Polyhydroxy starch with abundant hydroxyls and a unique structure enables uniform Zn deposition†
Ming
Song‡
a,
Zhaohe
Guo‡
a,
Yan
Xu
 *a,
Xueyao
Mo
a,
Xuena
Xu
b,
Limei
Sun
a,
Wenyi
Tan
a,
Dongliang
Chao
*a,
Xueyao
Mo
a,
Xuena
Xu
b,
Limei
Sun
a,
Wenyi
Tan
a,
Dongliang
Chao
 c and
Wanhai
Zhou
c and
Wanhai
Zhou
 *c
*c
aSchool of Materials and Chemical Engineering, Xuzhou University of Technology, Xuzhou 221018, China. E-mail: xuyan8787@163.com
bDepartment of Mechanical Engineering, Tsinghua University, Beijing 100084, China
cLaboratory of Advanced Materials, Aqueous Batteries Center, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Shanghai, 200433, China. E-mail: zhouwh@fudan.edu.cn
First published on 3rd January 2025
Abstract
Zinc metal is a promising anode material for zinc-ion batteries (ZIBs), but severe side reactions and dendrite formation hinder its commercialization. In this study, starch is introduced into the ZnSO4 electrolyte for stabilizing the Zn anode. With abundant hydroxyl groups, starch can reconstruct the H-bond system in the electrolyte, suppressing side reactions. Moreover, the unique ring and double-helix structures of starch show strong interactions with Zn2+ ions. The large molecule structure also exhibits a steric hindrance effect, regulating the diffusion and reduction of Zn2+. Additionally, starch molecules can adsorb onto the Zn anode, promoting the formation of a solid–electrolyte interphase (SEI) and resulting in uniform Zn deposition along the (002) plane. Consequently, this approach enhances the stability and reversibility of the Zn anode.
The increasing importance of renewable energy sources underscores the urgent need for effective energy storage solutions.1 Aqueous Zn-ion batteries (ZIBs) have emerged as promising candidates owing to their safety and affordability.2,3 Zinc metal, a key component of ZIBs, boasts non-toxicity, abundant reserves, and high theoretical capacity. However, the commercial viability of ZIBs is impeded by parasitic reactions and dendrite formation.4,5
Various strategies have been proposed to address these issues.6–8 Electrolyte engineering is particularly appealing due to its simplicity, cost-effectiveness, and multifunctionality.9 Existing additives encompass a range of materials such as inorganic salts, organic solvents, and polymers.10 Polymer additives, in particular, are characterized by multiple functional groups that can strongly interact with Zn2+, thereby influencing the transfer behavior. Moreover, they have the capability to disrupt original H-bonds, which can reduce the content of active water. For example, hyaluronic acid (HA) with abundant –COOH and –OH groups, could disrupt H-bonds among water molecules, thereby inhibiting side reactions.11 Additionally, Zn dendrite growth is believed to be influenced by the preferential growth of specific crystal planes. The preferred Zn(002) plane growth makes crystals grow at 0–30° from the Zn anode.12 However, Zn atoms tend to deposit on the (101) plane due to their strong binding energy, leading to preferential growth of the (101) plane at an angle of 30–70° and forming Zn dendrites.13 Introducing additives with different functional groups into the electrolyte, such as triethanolamine,14 and N,S-CDs,15 has been reported to induce Zn(002) plane deposition.
Starch, a low-cost natural biopolymer, has been used to prepare a gel electrolyte for zinc-air batteries due to its hydrophilicity and adhesion.16 Here, it is introduced into the ZnSO4 electrolyte for stabilizing the Zn anode. The starch (farina starch) consists of α-D-glucopyranosyl units linked via α-1,4-glucosidic linkages (Fig. S1, ESI†). Due to the ability of hydroxyl groups to form H-bonds, starch exhibits helical polymer chains. These chains intertwine in an anti-parallel manner, forming a characteristic double-helix structure.17 It also can reconstruct the H-bonds in the electrolyte, suppressing water-induced reactions. Due to the intense electron density, the ring and double-helix structure with obvious steric hindrance effect has strong zincophilicity, regulating the deposition behaviour of Zn2+ ions. Moreover, a solid electrolyte interface (SEI) is formed. Uniform Zn deposition with exposure of the Zn(002) plane is achieved, yielding an enhanced long-term cycling stability in Zn‖Zn, Zn‖Cu and Zn‖VO2 cells.
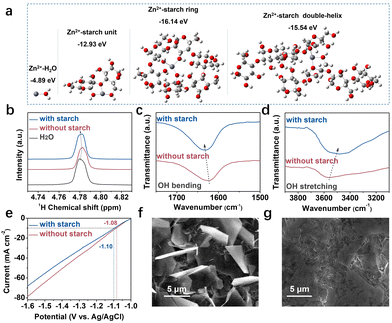
Generally, in a dilute electrolyte, Zn2+ tends to interact with surrounding electron donors, forming a Zn[H2O]62+ structure.18 When the starch is introduced, the coordination environment of Zn2+ can be altered due to the dense electron density from hydroxyl groups. The interaction between Zn2+ and starch (including units, rings, and double helices) has been calculated using density functional theory (DFT). As illustrated in Fig. 1a, a stronger interaction is formed between Zn2+ and starch, particularly with the ring and helix structure, which potentially alters the Zn[H2O]62+ solvation structure.
Nuclear magnetic resonance (NMR) analysis has been conducted to examine the changed solvation structure of Zn2+ (Fig. 1b). The 1H peak shifts to a lower field when ZnSO4 is introduced. This indicates the formation of Zn[H2O]62+, which can decrease the surrounding electron density and weaken the electrostatic shielding effect on protons.19 Upon the introduction of starch, the 1H peak shifted back to a higher field, indicating a changed coordination structure and formed H-bonds.20
The impact of starch on H-bonds has been confirmed using Fourier transform infrared spectroscopy (FTIR). A blue shift is observed in the bending vibration of H2O (1600–1700 cm−1), while the stretching vibration of H2O (3000–3500 cm−1) exhibits a notable red shift upon the introduction of starch (Fig. 1c and d). This shift indicates the formation of H-bonds between starch and water, leading to a reconstruction of the H-bond network in the electrolyte.21 Hydrogen evolution reaction (HER) is a competitive process with the formation of H-bonds.22 More energy may be needed to decouple the H-bonds in the reconstructed system, suppressing side reactions.
The HER and corrosion of the Zn anode in different electrolytes have been conducted using linear sweep voltammetry (LSV) and linear polarization experiments (Tafel). At 10 mA cm−2, a larger overpotential (−1.10 V) is observed in the electrolyte with starch, which is 19 mV higher than that in the bare ZnSO4 electrolyte. This result demonstrates the inhibitory effect of starch on H2 generation. Additionally, a wider electrochemical window is obtained in the starch-containing electrolyte in Fig. S2 (ESI†). The Tafel plot in Fig. S3 (ESI†) indicates a lower corrosion current in the starch-containing electrolyte, suggesting the mitigation of the self-corrosion issue of the Zn anode.
The starch in inhibiting side reactions has been further investigated by immersing Zn foil in the electrolyte for 6 hours. From X-ray powder diffraction (XRD) results (Fig. S4, ESI†), Zn4SO4(OH)6·4H2O (PDF#44-067323) is observed on the soaked Zn foil. Notably, the intensity is weak in the electrolyte containing starch, indicating suppressed formation of by-products. Moreover, the intensity of the Zn(002) plane is significantly enhanced in the starch-containing electrolyte compared with that in the bare ZnSO4 electrolyte, with I(002)/I(101) ratios of 3.9 and 1.8, respectively. The exposure of the Zn(002) plane facilitates horizontal Zn deposition, which is parallel to the surface of the electrode (Fig. S5, ESI†). Additionally, Zn(002) with the lowest surface energy is less reactive, contributing to the attenuation of side reactions.24 Scanning electron microscopy (SEM) images in Fig. 1f and g show that numerous irregular flake-like Zn dendrites are present in the bare ZnSO4 electrolyte, whereas the surface appears dense and flat in the starch-containing electrolyte. These SEM images corroborate the findings from the XRD analysis.
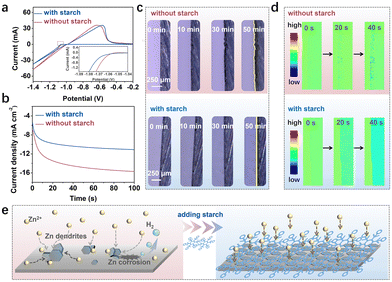
It is widely acknowledged that the morphology of initial nucleation is closely linked to the generation and growth of Zn dendrites. Uneven Zn nucleation can lead to surface protrusions on the Zn electrode, exacerbating electric field irregularities and ultimately promoting Zn dendrite formation through a “tip effect” mechanism.25 The nucleation overpotential, which influences Zn nucleation, has been assessed by the cyclic voltammetry (CV) method. As illustrated in Fig. 2a, a higher nucleation overpotential is attained in the electrolyte containing starch, which can foster the formation of smaller Zn nuclei.26
The diffusion behavior of Zn2+ has been examined using the chronoamperometry (CA) method at −150 mV (Fig. 2b). The rapid initial increase in current density observed in both electrolytes indicates the uncontrolled 2-dimensional (2D) diffusion.27 The continuous upward trend of current in the bare ZnSO4 electrolyte suggests sustained 2D diffusion, where Zn2+ diffuses laterally along the Zn surface and accumulates at energetically favourable sites (protrusions, lattice defects, boundaries, impurities, etc.) to form initial Zn nuclei.28 In contrast, with starch, the current stabilizes faster. The constrained 2D diffusion means that Zn2+ can deposit near the initial adsorption sites, resulting in more nucleation sites and uniform Zn deposition.29
The actual Zn deposition process has been observed using an in situ optical microscope. Noticeable protrusions appear in the ZnSO4 electrolyte after 10 minutes, followed by uncontrolled growth of Zn dendrites (Fig. 2c). In contrast, the starch-containing electrolyte exhibits uniform and stable Zn deposition, with no apparent protrusions even after 50 minutes. The in situ electrochemical digital holography (EDH) technique has been employed to monitor the evolution of ion concentration during the initial Zn2+ reduction (Fig. 2d). The change of Zn2+ ion concentration can lead to a shift in colour. After 20 seconds, a local colour change is observed in both electrolytes. In the ZnSO4 electrolyte, an uneven Zn2+ concentration is evident at the interface, which can lead to the formation of Zn dendrites. The uniform Zn2+ concentration in the starch-containing electrolyte indicates a uniform Zn2+ flux. The decreased Zn2+ concentration at the interface in starch-containing electrolyte should result from the adsorption of starch on the Zn anode, showing the steric hindrance effect. Due to the restrained ion and electron transfer, the Zn deposition process can be modulated. This speculation is verified by the electrochemical impedance spectroscopy (EIS) and the contact angle tests. The EIS results in Fig. S6 (ESI†) confirm the restrained reduction of Zn2+ ions. The lower contact angle between the Zn anode and the electrolyte containing starch in Fig. S7 (ESI†) indicates the higher surface energy at the interface between the Zn anode and electrolyte with starch, indicating the adsorption of starch molecules on the Zn anode.30
Based on the above analysis, it can be speculated that the starch molecule can reconstruct the H-bond system, suppressing side reactions. Moreover, it can interact with Zn2+ and adsorb on the electrode, directing the Zn2+ diffusion and reduction (Fig. 2e).
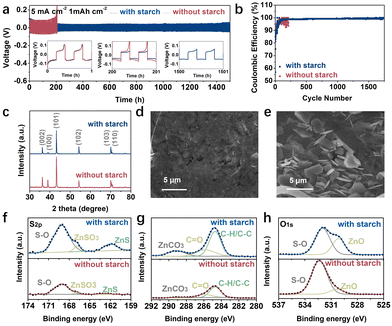
The stability of the Zn anode has been investigated using Zn‖Zn symmetric cells. As presented in Fig. 3a and Fig. S8 (ESI†), the cells in the ZnSO4 electrolyte can only work steadily for about 200 h (5 mA cm−2 and 1 mA h cm−2), 50 h (5 mA cm−2 and 5 mA h cm−2), and 110 h (10 mA cm−2 and 5 mA h cm−2) under various conditions. When the starch is introduced, there is an apparent improvement. The cycling life of Zn‖Zn cells increased to 1500 h, 225 h, and 350 h. The Zn‖Zn cells in the electrolyte with 0.1 and 0.4 mg mL−1 starch also exhibit an improved performance (Fig. S9 and S10, ESI†) compared to the bare ZnSO4 electrolyte. As starch has a steric hindrance effect, 0.2 mg mL−1 is the optimum concentration. It shows a comparable performance with the reported results (Table S1, ESI†). Besides, the better rate performance and cycling stability at 30 mA cm−2 of Zn‖Zn cells in electrolyte with additive corroborate the effectiveness of starch (Fig. S11 and S12, ESI†). The coulombic efficiency (CE) has been confirmed by the Zn‖Cu cells. As shown in Fig. 3b and Fig. S13 (ESI†), a more stable CE is achieved in the electrolyte with starch. The average CE is 99% under the condition of 1 mA cm−2 and 0.5 mA h cm−2 for 1750 cycles. In sharp contrast, in the ZnSO4 electrolyte, there are fluctuant voltage profiles with a low CE of 97% for about 210 cycles. The Zn deposition behaviour (plating and stripping) on Cu foil at 20 mA cm−2 has been studied. As shown in Fig. S14 (ESI†), there are flat Zn deposits on Cu foil in the electrolyte with starch.
The morphology and structure of the cycled Zn electrolyte after 50 cycles have also been analyzed. The XRD results in Fig. 3c exhibit a higher I(002)/I(101) ratio of 3.87 for the electrolyte with starch, while it is 1.50 in the ZnSO4 electrolyte, indicating a preferential exposure of the (002) plane. Compared to Fig. 3d and e, the compact, flat, and smooth Zn anode is achieved in electrolyte with starch, consistent with the XRD results.
The surface chemistry of the cycled Zn anode has been investigated by X-ray photoelectron spectroscopy (XPS) after Ar+ sputtering (5 nm). The S 2p spectra indicate the presence of S–O, ZnSO3, and ZnS components, with the S–O component from SO42− being the main component.31 The prominent ZnS peak in the electrolyte with starch indicates the formation of solid electrolyte interphase (SEI). In the C 1s spectra, C–C/C–H, C–O, and ZnCO3 can be observed,32 derived from CO2 and starch. The much higher intensity in the electrolyte with starch confirms the contribution of partial decomposition of starch. Additionally, as further confirmed by O 1s spectra, more ZnO is formed in the electrolyte with starch.33 Therefore, it can be speculated that the SEI layer consisting of ZnCO3, ZnS, and ZnO is formed on the Zn surface in the electrolyte with starch. The XPS spectrum of Zn 2p in Fig. S15 (ESI†) also affirms the influence of starch on the surface chemistry of the Zn electrode.
The inclusion of starch in the electrolyte is demonstrated to enhance the performance of Zn‖VO2 full cells, highlighting the significant potential for practical ZIBs. The synthesized VO2 is confirmed via XRD (JCPDF# 31-1438), as depicted in Fig. S16 (ESI†). The SEM result in Fig. S17 (ESI†) reveals its rod-like morphology. The rate capability of Zn‖VO2 cells in both electrolytes is depicted in Fig. 4a–c at 0.1–2 A g−1. Remarkably, the cells utilizing the electrolyte with starch exhibit a higher capacity compared to those using the bare ZnSO4 electrolyte. The cyclic voltammetry (CV) curves presented in Fig. 4d exhibit similar profiles in both electrolytes, confirming an unaffected charge storage mechanism. Noteworthy is the lower voltage polarization (0.16 V) observed in the electrolyte with starch compared to the bare ZnSO4 electrolyte (0.22 V). The long-term cycling performance shown in Fig. 4e demonstrates the full cells in the starch-containing electrolyte maintaining a more stable performance, retaining a capacity of 155 mA h g−1 after 250 cycles at 0.5 A g−1. Interestingly, the battery capacity in the bare ZnSO4 electrolyte is higher during the initial 10 cycles, possibly attributed to proton embedding, by-product contributions to capacity, and the rapid charge transfer facilitated by the Zn anode's rough surface.
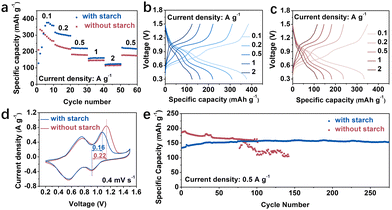 | ||
| Fig. 4 (a) Rate performance. GCD curves in electrolyte (b) with and (c) without starch. (d) CV curves. (e) Long-term stability of the Zn‖VO2 cell. | ||
In summary, starch is introduced into the ZnSO4 electrolyte. Due to the hydroxyl groups, the original H-bond system is altered, inhibiting the water-triggered reactions. Moreover, the large molecule structure enables starch with a steric hindrance effect, and the ring and helix structure has a stronger interaction with Zn2+, which can modulate the diffusion and reduction of Zn2+. In addition, SEI is formed on the Zn surface in the electrolyte with starch, facilitating the Zn deposition along with the (002) plane. Consequently, highly stable Zn‖Zn, Zn‖Cu, and Zn‖VO2 cells are achieved. This study offers a cost-effective and environmentally friendly electrolyte additive for stabilizing Zn anodes, thus advancing the development of ZIBs. The long-term stability of starch, as well as its stability under extreme conditions, should be studied in the future.
This work was financially supported by the National Natural Science Foundation of China (No. 22279023, 22075115, and 22309031), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 23KJB480009), the Xuzhou Science and Technology Project (No. KC23033), and the Shanghai Science and Technology Innovation Action Plan Morning Star Project (Sail Special) (No. 23YF1401800).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- A. Yu, W. Zhang, N. Joshi and Y. Yang, Energy Storage Mater., 2024, 64, 103075 CrossRef.
- M. Al-Abbasi, Y. Zhao, H. He, H. Liu, H. Xia, T. Zhu, K. Wang, Z. Xu, H. Wang, W. Zhang, Y. Lai and M. Ge, Carbon Neutralization, 2024, 3, 108–141 CrossRef CAS.
- W. Zhang, Y. Chen, H. Gao, W. Xie, P. Gao, C. Zheng and P. Xiao, Mater. Futures, 2023, 2, 042102 CrossRef CAS.
- J. Yang, R. Zhao, Y. Wang, Z. Hu, Y. Wang, A. Zhang, C. Wu and Y. Bai, Adv. Funct. Mater., 2023, 33, 2213510 CrossRef CAS.
- Y. Zuo, K. Wang, P. Pei, M. Wei, X. Liu, Y. Xiao and P. Zhang, Mater. Today Energy, 2021, 20, 100692 CrossRef CAS.
- G. Li, L. Sun, S. Zhang, C. Zhang, H. Jin, K. Davey, G. Liang, S. Liu, J. Mao and Z. Guo, Adv. Funct. Mater., 2023, 34, 2301291 CrossRef.
- X. He, M. Kwon, J. Chung, K. Lee, Y. Choi, Y. Im, J. Jang, Y. Choi and H. J. Yoon, Small, 2024, 20, 2403537 CrossRef CAS PubMed.
- H. Li, Z. Liu, Y. Tang, S. Liang and G. Fang, cMat, 2024, 1, e25 CrossRef.
- H. Li, S. Guo and H. Zhou, Energy Storage Mater., 2023, 56, 227–257 CrossRef.
- Y. Li, H. Yao, X. Liu, X. Yang and D. Yuan, Nano Res., 2023, 16, 9179–9194 CrossRef CAS.
- B. Wang, R. Zheng, W. Yang, X. Han, C. Hou, Q. Zhang, Y. Li, K. Li and H. Wang, Adv. Funct. Mater., 2022, 32, 2112693 CrossRef CAS.
- X. Liang, X. Chen, Z. Zhai, R. Huang, T. Yu and S. Yin, Chem. Eng. J., 2024, 480, 148040 CrossRef CAS.
- H. Zhang, Y. Zhong, J. Li, Y. Liao, J. Zeng, Y. Shen, L. Yuan, Z. Li and Y. Huang, Adv. Energy Mater., 2022, 13, 2203254 CrossRef.
- W. Ge, H. Peng, J. Dong, G. Wang, L. Cui, W. Sun, X. Ma and J. Yang, Chem. Commun., 2024, 60, 750–753 RSC.
- Z. Xu, H. Li, Y. Liu, K. Wang, H. Wang, M. Ge, J. Xie, J. Li, Z. Wen, H. Pan, S. Qu, J. Liu, Y. Zhang, Y. Tang and S. Chen, Mater. Horiz., 2023, 10, 3680–3693 RSC.
- Y. Zuo, K. Wang, M. Wei, S. Zhao, P. Zhang and P. Pei, Cell Rep. Phys. Sci., 2022, 3, 100687 CrossRef CAS.
- S. J. Zhang, J. Hao, H. Li, P. F. Zhang, Z. W. Yin, Y. Y. Li, B. Zhang, Z. Lin and S. Z. Qiao, Adv. Mater., 2022, 34, 2201716 CrossRef CAS PubMed.
- J. Cao, D. Zhang, X. Zhang, Z. Zeng, J. Qin and Y. Huang, Energy Environ. Sci., 2022, 15, 499–528 RSC.
- Z. Hu, F. Zhang, A. Zhou, X. Hu, Q. Yan, Y. Liu, F. Arshad, Z. Li, R. Chen, F. Wu and L. Li, Nano-Micro Lett., 2023, 15, 171 CrossRef CAS PubMed.
- H. Wang, W. Ye, B. Yin, K. Wang, M. S. Riaz, B. B. Xie, Y. Zhong and Y. Hu, Angew. Chem., Int. Ed., 2023, 62, e202218872 CrossRef CAS.
- K. Bao, M. Wang, Y. Zheng, P. Wang, L. Yang, Y. Jin, H. Wu and B. Sun, Nano Energy, 2024, 120, 109089 CrossRef CAS.
- T. Wang, Z. Tian, Z. You, Z. Li, H. Cheng, W. Li, Y. Yang, Y. Zhou, Q. Zhong and Y. Lai, Energy Storage Mater., 2022, 45, 24–32 CrossRef.
- P. Wang, X. Xie, Z. Xing, X. Chen, G. Fang, B. Lu, J. Zhou, S. Liang and H. J. Fan, Adv. Energy Mater., 2021, 11, 2101158 CrossRef CAS.
- M. Zhou, S. Guo, J. Li, X. Luo, Z. Liu, T. Zhang, X. Cao, M. Long, B. Lu, A. Pan, G. Fang, J. Zhou and S. Liang, Adv. Mater., 2021, 33, 2100187 CrossRef CAS.
- Y. Zhang, X. Fu, Y. Ding, Y. Liu, Y. Zhao and S. Jiao, Small, 2024, 20, 2311407 CrossRef CAS.
- C. Meng, W. He, L. Jiang, Y. Huang, J. Zhang, H. Liu and J. J. Wang, Adv. Funct. Mater., 2022, 32, 2207732 CrossRef CAS.
- Y. Liang, M. Qiu, P. Sun and W. Mai, Chem. Sci., 2024, 15, 1488 RSC.
- C. Nie, G. Wang, D. Wang, M. Wang, X. Gao, Z. Bai, N. Wang, J. Yang, Z. Xing and S. Dou, Adv. Energy Mater., 2023, 13, 2300606 CrossRef CAS.
- L. Zhang, L. Miao, W. Xin, H. Peng, Z. Yan and Z. Zhu, Energy Storage Mater., 2022, 44, 408–415 CrossRef.
- X. Shi, J. Wang, F. Yang, X. Liu, Y. Yu and X. Lu, Adv. Funct. Mater., 2022, 33, 2211917 CrossRef.
- L. Cao, D. Li, E. Hu, J. Xu, T. Deng, L. Ma, Y. Wang, X.-Q. Yang and C. Wang, J. Am. Chem. Soc., 2020, 142, 21404–21409 CrossRef CAS PubMed.
- C. Meng, W. He, H. Tan, X. Wu, H. Liu and J. Wang, Energy Environ. Sci., 2023, 16, 3587–3599 RSC.
- H. Dou, X. Wu, M. Xu, R. Feng, Q. Ma, D. Luo, K. Zong, X. Wang and Z. Chen, Angew. Chem., Int. Ed., 2024, 63, e202401974 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc06288d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |