Open Access Article
Open Access ArticleIsolation and oxygen activation of electron-rich CoII4O metallic clusters having a 3-fold symmetry†
Maria
Francis
 a,
Asutosh
Patra
a,
Asutosh
Patra
 a,
Farsana Abdul
Salam
a,
Farsana Abdul
Salam
 a,
Liviu
Ungur
a,
Liviu
Ungur
 b,
Björn
Schwarz
b,
Björn
Schwarz
 *c and
Sudipta
Roy
*c and
Sudipta
Roy
 *a
*a
aDepartment of Chemistry, Indian Institute of Science Education and Research (IISER) Tirupati, Tirupati – 517619, India. E-mail: roy.sudipta@iisertiruapti.ac.in
bDepartment of Chemistry, National University of Singapore, Singapore, Singapore
cInstitute for Applied Materials (IAM), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, Karlsruhe, D-76344, Germany. E-mail: bjoern.schwarz@kit.edu
First published on 2nd April 2025
Abstract
Two novel atomically precise tetra-nuclear cobalt clusters (3a–3b) having a CoII4O core with 3-fold symmetry, and three short Co–Co distances of 2.7046(4) Å are isolated with three chelating NP-donor mono-anionic ligands. 3a–3b are shown to react with O2, producing dinuclear cobalt complexes (4a–4b), where the P-centres of the ligands are oxygenated. All complexes are structurally characterized by single-crystal X-ray diffraction, and further studied by UV-vis, IR spectroscopy, CV, magnetic susceptibility measurements, and DFT calculations.
Metallic cobalt-clusters [Con/ConO] are known to exhibit numerous applications. Cobalt-carbonyls and their polynuclear analogues (Co4, Co10, etc.) have attracted chemists for their structural aspects, bonding, and unusually higher stability.1 Co2(CO)8 is known for hydroformylation of olefins.1 The stability, and bonding of metallic Con clusters (n = 4, 13) on the graphene surface have been studied by DFT calculation.2 The Co13 cluster is known to absorb CO gas.2d The Co4 coordination cluster is found to be effective in boosting the oxygen reduction of Fe−N−C catalysts with single-atom Fe–N4 configurations.3 Adsorption of H2 on cobalt clusters Co6 and Co13 has been studied by DFT calculations.4 A hydrocarbon-soluble CoI4 nano-catalyst having the spin crossover property5 is known to display excellent catalytic hydrogenation of unsaturated hydrocarbons at low temperature/pressure,6 while the corresponding cation exhibited slow relaxation of magnetization having S = 9/2 spin ground state.5 A Co4 cluster on a graphdiyne surface acts as a catalyst for electro-chemical N2 reduction producing ammonia.7 Con2a–c,8a,b (n = 4, 6, 13), and ConO8c clusters (n = 2, 3, 11) have been deposited on surfaces, followed by utilization in hydrogenation, and oxygenation reactions.8 Decomposition of N2O/NO on Con clusters has been theoretically investigated.9 Co-clusters confined in mesoporous silica nanospheres containing N-doped carbon have been shown to efficiently dissociate the O–O bond of peroxymonosulphate, producing HO˙, and SO4˙− radicals.10 The sub-nanocluster Co4-catalysts deposited on silanol nests containing both ionic Co–O, and metallic Co–Co bonds are known for efficient dehydrogenation of propane.11
Herein, we report on the solid-state isolation of highly air, and moisture-sensitive structurally well-defined organic solvent-soluble tetranuclear CoII4O metal-clusters 3a–3b, and the corresponding oxygen-activated dimeric CoII2 complexes 4a–4b.
A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar mixture of the orange crystals of Cs-(Dipp)-cyclic alkyl(imino) phosphide (1a),12 and anhydrous CoCl2 was stirred in THF at rt for 12 h under an argon atmosphere. Upon filtration, the insoluble black precipitate was separated, and the concentrated dark bluish-black filtrate was stored at −40 °C in a freezer. After 3–4 weeks, dark bluish-black hexagonal crystals of [((Dipp)(Et2-cAI)P(CoCl))3(Co)O] (3a) were obtained in 35% yield (Scheme 1).
2 molar mixture of the orange crystals of Cs-(Dipp)-cyclic alkyl(imino) phosphide (1a),12 and anhydrous CoCl2 was stirred in THF at rt for 12 h under an argon atmosphere. Upon filtration, the insoluble black precipitate was separated, and the concentrated dark bluish-black filtrate was stored at −40 °C in a freezer. After 3–4 weeks, dark bluish-black hexagonal crystals of [((Dipp)(Et2-cAI)P(CoCl))3(Co)O] (3a) were obtained in 35% yield (Scheme 1).
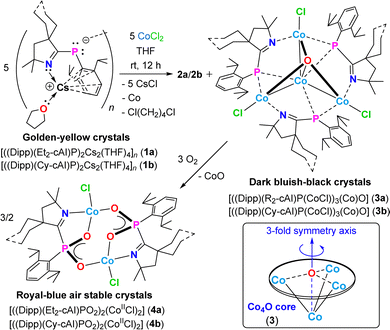 | ||
| Scheme 1 Syntheses of CoII4O clusters 3a–3b [Co1−Co2 2.7046(4)], and their reactivity with O2 affording dimeric CoII2 complexes 4a–4b. 2a–2b are the respective bis-(Dipp)(imino)-phosphene by-products, isolated separately as white needles (see ESI†). | ||
Colorless, manually separable needles of bis-(Dipp)(imino)-phosphene (2a) were produced after 2–3 days as the by-product in 29% isolated yield. When a similar reaction mixture as stated above with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of 1a and anhydrous CoCl2, following 12 h of stirring at rt, was exposed to air for 15 min, bright blue blocks of the oxygen activated dimeric CoII2 complex [((Dipp)(Et2-cAI)PO2)2(CoIICl)2] (4a) were isolated in 30% yield after 2–3 days of storing the concentrated reaction mixture at −40 °C in a freezer (Scheme 1). A 1
2 molar ratio of 1a and anhydrous CoCl2, following 12 h of stirring at rt, was exposed to air for 15 min, bright blue blocks of the oxygen activated dimeric CoII2 complex [((Dipp)(Et2-cAI)PO2)2(CoIICl)2] (4a) were isolated in 30% yield after 2–3 days of storing the concentrated reaction mixture at −40 °C in a freezer (Scheme 1). A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of 1b, and anhydrous CoCl2 in THF at rt afforded highly crystalline tetranuclear CoII4 metal cluster [((Dipp)(Cy-cAI)P(CoCl))3(Co)O] (3b) in 39% isolated yield (Scheme 1). Several attempts failed to produce good quality single crystals of 3b, suitable for X-ray diffraction. Exposure of the above-mentioned reaction mixture to air afforded air-stable royal blue blocks of the CoII2 complex [((Dipp)(Cy-cAI)PO2)2(CoIICl)2] (4b) from a freezer at −40 °C in 30% yield. The purity of the isolated crystals of 3a–3b, and 4a–4b has been confirmed by elemental analyses (see ESI†).
2 molar ratio of 1b, and anhydrous CoCl2 in THF at rt afforded highly crystalline tetranuclear CoII4 metal cluster [((Dipp)(Cy-cAI)P(CoCl))3(Co)O] (3b) in 39% isolated yield (Scheme 1). Several attempts failed to produce good quality single crystals of 3b, suitable for X-ray diffraction. Exposure of the above-mentioned reaction mixture to air afforded air-stable royal blue blocks of the CoII2 complex [((Dipp)(Cy-cAI)PO2)2(CoIICl)2] (4b) from a freezer at −40 °C in 30% yield. The purity of the isolated crystals of 3a–3b, and 4a–4b has been confirmed by elemental analyses (see ESI†).
The CoII4 clusters 3a–3b were highly air and moisture-sensitive. 3a–3b were highly soluble in polar solvents, such as THF and DCM. The crystalline solids, and THF and/or DCM solutions of 3a–3b were stable at rt under an argon atmosphere for more than 6 months. The powders of 3a–3b were melted to yellow liquids at 209–210, and 211–212 °C, respectively. The dimeric CoII2 complexes 4a–4b were air stable for a week, and sparingly soluble in THF. The powders of 4a–4b were melted to yellow liquids under an argon atmosphere at 175–177, and 174–176 °C, respectively.
3a–3b have been characterized by IR, and UV-vis spectroscopy (see ESI†). The UV-vis absorption spectrum of the DCM solution of 3a showed broad absorption bands with the corresponding maxima at 596 nm, and 597 nm with molar absorption coefficient (ε) values of 4862.38 and 229.35 M−1 cm−1, respectively. Whereas, 4a exhibited the absorption maxima at 289, 589, and 657 nm with ε values of 3283.01, 113.20, and 273.58 M−1 cm−1, respectively (see ESI†). The computed IRP−O stretching frequencies for the PO2− moiety in the model complex 4b′ were found to be 1067.16 cm−1, and 1213.93 cm−1 for the symmetric and asymmetric modes, respectively, which were comparable with the experimental IRP−O stretching frequencies observed for 4a–4b (1070, 1210 cm−1 for 4a; 1084, 1259 cm−1 for 4b). These frequencies can be well compared with the reported stretching frequencies observed in L:(O)2P–P(O)2:L (L:=:C{N(2,6-Pri2C6H3)CH}2) (1279 cm−1, 1061 cm−1),13 [(PO2){Re(PyrPz)(PNP)}] (1263 cm−1, 1086 cm−1),14 and the reported data for the free PO2− anion in a KCl matrix (1097 cm−1, 1207 cm−1).15
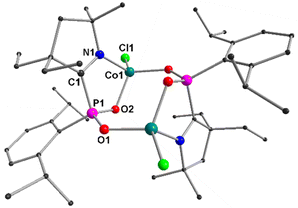
3a and 4a–4b have been structurally characterized by single-crystal X-ray diffraction (Fig. 1 and 2, see ESI†). 3a crystallizes in the trigonal R3c space group with a 3-fold rotational axis of symmetry passing through the Co2–O1 bond (2.036(2) Å) (Fig. 1). The structural feature of 3a can be correlated with a stemless mushroom possessing three CoII ions (three Co1 centres; Co–O distance of 1.9589(3) Å) in the periphery and a CoII centre (Co2) at the top. All four CoII atoms are connected through the μ4-O atom, which lies 0.16 Å above the plane of the three peripheral CoII ions (Co1), whereas the central Co2 atom is 1.87 Å below this plane. The Co2 atom is also 0.09 Å away from the plane containing three P1 atoms of the three (Dipp)-cyclic alkyl(imino) phosphide [(Dipp)(cAI)P−] ligands. The Co1–Co2 distance in 3a is found to be 2.7046(3) Å, which is significantly longer than that of the Co–Co distances present in the previously reported complexes [(IMe4)2M(μ-PMes)]2 (M = Co) (2.5241(9) Å),16 and triply μ-Oalkoxy bridged CoIII-complex [[CoIII2(hep)3(N3)3]·DMF] (2.595(6) Å),17 but slightly longer than that of (Me2-cAAC:)2Co2 (2.6550(6) Å).18 The Co1–P1, and Co2–P1 bond distances in 3a are found to be 2.4171(5) Å, and 2.2808(4) Å, respectively, in which the former one is comparable with that of the previously reported [Co{PH(IDipp)}{N(SiMe3)2}2] complex (2.4572(8) Å).19
The P1–C10 bond distance in 3a is found to be 1.8397(17) Å, which is typical of a P–C single bond. The N1–C10 bond length in complex 3a is found to be 1.296(2) Å, which is comparable to that of complex 1a (1.301(3) Å).12
The molecular structure of 4a has been depicted in Fig. 2 (see ESI† for 4b). 4a crystallized in the monoclinic space group P21/n. The asymmetric unit of 4a is composed of the monomeric unit [((Dipp)(cAI)PO2)CoIICl]. The two CoII ions in 4a are bridged by the two μ1,3-Dipp-PO2− moieties of the two PO2− ligands through two O atoms of the PO2 unit [syn–anti-bridging mode; PO2CoII2] displaying the η1:η1:η1:μ2 bridging mode by the PO2N donor set of the ligand. Each CoII ion of 4a is coordinated by one N atom of the [(Dipp)(cAI)P−] ligand, while two O atoms of this ligand act as a cis-, anti-bridge between two CoII ions. Finally, one terminal Cl atom completes the distorted tetrahedral geometry of each CoII centre. The bond distances between Co1–O1/O2 are 1.941(2) Å, and 1.993(3) Å, respectively, where the former one is much shorter than the Coperipheral–O distance (1.9589(3) Å), whereas, the latter one is comparable with the Coapex–O distance (2.036(2) Å) observed in 3a. The P–C bond distances [(1.843(4) Å] in 4a correspond to the typical P–C single bond [P–C bond distance in NHC: → PCl3 adduct is reported as 1.871(11) Å)], and are comparable to that of 3a (1.8397(17) Å),20 and slightly shorter than that of 2a (1.8550(12) Å). The two P–O bond distances in 4a are found to be almost identical (1.512(3) Å, 1.514(3) Å) representing the delocalization, which is also visible from the molecular orbitals in α-SOMO−58 and β-SOMO−52 (see ESI†). These bond lengths are slightly longer than the P–O distances found in NHC2(PO2)2 (1.470(2)), (1.466(3) Å).20 The distances between the two Co ions in 4a and 4b are found to be 4.247 and 4.40 Å, respectively, which are significantly longer than that in 3a (2.7046(4) Å).
The electron paramagnetic resonance (EPR) spectrum of 3a in DCM at 77 K exhibited a broad signal presumably due to the distorted tetrahedral geometry of Co1 (side), and Co2 (central) ions. The broadening of the EPR signal can be rationalized by the positive D values (−14.4, +32.4 cm−1), and the rhombic nature of the Co(II) ions (see ESI†). The Mulliken spin density calculations (computed at UB3LYP-D3(BJ)/def2-TZVP level of theory) showed that the majority of the α-spin density of the model complex 3a′ (replacing Dipp groups by Me groups) is delocalized across the Co atoms (31.0%) located in the periphery, along with contributions from the central O atom (2.0%) and Cl atoms (1.2%) (Fig. 3).
 | ||
| Fig. 3 Mulliken α-(blue), β-spin (green) densities of the model complex [((Me)(Et2-cAI)P(CoCl))3O] (3a′) at S = 3 (computed at the UB3LYP-D3(BJ)/def2-TZVP level of theory). | ||
In contrast, the β-spin density is primarily distributed over the Co (axial) and P atoms, with the largest contribution coming from the Co atom (95.0%).
The temperature-dependent magnetic susceptibility measurements performed on 3a within the temperature range of 2–300 K revealed that the experimentally observed χT susceptibility vs. temperature values are slightly above the calculated molar Curie constant C4Co(II) = 7.50 cm3 K mol−1 (dashed line in Fig. 4(a)) for four free high spin CoII ions, each with spin S = 3/2 within a formula unit (f.u.), and without any interactions between each other. The slightly increased value is ascribed to weak dominant ferromagnetic (FM) interaction as already indicated by the positive Weiss constant obtained from the Curie–Weiss fit (see ESI†).
For the magnetic model, a total spin S = 3/2 was ascribed to each Co site. The three Co1 (side) are exchange coupled via the isotropic exchange constant J1 with each other, and each Co1 is coupled via J2 to the central Co2 ion. Utilizing the program PHI,21 isotropic exchange parameters were refined to J1 = 18.52(5) cm−1, and J2 = −15.15(5) cm−1, i.e. with a slightly dominant FM exchange interaction as also found by Curie–Weiss fit. Furthermore, the ZFS parameters (only the axial D parameter has been used for the applied model here) were refined to D(Co1) = −14.4(1) cm−1, and D(Co2) = 32.4(1) cm−1. The antiferromagnetic coupling between Co1 (side) and Co2 (central) creates a ground state with reduced magnetic moment due to the mutual partial cancelling out, and furthermore, the ZFS reduces the measured magnetization, especially at low temperature due to the single-ion anisotropy that forces the magnetic moments to be aligned along the statistically distributed (polycrystalline sample) uniaxial anisotropy axes (D).22 The spin ground state of the previously reported (NHC)4CoII4S4 cluster with Co–Co distances of ∼2.69 Å was found to be S = 3 (see ESI†).23
The natural bond orbital (NBO) analysis performed on model complex 3a′ (calculated at UB3LYP/def2-TZVP level of theory) in the septet state revealed that the α-SOMO corresponds to the delocalization of electrons on Co and O on the axial position, and P atoms on the periphery (Fig. 5). The α-SOMO−1 corresponds to the 3-centered electron delocalization over the Coperiphery–P–Coaxial moiety, where the major coefficient resides on the p-orbital of P (75.6%). The α-SOMO−5 corresponds to the σ-electron donation from O atom (83.5%) to the Co present at the axial position.
 | ||
| Fig. 5 Selected Kohn–Sham orbitals of the model complex [((Me)(Et2-cAI)P(CoCl))3O] (3a′) at the S = 3 (at UB3LYP-D3(BJ)/def2-TZVP level of theory; energies given in parentheses are in eV). | ||
The EPR spectrum of 4a–4b in DCM at 298 K exhibited the signal with a geff value of 2.0053 and 2.0829, respectively (see ESI†). The α-spin density of 4b is predominantly found on the Co ions (45.6%), where the unpaired electron exists with only minimal contributions from O (0.69%, 0.62%), P (0.3%), and the ligand (C 0.1%; N 0.6%) (see ESI†).
The DC magnetic susceptibility measurements of 4b showed that the magnetic momentum per Co-ion is significantly higher than that of 3a (see ESI†). The χT product is 7.65 cm3 K mol−1 at 300 K, which slowly decreases to 6.77 cm3 K mol−1 at 28 K due to the spin–orbit-coupling of CoII ions (see ESI†).
In conclusion, we have developed a novel strategy for solid state isolation of the highly moisture, and oxygen-sensitive structurally well-defined CoII4 metal clusters, 3a–3b with the Co4O core by reacting the Cs-salts 1a–1b with anhydrous CoCl2 at rt under an argon atmosphere. The Mulliken spin density calculations on 3a′ revealed delocalized α-spin density across the peripheral Co ions with smaller contributions from central O and Cl atoms. 3a–3b were successfully utilized for the activation of aerial O2 affording air-stable dimeric CoII complexes 4a–4b.
SR gratefully acknowledges STARS-IISC-MoE, (MoE-STARS/STARS-2/2023-0666), and IISER Tirupati for financial support. We acknowledge Subuhan Ahamed (IIT Madras) for generating the Hirshfeld plots, and EPR simulation.
Data availability
The data supporting this article (syntheses, UV-Vis, EPR, XPS, single-crystal X-ray data, magnetic data, and computational details) are included as part of the ESI.† Crystallographic data for 2a–2b, 3a, and 4a–4b have been deposited at the CCDC (2324725–2324727, 2344451 and 2344452†), which can be obtained from https://www.ccdc.cam.ac.uk/.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) L. P. Battaglia, D. Delledonne, M. Nardelli, G. Predieri, G. P. Chiusoli, M. Costa and C. Pelizzi, J. Organomet. Chem., 1898, 363, 209 CrossRef; (b) C. Xia, K. Yang, S. G. Bott and M. G. Richmond, Organometallics, 1996, 15, 4480 CrossRef CAS; (c) M. Costa, G. Gervasio, D. Marabello and E. Sappa, J. Organomet. Chem., 2002, 656, 57 CrossRef CAS; (d) F. Mafuné, Y. Wu, M. Yamaguchi and S. Kudoh, J. Phys. Chem. A, 2024, 128, 3516 CrossRef PubMed.
- (a) L. Liu, Y. Su, J. Gao and J. Zhao, Physica E, 2012, 46, 11 Search PubMed; (b) T. Alonso-Lanza, A. Ayuela and F. Aguilera-Granja, ChemPhysChem, 2015, 16, 3700 CrossRef CAS PubMed; (c) K. García-Díez, J. Fernández-Fernández, J. A. Alonso and M. J. López, Phys. Chem. Chem. Phys., 2018, 20, 21163 RSC; (d) Y. Wang, L. Wang and S. Ma, Appl. Surf. Sci., 2019, 481, 1080 CrossRef CAS.
- A. Han, W. Sun, X. Wan, D. Cai, X. Wang, F. Li, J. Shui and D. Wang, Angew. Chem., Int. Ed., 2023, 62, e202303185 Search PubMed.
- K. García-Díez, J. Fernández-Fernández, J. A. Alonso and M. J. López, Phys. Chem. Chem. Phys., 2018, 20, 21163 Search PubMed.
- K. Chakarawet, P. C. Bunting and J. R. Long, J. Am. Chem. Soc., 2018, 140, 2058 CrossRef CAS PubMed.
- J. Camacho-Bunquin, M. J. Ferguson and J. M. Stryker, J. Am. Chem. Soc., 2013, 135, 5537 CrossRef CAS.
- Y. Luo, M. Li, Y. Dai, R. Zhao, F. Jiang, S. Wang and Y. Huang, Inorg. Chem., 2021, 60, 18251 CrossRef CAS.
- (a) J. L. Rodríguez-López, F. Aguilera-Granja, K. Michaelian and A. Vega, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 174413 CrossRef; (b) F. Mafuné, Y. Wu, M. Yamaguchi and S. Kudoh, J. Phys. Chem. A, 2024, 128, 3516 CrossRef; (c) S. Lee, A. Halder, G. A. Ferguson, S. Seifert, R. E. Winans, D. Teschner, R. Schlögl, V. Papaefthimiou, J. Greeley, L. A. Curtiss and S. Vajda, Nat. Commun., 2019, 10, 954 CrossRef.
- J. Facio-Muñoz, D. Hernández-Velázquez, G. Guzmán-Ramírez, R. Flores-Moreno, J. Rodriguez-Zavala and F. Tenorio, J. Mol. Model., 2022, 28, 197 CrossRef PubMed.
- X. Xie, M. Zhu, F. Xiao, Y. Xiang, H. Zhong, Z. Ao and H. Huang, JACS Au, 2023, 3, 1496 CrossRef CAS PubMed.
- W. Deng, D. He, D. Chen, Z. Huang, J. Deng and Y. Luo, Commun. Mater., 2024, 5, 215 CrossRef CAS.
- E. Nag, M. Francis, D. Putta and S. Roy, Chem. – Eur. J., 2023, e202302120 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, P. Wie, H. F. Schaefer III, P. V. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2013, 135, 19139 CrossRef CAS PubMed.
- J. Abbenseth, F. Wätjen, M. Finger and S. Schneider, Angew. Chem., Int. Ed., 2020, 59, 23574 CrossRef CAS PubMed.
- S. J. Hunter, K. W. Hipps and A. H. Francis, Chem. Phys., 1979, 39, 209 CrossRef CAS.
- K. Pal, O. B. Hemming, B. M. Day, T. Pugh, D. J. Evans and R. A. Layfield, Angew. Chem., Int. Ed., 2016, 55, 1690 CrossRef CAS PubMed.
- J. Kumar, N. S. M. Gorantla, S. Roy, A. N. Paesch, R. Herbst-Irmer, D. Stalke, C. Anusha, S. De, P. Parameswaran, H. W. Roesky and K. C. Mondal, ChemistrySelect, 2018, 3, 8221 CrossRef CAS.
- K. C. Mondal, P. P. Samuel, H. W. Roesky, E. Carl, R. HerbstIrmer, D. Stalke, B. Schwederski, W. Kaim, L. Ungur, L. F. Chibotaru, M. Hermann and G. Frenking, J. Am. Chem. Soc., 2014, 136, 1770 CrossRef CAS PubMed.
- R. Weller, A. Gonzalez, H. Gottschling, C. von Hänisch and W. C. Gunnar, Z. Anorg. Allg. Chem., 2022, 648, e202100338 CrossRef CAS.
- Y. Wang, Y. Xie, M. Y. Abraham, R. J. Jr, P. Wei, H. F. III Paul and G. H. Robinson, Organometallics, 2010, 29, 4778 CrossRef CAS.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef CAS PubMed.
- B. Schwarz and Q. Fu, Eur. J. Inorg. Chem., 2024, e202400162 CrossRef CAS.
- L. Deng, E. Bill, K. Wieghardt and R. H. Holm, J. Am. Chem. Soc., 2009, 131, 11213 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC: 2324725 (2a), 2324727 (3a), 2324726 (4a), 2344451 (2b), 2344452 (4b). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc06550f |
| This journal is © The Royal Society of Chemistry 2025 |