Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of fully fused tetrapyrazinoporphyrazine polymers bearing three-dimensional structures controlled by steric repulsion†
Kosuke
Watanabe
 ab,
Teruki
Toya
c,
Yuto
Toyota
c,
Yoichi
Kobayashi
ab,
Teruki
Toya
c,
Yuto
Toyota
c,
Yoichi
Kobayashi
 c,
Junichi
Usuba
d,
Yuh
Hijikata
c,
Junichi
Usuba
d,
Yuh
Hijikata
 d,
Ryotaro
Matsuda
de,
Katsuyuki
Nishimura
ab,
Haruki
Sugiyama
abf and
Yasutomo
Segawa
d,
Ryotaro
Matsuda
de,
Katsuyuki
Nishimura
ab,
Haruki
Sugiyama
abf and
Yasutomo
Segawa
 *ab
*ab
aInstitute for Molecular Science, Myodaiji, Okazaki, 444-8787, Japan. E-mail: segawa@ims.ac.jp
bThe Graduate University for Advanced Studies, SOKENDAI, Myodaiji, Okazaki, 444-8787, Japan
cDepartment of Applied Chemistry, College of Life Sciences, Ritsumeikan University, 1-1-1 Nojihigashi, Kusatsu, Shiga 525-8577, Japan
dResearch Center for Net Zero Carbon Society, Institute of Innovation for Future Society, Nagoya university, Nagoya, 464-8601, Japan
eDepartment of Chemistry and Biotechnology, School of Engineering, and Department of Materials Chemistry, Graduate School of Engineering, Nagoya University, Chikusa-ku, Nagoya, 464-8603, Japan
fNeutron Industrial Application Promotion Center, Comprehensive Research Organization for Science and Society, Tokai, Ibaraki 319-1106, Japan
First published on 16th January 2025
Abstract
The synthesis and characterization of fused aromatic networks composed of zinc tetrapyrazinoporphyrazines are reported. The steric repulsion of bulky substituents induced the formation of three-dimensional structures. Thus-obtained insoluble polymers adsorbed CO2 and had near-infrared absorption indicating their porosity and extended π-conjugation.
Fused aromatic networks (FANs), the network polymers consisting of fully fused aromatic rings, have been intensively studied due to their unique structural, optical, and electronic properties.1 While many types of two-dimensional (2D) FANs have been reported, 3D FANs have not been well studied due to the difficulty of constructing 3D networks with fused aromatic structures. To date, several examples of 3D FANs have been synthesized using nonplanar conjugated units and their structural features, thermal stability, porosity, and semiconductor properties have been studied. The 3D FANs with saddle-shaped cyclooctatetraene derivatives as nonplanar conjugated units exhibited physical and chemical stability, permanent porosity, high surface area, and semiconductor properties.2 Triptycenes were also used as nonplanar conjugated units to form 3D FANs that exhibited high gas adsorption properties and thermal stability.3
Considering the high stability and electronic properties, phthalocyanines and their analogues are useful π-conjugated units. Phthalocyanine-based polymers have been studied for many years.4 Based on band structure calculations, 1D and 2D fully fused polymers were predicted to exhibit electrical conductivity.5 The synthesis of 2D phthalocyanine polymers was carried out both on metal surfaces and in solution.6 However, only a few examples of 3D polymers have been reported. The 3D frameworks containing phthalocyanines reported so far were not conjugated because they were linked by sp3 carbon or boron atoms.7 An electrically neutral, spiro-linked phthalocyanine-containing 3D polymer was synthesized by McKeown and co-workers in 2004.8 Later in 2017, Kimura and co-workers reported the synthesis of a spiro-conjugated 3D polymer using spirobifluorenes.9
To synthesize 3D FANs containing phthalocyanine analogues, we devised a strategy of using tetrapyrazinoporphyrazine (TPyzPz) and bending it with bulky substituents. TPyzPz is an analogue in which the benzene rings of phthalocyanine are replaced with pyrazine rings, and 2D FANs based on TPyzPz units have been synthesized (Fig. 1a).10 Since the introduction of bulky substituents distorts phthalocyanines to be saddle-shaped structures,11 we assumed that 2D and 3D FANs can be synthesized from tetracyanodihydrodipyrazinopyrazines (TCDP) depending on the size of the substituents (Fig. 1b). Herein, we report the synthesis of Zn TPyzPz FANs bearing 1-ethylpropyl and 2,4,6-trimethylphenyl (mesityl) groups. The crystallinity of the obtained solids was investigated by X-ray diffraction and the progress of the polymerization reaction was confirmed by using Fourier transform infrared (FT-IR) spectroscopy, solid-state 13C nuclear magnetic resonance (NMR) spectroscopy, and diffuse reflectance spectroscopy. The thermal stability and porosity of the polymers were also measured by Thermogravimetric analysis (TG) measurements and adsorption–desorption experiments, respectively.
First, the structures of Zn TPyzPz dimers were optimized by density functional theory (DFT) calculations to investigate the influence of the substituent size on polymer networks. For the calculation, B3LYP method was used and LANL2DZ and 6-31G(d) basis sets were applied for Zn and other atoms, respectively (Fig. 2a). As shown in Fig. 2b, the dimer having 1-ethylpropyl groups (DiEtPr) adopts a planar structure. On the other hand, the optimized structures of the dimers having mesityl and 3,5-di-t-butylphenyl groups (DiMes and DiBuPh) are nonplanar in which the TPyzPz are distorted to be saddle-shaped structures. The bent angles of DiMes and DiBuPh are 145.3° and 123.3°, respectively, indicating that the bent angles increase with the substituent size caused by the steric repulsion of substituents. The dimer structure of DiMes was then extended to a 3D periodic structure and structural relaxation was performed by Quantum Espresso to obtain the framework structure with dia-f topology (see Fig. S1 for details, ESI†).12 These calculations indicate that the TPyzPz polymers with bulky substituents hinder the formation of 2D sheet and result in a 3D network structure.
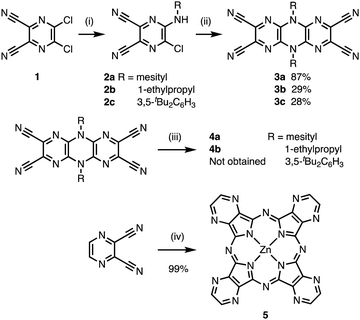
Monomers 3a–c were prepared according to reported procedures with modifications (Fig. 3a).13 The SNAr reactions of dichlorodicyanopyrazine 1 with corresponding alkyl- and arylamines were carried out to produce monoaminated products 2a–c. Dimerization of 2a–c using triethylamine was performed in refluxing N,N-dimethylformamide (DMF) to obtain desired monomers 3a–c. The monomers 3a–c were identified by 1H and 13C NMR spectroscopy, mass spectrometry, and X-ray crystallography (see ESI† for details).
With three monomers in hand, we investigated the polymerization reactions. As shown in Fig. 3, polymer 4a was synthesized by the cyclotetramerization of the dicyanopyrazine moieties of monomer 3a in the presence of anhydrous zinc chloride (0.5 equiv.), n-pentanol, and a catalytic amount of triethylamine in N,N-dimethylacetamide (DMAc).10 The mixture was heated without stirring and the solution turned dark green as the reaction progressed. Polymer 4a was obtained as an insoluble black solid and thoroughly washed with organic solvents and water. The use of diphenyl ether instead of DMAc as solvent afforded a brittle solid. In the reaction conditions using 1,8-diazabicyclo[5.4.0]-7-undecene as a base, no insoluble solid product was obtained. The optimized procedure using DMAc and triethylamine was also applied to 3b and 3c, and as a result, an insoluble black solid (polymer 4b) was obtained from 3b whereas no solid was formed from 3c. These results indicate that 3a and 3b were polymerized to form highly crosslinked polymers. As described in Fig. 2b, the oligomers of 3a cannot form a planar 2D sheet, suggesting the formation of 3D structures. On the other hand, no polymer was obtained from 3c. Considering that the electronic effects of the mesityl and 3,5-di-tert-butylphenyl groups are comparable, the size of the substituent causes a significant difference in the reactivity of polymerization. The formation of TPyzPz structure was confirmed by the cyclotetramerization of 2,3-dicyanopyrazine under the reaction conditions using zinc chloride, n-pentanol and triethylamine in DMAc to obtain compound 5 in 99% isolated yield (Fig. 3).
To confirm the polymer structures in detail, a series of analyses were performed including FT-IR spectroscopy, solid-state 13C NMR spectroscopy, energy dispersive spectroscopy (EDS), and powder X-ray diffraction (PXRD) measurement. FT-IR measurements of 4a, 4b and their monomers (3a, 3b) were performed in air using an attenuated total reflectance method. Because the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching peak at about 2230 cm−1 observed in the FT-IR spectra of monomers 3a and 3b disappeared in those of polymers 4a and 4b, it is inferred that the cyano groups were consumed (Fig. 4a). Solid-state 13C NMR spectra of 4a and 4b using the cross polarization magic angle spinning with total suppression of sidebands (CP-MAS TOSS) method are shown in Fig. S9 and S10 (ESI†), respectively. In the spectrum of 4a, aliphatic and aromatic signals derived from mesityl groups and TPyzPz moieties were observed. Signals attributed to DMAc were also observed at 160–170 ppm and 25–40 ppm. The spectrum of 4b shows the signals corresponding to 1-ethylpropyl, TPyzPz, and DMAc. For 4b, a small signal at 115 ppm was observed, which could be unreacted cyano groups. The EDS of 4a and 4b were performed using a Schottky emission scanning electron microscope. As shown in Fig. S11 and S12 (ESI†), C, N, and Zn elements are distributed throughout the surface of the solids. PXRD results of 4a and 4b supported the formation of partially ordered polymers (see ESI† for details).
N stretching peak at about 2230 cm−1 observed in the FT-IR spectra of monomers 3a and 3b disappeared in those of polymers 4a and 4b, it is inferred that the cyano groups were consumed (Fig. 4a). Solid-state 13C NMR spectra of 4a and 4b using the cross polarization magic angle spinning with total suppression of sidebands (CP-MAS TOSS) method are shown in Fig. S9 and S10 (ESI†), respectively. In the spectrum of 4a, aliphatic and aromatic signals derived from mesityl groups and TPyzPz moieties were observed. Signals attributed to DMAc were also observed at 160–170 ppm and 25–40 ppm. The spectrum of 4b shows the signals corresponding to 1-ethylpropyl, TPyzPz, and DMAc. For 4b, a small signal at 115 ppm was observed, which could be unreacted cyano groups. The EDS of 4a and 4b were performed using a Schottky emission scanning electron microscope. As shown in Fig. S11 and S12 (ESI†), C, N, and Zn elements are distributed throughout the surface of the solids. PXRD results of 4a and 4b supported the formation of partially ordered polymers (see ESI† for details).
 | ||
| Fig. 4 (a) IR spectra of 3a, 3b, 4a, and 4b. (b) TG curves of 4a and 4b. (c) Diffuse reflectance spectra of 4a and 4b, and absorption spectrum of 5 in DMSO. (d) Adsorption isotherms of 4a and 4b. | ||
TG was performed to investigate the thermal stability of polymers 4a and 4b. Before the measurement, polymers were soaked in diethyl ether and dried under vacuum. The weight loss derived from decomposition of 4b was observed at around 300 °C, whereas no decomposition of 4a was observed up to 500 °C (Fig. 4b). These differences in thermal stability are probably due to the 2D and 3D network structures.
In order to gain insight into the photophysical properties of 4a and 4b, diffuse reflectance spectroscopies were carried out. Each polymer was dispersed to 1 w% in magnesium oxide as dispersant and ground for 30 min prior to the measurements. The diffuse reflectance spectra of 4a and 4b are shown in Fig. 4c with the absorption spectrum of 5 in dimethyl sulfoxide (DMSO) solution as a reference. Both spectra show two broad peaks that can be assigned as Soret bands (4a: 436 nm, 4b: 430 nm) and Q bands (4a: 667 nm, 4b: 664 nm). In comparison to the absorption spectrum of 5 (soret: 335 nm, Q: 634 nm) in DMSO, the Soret and Q bands of 4a were red-shifted by 101 and 33 nm (6.91 × 103 and 7.80 × 102 cm−1), respectively, indicating the extension of π-conjugation through polymerization. Similar results were observed for 4b (6.59 × 103 cm−1 for soret band and 7.13 × 102 cm−1 for Q band).
Adsorption–desorption experiments were carried out to investigate the porous properties of 4a and 4b. As shown in Fig. 4d, 4a and 4b showed adsorption behavior for CO2. These results show the porous properties of 4a and 4b. The BET surface and Langmuir surface area of 4a calculated from CO2 adsorption are 581 and 576 m2 g−1, respectively, are slightly larger than those of 4b (386 and 446 m2 g−1).
In summary, we have synthesized fused aromatic network polymers 4a and 4b and investigated their optical properties and porosity. Monomers 3a–3c were prepared from dichlorodicyanopyrazine and aryl- or alkylamines in two steps. Polymerization of monomers 3a and 3b afforded insoluble black solids 4a and 4b, while no solid was obtained from 3c indicating that 3,5-di-tert-butylphenyl groups were too bulky to polymerize. The consumption of cyano groups and the formation of Zn TPyzPz moieties were confirmed by IR spectra and solid-state 13C NMR spectra. The formation of partially ordered polymers was supported by PXRD measurements. Differences in thermal stability, possibly due to the 2D and 3D network structures, were observed in TG-DTA measurements. The extension of π-conjugation through polymerization and the porous properties of the 4a and 4b were confirmed by diffuse reflectance spectroscopies and adsorption–desorption measurements, respectively. This work demonstrated the novel strategy for the synthesis of 3D FANs to create optically unique and porous organic materials.
This work was supported by FOREST program (JPMJFR211R to Y. S.) from JST, JSPS KAKENHI (JP22K19038 and JP22H02068 to Y. S.), Tatematsu Foundation and Murata Science and Education Foundation. We thank Prof. Yasuhiro Uozumi, Dr Taketoshi Minato, Ms Aya Tazawa (Institute for Molecular Science (IMS)), Dr Hirotoshi Sakamoto (Kyoto University) and Dr Masato Taki (Nagoya Institute of Technology) for their support of experiments and fruitful advices. K. W. is a recipient of JSPS Research Fellowship for Young Scientists (DC1). This work was conducted in IMS supported by “Advanced Research Infrastructure for Materials and Nanotechnology in Japan (ARIM)” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (JPMXP1224MS5001, JPMXP1223MS5011). Calculations were performed using the resources of the Research Center for Computational Science, Okazaki, Japan (24-IMS-C232). The solid-state NMR spectroscopy was supported by the Equipment Sharing Division, Organization for Co-Creation Research and Social Contributions, Nagoya Institute of Technology.
Data availability
Crystallographic data have been deposited at the CCDC under 2403177 (3a), 2403178 (3b), 2403179 (3c) and 2404808 (5). The data supporting this article have been included as the ESI† (pdf) and CartesianCoordinates.xyz (xyz) files.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) S. Che and L. Fang, Chem, 2020, 6, 2558–2590 CrossRef CAS; (b) J. Mahmood, M. A. R. Anjum and J. B. Baek, Adv. Mater., 2018, 31, 1805062 CrossRef; (c) I. Ahmad, J. Mahmood and J. B. Baek, Small Sci., 2021, 1, 2000007 CrossRef CAS.
- (a) S. N. Talapaneni, J. Kim, S. H. Je, O. Buyukcakir, J. Oh and A. Coskun, J. Mater. Chem. A, 2017, 5, 12080–12085 RSC; (b) Y. Byun, L. S. Xie, P. Fritz, T. Ashirov, M. Dinca and A. Coskun, Angew. Chem., Int. Ed., 2020, 59, 15166–15170 Search PubMed; (c) T. Ashirov, P. W. Fritz, Y. Lauber, C. E. Avalos and A. Coskun, Chem. – Eur. J., 2023, 29, e202301053 CrossRef CAS PubMed.
- J. Mahmood, S. J. Kim, H. J. Noh, S. M. Jung, I. Ahmad, F. Li, J. M. Seo and J. B. Baek, Angew. Chem., Int. Ed., 2018, 57, 3415–3420 CrossRef CAS PubMed.
- N. B. McKeown, J. Mater. Chem., 2000, 10, 1979–1995 RSC.
- P. Gomez-Romero, Y. S. Lee and M. Kertesz, Inorg. Chem., 2002, 27, 3672–3675 CrossRef.
- (a) E. Nardi, M. Koudia, S. Kezilebieke, J.-P. Bucher and M. Abel, in On-surface synthesis, ed. A. Gourdon, On-Surface Synthesis of Phthalocyanine Compounds, Springer, Cham, 2016, pp. 115–129 Search PubMed; (b) K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed; (c) Q. Guan, L. L. Zhou and Y. B. Dong, Chem. Soc. Rev., 2022, 51, 6307–6416 RSC.
- (a) B. Han, L. Zhang, L. Yunfei, B. Bin, E. Jie, L. Q. Zhang, Z. Xie, H. Wang and J. Jiang, Adv. Funct. Mater., 2024, 2404289 Search PubMed; (b) Q. Zhang, B. Han, Y. Jin, M. Li, E. Zhang and J. Jiang, Chin. Chem. Lett., 2024, 110330 Search PubMed; (c) X. Wang, M. Bahri, Z. Fu, M. A. Little, L. Liu, H. Niu, N. D. Browning, S. Y. Chong, L. Chen, J. W. Ward and A. I. Cooper, J. Am. Chem. Soc., 2021, 143, 15011–15016 CrossRef CAS PubMed; (d) X. Wang, T. Fellowes, M. Bahri, H. Qu, B. Li, H. Niu, N. D. Browning, W. Zhang, J. W. Ward and A. I. Cooper, J. Am. Chem. Soc., 2024, 146, 14128–14135 CrossRef CAS.
- (a) P. M. Budd, S. M. Makhseed, B. S. Ghanem, K. J. Msayib, C. E. Tattershall and N. B. McKeown, Mater. Today, 2004, 7, 40–46 CrossRef CAS; (b) H. J. Mackintosh, P. M. Budd and N. B. McKeown, J. Mater. Chem., 2008, 18, 573–578 RSC.
- R. Tamura, T. Kawata, Y. Hattori, N. Kobayashi and M. Kimura, Macromolecules, 2017, 50, 7978–7983 CrossRef CAS.
- (a) Y.-K. Im, D.-G. Lee, H.-J. Noh, S.-Y. Yu, J. Mahmood, S. Lee and J.-B. Baek, Angew. Chem., Int. Ed., 2022, 61, e202203250 CrossRef CAS; (b) Y. Wang, M. Wang, T. Chen, W. Yu, H. Liu, H. Cheng, W. Bi, M. Zhou, Y. Xie and C. Wu, Angew. Chem., Int. Ed., 2023, 47, e202308070 Search PubMed.
- S. Shimizu, A. Miura and N. Kobayashi, CrystEngComm, 2013, 15, 3759–3762 RSC.
- (a) O. Delgado-Friedrichs, M. O’Keeffe and O. M. Yaghi, Acta Crystallogr., Sect. A: Found. Crystallogr., 2003, 59, 515–525 CrossRef; (b) V. A. Blatov, L. Carlucci, G. Ciani and D. M. Proserpio, CrystEngComm, 2004, 6, 377–395 RSC.
- J. Y. Jaung, K. Fukunishi and M. Matsuoka, J. Heterocycl. Chem., 1997, 34, 653–657 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2403177–2403179 and 2404808. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc06293k |
| This journal is © The Royal Society of Chemistry 2025 |