Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lewis acid-catalyzed [2π+2σ] cycloaddition of dihydropyridines with bicyclobutanes†
Yujie
Liang
 ,
Ronewa
Nematswerani
,
Ronewa
Nematswerani
 ,
Constantin G.
Daniliuc
,
Constantin G.
Daniliuc
 and
Frank
Glorius
and
Frank
Glorius
 *
*
Organisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149 Münster, Germany. E-mail: glorius@uni-muenster.de
First published on 7th January 2025
Abstract
Herein we report a simple BF3-catalyzed cycloaddition of dihydropyridines with bicyclobutanes for the expedient synthesis of novel three-dimensional azacycle-fused bicyclo[2.1.1]hexane scaffolds. The reaction utilizes easily accessible starting materials and proceeds under mild, metal-free conditions with high atom efficiency.
Building on the success of the “escape from flatland” concept in pharmaceutical development, there is growing interest among chemists in creating efficient methods to rapidly construct conformationally rigid, C(sp3)-enriched scaffolds. These structures hold immense potential for phenyl or pyridinyl bioisosterism, with a higher proportion of sp3-hybridized carbon atoms that is often linked to enhanced physicochemical and pharmacokinetic properties of drug candidates.1 This is particularly true for bicyclo[2.1.1]hexanes (BCHs), which have shown promise as benzene bioisosteres due to their rigid conformation.2 Consequently, there is a continual pursuit of new methods for the rapid construction of these ring systems with diverse substitution patterns.
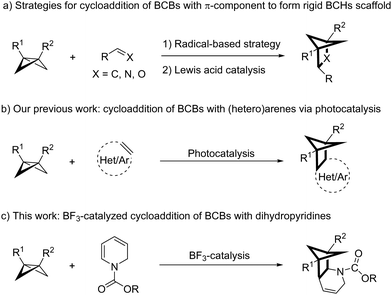
To synthesize these coveted BCH building blocks in a straightforward and atom-economical way, the direct cycloaddition of bicyclobutanes (BCBs) with π-components has been the method of choice in recent years. Accordingly, these methods can be categorized into two main reaction pathways: those that proceed via a radical-based mechanism3 and those that utilize Lewis acid catalysis4 (Scheme 1a). Given the ubiquity of (hetero)arenes, the dearomative cycloaddition of (hetero)arenes with BCBs represents an attractive approach to their synthesis. In this context, Deng5 and Feng6 independently reported the Lewis acid-catalyzed [2π+2σ] cycloaddition reactions of BCBs with indole derivatives to afford indoline fused BCHs. In addition, through photocatalysis strategies, our group has achieved the direct cycloaddition reactions of BCBs with diverse (hetero)arenes (e.g. indoles, coumarins, flavones, (iso)quinolines, quinazolines and phenols) to produce highly substituted BCHs (Scheme 1b).7 Pyridines are readily available and abundant feedstock chemicals, and the development of new cycloaddition reactions could facilitate their use in rapidly building up molecular complexity. However, due to their inherent stability from aromaticity, cycloaddition of these aromatic cores with BCBs remains challenging.8 Inspired by the elegant work on cycloaddition reactions of dihydropyridines (synthesized in one step from pyridines) with α-substituted acroleins,9 as well as recent advancements in Lewis acid-catalyzed BCB-based cycloaddition chemistry,4–6 we envisioned that a Lewis acid catalytic strategy would be capable of achieving [2π+2σ] cycloaddition of dihydropyridines with BCBs (Scheme 1c). Such a method could provide straightforward access to BCHs directly fused to azacycles, which are among the most frequently encountered structural motifs in FDA-approved pharmaceuticals.10 Driven by our ongoing interest in dearomative cycloaddition reactions of (hetero)arenes with BCBs7 and prompted by the growing demand of conformationally restricted, saturated scaffolds in drug discovery, herein, we report that the direct cycloaddition of dihydropyridines with BCBs can be smoothly executed under simple BF3 catalysis (Scheme 1c).
Initially, we chose BCB 1a and dihydropyridine 2a as the model substrates, and we were pleased to find that, after stirring them in DCM at room temperature for 24 h with BF3·Et2O as catalyst, the corresponding [2π+2σ] cycloaddition product 3a was furnished in 56% yield, with no formation of a [4π+2σ] cycloaddition product observed (Table 1, entry 1). The structure of 3a was confirmed by single-crystal X-ray diffraction analysis (CCDC: 2400706†). Other Lewis acid catalysts were also tested but gave poorer results (Table 1, entries 2–6). A control experiment showed that the Lewis acid catalyst was crucial for this transformation (Table 1, entry 7). Other solvents were subsequently investigated, and MeCN was found to be superior (Table 1, entries 8–14). Performing the reaction with 1.5 equivalent of 2a gave a similar yield (Table 1, entry 15). Using dihydropyridine 2a as limiting reagent and slightly excess BCB did not improve the yield significantly (Table 1, entry 16). Furthermore, using a higher loading of Lewis acid catalyst or conducting the reaction at 50 °C afforded the product 3a in comparable yields (Table 1, entries 17 and 18). Finally, we conducted the reaction on a 0.2 mmol scale and the product 3a can be isolated in 60% yield (Table 1, entry 19). Notably, condition-based sensitivity assessment11 indicated that moisture supressed the reaction, while other reaction parameters such as concentration, O2 level, reaction temperature and scale showed only negligible effects on the reaction outcome (Table 1).
| Entry | Catalyst | Solvent | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.12 mmol), catalyst (10 mol%), solvent (1 mL), Ar, rt, 24 h. Yields were determined by 1H NMR analysis of the crude mixture using CH2Br2 as an internal standard. b Using 2a (0.15 mmol). c Using 1a (0.12 mmol) and 2a (0.1 mmol). d Using catalyst (20 mol%). e Reaction conducted at 50 °C. f Reaction condition: 1a (0.2 mmol), 2a (0.24 mmol), catalyst (10 mol%), solvent (2 mL), Ar, rt, 24 h. Isolated yield is showed. | |||
| 1 | BF3·Et2O | DCM | 56 |
| 2 | Sc(OTf)3 | DCM | 42 |
| 3 | Eu(OTf)3 | DCM | 11 |
| 4 | AlCl3 | DCM | <5 |
| 5 | TMSOTf | DCM | 16 |
| 6 | Y(OTf)3 | DCM | <5 |
| 7 | — | DCM | — |
| 8 | BF3·Et2O | MeCN | 69 |
| 9 | BF3·Et2O | THF | 35 |
| 10 | BF3·Et2O | PhMe | 66 |
| 11 | BF3·Et2O | EtOAc | 68 |
| 12 | BF3·Et2O | Acetone | 67 |
| 13 | BF3·Et2O | DME | 43 |
| 14 | BF3·Et2O | CHCl3 | 50 |
| 15b | BF3·Et2O | MeCN | 70 |
| 16c | BF3·Et2O | MeCN | 70 |
| 17d | BF3·Et2O | MeCN | 72 |
| 18e | BF3·Et2O | MeCN | 70 |
| 19f | BF3·Et2O | MeCN | 60 |
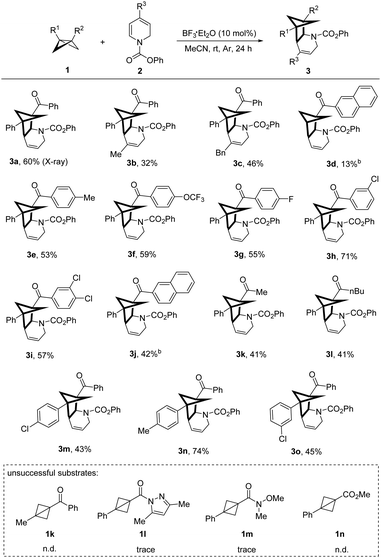
With the optimized conditions in hand, an initial survey of the dihydropyridines and BCBs substrate scope was investigated (Table 2). To our delight, alkyl-substituted dihydropyridines can be accepted in this protocol (3b, 3c). Although the use of a mono-substituted BCB resulted in a lower yield of product (3d) due to decomposition under the reaction conditions, various disubstituted ketone BCBs with electron-donating or electron-withdrawing groups reacted smoothly, producing cycloadducts in moderate to good yields (3e–3o). Among them, medicinally relevant trifluoromethyl and fluoro groups can be tolerated (3f, 3g). Pleasingly, alkyl ketone BCBs can also be accommodated in this protocol (3k, 3l). Other BCB substrates were also tested, for examples, substrates 1k, 1l, and 1m produced little or no product, with a significant amount of starting material remaining. Substrate 1n, however, decomposed under the reaction conditions, resulting in an uncharacterized complex mixture. In addition to dihydropyridines, we were delighted to find that, dihydroisoquinoline could also undergo the corresponding [2π+2σ] cycloaddition (Scheme 2).
Based on the observed regioselectivity and literature reports,4–6 a plausible reaction mechanism is proposed (Scheme 3). Initially, the BCB substrate 1a coordinates with the Lewis acid catalyst to form complex A, which then undergoes enolization to yield species B. Subsequently, the electron-rich dihydropyridine 2a nucleophilically attacks B to generate enolate and iminium intermediate C. Finally, intramolecular cyclization gives product 3a and regenerates the BF3 catalyst.
In conclusion, we have developed a new BF3-catalyzed cycloaddition reaction between dihydropyridines and bicyclobutanes to create novel azacycle-fused BCH scaffolds. The reaction utilizes easily accessible starting materials and proceeds under mild conditions, further enriching the synthetic toolkit for rapid access to structurally diverse rigid scaffolds.
Generous financial support by the Alexander von Humboldt Foundation (Y. L.), the Deutsche Forschungsgemeinschaft (Chembion) and the ERC Advanced Grant (Agreement No. 101098156, HighEnT) is gratefully acknowledged.
Data availability
The data supporting this article has been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed; (b) T. J. Ritchie and S. J. F. Macdonald, Drug Discovery Today, 2009, 14, 1011–1020 CrossRef CAS PubMed; (c) F. Lovering, Med. Chem. Commun., 2013, 4, 515–519 RSC; (d) D. Trauner, Angew. Chem., Int. Ed., 2018, 57, 4177–4191 CrossRef CAS PubMed; (e) P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC; (f) M. A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 14046–14128 CrossRef CAS PubMed.
- (a) A. Denisenko, P. Garbuz, S. V. Shishkina, N. M. Voloshchuk and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2020, 59, 20515–20521 CrossRef CAS PubMed; (b) A. Denisenko, P. Garbuz, N. M. Voloshchuk, Y. Holota, G. Al-Maali, P. Borysko and P. K. Mykhailiuk, Nat. Chem., 2023, 15, 1155–1163 CrossRef CAS PubMed; (c) K. J. Woelk, K. Dhake, N. D. Schley and D. C. Leitch, Chem. Commun., 2023, 59, 13847–13850 RSC; (d) M. Zanini, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2024, 63, e202410207 CrossRef CAS PubMed; (e) J. Tsien, C. Hu, R. R. Merchant and T. Qin, Nat. Rev. Chem., 2024, 8, 605–627 CrossRef CAS PubMed; (f) T. Qin, M. He and W. Zi, Nat. Synth., 2024 DOI:10.1038/s44160-024-00659-6; (g) E. F. Plachinski, R. Z. Qian, R. Villanueva, D. L. Poole, T. Rosenthal and T. P. Yoon, J. Am. Chem. Soc., 2024, 146, 31400–31404 CrossRef CAS PubMed.
- (a) R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef CAS PubMed; (b) M. Xu, Z. Wang, Z. Sun, Y. Ouyang, Z. Ding, T. Yu, L. Xu and P. Li, Angew. Chem., Int. Ed., 2022, 61, e202214507 CrossRef CAS PubMed; (c) Y. Liang, R. Kleinmans, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2022, 144, 20207–20213 CrossRef CAS PubMed; (d) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 535–541 CrossRef CAS PubMed; (e) Y. Liu, S. Lin, Y. Li, J.-H. Xue, Q. Li and H. Wang, ACS Catal., 2023, 13, 5096–5103 CrossRef CAS; (f) H. Yan, Y. Liu, X. Feng and L. Shi, Org. Lett., 2023, 25, 8116–8120 CrossRef CAS PubMed; (g) M. Robichon, T. Kratz, F. Beyer, J. Zuber, C. Merten and T. Bach, J. Am. Chem. Soc., 2023, 145, 24466–24470 Search PubMed; (h) J. L. Tyler, F. Schäfer, H. Shao, C. Stein, A. Wong, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 16237–16247 CrossRef CAS PubMed; (i) Q. Fu, S. Cao, J. Wang, X. Lv, H. Wang, X. Zhao and Z. Jiang, J. Am. Chem. Soc., 2024, 146, 8372–8380 CrossRef CAS PubMed; (j) H. Ren, T. Li, J. Xing, Z. Li, Y. Zhang, X. Yu and J. Zheng, Org. Lett., 2024, 26, 1745–1750 CrossRef CAS PubMed; (k) J. Jeong, S. Cao, H.-J. Kang, H. Yoon, J. Lee, S. Shin, D. Kim and S. Hong, J. Am. Chem. Soc., 2024, 146, 27830–27842 CrossRef CAS PubMed; (l) Y. Liu, Z. Wu, J.-R. Shan, H. Yan, E.-J. Hao and L. Shi, Nat. Commun., 2024, 15, 4374–4383 CrossRef CAS PubMed; (m) S. Dutta, Y.-L. Lu, J. E. Erchinger, H. Shao, E. Studer, F. Schäfer, H. Wang, D. Rana, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 5232–5241 CrossRef CAS PubMed.
- (a) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. C. Leitch, Angew. Chem., Int. Ed., 2022, 61, e202204719 CrossRef CAS PubMed; (b) Y. Liang, F. Paulus, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2023, 62, e202305043 CrossRef CAS PubMed; (c) N. Radhoff, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2023, 62, e202304771 CrossRef CAS PubMed; (d) Z. Lin, H. Ren, X. Lin, X. Yu and J. Zheng, J. Am. Chem. Soc., 2024, 146, 18565–18575 CrossRef CAS PubMed; (e) S. Hu, Y. Pan, D. Ni and L. Deng, Nat. Commun., 2024, 15, 6128–6137 CrossRef CAS PubMed; (f) Q.-Q. Hu, L.-Y. Wang, X.-H. Chen, Z.-X. Geng, J. Chen and L. Zhou, Angew. Chem., Int. Ed., 2024, 63, e202405781 CrossRef CAS PubMed; (g) J.-Y. Su, J. Zhang, Z.-Y. Xin, H. Li, H. Zheng and W.-P. Deng, Org. Chem. Front., 2024, 11, 4539–4545 RSC; (h) K. Zhang, S. Tian, W. Li, X. Yang, X.-H. Duan, L.-N. Guo and P. Li, Org. Lett., 2024, 26, 5482–5487 CrossRef CAS PubMed; (i) D. Sarkar, S. Deswal, R. C. Das and A. T. Biju, Chem. Sci., 2024, 15, 16243–16249 RSC; (j) S. Nicolai and J. Waser, Chem. Sci., 2024, 15, 10823–10829 RSC; (k) J.-J. Wang, L. Tang, Y. Xiao, W.-B. Wu, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202405222 CrossRef CAS PubMed; (l) S. Dutta, C. G. Daniliuc, C. Mück-Lichtenfeld and A. Studer, J. Am. Chem. Soc., 2024, 146, 27204–27212 CrossRef CAS PubMed; (m) W.-B. Wu, B. Xu, X.-C. Yang, F. Wu, H.-X. He, X. Zhang and J.-J. Feng, Nat. Commun., 2024, 15, 8005–8014 CrossRef CAS PubMed; (n) X.-G. Zhang, Z.-Y. Zhou, J.-X. Li, J.-J. Chen and Q.-L. Zhou, J. Am. Chem. Soc., 2024, 146, 27274–27281 CrossRef CAS PubMed; (o) Y. Xiao, F. Wu, L. Tang, X. Zhang, M. Wei, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202408578 CAS; (p) J. Zhang, J.-Y. Su, H. Zheng, H. Li and W.-P. Deng, Angew. Chem., Int. Ed., 2024, 63, e202318476 CrossRef CAS PubMed; (q) F. Wu, W.-B. Wu, Y. Xiao, Z. Li, L. Tang, H.-X. He, X.-C. Yang, J.-J. Wang, Y. Cai, T.-T. Xu, J.-H. Tao, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202406548 CrossRef CAS PubMed; (r) X. Wang, R. Gao and X. Li, J. Am. Chem. Soc., 2024, 146, 21069–21077 CrossRef CAS PubMed.
- D. Ni, S. Hu, X. Tan, Y. Yu, Z. Li and L. Deng, Angew. Chem., Int. Ed., 2023, 62, e202308606 CrossRef CAS PubMed.
- L. Tang, Y. Xiao, F. Wu, J.-L. Zhou, T.-T. Xu and J.-J. Feng, Angew. Chem., Int. Ed., 2023, 62, e202310066 CrossRef CAS PubMed.
- (a) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc and F. Glorius, Nature, 2022, 605, 477–482 CrossRef CAS PubMed; (b) R. Kleinmans, S. Dutta, K. Ozols, H.-L. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2023, 145, 12324–12332 CrossRef CAS PubMed; (c) S. Dutta, D. Lee, K. Ozols, C. G. Daniliuc, R. Shintani and F. Glorius, J. Am. Chem. Soc., 2024, 146, 2789–2797 CrossRef CAS PubMed.
- J. Ma, F. Strieth-Kalthoff, T. Dalton, M. Freitag, J. L. Schwarz, K. Bergander, C. Daniliuc and F. Glorius, Chem, 2019, 5, 2854–2864 CAS.
- M. Hatano, Y. Goto, A. Izumiseki, M. Akakura and K. Ishihara, J. Am. Chem. Soc., 2015, 137, 13472–13475 CrossRef CAS PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- L. Pitzer, F. Schäfers and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 8572–8576 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2400706. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc06133k |
| This journal is © The Royal Society of Chemistry 2025 |