. Yao, K. Yan, G. Zheng, Z. Liang, Y.-M. Chiang and Y. Cui, Nat. Commun., 2015, 6, 7436 CrossRef CAS PubMed.
- Y. Lei, Y. Xie, Y. Huang, Q. Wang, Z. Li, X. Wu, Y. Qiao, P. Dai, L. Huang and Y. Hua, et al. , Chem. Commun., 2021, 57, 10055–10058 RSC.
- S. Wang, L. He, M. Wang, X. Guo, X. Qiu, S. Xu, P. Senin, T. Bian and T. Wei, Particuology, 2024, 93, 203–210 CrossRef CAS.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef CAS.
- Z. Wang, Z. Du, Y. Liu, C. E. Knapp, Y. Dai, J. Li, W. Zhang, R. Chen, F. Guo, W. Zong, X. Gao, J. Zhu, C. Wei and G. He, eScience, 2024, 4, 100189 CrossRef.
- D. Li, S. Zhang, Q. Zhang, P. Kaghazchi, H. Qi, J. Liu, Z. Guo, L. Wang and Y. Wang, Energy Storage Mater., 2020, 26, 593–603 CrossRef.
- C. Wang, S. Liu, H. Xu, X. Wang, G. Tian, F. Fan, P. Liu, S. Wang, C. Zeng and C. Shu, Small, 2024, 20, 2308995 CrossRef CAS PubMed.
- C. Wang, T. Ouyang, X. Wang, S. Liu, G. Tian, F. Fan, P. Liu, S. Wang, C. Zeng and C. Shu, J. Energy Chem., 2024, 99, 384–392 CrossRef CAS.
- C. Wang, S. Liu, X. Wang, G. Tian, F. Fan, P. Liu, S. Wang, C. Zeng and C. Shu, Chem. Commun., 2024, 60, 7045–7048 RSC.
- L. Grande, E. Paillard, J. Hassoun, J. B. Park, Y. J. Lee, Y. K. Sun, S. Passerini and B. Scrosati, Adv. Mater., 2015, 27, 784–800 CrossRef CAS PubMed.
- M. Winter, B. Barnett and K. Xu, Chem. Rev., 2018, 118, 11433–11456 CrossRef CAS PubMed.
- L. Wang, S. Xu, Z. Song, W. Jiang, S. Zhang, X. Jian and F. Hu, InfoMat, 2024, 6, e12551 CrossRef CAS.
- H. Zhang, L. Huang, H. Xu, X. Zhang, Z. Chen, C. Gao, C. Lu, Z. Liu, M. Jiang and G. Cui, eScience, 2022, 2, 201–208 CrossRef.
- Y. Liang, J. Lu, Y. Zhao, R. Chen, X. Guo, M. Wang, S. Wang, Q. Huang, W. Zhao, C. Xu and T. Wei, Ionics, 2024, 30, 709–717 CrossRef CAS.
- T. Wei, Q. Zhang, S. Wang, M. Wang, Y. Liu, C. Sun, Y. Zhou, Q. Huang, X. Qiu and F. Tian, Int. J. Miner., Metall. Mater., 2023, 30, 1897–1905 CrossRef CAS.
- D. Hu, G. Chen, J. Tian, N. Li, L. Chen, Y. Su, T. Song, Y. Lu, D. Cao, S. Chen and F. Wu, J. Energy Chem., 2021, 60, 104–110 CrossRef CAS.
- D. Chen, Y. Liu, C. Xia, Y. Han, Q. Sun, X. Wang, W. Chen, X. Jian, W. Lv, J. Ma and W. He, InfoMat, 2022, 4, e12247 CrossRef CAS.
- H. Xiao, Y. Li, R. Chen, T. Xie, P. Xu, H. Zhu, J. He, W. Zheng and S. Huang, eScience, 2023, 3, 100134 CrossRef.
- S. Wang, L. He, M. Wang, X. Guo, R. Chen, X. Qiu, S. Kudashev, T. Wei and Q. Wang, J. Mater. Sci., 2024, 59, 8650–8668 CrossRef CAS.
- C. Fang, B. Lu, G. Pawar, M. Zhang, D. Cheng, S. Chen, M. Ceja, J.-M. Doux, H. Musrock and M. Cai, et al. , Nat. Energy, 2021, 6, 987–994 CrossRef CAS.
- T. Wei, X. Guo, Y. Zhou, M. Wang, P. Hao, X. Jiang, J. Lu, R. Li, X. Ye and R. Yang, Next Energy, 2025, 7, 100237 CrossRef.
- K. Lee, S.-H. Kwon, J. Kim, E. Park, I. Kim, H. C. Ahn, A. Coskun and J. W. Choi, ACS Energy Lett., 2024, 9, 2201–2211 CrossRef CAS.
- S. Liu, X. Yu, Y. Yan, T. Zeng, X. Wang, G. Tian, C. Wang, S. Wang, Y. Zeng and C. Shu, Energy Storage Mater., 2023, 62, 102959 CrossRef.
- T. Liu, J. Wang, Y. Xu, Y. Zhang and Y. Wang, Nano-Micro Lett., 2021, 13, 170 CrossRef CAS PubMed.
- L. Zheng, F. Guo, T. Kang, J. Yang, Y. Liu, W. Gu, Y. Zhao, H. Lin, Y. Shen, W. Lu and L. Chen, Nano Res., 2020, 13, 1324–1331 CrossRef CAS.
- H. Liu, X. B. Cheng, R. Xu, X. Q. Zhang, C. Yan, J. Q. Huang and Q. Zhang, Adv. Energy Mater., 2019, 9, 1902254 CrossRef CAS.
- H. Xiao, Y. Li, R. Chen, T. Xie, P. Xu, H. Zhu, J. He, W. Zheng and S. Huang, eScience, 2023, 3, 2667 CrossRef.
- D. Zhang, Y. Yin, C. Liu and S. Fan, Chem. Commun., 2015, 51, 322–325 RSC.
- Z. Yu, Q. Yang, W. Xue, J. Shen, J. Zhang, S. Zhu, S. Li and Y. Li, Nanoscale, 2023, 15, 4529–4535 RSC.
- Z. Guo, Q. Zhang, C. Wang, Y. Zhang, S. Dong and G. Cui, Adv. Funct. Mater., 2022, 32, 2108993 CrossRef CAS.
- Q. Wang, C. Yang, J. Yang, K. Wu, C. Hu, J. Lu, W. Liu, X. Sun, J. Qiu and H. Zhou, Adv. Mater., 2019, 31, 1903248 CrossRef CAS PubMed.
- H. Wang, Q. Wang, X. Cao, Y. He, K. Wu, J. Yang, H. Zhou, W. Liu and X. Sun, Adv. Mater., 2020, 32, 2001259 CrossRef CAS PubMed.
- S. Mao, Q. Wu, F. Ma, Y. Zhao, T. Wu and Y. Lu, Chem. Commun., 2021, 57, 840–858 RSC.
- Z. Wang, Z. Du, Y. Liu, C. E. Knapp, Y. Dai, J. Li, W. Zhang, R. Chen, F. Guo and W. Zong, et al. , eScience, 2024, 4, 100189 CrossRef.
- J. Cao, Y. Shi, A. Gao, G. Du, M. Dilxat, Y. Zhang, M. Cai, G. Qian, X. Lu and F. Xie, et al. , Nat. Commun., 2024, 15, 1354 CrossRef CAS PubMed.
- C. Wei, Z. Yao, J. Ruan, Z. Song, A. Zhou, Y. Song, D. Wang, J. Jiang, X. Wang and J. Li, Chin. Chem. Lett., 2024, 35, 109330 CrossRef CAS.
- S. Li, J. Huang, Y. Cui, S. Liu, Z. Chen, W. Huang, C. Li, R. Liu, R. Fu and D. Wu, Nat. Nanotechnol., 2022, 17, 613–621 CrossRef CAS PubMed.
- Y. Cheng, X. Ke and Z. Shi, Acta Metall. Sin., 2020, 34, 354–358 CrossRef.
- J. Pokharel, A. Cresce, B. Pant, M. Y. Yang, A. Gurung, W. He, A. Baniya, B. S. Lamsal, Z. Yang and S. Gent, et al. , Nat. Commun., 2024, 15, 3085 CrossRef CAS PubMed.
- H. Kitaura, E. Hosono and H. Zhou, Energy Environ. Sci., 2021, 14, 4474–4480 RSC.
- Z. Peng, N. Zhao, Z. Zhang, H. Wan, H. Lin, M. Liu, C. Shen, H. He, X. Guo, J.-G. Zhang and D. Wang, Nano Energy, 2017, 39, 662–672 CrossRef CAS.
- W. Tang, X. Yin, S. Kang, Z. Chen, B. Tian, S. L. Teo, X. Wang, X. Chi, K. P. Loh, H. W. Lee and G. W. Zheng, Adv. Mater., 2018, 30, 1801745 CrossRef PubMed.
- R. Xu, F. Liu, Y. Ye, H. Chen, R. R. Yang, Y. Ma, W. Huang, J. Wan and Y. Cui, Adv. Mater., 2021, 33, e2104009 CrossRef PubMed.
- J. Zhang, J. Zhu, R. Zhao, J. Liu, X. Song, N. Xu, Y. Liu, H. Zhang, X. Wan and Y. Ma, et al. , Energy Environ. Sci., 2024, 17, 7119–7128 RSC.
- T. Wei, Z. H. Zhang, Q. Zhang, J. H. Lu, Q. M. Xiong, F. Y. Wang, X. P. Zhou, W. J. Zhao and X. Y. Qiu, Int. J. Miner., Metall. Mater., 2021, 28, 1636–1646 CrossRef CAS.
- Q. Zhang, T. Wei, J. Lu, C. Sun, Y. Zhou, M. Wang, Y. Liu, B. Xiao, X. Qiu and S. Xu, J. Electroanal. Chem., 2022, 926, 116935 Search PubMed.
- P. Qing, Z. Wu, A. Wang, S. Huang, K. Long, T. Naren, D. Chen, P. He, H. Huang and Y. Chen, et al. , Adv. Mater., 2023, 35, e2211203 CrossRef PubMed.
- Z. Yan, J. Liu, Y. Lin, Z. Deng, X. He, J. Ren, P. He, C. Pang, C. Xiao and D. Yang, et al. , Electrochim. Acta, 2021, 390, 138814 CrossRef CAS.
- K. Wu, B. Zhao, C. Yang, Q. Wang, W. Liu and H. Zhou, J. Energy Chem., 2020, 43, 16–23 CrossRef.
- T. Li, H. Zhou, W. Liu, J. Gao, Z. Guo, Z. Su, Y. Yan, S. Su, H. Xie, G. Peng and M. Qu, Chemistry, 2023, 29, e202301991 CrossRef CAS PubMed.
- P. Bai, J. Li, F. R. Brushett and M. Z. Bazant, Energy Environ. Sci., 2016, 9, 3221–3229 RSC.
- Y. Zhang, F. M. Heim, J. L. Bartlett, N. Song, D. Isheim and X. Li, Sci. Adv., 2019, 5, eaav5577 CrossRef CAS PubMed.
- J. Zheng, M. H. Engelhard, D. Mei, S. Jiao, B. J. Polzin, J.-G. Zhang and W. Xu, Nat. Energy, 2017, 2, 17012 CrossRef CAS.
- R. Weber, M. Genovese, A. J. Louli, S. Hames, C. Martin, I. G. Hill and J. R. Dahn, Nat. Energy, 2019, 4, 683–689 CrossRef CAS.
- J. I. Yamaki, S. I. Tobishima, K. Hayashi, K. Saito, Y. Nemoto and M. Arakawa, J. Power Sources, 1998, 74, 219–227 CrossRef CAS.
- F. Shi, A. Pei, A. Vailionis, J. Xie, B. Liu, J. Zhao, Y. Gong and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 12138–12143 CrossRef CAS PubMed.
- A. Pei, G. Zheng, F. Shi, Y. Li and Y. Cui, Nano Lett., 2017, 17, 1132–1139 CrossRef CAS PubMed.
- B. Liu, J.-G. Zhang and W. Xu, Joule, 2018, 2, 833–845 CrossRef CAS.
- Y. Liu, D. Lin, Y. Li, G. Chen, A. Pei, O. Nix, Y. Li and Y. Cui, Nat. Commun., 2018, 9, 3656 CrossRef PubMed.
- X. B. Cheng, R. Zhang, C. Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- 62 Y. Wang, Z. Wang, D. Lei, W. Lv, Q. Zhao, B. Ni, Y. Liu, B. Li, F. Kang and Y. B. He, ACS Appl. Mater. Interfaces, 2018, 10, 20244–20249 CrossRef CAS PubMed.
- Q. He, Z. Li, M. Wu, M. Xie, F. Bu, H. Zhang, R. Yu, L. Mai and Y. Zhao, Adv. Mater., 2023, 35, 2302418 CrossRef CAS PubMed.
- C. Wang, C. Yang, Y. Du, Z. Guo and H. Ye, Adv. Funct. Mater., 2023, 33, 2303427 CrossRef CAS.
- S. Chandrashekar, N. M. Trease, H. J. Chang, L. S. Du, C. P. Grey and A. Jerschow, Nat. Mater., 2012, 11, 311–315 CrossRef CAS PubMed.
- G. Li, Adv. Energy Mater., 2021, 11, 2002891 CrossRef CAS.
- W. Ren, Y. Zheng, Z. Cui, Y. Tao, B. Li and W. Wang, Energy Storage Mater., 2021, 35, 157–168 CrossRef.
- J. Zheng, P. Yan, D. Mei, M. H. Engelhard, S. S. Cartmell, B. J. Polzin, C. Wang, J.-G. Zhang and W. Xu, Adv. Energy Mater., 2016, 6, 1502151 CrossRef.
- S. Rajendran, Z. Tang, A. George, A. Cannon, C. Neumann, A. Sawas, E. Ryan, A. Turchanin and L. M. R. Arava, Adv. Energy Mater., 2021, 11, 2100666 CrossRef CAS.
- H. J. S. Sand, Proc. Phys. Soc. Lond., 1899, 17, 496 CrossRef.
- C. Brissot, M. Rosso, J. N. Chazalviel and S. Lascaud, J. Power Sources, 1999, 81–82, 925–929 CrossRef CAS.
- J. N. Chazalviel, Phys. Rev. A:At., Mol., Opt. Phys., 1990, 42, 7355–7367 CrossRef CAS PubMed.
- X. Chen, X. R. Chen, T. Z. Hou, B. Q. Li, X. B. Cheng, R. Zhang and Q. Zhang, Sci. Adv., 2019, 5, eaau7728 CrossRef CAS PubMed.
- Y. Yan, C. Shu, R. Zheng, M. Li, Z. Ran, M. He, A. Hu, T. Zeng, H. Xu and Y. Zeng, Nano Res., 2022, 15, 3150–3160 CrossRef CAS.
- J. Chazalviel, Phys. Rev. A: At., Mol., Opt. Phys., 1990, 42, 7355–7367 CrossRef CAS PubMed.
- V. Fleury, J. N. Chazalviel, M. Rosso and B. Sapoval, J. Electroanal. Chem. Interfacial Electrochem., 1990, 290, 249–255 CrossRef CAS.
- M. D. Tikekar, S. Choudhury, Z. Tu and L. A. Archer, Nat. Energy, 2016, 1, 16114 CrossRef CAS.
- Q. Yun, Y.-B. He, W. Lv, Y. Zhao, B. Li, F. Kang and Q.-H. Yang, Adv. Mater., 2016, 28, 6932–6939 CrossRef CAS PubMed.
- C.-P. Yang, Y.-X. Yin, S.-F. Zhang, N.-W. Li and Y.-G. Guo, Nat. Commun., 2015, 6, 8058 CrossRef CAS PubMed.
- X. R. Chen, B. C. Zhao, C. Yan and Q. Zhang, Adv. Mater., 2021, 33, e2004128 CrossRef PubMed.
- B. Thirumalraj, T. T. Hagos, C. J. Huang, M. A. Teshager, J. H. Cheng, W. N. Su and B. J. Hwang, J. Am. Chem. Soc., 2019, 141, 18612–18623 CrossRef CAS PubMed.
- D. R. Ely and R. E. García, J. Electrochem. Soc., 2013, 160, A662 CrossRef CAS.
- X. Shen, S. Shi, B. Li, S. Li, H. Zhang, S. Chen, H. Deng, Q. Zhang, J. Zhu and X. Duan, Adv. Funct. Mater., 2022, 32, 2206388 CrossRef CAS.
- Y. Yao, J. Wan, N.-Y. Liang, Y. Chong, R. Wen and Q. Zhang, J. Am. Chem. Soc., 2023, 145, 8001–8006 CrossRef CAS PubMed.
- J. A. Sethian and J. Straint, J. Comput. Phys., 1992, 98, 231–253 CrossRef CAS.
- J. Steiger, G. Richter, M. Wenk, D. Kramer and R. Mönig, Electrochem. Commun., 2015, 50, 11–14 CrossRef CAS.
- J. Steiger, D. Kramer and R. Mönig, J. Power Sources, 2014, 261, 112–119 CrossRef CAS.
- P. Zou, Y. Sui, H. Zhan, C. Wang, H. L. Xin, H.-M. Cheng, F. Kang and C. Yang, Chem. Rev., 2021, 121, 5986–6056 CrossRef CAS PubMed.
- E. Kazyak, K. N. Wood and N. P. Dasgupta, Chem. Mater., 2015, 27, 6457–6462 CrossRef CAS.
- Z. Tu, S. Choudhury, M. J. Zachman, S. Wei, K. Zhang, L. F. Kourkoutis and L. A. Archer, Nat. Energy, 2018, 3, 310–316 CrossRef CAS.
- V. Yurkiv, T. Foroozan, A. Ramasubramanian, R. Shahbazian-Yassar and F. Mashayek, Electrochim. Acta, 2018, 265, 609–619 CrossRef CAS.
- J. Steiger, D. Kramer and R. Mönig, Electrochim. Acta, 2014, 136, 529–536 CrossRef CAS.
- A. Pei, G. Zheng, F. Shi, Y. Li and Y. Cui, Nano Lett., 2017, 17, 1132–1139 CrossRef CAS PubMed.
- F. Orsini, A. du Pasquier, B. Beaudouin, J. M. Tarascon, M. Trentin, N. Langenhuizen, E. de Beer and P. Notten, J. Power Sources, 1999, 81–82, 918–921 CrossRef CAS.
- H. Jing, H. Xing, B. Li and Y. Han, Energy Mater. Adv., 2023, 4, 0018 CrossRef CAS.
- H. Ye, S. Xin, Y.-X. Yin, J.-Y. Li, Y.-G. Guo and L.-J. Wan, J. Am. Chem. Soc., 2017, 139, 5916–5922 CrossRef CAS PubMed.
- Z. Wang, C. Sun, Y. Shi, F. Qi, Q. Wei, X. Li, Z. Sun, B. An and F. Li, J. Power Sources, 2019, 439, 227073 CrossRef CAS.
- M. Zhu, K. Xu, D. Li, T. Xu, W. Sun, Y. Zhu and Y. Qian, ACS Appl. Mater. Interfaces, 2020, 12, 38098–38105 CrossRef CAS PubMed.
- T.-S. Wang, X. Liu, X. Zhao, P. He, C.-W. Nan and L.-Z. Fan, Adv. Funct. Mater., 2020, 30, 2000786 CrossRef CAS.
- Y. Shi, G.-X. Liu, J. Wan, R. Wen and L.-J. Wan, Sci. China Chem., 2021, 64, 734–738 CrossRef CAS.
- Q.-Y. Yang, Z. Yu, Y. Li, W. Zhang, H.-W. Yuan, H.-J. Li, W. Ma, S.-M. Zhu and S. Li, Rare Met., 2022, 41, 2800–2818 CrossRef CAS.
- Y. Zhao, L. Li, D. Zhou, Y. Shan, X. Chen and W. Cui, Energy Environ. Mater., 2023, 6, e12463 CrossRef CAS.
- M. Han, G. Liu, J. Jiang, S. Lu, Y. Jiang, Y. Liu, B. Zhao and J. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 48828–48837 CrossRef CAS PubMed.
- J. Park, J. I. Jung, S. Ha, D. H. Kim, H.-S. Jang, B. H. Kim, H.-K. Lim, H.-J. Jin and Y. S. Yun, Angew. Chem., Int. Ed., 2024, 63, e202409992 CrossRef CAS PubMed.
- C. Wang, H. Wang, L. Tao, X. Wang, P. Cao, F. Lin and H. L. Xin, ACS Energy Lett., 2023, 8, 1929–1935 CrossRef CAS.
- M. Tao, X. Chen, H. Lin, Y. Jin, P. Shan, D. Zhao, M. Gao, Z. Liang and Y. Yang, ACS Nano, 2023, 17, 24104–24114 CrossRef CAS PubMed.
- A. Jana, D. R. Ely and R. E. García, J. Power Sources, 2015, 275, 912–921 CrossRef CAS.
- J. Sun, J. Peng, T. Ring, L. Whittaker-Brooks, J. Zhu, D. Fraggedakis, J. Niu, T. Gao and F. Wang, Energy Environ. Sci., 2022, 15, 5284–5299 RSC.
- Y. Xu, H. Wu, Y. He, Q. Chen, J.-G. Zhang, W. Xu and C. Wang, Nano Lett., 2020, 20, 418–425 CrossRef CAS PubMed.
- A. C. Thenuwara, P. P. Shetty, N. Kondekar, S. E. Sandoval, K. Cavallaro, R. May, C.-T. Yang, L. E. Marbella, Y. Qi and M. T. McDowell, ACS Energy Lett., 2020, 5, 2411–2420 CrossRef CAS.
- W. Fang, Z. Wen, L. Chen, Z. Qin, J. Li, Z. Zheng, Z. Weng, G. Wu, N. Zhang and X. Liu, et al. , Nano Energy, 2022, 104, 107881 CrossRef CAS.
- F. Shi, A. Pei, A. Vailionis, J. Xie, B. Liu, J. Zhao, Y. Gong and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 12138–12143 CrossRef CAS PubMed.
- J. Lu, L. Li, J.-B. Park, Y.-K. Sun, F. Wu and K. Amine, Chem. Rev., 2014, 114, 5611–5640 CrossRef CAS PubMed.
- J. Liu, S. Ihuaenyi, R. Kuphal, J. Salinas, L. Xie, L. Yang, U. Janakiraman, M. E. Fortier and C. Fang, J. Electrochem. Soc., 2023, 170, 010535 CrossRef CAS.
- D. Aurbach, O. Youngman, Y. Gofer and A. Meitav, Electrochim. Acta, 1990, 35, 625–638 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303–4418 CrossRef CAS PubMed.
- Y.-K. Sun, S.-T. Myung, B.-C. Park, J. Prakash, I. Belharouak and K. Amine, Nat. Mater., 2009, 8, 320–324 CrossRef CAS PubMed.
- J. Qian, W. A. Henderson, W. Xu, P. Bhattacharya, M. Engelhard, O. Borodin and J.-G. Zhang, Nat. Commun., 2015, 6, 6362 CrossRef CAS PubMed.
- L. Suo, W. Xue, M. Gobet, S. G. Greenbaum, C. Wang, Y. Chen, W. Yang, Y. Li and J. Li, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 1156–1161 CrossRef CAS PubMed.
- C. Yan, Y.-X. Yao, X. Chen, X.-B. Cheng, X.-Q. Zhang, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2018, 57, 14055–14059 CrossRef CAS PubMed.
- Z. Jin, Y. Liu, H. Xu, T. Chen and C. Wang, Angew. Chem., Int. Ed., 2024, 63, e202318197 CrossRef CAS PubMed.
- Y. Liang, W. Wu, D. Li, H. Wu, C. Gao, Z. Chen, L. Ci and J. Zhang, Adv. Energy Mater., 2022, 12, 2202493 CrossRef CAS.
- S. Liu, J. Xia, W. Zhang, H. Wan, J. Zhang, J. Xu, J. Rao, T. Deng, S. Hou, B. Nan and C. Wang, Angew. Chem., Int. Ed., 2022, 61, e202210522 CrossRef CAS PubMed.
- F. Wang, Z. Wen, Z. Zheng, W. Fang, L. Chen, F. Chen, N. Zhang, X. Liu, R. Ma and G. Chen, Adv. Energy Mater., 2023, 13, 2203830 CrossRef CAS.
- Y. Luo, T. Li, X. Yang, H. Zhang, Z. Jia, J. Yan and X. Li, Adv. Energy Mater., 2022, 12, 2103503 CrossRef CAS.
- S. H. Park and Y. J. Lee, J. Mater. Chem. A, 2021, 9, 1803–1811 RSC.
- K. Dong, Y. Xu, J. Tan, M. Osenberg, F. Sun, Z. Kochovski, D. T. Pham, S. Mei, A. Hilger and E. Ryan, et al. , ACS Energy Lett., 2021, 6, 1719–1728 CrossRef CAS.
- X.-R. Chen, Y.-X. Yao, C. Yan, R. Zhang, X.-B. Cheng and Q. Zhang, Angew. Chem., Int. Ed., 2020, 59, 7743–7747 CrossRef CAS PubMed.
- S. Jo, B. Kwon, J. Oh, J. Lee, K. Park and K. T. Lee, J. Mater. Chem. A, 2022, 10, 5520–5529 RSC.
- G. Jiang, N. Jiang, N. Zheng, X. Chen, J. Mao, G. Ding, Y. Li, F. Sun and Y. Li, Energy Storage Mater., 2019, 23, 181–189 CrossRef.
- Q. Li, J. Zhang, Y. Zeng, Z. Tang, D. Sun, Z. Peng, Y. Tang and H. Wang, Chem. Commun., 2022, 58, 2597–2611 RSC.
- C. P. Yang, Y. X. Yin, S. F. Zhang, N. W. Li and Y. G. Guo, Nat. Commun., 2015, 6, 8058 CrossRef CAS PubMed.
- H. Liu, X. Yue, X. Xing, Q. Yan, J. Huang, V. Petrova, H. Zhou and P. Liu, Energy Storage Mater., 2019, 16, 505–511 CrossRef.
- W. Jia, J. Zhang, L. Zheng, H. Zhou, W. Zou and L. Wang, eScience, 2024, 4, 100266 CrossRef.
- Y. Liu, D. Lin, Z. Liang, J. Zhao, K. Yan and Y. Cui, Nat. Commun., 2016, 7, 10992 CrossRef CAS PubMed.
- D. Lin, Y. Liu, Z. Liang, H. W. Lee, J. Sun, H. Wang, K. Yan, J. Xie and Y. Cui, Nat. Nanotechnol., 2016, 11, 626–632 CrossRef CAS PubMed.
- Z. Liang, D. Lin, J. Zhao, Z. Lu, Y. Liu, C. Liu, Y. Lu, H. Wang, K. Yan, X. Tao and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2862–2867 CrossRef CAS PubMed.
- S. S. Chi, Y. Liu, W. L. Song, L. Z. Fan and Q. Zhang, Adv. Funct. Mater., 2017, 27, 1700348 CrossRef.
- S. Huang, H. Yang, J. Hu, Y. Liu, K. Wang, H. Peng, H. Zhang and L. Z. Fan, Small, 2019, 15, e1904216 CrossRef PubMed.
- C. Yan, X. B. Cheng, Y. Tian, X. Chen, X. Q. Zhang, W. J. Li, J. Q. Huang and Q. Zhang, Adv. Mater., 2018, 30, e1707629 CrossRef PubMed.
- T. Wei, M. Wang, Y. Zhou, X. Guo, S. Wang, Y. Liu, C. Sun and Q. Wang, Chin. J. Chem. Eng., 2024, 71, 13–23 CrossRef CAS.
- Y. Liu, X. Guo, Y. Zhou, M. Wang, C. Sun, S. Xu, X. Qiu, Q. Huang and T. Wei, CrystEngComm, 2024, 26, 681–690 RSC.
- T. Wei, Y. Zhou, C. Sun, L. Liu, S. Wang, M. Wang, Y. Liu, Q. Huang, Q. Zhuang and Y. Tang, Particuology, 2024, 84, 89–97 CrossRef CAS.
- X. Hao, Q. Zhao, S. Su, S. Zhang, J. Ma, L. Shen, Q. Yu, L. Zhao, Y. Liu, F. Kang and Y. B. He, Adv. Energy Mater., 2019, 9, 1901604 CrossRef.
- Z. Huang, G. Zhou, W. Lv, Y. Deng, Y. Zhang, C. Zhang, F. Kang and Q.-H. Yang, Nano Energy, 2019, 61, 47–53 CrossRef CAS.
- M. Cai, Y. Lu, L. Yao, J. Jin and Z. Wen, Chem. Eng. J., 2021, 417, 129158 CrossRef CAS.
- T. Deng, X. Ji, Y. Zhao, L. Cao, S. Li, S. Hwang, C. Luo, P. Wang, H. Jia and X. Fan, et al. , Adv. Mater., 2020, 32, e2000030 CrossRef PubMed.
- F. Zhao, X. Zhou, W. Deng and Z. Liu, Nano Energy, 2019, 62, 55–63 CrossRef CAS.
- T. Wei, J. Lu, M. Wang, C. Sun, Q. Zhang, S. Wang, Y. Zhou, D. Chen and Y. Q. Lan, Chin. J. Chem., 2023, 41, 1861–1874 CrossRef CAS.
- T. Wei, J. Lu, P. Zhang, G. Yang, C. Sun, Y. Zhou, Q. Zhuang and Y. Tang, Chin. Chem. Lett., 2023, 34, 107947 CrossRef CAS.
- Z. Zhuang, F. Zhang, D. Gandla, V. V. Jadhav, Z. Liu, L. Hu, F. Lu and D. Q. Tan, ACS Appl. Mater. Interfaces, 2023, 15, 38530–38539 CrossRef CAS PubMed.
- T. Wei, C. Sun, X. Guo, Y. Zhou, M. Wang, X. Qiu, Q. Wang and Y. Tang, J. Colloid Interface Sci., 2024, 664, 596–606 CrossRef CAS PubMed.
- M. Wang, T. Wei, J. Lu, X. Guo, C. Sun, Y. Zhou, C. Su, S. Chen, Q. Wang and R. Yang, ChemSusChem, 2024, 17, e202400569 CrossRef CAS PubMed.
- C. Sun, J. Lu, X. Guo, Y. Zhou, M. Wang, X. Qiu, Q. Wang, R. Yang and T. Wei, J. Power Sources, 2024, 607, 234597 CrossRef CAS.
- G. Huang, P. Guo, J. Wang, S. Chen, J. Liang, R. Tao, S. Tang, X. Zhang, S. Cheng, Y.-C. Cao and S. Dai, Chem. Eng. J., 2020, 384, 123313 CrossRef CAS.
- J. Sun, Y. Cheng, H. Zhang, X. Yan, Z. Sun, W. Ye, W. Li, M. Zhang, H. Gao and J. Han, et al. , Nano Lett., 2022, 22, 5874–5882 CrossRef CAS PubMed.
- Z. Zhuang, C. Liu, Y. Yan, P. Ma and D. Q. Tan, J. Mater. Chem. A, 2021, 9, 27095–27101 RSC.
- J. Yun, B.-K. Park, E.-S. Won, S. H. Choi, H. C. Kang, J. H. Kim, M.-S. Park and J.-W. Lee, ACS Energy Lett., 2020, 5, 3108–3114 CrossRef CAS.
- Y. Zhao, L. Wang, J. Zou, Q. Ran, L. Li, P. Chen, H. Yu, J. Gao and X. Niu, J. Energy Chem., 2022, 65, 666–673 CrossRef CAS.
- J. Pu, J. Li, K. Zhang, T. Zhang, C. Li, H. Ma, J. Zhu, P. V. Braun, J. Lu and H. Zhang, Nat. Commun., 2019, 10, 1896 CrossRef PubMed.
- K. H. Park, D. W. Kang, J.-W. Park, J.-H. Choi, S.-J. Hong, S. H. Song, S.-M. Lee, J. Moon and B. G. Kim, J. Mater. Chem. A, 2021, 9, 1822–1834 RSC.
- T. Li, S. Gu, L. Chen, L. Zhang, X. Qin, Z. Huang, Y. B. He, W. Lv and F. Kang, Small, 2022, 18, e2203273 CrossRef PubMed.
- T. Wei, J. Lu, P. Zhang, Q. Zhang, G. Yang, R. Yang, D. Chen, Q. Wang and Y. Tang, Chin. Chem. Lett., 2024, 35, 109122 CrossRef CAS.
- T. Wei, Y. Zhou, C. Sun, X. Guo, S. Xu, D. Chen and Y. Tang, Nano Res., 2023, 17, 2763–2769 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. see DOI: https://doi.org/10.1039/d4cc06729k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |
