Open Access Article
Open Access ArticleThree-step click assembly using trivalent platforms bearing azido, ethynyl, and fluorosulfonyl groups†
Takahiro
Yasuda
,
Gaku
Orimoto
and
Suguru
Yoshida
 *
*
Department of Biological Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku Tokyo 125-8585, Japan. E-mail: s-yoshida@rs.tus.ac.jp
First published on 7th January 2025
Abstract
Divergent synthesis of triazoles was achieved using newly designed platform molecules possessing azide, alkyne, and fluorosulfonyl moieties. Consecutive conjugations by the sulfur(VI) fluoride exchange and following consecutive triazole formations allowed us to prepare a wide variety of bis(triazole)s by virtue of selective transformations. One-pot triple-click assembly of easily accessible modules led to the facile synthesis of middle-molecular-weight triazoles with various functional moieties.
Triple click assembly using trivalent platforms has gained attention from a wide range of research fields including pharmaceutical sciences, peptide chemistry, and chemical biology (Fig. 1A).1–4 Although a variety of trivalent platforms have been developed so far, it is not easy to realize efficient click assembly using platforms bearing azide and alkyne moieties due to the challenging selective triazole formation without deprotection steps (Fig. 1B and C).3 Divergent synthesis of highly functionalized molecules using platforms bearing azide and alkyne moieties is awaited. We herein describe new trivalent platforms with azide, alkyne, and fluorosulfonyl moieties, allowing us to accomplish efficient triple-click assembly (Fig. 1D).
 | ||
| Fig. 1 (A) Examples of trivalent platforms. (B) Bis(triazole) synthesis by Kalippan. (C) Tris(triazole) synthesis by Aucagne. (D) This work. | ||
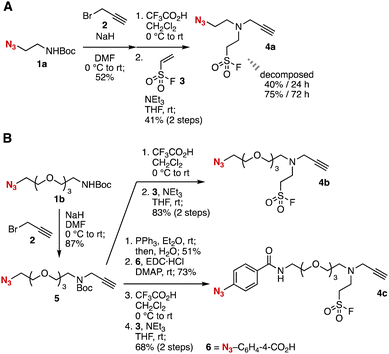
During our studies in click chemistry,4 we planned to develop new trivalent platforms 4 for triple click assembly involving sequential triazole formations (Fig. 2). Considering recent great achievements in triazole formations in terms of efficiency and selectivity,5 a wide variety of bis(triazole)s are expected to be prepared by sequential triazole formations and the sulfur–fluoride exchange (SuFEx) reaction6 using new platforms 4 possessing azide, alkyne, and fluorosulfonyl moieties. Thus, we started synthesizing new trivalent platforms 4a–4c from azides bearing N-Boc amino groups. Synthesis of trivalent platform 4a was achieved from azide 1a through N-propargylation with 2, deprotection of the Boc group, and 1,4-addition to ethenesulfonyl fluoride (3) (Fig. 2A). However, gradual decomposition of trivalent platform 4a was observed after the purification with silica gel probably due to the side reactions involving intramolecular triazole formation.7 In contrast, trivalent platforms 4b and 4c with long linkers were significantly stable under ambient conditions (Fig. 2B). The synthesis of 4b was accomplished from azide 1b by N-propargylation, deprotection, and the introduction of 2-(fluorosulfonyl)ethyl group. We also accomplished the preparation of 4c from 4bvia the reduction of the azido group, condensation with 6, deprotection, and 1,4-addition. Since trivalent platforms 4b and 4c show good stability at room temperature, we found that long linkers prevent decomposition through the intramolecular reaction between the azide and alkyne moiety.
Then, the SuFEx reaction of trivalent platform 4b enabled us to conjugate a range of functionalized alcohols (Fig. 3A).6 For example, treatment of 4b with phenol (7a) in the presence of cesium carbonate in acetonitrile afforded phenyl sulfonate 8a in good yield, in which azide and alkyne moieties were tolerated under the basic conditions. We also succeeded in the synthesis of 8b and 8c in high yields without damaging hydroxy, amino, and methoxycarbonyl groups. Moreover, perfluoroalkyl ester 8d was prepared efficiently by the SuFEx reaction with n-C5F11CH2OH, where side products were not observed through the substitution of the alkyl sulfonate moiety.
 | ||
| Fig. 3 (A) SuFEx reaction of 4b. (B) Transformations of azide 8a. (C) Transformations of alkyne 10a. See the ESI,† for details. | ||
The versatility in the second step was demonstrated by diverse transformations of azide 8a (Fig. 3B). The copper-catalyzed azide–alkyne cycloaddition (CuAAC) with 1-ethynyl-1H-benzimidazole (9a) proceeded smoothly to furnish triazole 10a in good yield leaving the alkyne moiety intact.8 Selective triazole formation was realized due to the higher reactivity of ynamide 9a than that of the ethynyl group of 4b under the CuAAC conditions. Triazole 10b was efficiently synthesized from azide 8a and alkynyl sulfide 9b by the iridium-catalyzed conditions, in which the regioisomer was not detected.9 Triazole formation of azide 8a with dimethyl acetylenedicarboxylate (9c) also occurred at room temperature.10 Cycloalkyne 9d rapidly reacted with azide 8a to furnish triazole 10d in an excellent yield through the strain-promoted azide–alkyne cycloaddition (SPAAC).11,12 Furthermore, the Bertozzi–Staudinger ligation of azide 8a using phosphine 11a allowed us to prepare amide 12 quantitatively.13 Since various selective transformations of azide 8a resulted in the synthesis of 10a–10d and 12 without damaging the alkyne moiety, a wide variety of modules will participate in the click conjugation.
A broad range of third transformations enabled us to synthesize diverse triazoles from alkyne 10a (Fig. 3C). Indeed, CuAAC reaction of alkyne 10a efficiently proceeded with azide 13a to provide bis(triazole) 14a in good yield without damaging amine, triazolylimidazole, and sulfonyl ester moieties.5 We also synthesized amidine 15 from alkyne 10a with tosyl azide (13b) catalyzed by copper iodide.14 Moreover, alkyne 10a efficiently reacted with tetrazine 16 in toluene at 110 °C to afford pyridazines 17 and 17′ in moderate selectivity.15 In contrast, the pyridazine formation did not proceed when conducting the reaction in HFIP at 40 °C according to our previous study. We also achieved the preparation of arylalkyne 19 by the Sonogashira coupling of alkyne 10a and aryl iodide 18. Thus, a wide range of modules can be assembled onto trivalent platform 4b by the SuFEx reaction followed by various transformations such as sequential CuAAC reactions. It is worth noting that consecutive triazole formations were accomplished selectively at the azide and alkyne moieties of trivalent platforms 4, allowing us to construct a vast chemical library from simple modules.
The triple-click assemblies using trivalent platforms 4b and 4c were achieved in a one-pot manner (Fig. 4). For instance, the SuFEx reaction with phenol (7a), SPAAC reaction with cycloalkyne 9d, and CuAAC reaction with benzyl azide (13a) occurred in one-pot when using acetonitrile as a solvent (Fig. 4A). Selective transformations of not only alkyl azide 4b but also aromatic azide 4c16 were realized in a one-pot fashion through the SuFEx and twice triazole formations, where tris(triazole)-type ligand THPTA17 was added in the second triazole formation (Fig. 4B). Thus, the one-pot assembly of functional modules 7, 9, and 13 onto trivalent platforms 4 enabled us to prepare highly functionalized bis(triazole)s. In particular, HaloTag ligand, fluorescent dansyl, and biotin moieties were uneventfully conjugated onto platform 4b in a one-pot manner without damaging various functional groups (Fig. 5).
The assembly order using trivalent platform 4b was switchable (Fig. 6A). First, the CuAAC reaction with picolyl azide 13d took place selectively at the alkyne moiety leaving azide and sulfonyl fluoride moieties untouched.18 Second, the remaining azido group of 21 efficiently reacted with alkyne 9e in the presence of a catalytic amount of copper complex and THPTA without damaging the fluorosulfonyl group. Due to the good functional group tolerance, a wide variety of sulfonyl fluorides would be accessible by the sequential triazole formations.4b,4c,19 Third, we succeeded in the SuFEx reaction of the resulting sulfonyl fluoride 22 to furnish bis(triazole) 14d in an excellent yield.
 | ||
| Fig. 6 (A) Synthesis of 14d. (B) Synthesis of tris(triazole) 23. See the ESI,† for details. | ||
The significant modular synthesis of tris(triazole) 23 was achieved using trivalent platforms 4b and 4c with 7a, 7d, 9a, and 13d (Fig. 6B). Indeed, the SuFEx reaction of 4c with alcohol 7d followed by selective CuAAC reaction with 1-ethynyl-1H-benzimidazole (9a) occurred with the remaining azido group. The resulting alkyne efficiently reacted with an azide prepared by the selective CuAAC reaction of 8a and azide 13d, to provide middle-molecular-weight tris(triazole) 23. Thus, the switchable order of trivalent platforms enabled the click assembly of six modules, serving in the synthesis of highly functionalized molecules by sequential click reactions.
In summary, we have developed divergent synthetic methods for middle-molecular-weight triazoles using newly designed trivalent platforms with azide, alkyne, and fluorosulfonyl moieties. The key to successful selective triazole formations lies in choosing the preferred alkynes or azides under appropriate conditions. One-pot triple-click assembly of easily accessible modules onto the trivalent platforms enabled us to synthesize a broad range of triazoles. Our research group is undertaking further studies such as applications to iterative multi(triazole) synthesis using trivalent platforms.
This work was supported by JSPS KAKENHI Grant Number 23K179200 (S.Y.) and Asahi Glass Foundation (S.Y.).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- For reviews of sequential click reactions, see: (a) D. M. Beal and L. H. Jones, Angew. Chem., Int. Ed., 2012, 51, 6320 CrossRef PubMed; (b) A.-C. Knall and C. Slugovc, Chem. Soc. Rev., 2013, 42, 5131 RSC; (c) Z.-J. Zheng, D. Wang, Z. Xu and L.-W. Xu, Beilstein J. Org. Chem., 2015, 11, 2557 CrossRef PubMed; (d) A. Maruani, D. A. Richards and V. Chudasam, Org. Biomol. Chem., 2016, 14, 6165 RSC; (e) S. Yoshida, Org. Biomol. Chem., 2020, 18, 1550 RSC; (f) D. Sato, Z. Wu, H. Fujita and J. S. Lindsey, Organics, 2021, 2, 161 CrossRef; (g) H. Tanimoto and T. Tomohiro, Chem. Commun., 2024, 60, 12062 RSC.
- For recent examples of sequential click reactions using trivalent platforms, see: (a) V. Vaněk, J. Pícha, B. Fabre, M. Buděšínský, M. Lepšík and J. Jiráček, Eur. J. Org. Chem., 2015, 3689 CrossRef; (b) B. Fabre, J. Pícha, V. Vaněk, M. Buděšínský and J. Jiráček, Molecules, 2015, 20, 19310 CrossRef PubMed; (c) B. Fabre, J. Pícha, V. Vaněk, I. Selicharová, M. Chrudinová, M. Collinsová, L. Žáková, M. Buděšínský and J. Jiráček, ACS Comb. Sci., 2016, 18, 710 CrossRef PubMed; (d) A.-C. Knall, M. Hollauf, R. Saf and C. Slugovc, Org. Biomol. Chem., 2016, 14, 10576 RSC; (e) T. Yokoi, H. Tanimoto, T. Ueda, T. Morimoto and K. Kakiuchi, J. Org. Chem., 2018, 83, 12103 CrossRef PubMed; (f) T. Yokoi, T. Ueda, H. Tanimoto, T. Morimoto and K. Kakiuchi, Chem. Commun., 2019, 55, 1891 RSC; (g) K. Maegawa, H. Tanimoto, S. Onishi, T. Tomohiro, T. Morimoto and K. Kakiuchi, Org. Chem. Front., 2021, 8, 5793 RSC.
- (a) K. P. Kaliappan, P. Kalanidhi and S. Mahapatra, Synlett, 2009, 2162 CrossRef; (b) I. E. Valverde, A. F. Delmas and V. Aucagne, Tetrahedron, 2009, 65, 7597 CrossRef; (c) H. S. G. Beckmann, F. Nie, C. E. Hagerman, H. Johansson, Y. S. Tan, D. Wilcke and D. R. Spring, Nat. Chem., 2013, 5, 861 CrossRef PubMed; (d) M.-L. Winz, E. C. Linder, J. Becker and A. Jäschke, Chem. Commun., 2018, 54, 11781 RSC; (e) K. Hema and K. M. Sureshan, Acc. Chem. Res., 2019, 52, 3149 CrossRef PubMed.
- For examples of recent our studies on sequential click reactions using trivalent platforms, see: (a) H. Takemura, S. Goto, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 15541 RSC; (b) H. Takemura, G. Orimoto, A. Kobayashi, T. Hosoya and S. Yoshida, Org. Biomol. Chem., 2022, 20, 6007 RSC; (c) G. Orimoto and S. Yoshida, Chem. Commun., 2024, 60, 4545 RSC; (d) M. Hamada, G. Orimoto and S. Yoshida, Chem. Commun., 2024, 60, 7930 RSC.
- A. K. Agrahari, P. Bose, M. K. Jaiswal, S. Rajkhowa, A. S. Singh, S. Hotha, N. Mishra and V. K. Tiwari, Chem. Rev., 2021, 121, 7638 CrossRef CAS PubMed.
- For reviews of the SuFEx chemistry, see: (a) J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9430 CrossRef CAS; (b) A. S. Barrow, C. J. Smedley, Q. Zheng, S. Li, J. Dongd and J. E. Moses, Chem. Soc. Rev., 2019, 48, 4731 RSC; (c) Y.-P. Meng, S.-M. Wang, W.-Y. Fang, Z.-Z. Xie, J. Leng, H. Alsulami and H.-L. Qin, Synthesis, 2020, 673 Search PubMed.
- Intramolecular reactions between azides and alkynes, see: (a) R. Li, D. J. Jansen and A. Datta, Org. Biomol. Chem., 2009, 7, 1921 RSC; (b) Z. Shan, M. Peng, H. Fan, Q. Lu, P. Lu, C. Zhao and Y. Chen, Bioorg. Med. Chem. Lett., 2011, 21, 1731 CrossRef CAS PubMed.
- (a) M. Z. C. Hatit, J. C. Sadler, L. A. McLean, B. C. Whitehurst, C. P. Seath, L. D. Humphreys, R. J. Young, A. J. B. Watson and G. A. Burley, Org. Lett., 2016, 18, 1694 CrossRef CAS PubMed; (b) M. Z. C. Hatit, C. P. Seath, A. J. B. Watson and G. A. Burley, J. Org. Chem., 2017, 82, 5461 CrossRef CAS; (c) F. Peschke, A. Taladriz-Sender, M. J. Andrews, A. J. B. Watson and G. A. Burley, Angew. Chem., Int. Ed., 2023, 62, e202313063 CrossRef CAS; (d) R. P. Bunschoten, F. Peschke, A. Taladriz-Sender, E. Alexander, M. J. Andrews, A. R. Kennedy, N. J. Fazakerley, G. C. L. Jones, A. J. B. Watson and G. A. Burley, J. Am. Chem. Soc., 2024, 146, 13558 CrossRef CAS PubMed; (e) F. Peschke, A. Taladriz-Sender, A. J. B. Watson and G. A. Burley, Bioconjugate Chem., 2024, 35, 1788 CrossRef CAS.
- S. Ding, G. Jia and J. Sun, Angew. Chem., Int. Ed., 2014, 53, 1877 CrossRef CAS PubMed.
- S. K. Khetan and M. V. George, Can. J. Chem., 1967, 45, 1993 CrossRef CAS.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422 CrossRef CAS.
- J. C. Jewett and C. R. Bertozzi, Chem. Soc. Rev., 2010, 39, 1272 RSC.
- E. Saxon and C. R. Bertozzi, Science, 2000, 287, 2007 CrossRef CAS.
- D. P. Chauhan, S. J. Varma, A. Vijeta, P. Banerjee and P. Talukdar, Chem. Commun., 2014, 50, 323 RSC.
- (a) C. Yamamoto, M. Suzuki and S. Yoshida, Bull. Chem. Soc. Jpn., 2022, 95, 1741 CrossRef CAS; (b) C. Yamamoto, K. Numata, M. Suzuki and S. Yoshida, Org. Chem. Front., 2024, 11, 6159 RSC.
- Since aromatic azides also serve in various triazole formations and show unique selectivity in base-catalyzed 1,5-triazole formation, a wide range of triazoles would be accessible using trivalent platform 4c. See, ref. 1e. For an example of a base-catalyzed reaction, see: S. W. Kwok, J. R. Fotsing, R. J. Fraser, V. O. Rodionov and V. V. Fokin, Org. Lett., 2010, 12, 4217 CrossRef CAS.
- (a) T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Org. Lett., 2004, 6, 2853 CrossRef CAS; (b) X.-M. Liu, A. Thakur and D. Wang, Biomacromolecules, 2007, 8, 2653 CrossRef CAS.
- (a) G.-C. Kuang, H. A. Michaels, J. T. Simmons, R. J. Clark and L. Zhu, J. Org. Chem., 2010, 75, 6540 CrossRef PubMed; (b) Z. Yuan, G.-C. Kuang, R. J. Clark and L. Zhu, Org. Lett., 2012, 14, 2590 CrossRef.
- (a) X. Zhang, B. Moku, J. Leng, K. P. Rakesh and H.-L. Qin, Eur. J. Org. Chem., 2019, 1763 CrossRef; (b) Z. Li, X. Ren, P. Sun, H. Ding, S. Li, Y. Zhao and K. Zhang, ACS Macro Lett., 2021, 10, 223 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization for new compounds including NMR spectra. See DOI: https://doi.org/10.1039/d4cc06585a |
| This journal is © The Royal Society of Chemistry 2025 |