Open Access Article
Open Access ArticleIonic gradients in flow to control transport of emissive ions†
Lucy L.
Fillbrook‡
 a,
Isis A.
Middleton‡
a,
Isis A.
Middleton‡
 a,
Hamid
Rashidnejad
a,
Hamid
Rashidnejad
 a,
Aditya
Sapre
b,
Timothy W.
Schmidt
a,
Aditya
Sapre
b,
Timothy W.
Schmidt
 a,
Ayusman
Sen
a,
Ayusman
Sen
 b and
Jonathon E.
Beves
b and
Jonathon E.
Beves
 *a
*a
aSchool of Chemistry, UNSW, Sydney, NSW 2052, Australia. E-mail: j.beves@unsw.edu.au
bDepartments of Chemical Engineering and Chemistry, The Pennsylvania State University, University Park, Pennsylvania 16802, USA
First published on 31st January 2025
Abstract
Concentration gradients of simple salts in microfluidic channels control the transport of a common photoredox catalyst.
Controlling how species move in response to chemical stimuli is key for understanding complex biological systems. Microfluidics experiments have provided significant insight into how biologically relevant species spatially respond to changes in their chemical environment, including for nucleic acids,1,2 proteins,3 and bacteria.4 Flow experiments have also been used to monitor the movement of small ionic molecules in response to salt gradients.5 Suspended particles can respond to salt gradients,6–9 such as the movement of charged colloids10,11 in solutions of chloride salts. Salt gradients can also direct and aggregate surfactant vesicles,12 as well as controlling the formation and movement of DNA and protein-based condensates.13 Magnetic fields have been used to precisely control the movement of microparticles,14 and supramolecular nanomotors have been developed with temperature-controlled speeds.15 Chemotactic studies using host–guest interactions16,17 or catalysis18,19 have manipulated macromolecules,20,21 while other aspects of small molecule diffusion during chemical reactions remain controversial.22 Despite these examples, control of small molecule movement remains a significant challenge.
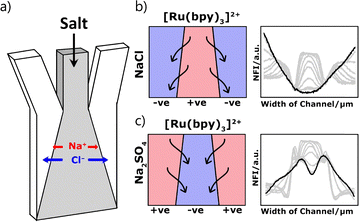
Herein we report a simple microfluidics system using the variable diffusivities of ions in sodium salts to generate liquid junction potentials and control the movement of a small cationic dye. We used a microfluidic device with three-inlets (Fig. 1a), based on an established design used for chemotaxis studies.23 The known diffusivities24 of Na+ (1.33 × 10−9 m2 s−1), Cl− (2.03 × 10−9 m2 s−1), and SO42− (1.07 × 10−9 m2 s−1) cause the cation and anion to disperse at different rates and can create a liquid junction potential (Fig. 1b and c). Such liquid junction potentials can be used to control the migration of charged species.
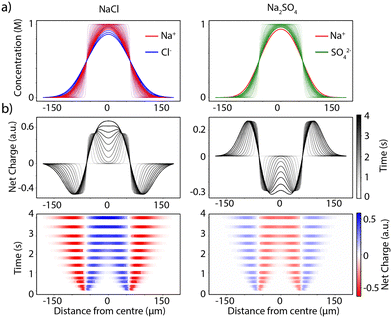
We first modelled the diffusion of ions when sodium chloride or sodium sulfate are added to the central inlet of a device, assuming the movement of each ion follows a simple Fickian diffusion profile.17 A full description of the model is provided in ESI,† S3. Fig. 2a shows a simulation of the diffusion of the ions across the channel where the salt is added to the central inlet. Each ion carries a charge, and the different concentration profiles of the cation and anion (Fig. 2b) result in a net charge difference across the channel. At the initial time points the net charge distribution is localised to the boundaries of the channel centre. For sodium chloride, the chloride ions diffuse slightly faster than the sodium ions, generating an area of negative charge just outside the flow of the salt. For sodium sulfate the opposite effect is observed as the sulfate ions diffuse more slowly than sodium ions. At the later time points, the ions have diffused further across the channel, and a liquid junction potential is formed. With the sodium chloride salt a net positive charge is formed in the centre of the channel, and with sodium sulfate a net negative charge is formed. This effect is shown in Fig. 2b for comparison with the experimental data.
With this data in hand, we set to monitor the formation of such liquid junction potentials experimentally. We aimed to use optical microscopy with a luminescent dye to measure concentration changes. We selected [Ru(bpy)3]Cl2 as a small, water-soluble dye which does not readily aggregate in dilute solution and has well-defined photophysical properties.25 Critically, the visible absorption and emission of this complex are due to MLCT transitions that are only weakly influenced by the ionic strength of the solution. Static absorbance and emission measurements of [Ru(bpy)3]Cl2 (50 μM) in 2-(N-morpholino)ethanesulfonic acid buffer (MES, 50 mM, pH 6) in the absence and presence of salt (1 M NaCl or 1 M Na2SO4), confirmed the emission of [Ru(bpy)3]Cl2 is a reliable measure of the concentration of the complex under this range of conditions (see ESI,† S4).
We used polydimethylsiloxane (PDMS) based microfluidics devices, mounted on 1 mm glass slides to enable optical monitoring with a high-sensitivity laser scanning confocal microscope. The channel width of the device is 360 μm, depth is 50 μm and length is 4 cm, corresponding to a cross-section of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm2. The emission intensity was measured across the channel width, always at 2 mm from the channel entry point to ensure a stable concentration gradient. Flow rates were varied from 1–20 μL min−1 to give residence times in the channel before detection of 0.11–2.16 s. Solutions were flowed through the devices via all three inlets simultaneously with identical flow rates. In all cases the emission intensity was normalised across the width of the channel for each time point (see ESI,† S5).
000 μm2. The emission intensity was measured across the channel width, always at 2 mm from the channel entry point to ensure a stable concentration gradient. Flow rates were varied from 1–20 μL min−1 to give residence times in the channel before detection of 0.11–2.16 s. Solutions were flowed through the devices via all three inlets simultaneously with identical flow rates. In all cases the emission intensity was normalised across the width of the channel for each time point (see ESI,† S5).
Solutions of [Ru(bpy)3]Cl2 (50 μM) in MES buffer (50 mM, pH 6) were flowed through all three inlets at variable rates (1–20 μL min−1). The salt was added to the dye stock solution and flowed either through only the central inlet, or through the two external inlets. The experimental data is shown in Fig. 3. Where sodium chloride was added to the central inlet the dye was displaced from the centre of the channel towards the edges (Fig. 3a). Where sodium sulfate was added to the central channel, the dye concentrated in the centre, moving in from the edges (Fig. 3b). These observations match the expected behaviour shown in Fig. 2. The opposite effect was observed when flowing the salt solutions through the two outer inlets rather than the central inlet. That is, where sodium chloride was added to the outer inlets the emission intensity increases in the centre of the channel as the dye is concentrated into the centre. The focusing of the dye into the middle of the channel is more pronounced as twice as much salt is added when two inlets are used compared to the single central inlet. When sodium sulfate was added to the external inlets, propagating waves of the dye are observed on either side of the centre as the dye migrates towards the edges.
Increasing the salt concentration added to the central inlet (0.1, 0.25, 0.5 and 1.0 M) increased the displacement of the dye (see ESI,† S6). These experiments establish that simple salt gradients can control the migration of emissive ions over hundreds of micrometres, distances which enable controlled movement in microfluidic systems (Fig. 4). The three-inlet design allows both the focusing and dispersion of the emissive ion. The estimated potential based on the simulated ion concentrations is 8 mV, similar in magnitude to the membrane potential of erythrocytes (red blood cells) (see ESI,† S3).26
We then applied the system to separate species with different charges (Fig. 5). Solutions of [Ru(bpy)3]Cl2 and fluorescein were flowed through all three inlets and then sodium chloride was added to the central inlet, forming the liquid junction potential. The flow rates were varied from 20–1.2 μL min−1 and imaged using 458 nm and 488 nm excitation optimised using ZEN Black 2010 for the two dyes (see ESI,† S7). At pH 6.5, the two dyes are oppositely charged, the [Ru(bpy)3]Cl2 migrates to the edges of the channel, and the fluorescein is concentrated into the centre. The spectral properties of fluorescein did not significantly change in the presence of salt (see ESI,† S4).
The experiment was repeated with just fluorescein in the absence of [Ru(bpy)3]Cl2 (see ESI,† S8). While such movement in response to ionic gradients is obvious, microfluidic devices allow stable gradients to be generated under conditions suitable for the direct observation of molecular transport. Regulating the movement of photoredox catalysts like Ru(bpy)3Cl2 could allow spatial control of reactions. The generated liquid junction potentials could control the formation of concentration-dependent self-assemblies27 and offer insight into how concentration gradients control the movement of species in biology.
This work was supported by the Australian Research Council (DP220101847). A. Sen acknowledges support by the Sloan Foundation. We acknowledge the Mark Wainwright Analytical Centre at UNSW Sydney for access to the NMR facility and the UNSW node of the NCRIS-enabled Australian National Fabrication Facility (ANFF) and Dr Jasper Fried for device fabrication. The imaging component of this study was carried out using instruments situated in, and maintained by, the Katharina Gaus Light Microscopy Facility (KGLMF) at UNSW.
Data availability
All data for this work is deposited on the ChemRxiv server, see L. L. Fillbrook, I. A. Middleton, H. Rashidnejad, A. Sapre, A. Sen, J. E. Beves, T. W. Schmidt. DOI: 10.26434/chemrxiv-2024-tvmn2-v3.Conflicts of interest
There are no conflicts to declare.References
- S. Sengupta, M. M. Spiering, K. K. Dey, W. Duan, D. Patra, P. J. Butler, R. D. Astumian, S. J. Benkovic and A. Sen, ACS Nano, 2014, 8, 2410–2418 CrossRef CAS PubMed.
- M. Wanunu, W. Morrison, Y. Rabin, A. Y. Grosberg and A. Meller, Nat. Nanotechnol., 2010, 5, 160–165 CrossRef CAS PubMed.
- Y. A. Song, S. Hsu, A. L. Stevens and J. Han, Anal. Chem., 2006, 78, 3528–3536 CrossRef CAS PubMed.
- V. S. Doan, P. Saingam, T. Yan and S. Shin, ACS Nano, 2020, 14, 14219–14227 CrossRef CAS PubMed.
- M. S. Munson, C. R. Cabrera and P. Yager, Electrophoresis, 2002, 23, 2642–2652 CrossRef CAS PubMed.
- J. P. Ebel, J. L. Anderson and D. C. Prieve, Langmuir, 1988, 4, 396–406 CrossRef CAS.
- A. Kar, T. Y. Chiang, I. Ortiz Rivera, A. Sen and D. Velegol, ACS Nano, 2015, 9, 746–753 CrossRef CAS PubMed.
- J. Deseigne, C. Cottin-Bizonne, A. D. Stroock, L. Bocquet and C. Ybert, Soft Matter, 2014, 10, 4795–4799 RSC.
- S. Vrhovec Hartman, B. Bozic and J. Derganc, New Biotechnol., 2018, 47, 60–66 CrossRef CAS PubMed.
- B. Abécassis, C. Cottin-Bizonne, C. Ybert, A. Ajdari and L. Bocquet, Nat. Mater., 2008, 7, 785–789 CrossRef PubMed.
- B. Abécassis, C. Cottin-Bizonne, C. Ybert, A. Ajdari and L. Bocquet, New J. Phys., 2009, 11 CrossRef.
- R. Yadav, N. Sivoria and S. Maiti, J. Phys. Chem. B, 2024, 128, 9573–9585 CrossRef CAS PubMed.
- V. S. Doan, I. Alshareedah, A. Singh, P. R. Banerjee and S. Shin, Nat. Commun., 2024, 15, 7686 CrossRef CAS PubMed.
- S. Sanchez, A. A. Solovev, S. M. Harazim and O. G. Schmidt, J. Am. Chem. Soc., 2011, 133, 701–703 CrossRef CAS PubMed.
- Y. Tu, F. Peng, X. Sui, Y. Men, P. B. White, J. C. M. van Hest and D. A. Wilson, Nat. Chem., 2017, 9, 480–486 CrossRef CAS PubMed.
- N. S. Mandal and A. Sen, Langmuir, 2021, 37, 12263–12270 CrossRef CAS PubMed.
- K. T. Krist, A. Sen and W. G. Noid, J. Chem. Phys., 2021, 155, 164902 CrossRef CAS PubMed.
- N. S. Mandal, A. Sen and R. D. Astumian, J. Am. Chem. Soc., 2023, 145, 5730–5738 CrossRef CAS PubMed.
- N. S. Mandal, A. Sen and R. D. Astumian, Chem, 2024, 10, 1147–1159 CAS.
- J. M. Schurr, B. S. Fujimoto, L. Huynh and D. T. Chiu, J. Phys. Chem. B, 2013, 117, 7626–7652 CrossRef CAS PubMed.
- J. Agudo-Canalejo, P. Illien and R. Golestanian, Nano Lett., 2018, 18, 2711–2717 CrossRef CAS PubMed.
- L. L. Fillbrook, J. P. Gunther, G. Majer, D. J. O’Leary, W. S. Price, H. Van Ryswyk, P. Fischer and J. E. Beves, J. Am. Chem. Soc., 2021, 143, 20884–20890 CrossRef CAS PubMed.
- F. Mohajerani, X. Zhao, A. Somasundar, D. Velegol and A. Sen, Biochemistry, 2018, 57, 6256–6263 CrossRef CAS PubMed.
- D. R. Lide, CRC Handbook of Chemistry and Physics: A Ready-reference Book of Chemical and Physical Data, CRC-Press, 1995 Search PubMed.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85–277 CrossRef CAS.
- U. V. Lassen and O. Stenknud, J. Physiol., 1968, 195, 681–694 CrossRef CAS PubMed.
- W. Wang, Y.-X. Wang and H.-B. Yang, Chem. Soc. Rev., 2016, 45, 2656–2693 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and additional data are supplied. See DOI: https://doi.org/10.1039/d4cc06182a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |