Recent developments in pillar[5]arene-based nanomaterials for cancer therapy
Yu
Dai†
,
Wenqiang
Yu†
,
Yushan
Cheng
,
Yao
Zhou
,
Jiaye
Zou
,
Yujia
Meng
,
Feiyu
Chen
,
Yihan
Qian
and
Yong
Yao
 *
*
School of Chemistry and Chemical Engineering, Nantong University, Nantong, Jiangsu 226019, P. R. China. E-mail: yaoyong1986@ntu.edu.cn
First published on 3rd January 2025
Abstract
Nanomaterials possess unique size characteristics, enabling them to cross tissue gaps, penetrate the blood–brain barrier and endothelial cells, and release drugs at the cellular level. Additionally, the surface of nanomaterials is readily functionalized, endowing them with good biocompatibility, low biotoxicity, and specific targeting. All these advantages render nanomaterials broad application prospects in tumor therapy. Pillar[5]arenes are a new category of macrocyclic host compounds featuring rich host–guest properties and diverse environmental responses. In recent years, by combining the advantages of pillar[5]arenes and nanomaterials, the application of pillar[5]arene-based nanomaterials in tumor therapy has drawn extensive attention from scientists. In this review, we summarize five distinct types of pillar[5]arene-based nanomaterials: (1) pillar[5]arene-modified inorganic nanomaterials; (2) pillar[5]arene-modified organic porous materials; (3) pillar[5]arene-modified organic/inorganic hybrid materials; (4) nanomaterials self-assembled from pillar[5]arene-based host–guest complexes; (5) nanomaterials self-assembled from amphiphilic pillar[5]arenes. Moreover, the different tumor treatment modes of these nanomaterials, including chemotherapy, photodynamic therapy, photothermal therapy, gene therapy, and multimodal synergistic therapy, are also elaborated in detail.
1. Introduction
Cancer is one of the foremost causes of human death. In 2023, there were more than 20 million new cases and around 10 million deaths.1 Several methods for cancer treatment, such as surgery, radiation, and chemotherapy, have been developed.2 However, as of yet, no available approach has achieved complete therapeutic success. This is mainly because these methods are often nonspecific and have various side effects on healthy cells and tissues.3 In recent years, nanomaterials, typically in the size range of 1 to 100 nm, have garnered significant attention from scientists in cancer therapy due to their diverse electrical, optical, and magnetic properties.4 Furthermore, the high surface–volume ratio, high load capacity, the ability to synthesize nanomaterials with adjustable size, morphology, and surface chemistry, and the ease of functionalizing them with different biomolecules make them an excellent reagent for imaging, therapy, and drug delivery.5,6Supramolecular macrocycles are cyclic molecules formed through non-covalent interactions such as hydrogen bonding, π–π stacking, and host–guest interactions.7 Synthetic supramolecular macrocycles include cyclodextrins, cucurbiturils, calixarenes, and crown ethers.8–11 Synthetic supramolecular macrocycles offer unique advantages, such as controlled release, reduced toxicity, enhanced stability, and selective recognition, for the development of therapeutic nanomaterials, but there are also numerous challenges, like complex synthesis, uncertain biocompatibility, stability in biological environment, and scale-up challenges, that need to be addressed through further research and technological innovation to realize their full potential in the field of medicine.12,13
Pillar[5]arenes (P5s), the newest representative supramolecular macrocycles, which are composed of five hydroquinone units bridged by methylene at the p-position, were initially designed and synthesized in 2008 by Ogoshi.14P5 contains a symmetrical pillar-like cavity and ten functionalizable sites, enabling easy modification and endowing it with superiority over other macrocyclic hosts in host–guest chemistry.15–19 Over the past decade, their synthesis, configuration transformation, host–guest properties, and applications have been extensively investigated.20–27
Pillar[5]arene and its derivatives play different important roles in various nanomaterials, such as backbone building units, drug loading sites, and stimulus-responsive nano-valves, providing valuable insights for the design of multifunctional therapeutic nanomaterials.28–34 What's more, previous studies have shown that pillar[n]arenes exhibit very low cytotoxicity even at a high concentration (500 μmol L−1), which lays a foundation for their application in biological systems.35 By combining the abundant molecular recognition capability of P5s with the excellent optical, electrical, and magnetic properties of nanomaterials, as well as their good biocompatibility and low biotoxicity, significant research progress has been achieved in the field of cancer therapy through the use of P5-based nanomaterials in recent years.36–40 Thus, this Feature Article presents an overview of P5-based nanomaterials, including inorganic nanomaterials, organic porous materials, organic/inorganic hybrid materials, host–guest complex self-assembled nanomaterials, and amphiphilic P5s self-assembled nanomaterials, for multimodal cancer therapy such as chemotherapy, photodynamic therapy, photothermal therapy, and synergistic therapy in the recent five years.
2. P5 based inorganic nanomaterials
2.1 P5 based inorganic porous nanomaterials
As a potential alternative system for cancer treatment, gated nanocarriers for the smart controlled release of chemotherapeutic drugs have garnered widespread attention.41 These systems can be constructed by attaching supramolecular binding sites to mesoporous silica nanoparticles (MSNPs), which interact with mobile components and reversibly open and close the entrance of nano-channels of MSNPs, enabling the release of contents upon external stimuli.Santos et al. designed and constructed a novel pH-responsive nanocarrier loaded with DOX by combining negatively charged MSNPs with water-soluble cationic pillar[5]arene (CWP5) nano-gates, achieving smart controlled release.42 The results indicated that the release of DOX from both nanocarriers was greater at pH 2.0 than at pH 5.5. Moreover, due to the reversibility of electrostatic interactions, MCM-41-COO-DOX-CWP5 exhibited potential intelligent switch release behavior. At the same concentration, MCM-41-COO-CWP5 loaded with DOX showed higher cytotoxicity compared to free DOX. Confocal fluorescence microscopy demonstrated that the nanocarriers were capable of releasing DOX inside the cell nucleus. Therefore, this novel nanocarrier seems to be a potential candidate for an alternative system in cancer therapy (Fig. 1). Besides, Prof. Yang's group fabricated supramolecular nanomaterials based on hollow mesoporous drug carriers and WP5Na-capped CuS nanogates for synergistic chemo-photothermal therapy successfully.43
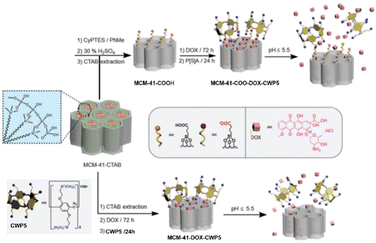 | ||
| Fig. 1 Schematic representation of the preparation of DOX-loaded MCM-41-COO- and its interaction with CWP5 nanogate. The same procedure was carried out for non-functionalized MCM-41. MCM-41-COO-DOX-CWP5 and MCM-41-DOX-CWP5 can be operated by chemical stimulus (pH changes) to regulate the release of doxorubicin (DOX). Reproduced with permission from ref. 42. Copyright 2020 Royal Society of Chemistry. | ||
2.2 P5 based inorganic nonporous nanomaterials
Inorganic metal nanoparticles possess numerous advantages, such as high chemical stability, ease of synthesis, biocompatibility, and excellent photothermal conversion ability.44,45 Recently, researchers have presented several types of P5-modified inorganic metal nanomaterials for cancer therapy. For example, in 2020, Zhang's group designed a mitochondria-targeted and pH-switchable supramolecular photosensitizer derived from pillar[5]arene-modified gold nanoparticles.46 As shown in Fig. 2, they attached water-soluble carboxyl pillar[5]arene sodium (WP5Na) and (5-carboxypentyl)triphenylphosphonium bromide (G1) to gold nanoparticles (AuNPs) through host–guest interaction to construct a mitochondria-targeting host motif (AuTP). Based on the AuTP host motif, they fabricated the mitochondria-targeted and pH-switchable supramolecular photosensitizer (AuTSP), in which tetrakis(4-pyridyl)porphyrin (TPyP) and terminal cyano-functionalized polyethylene glycol (PEG) chains served as guest motifs. The photodynamic therapy (PDT) effect of AuTSP is quenched during circulation via the Förster resonance energy transfer (FRET) effect. Once entering the acidic tumor microenvironment, AuTSP dissociates, activating the PDT effect. This activation is due to the host–guest interaction and pH responsiveness of WP5Na on the gold nanoparticles. Furthermore, the hybrid switchable supramolecular photosensitizer integrates mitochondria-targeting and reductive glutathione (GSH) scavenging, prolonging the lifetime of PDT-generated reactive oxygen species near mitochondria and enhancing the PDT efficacy.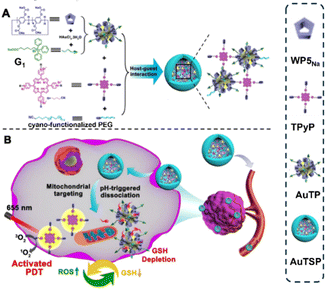 | ||
| Fig. 2 (A) Preparation of mitochondria-targeting and pH-switched supramolecular photosensitizers (AuTSP) through host–guest interaction among pillar[5]arene modified Au NPs (AuTP), TPyP, and PEG in aqueous solution. The photoactivity of TPyP was significantly quenched by AuTP. (B) Illustration of self-amplified and pH-activated PDT process. Reproduced with permission from ref. 46. Copyright 2020 American Chemical Society. | ||
Besides, they also constructed a hierarchical supramolecular multifunctional nanoplatform by using a reversible host–guest interaction between WP5Na-capped gold nanoparticles (AuPs) as the nanozyme and porphyrin-containing amphiphilic block copolymers (PS) as the photosensitizer, realizing specific and efficient programming tumor therapy.47
With the swift progress of nanotechnology, near-infrared (NIR) light-assisted therapy as a tumor treatment approach, especially photothermal therapy (PTT) based on NIR irradiation, which can convert NIR irradiation energy into heat therapy to eliminate cancer cells, has garnered widespread attention.48
Cu2−xSe nanoparticles were selected as the photothermal agent due to their remarkable photothermal conversion ability under NIR irradiation. Employing cationic pillar[5]arene (CWP5) as the stabilizer, a supramolecular nano-system based on CWP5 functionalized Cu2−xSe nanoparticles (Cu2−xSe@CWP5 NPs) was constructed, integrating ATP capture and mitochondria-targeting molecule (TPP).49 Under laser irradiation, Cu2−xSe@CWP5/TPP NPs showed significant photothermal ablation capability against cancer cells. As anticipated, Cu2−xSe@CWP5/TPP NPs also demonstrated excellent therapeutic effects because of the inhibition of ATP hydrolysis and targeted photothermal therapy (Fig. 3). Furthermore, Prof. Pei constructed synergistic and targeted drug delivery systems based on nano-CeO2 capped with galactose-functionalized pillar[5]arene via host–guest interactions.50 Professor Stoikov prepared stable pillar[5]arene/Ag+ nanoparticles, which consist of water-soluble pillar[5]arene containing γ-sulfobetaine fragments and Ag+ ions without Ag–Ag bonds for the treatment of the human lung cancer cell line A549.51 Prof. Yang constructed WP5Na-modified Au nanorods as nanocarriers for multi-modal imaging-guided synergistic PDT and PTT.52
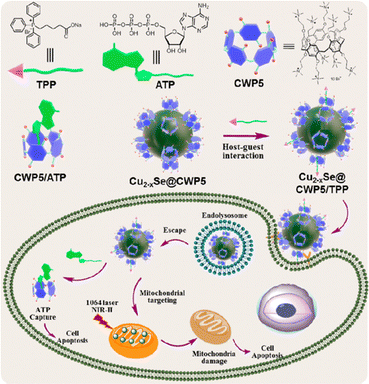 | ||
| Fig. 3 Chemical structures of mitochondria-targeted molecule (TPP), adenosine triphosphate (ATP), and cationic water-soluble pillar[5]arene (CWP5) and the schematic illustration of constructing Cu2−xSe@CWP5/TPP supramolecular NPs and their application in targeted photothermal therapy. Reproduced with permission from ref. 49. Copyright 2022 American Chemical Society. | ||
The advantages of pillar[5]arene-based inorganic nanomaterials lie in their relatively good stability and strong modifiability, which are conducive to drug loading and so on. The disadvantages are the relatively high preparation cost and complex manufacturing processes. The applicable cancer models include lung cancer and gastric cancer models. They can help with targeted drug delivery and diagnostic imaging by virtue of their unique properties, thus providing new ideas for cancer treatment.
3. P5 based organic porous nanomaterials
3.1 Covalent organic polymers
Covalent organic polymeric materials (COMs), a new kind of materials linked by covalent bonds and encompassing two-dimensional and three-dimensional network structures, have drawn extensive attention.53 Owing to their outstanding biocompatibility and diverse modifications, COMs with different structures have been widely applied in sensing, gas separation/storage, catalysis and other fields and have also found extensive usage in the biomedical field.54 In 2022, our group designed and fabricated a novel COMs material (P5COMs) by taking aldehyde-modified pillar[5]arene (P5CHO) and tetra-(4-aminophenyl)porphyrin (TAPP) as raw materials and connecting them through dynamic covalent imine bond as an efficient cancer treatment platform. P5COMs has the ability to target cancer cells and the capacity to load drugs (Fig. 4). Under near-infrared irradiation, P5COMs not only generates singlet oxygen (1O2) but also produces a photothermal effect, directly killing cancer cells. At the same time, the near-infrared-induced photothermal effect helps to accelerate the release of doxorubicin (DOX) loaded in P5COMs, enhancing the chemotherapy efficacy.55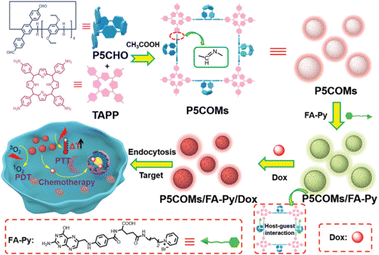 | ||
| Fig. 4 Schematic illustration of pillar[5]arene-based covalent organic polymers for targeted and combined cancer photo- and chemotherapy. Reproduced with permission from ref. 55. Copyright 2022 Royal Society of Chemistry. | ||
3.2 Covalent organic frameworks
Covalent organic frameworks (COFs) have drawn extensive attention because of their high designability, large specific surface area and porosity, favorable chemical stability, and adjustable physical and chemical properties. In this regard, the Yang research team has created a nanomaterial for the treatment of periodontitis. This nanomaterial is based on pillararene-embedded covalent organic frameworks (PCOFs) combined with the antibacterial prodrug thioacetal (TA).34 The drug-loaded nanoplatform, PCOF-TA, takes advantage of the self-amplifying reactive oxygen species (ROS) characteristic to improve the treatment effect. When serving as drug carriers, covalent organic frameworks possess outstanding photosensitivity and the capacity to produce reactive oxygen species. Once in the ROS environment, the thioacetal within the nanoplatform gets activated and breaks down into cinnamaldehyde (CA), which is a highly effective antibacterial substance. By using visible light to activate the targeting of specific infection areas, PCOF-TA successfully relieved periodontitis, thereby contributing to the progress of the antibacterial drug delivery systems field (Fig. 5).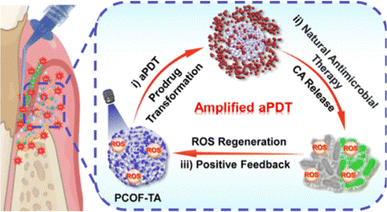 | ||
| Fig. 5 Schematic illustration of PCOF-TA as a self-supplied ROS drug delivery platform with anti-periodontitis efficacy. Reproduced with permission from ref. 34. Copyright 2024 American Chemical Society. | ||
Pillar[5]arene-based organic porous materials possess advantages like high specific surface area, multifunctional modification, good drug loading and controlled release, and regular pore structure with excellent selective adsorption. However, their synthesis conditions are harsh and stability sometimes insufficient. They’re applicable in liver and colon cancer models, helping with targeted drug delivery to improve cancer treatment efficacy.
4. P5 based hybrid organic/inorganic nanomaterials
Organic–inorganic hybrid materials, which incorporate different inorganic elements and organic ligands or polymers into the system, not only display the relevant properties of the inorganic active center or organic matter but also manifest numerous unique and novel properties through their interaction or energy transfer.56 In 2020, Professor Jung and colleagues prepared a dual-functional nano-carrier composed of fluorescein isothiocyanate (FITC)/pyridine-mesoporous silica (NP-3) and carboxylated pillar[5]arene-encapsulated gold nanoparticles (WP5Na-AuNPs), forming a host–guest complex that acts as a selective mitochondria-targeted drug delivery system (Fig. 6). After loading DOX and F16 onto NP-3 and WP5Na-AuNPs respectively, their selective accumulation in cancer cell mitochondria was observed. Co-assembled nanoparticles loaded with DOX and F16 (NP-1) were also delivered to mitochondria, indicating NP-1 as a dual drug carrier. Moreover, the anticancer activity of DOX concentrated in mitochondria was retained, suggesting that no structural deformation of DOX occurred during the carrier transport process.57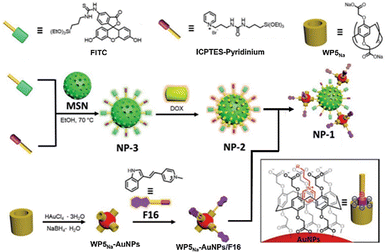 | ||
| Fig. 6 Fabrications of co-assembled silica–Au hybrid carriers for DOX and F16 (NP-1) consisted of DOX-loaded FITC/pyridinium-mesoporous silica nanoparticles (NP-2) and F16/WP5Na-AuNPs. Reprinted with permission from ref. 57. Copyright 2020 Wiley-VCH. | ||
Besides, by conjugating the thermal-responsive NO donor of S-nitrosothiols (RSNO) on the surface of mesoporous silica-coated gold nanorods (AuNRs@MSN) and coating a supramolecular complex consisting of a sugar-targeted NO nanogenerator modified with amino pillar[5]arene (NP5) and a half-lactose derivative (G3), Prof. Pei and colleagues designed and constructed a thermal-responsive radical initiator AIBI-loaded glycol-targeting NO nanogenerator (A-AuNRs@MSNSNO@NP5G).58 Under near-infrared (NIR) irradiation, the AuNRs in the nanogenerator can generate heat, inducing the decomposition of reactive RSNO and AIBI, achieving a comprehensive treatment that combines photothermal therapy, gas therapy, and alkyl radical therapy (Fig. 7).
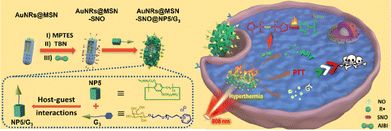 | ||
| Fig. 7 A scheme indicating the construction of AIBI-loaded glyco-targeting NO nanogenerator (A-AuNRs@MSN-SNO@CWP5/G3) and its triple-mode therapy of PTT, GT and ART. Reproduced with permission from ref. 58. Copyright 2022 American Chemical Society. | ||
The organic–inorganic hybrid materials based on pillar[5]arene combine the characteristics of organic and inorganic materials. They possess good modifiability and stability, have a strong drug-loading capacity and can achieve multi-modal synergistic effects. However, their preparation is complicated and the cost is relatively high. The applicable cancer models include breast cancer and pancreatic cancer models, etc., which can contribute to precise diagnosis and treatment and improve the effectiveness of anti-cancer therapies.
5. Nanomaterials self-assembled from P5 host–guest complexes
The superamphiphilic molecules based on pillar[5]arenes were constructed by means of host–guest interaction, resulting in the further self-assembly of nanomaterials.59–62 The different types of pillar[5]arenes comprise anionic water-soluble pillar[5]arene (WP5),63–65 cationic water-soluble pillar[5]arene (CWP5),66,67 neutral water-soluble pillar[5]arene (NWP5),68–70 A1/B1 type pillar[5]arenes,71–73 mono-functional pillar[5]arenes,74–77 and bridge-extend pillar[5]arenes.785.1 Anionic water-soluble P5 (WP5) based host–guest complex
The first water-soluble pillar[5]arene that was prepared is anionic water-soluble pillar[5]arene, which is characterized by simple synthesis, excellent water solubility, diverse host–guest properties and low cytotoxicity.79 As a result, it has wide applications in the construction of biological nanomaterials.In 2022, Prof. Liu's team successfully prepared water-soluble AIE photosensitizers (WAPS) with high targeting ability by supramolecular assembly-mediated methods.80 In neutral aqueous solutions, anionic water-soluble pillar[5]arene (WP5NH4) and WAPS can form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex through host–guest interactions, leading to significant changes in the donor–acceptor structure of WAPS and resulting in weaker photodynamic activity. However, under acidic conditions (pH 5.2), the binding interface between WAPS and WP5NH4 changes, enabling reversible control of singlet oxygen (1O2) generation, thus achieving targeted photodynamic therapy for cancer cells and achieving synergistic treatment effects. By combining AIE active photosensitizers with supramolecular assembly techniques, more precise targeted therapy can be achieved, providing a new pathway to improve the efficiency of cancer treatment and reduce side effects (Fig. 8).
1 complex through host–guest interactions, leading to significant changes in the donor–acceptor structure of WAPS and resulting in weaker photodynamic activity. However, under acidic conditions (pH 5.2), the binding interface between WAPS and WP5NH4 changes, enabling reversible control of singlet oxygen (1O2) generation, thus achieving targeted photodynamic therapy for cancer cells and achieving synergistic treatment effects. By combining AIE active photosensitizers with supramolecular assembly techniques, more precise targeted therapy can be achieved, providing a new pathway to improve the efficiency of cancer treatment and reduce side effects (Fig. 8).
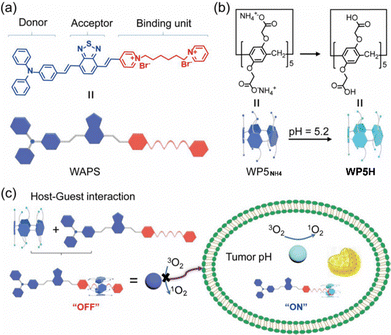 | ||
| Fig. 8 Chemical structures and cartoon representations of (a) WAPS, (b) anionic water-soluble pillar[5]arene (WP5NH4). (c) Schematic illustration of the targeted photodynamic therapy using a water-soluble aggregation-induced emission photosensitizer activated by an acidic tumor microenvironment. Reproduced with permission from ref. 80. Copyright 2022 Elsevier. | ||
Besides forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between guest molecule and pillar[5]arene, they can also form a 1
1 complex between guest molecule and pillar[5]arene, they can also form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
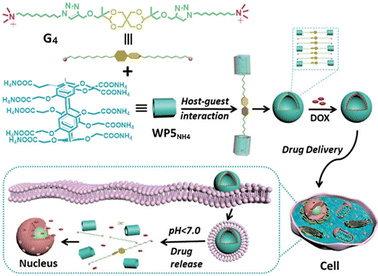
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex. For instance, in 2019, Prof. Wang and colleagues successfully constructed a dual-acid-responsive bola-type supramolecular vesicle for efficient intracellular delivery of anticancer drugs.81 The vesicle is composed of two WP5NH4 complexed with an acid-sensitive guest molecule (G4) containing a 2,4,8,10-tetraoxadecane segment (Fig. 9). Compared with the control system with only WP5NH4 single pH-responsive behavior, the dual-acid-responsive supramolecular vesicle showed higher drug release efficiency in the acidic microenvironment of cancer cells. Moreover, the DOX-loaded supramolecular vesicle not only significantly improved the anticancer efficacy of free DOX against tumor cells but also markedly reduced the side effects on normal cells, demonstrating its potential application prospects in cancer therapy (Fig. 9). Additionally, several other guests, such as bola-form naphthalimide guest and perylene diimide dye guest,82,83 can also form 1
2 host–guest complex. For instance, in 2019, Prof. Wang and colleagues successfully constructed a dual-acid-responsive bola-type supramolecular vesicle for efficient intracellular delivery of anticancer drugs.81 The vesicle is composed of two WP5NH4 complexed with an acid-sensitive guest molecule (G4) containing a 2,4,8,10-tetraoxadecane segment (Fig. 9). Compared with the control system with only WP5NH4 single pH-responsive behavior, the dual-acid-responsive supramolecular vesicle showed higher drug release efficiency in the acidic microenvironment of cancer cells. Moreover, the DOX-loaded supramolecular vesicle not only significantly improved the anticancer efficacy of free DOX against tumor cells but also markedly reduced the side effects on normal cells, demonstrating its potential application prospects in cancer therapy (Fig. 9). Additionally, several other guests, such as bola-form naphthalimide guest and perylene diimide dye guest,82,83 can also form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complexes with WP5NH4 to further self-assemble into nano-biomaterials.
2 host–guest complexes with WP5NH4 to further self-assemble into nano-biomaterials.
 | ||
| Fig. 9 Schematic illustration of constructing bola-type supramolecular vesicles based on anionic water-soluble pillar[5]arene host–guest complex and the application of supramolecular vesicles in anticancer drug delivery. Reproduced with permission from ref. 81. Copyright 2019 Royal Society of Chemistry. | ||
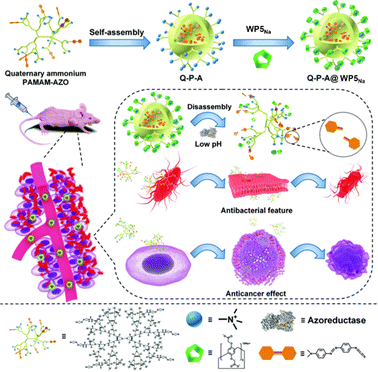
In the treatment of colorectal cancer (CRC), tumor resistance around the tumor is often caused by spore-forming bacteria, resulting in chemotherapy failure. Broad-spectrum antibiotics and multifunctional cationic quaternary ammonium materials have been widely utilized in the field of antibacterial and anticancer research. To achieve antibacterial and anticancer therapy, Prof. Liu designed an intelligent supramolecular quaternary ammonium nanocomposite named Quaternary Ammonium PAMAM-AZO@WP5Na (Q-P-A@WP5Na).84 This nanocomposite consists of azobenzene (AZO)-conjugated dendritic cationic polyamidoamine (PAMAM) as the core and carboxyl-functionalized pillar[5]arene (WP5Na) as the switchable component (Fig. 10). Quaternary ammonium salt-PAMAM-AZO (Q-P-A) is a quaternary ammonium compound with strong antibacterial and anticancer properties. Under normal circumstances, the –N+CH3 groups on the surface of Q-P-A are located inside the WP5Na cavity, thus improving the biocompatibility of Q-P-A@WP5Na. At the same time, WP5Na can effectively suppress the –N+CH3 groups under pathological conditions to achieve effective antibacterial and anticancer therapy. Experimental results indicate that Q-P-A@WP5Na shows good biocompatibility and antibacterial effects and can effectively treat colorectal cancer with minimal side effects.
 | ||
| Fig. 10 Schematic diagram for the fabrication of the dendritic supramolecular polymer Q-P-A@WP5Na from anionic water-soluble pillar[5]arene and its application for drug-resistant CRC therapy and representative components in this supramolecular system. Reproduced with permission from ref. 84. Copyright 2021 Royal Society of Chemistry. | ||
5.2 Cation water-soluble P5 (CWP5) based host–guest complex
The cationic water-soluble pillar[5]arenes (CWP5) modified with quaternary ammonium or pyridinium can further self-assemble to form nanomaterials by complexing with anionic guest molecules.85 Xanthate is a functional group with pH and H2O2 stimulus-responsive properties, having significant potential in constructing dual-responsive drug delivery systems. In 2022, Prof. Pei used sulfonated xanthate derivative (SXD) as the guest molecule and quaternary ammonium pillar[5]arene (CWP5) as the host molecule to propose a novel dual-stimuli-responsive supramolecular drug delivery system through host–guest interactions.86CWP5⊃SXD self-assembled into vesicles, effectively loading the anticancer drug DOX. In the tumor microenvironment, CWP5⊃SXD nanoparticles achieved efficient drug delivery and controlled release, significantly enhancing the anticancer efficacy of DOX against cancer cells (Fig. 11).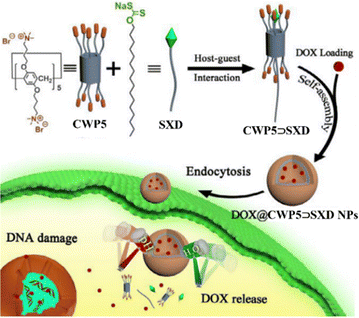 | ||
| Fig. 11 Schematic illustration of the construction of the supramolecular system based on cationic water-soluble pillar[5]arene (CWP5) and SXD and its application in controllable drug delivery. Reproduced with permission from ref. 86. Copyright 2022 Elsevier. | ||
5.3 Neutral water-soluble P5 based host–guest complex
In addition to ionic pillar[5]arenes, some neutral groups, such as glycosyl or glycol-chain modified pillar[5]arenes, can further self-assemble to form nanomaterials by complexing with specific guests in aqueous solution. The Pei research group has conducted a systematic study on the glycosyl modified water-soluble pillar[5]arene. In 2022, they successfully prepared a novel supramolecular drug delivery system (GP5Pro-ANI) based on the host–guest interaction between neutral glycosylated pillar[5]arene (GP5) and AIE PARP inhibitor prodrug (Pro-ANI).87 This system can self-assemble into nanovesicles with high DOX loading capacity, achieving targeted drug delivery, esterase-responsive release, drug uptake tracking, and chemosensitivity. Targeted drug delivery is accomplished through the specific binding of lactose clusters to overexpressed ASGPRs. Once internalized into tumor cells, high levels of esterase lead to the cleavage of amide bonds, resulting in vesicle rupture and efficient drug release. Moreover, the AIE effect of GP5Pro-ANI enables drug uptake tracking. DOX disrupts DNA structure, while ANI inhibits PARP activity, blocking the DNA damage repair pathway, inhibiting autophagy in cancer cells, inducing apoptosis, overcoming drug resistance, and inhibiting cancer cell migration. Therefore, the supramolecular nanodelivery system based on pillar[n]arene and PARP inhibitors provides an ideal example for visual drug uptake and resistant therapy (Fig. 12).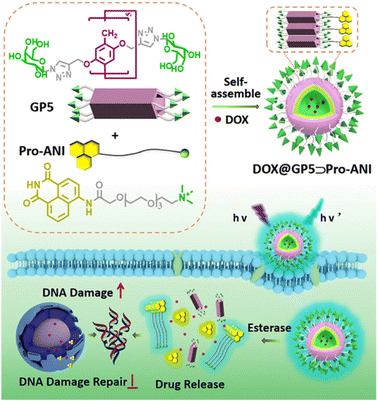 | ||
| Fig. 12 Schematic overview of the construction of a supramolecular nano-delivery system based on the PARP inhibitor prodrug and neutral water-soluble pillar[5]arene and the application for drug-resistance therapy. Reproduced with permission from ref. 87. Copyright 2022 Royal Society of Chemistry. | ||
Subsequently, in order to achieve real-time monitoring of the delivery of the non-fluorescent drug gemcitabine (GEM) to cancer cells, they prepared neutral mannose-functionalized pillar[5]arene (ManP5) and constructed a novel supramolecular fluorescent probe (ManP5⊃G5) from ManP5 and the guest molecule (G5) derived from the near-infrared fluorescent dye (4,4-dimethylaminostyryl-4H-pyran, DCM) through host–guest interactions.88 The research results show that ManP5⊃G5 self-assembles into nanovesicles, capable of loading GEM to form GEM@ManP5⊃G5 nanoparticles (NPs). These NPs exhibit good responsiveness to glutathione (GSH) and can recognize MCF-7 cells through mannose, achieving effective delivery of GEM and selective release under GSH stimulation. Meanwhile, the fluorescence recovery of DCM under GSH stimulation enables real-time monitoring of GEM release. Furthermore, GEM@ManP5⊃G5 NPs not only effectively reduce side effects on normal cells but also enhance the damage to cancer cells (Fig. 13).
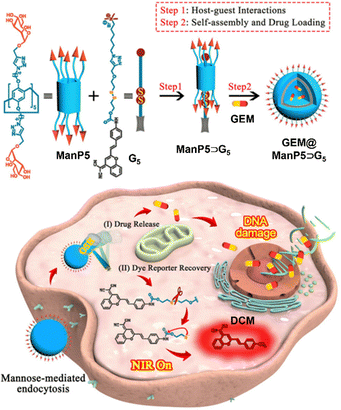 | ||
| Fig. 13 Schematic illustration of a neutral water-soluble mannose-functionalized pillar[5]arene-based supramolecular fluorescent probe and its application in the targeted delivery and real-time monitoring of gemcitabine. Reproduced with permission from ref. 88. Copyright 2023 Royal Society of Chemistry. | ||
Furthermore, their group also constructed a supramolecular nanoprodrug (DOX@GP5⊃Pro-NFA) based on the host–guest complexation of the chloride channel blocker prodrug (Pro-NFA) and glycosylated pillar[5]arene (GP5), which could target tumor cells via galactose and release DOX/NFA responsively under esterase stimulation.89
5.4 Mono-functionalized P5 based host–guest complex
When a functional group is modified on pillar[5]arene, the final self-assemblies can be endowed with more unique properties. In 2022, our group successfully designed and synthesized a pillar[5]arene modified with terpyridine (TP5).90TP5 could coordinate with Zn2+ to form a fluorescent pillar[5]arene–Zn complex (TP5/Zn), which was then used to interact with a guest molecule containing polyethylene glycol (PM) to successfully construct a supramolecular amphiphilic compound TP5/Zn/PM. Due to the amphiphilic nature and fluorescence properties of TP5/Zn/PM, it could self-assemble into fluorescent particles with a diameter of approximately 150 nm. These fluorescent particles could effectively load and control the release of anti-cancer drugs, achieving precise drug release and live cell imaging (Fig. 14).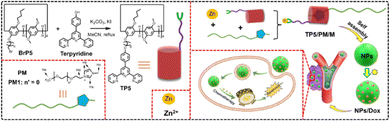 | ||
| Fig. 14 Synthesis route to mono-terpyridine-modified pillar[5]arenes (TP5) and cartoon representation of construction of supramolecular amphiphile based on TP5, guest molecule contains polyethylene glycol (PM) and Zn ions for cancer therapy. Reproduced with permission from ref. 90. Copyright 2023 MDPI. | ||
In addition, our group also prepared another mono-functionalized pillar[5]arene modified by peptide (P5-PLL-DMA).91 The prodrug SPP-DOX is constructed from P5-PLL-DMA and pyridine-terminated modified doxorubicin-dependent peptide (P-PLL-DOX) through host–guest recognition. Then, the host–guest complex encapsulates chlorin e6 (Ce6) to obtain the supramolecular peptide prodrug SPP-DOX/Ce6. At physiological pH 7.4, SPP-DOX/Ce6 exhibits high stability and a longer circulation time, effectively avoiding nonspecific protein adsorption and premature drug release. When it reaches the extracellular environment of tumor cells (pH 6.5), the P5-PLL-DMA group quickly hydrolyzes and undergoes a conversion from negative charge to positive charge, significantly enhancing cellular uptake and intracellular drug accumulation. Under 660 nm light irradiation, the generated reactive oxygen species can effectively cleave the TK linker, releasing activated DOX, thereby producing a combination effect of PDT and CT with good synergistic effects and enhancing the anti-tumor effect (Fig. 15).
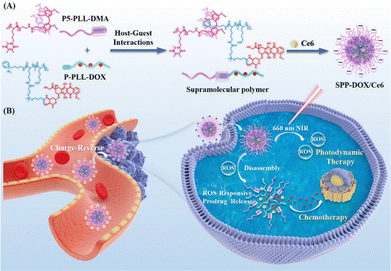 | ||
| Fig. 15 (A) Schematic illustration of the fabrication of supramolecular polymer from mono-functionalized pillar[5]arene and Ce6-loaded nanoparticles (SPP-DOX/Ce6); (B) the mechanism of charge-reverse and PDT-CT combination therapy of SPP-DOX/Ce6. Reproduced with permission from ref. 91. Copyright 2022 Elsevier. | ||
5.5 Bridge-extend P5 based host–guest complex
Pillar[5]arene can be modified not only on its phenolic hydroxyl group but also functionalized on its bridge-C.92 The bridge-extended pillar[5]arene obtained not only has the host–guest properties of a macrocycle but also introduces more functionality. By introducing an AIE-active TPE core and precisely designing orthogonal strategies to embed it into the water-soluble pillar[5]arene skeleton, a novel bridge-extended pillar[5]arene with TPE functionalized on the bridged methylene group of the water-soluble pillar[5]arene (m-TPEWP5) can be directly prepared. m-TPEWP5 simultaneously possesses host–guest recognition properties and AIE effects.93 Furthermore, the m-TPEWP5 host is orthogonally integrated with a custom-designed prodrug guest DNS-G to construct an AIE-active drug delivery system. The resulting supramolecular nanoparticles exhibit yellow emission and excellent GSH responsiveness, enabling effective release of SN-38 in the tumor cell microenvironment with high GSH concentration, making it a suitable candidate for cancer therapy. This supramolecular orthogonal strategy not only provides a simple and effective method for preparing mesoporous functionalized pillar[5]arene derivatives but also enriches the AIE-active water-soluble macrocyclic family. In addition to the above advantages, it can be anticipated that m-TPEWP5 with an interesting skeleton and excellent AIE performance has potential applications in photodynamic therapy, light harvesting functions, chemical sensing, and other fields (Fig. 16).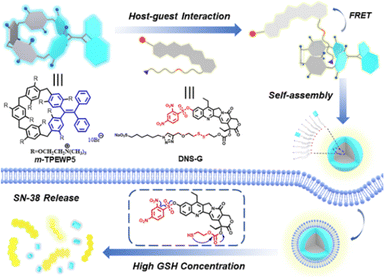 | ||
| Fig. 16 Schematic illustration of the formation of supramolecular nanoparticles from bridge-extend pillar[5]arene (m-TPEWP5) and their stimuli-responsive drug release. Reproduced with permission from ref. 93. Copyright 2021 American Chemical Society. | ||
As is known to all, supramolecular host–guest interactions offer an efficient and convenient way to control the “on–off” switch of ROS generation. By making use of the aggregation-induced emission (AIE) characteristics and Förster resonance energy transfer (FRET), attractive photosensitizer-related nanomaterials with controllable precise modulation functions can be developed. With bridge-extended pillar[5]arene m-TPEWP5 as an AIE host, combined with a spiropyran derivative (SP-G) as the guest and Nile blue (NiB) dye as the acceptor, a light-switchable two-step FRET process is achieved, making the system a suitable candidate for controllable PDT treatment responsive to changes in light wavelength. The reversible isomerization process of SP-G endows this PS with switchability between “on” and “off” states. Upon exposure to UV light, the non-emissive closed-form guest SP-G can be converted to the emissive open-form merocyanine MC-G (Fig. 17). The in situ formed MC-G serves as both the first energy acceptor and an efficient PS for ROS generation. Additionally, the introduction of NiB enhances synergistic ROS-generation activity, resulting in a two-step dynamic FRET process under UV and visible light irradiation. Furthermore, this photoswitchable system exhibits excellent controllable fluorescence performance and ROS-generation ability, making it suitable for use in cancer cell and bacteria inactivation.94
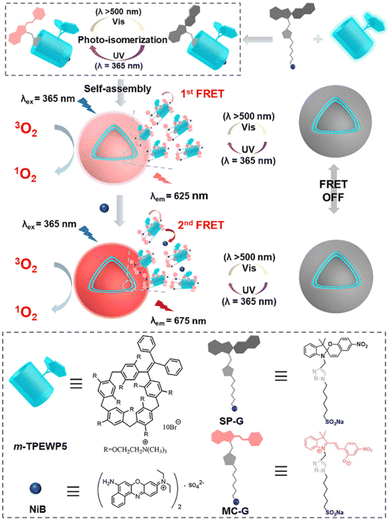 | ||
| Fig. 17 A schematic illustration of the construction of an AIE-based ROS-generation system in aqueous solution based on bridge-extend pillar[5]arene (m-TPEWP5). Reproduced with permission from ref. 94. Copyright 2023 Royal Society of Chemistry. | ||
The self-assembled materials of pillar[5]arene host–guest complexes can be precisely self-assembled through host–guest interactions. They have diverse structures and good stability and can carry functional molecules such as drugs as needed. Disadvantages: the assembly conditions are strict and reproducibility is difficult to control. Applicable cancer models include lung cancer and lymphoma models, which help with targeted drug delivery and improve the precision of cancer treatment.
6. Amphiphilic P5 based nanomaterials
In addition to the host–guest complex of pillar[5]arenes that can self-assemble to form biomedical nanomaterials, amphiphilic pillar[5]arenes can also self-assemble to form micelles or vesicles to further load drug molecules for achieving tumor therapy.95 In 2019, Prof. Zhang and colleagues prepared a charge-reversal amphiphilic pillar[5]arene (P5NH-DCA) with 10 charge-reversal head groups, targeting the cancer cell membrane for the selective eradication of cancer cells by disrupting the cell membrane.96 In the acidic tumor microenvironment, the head group charges of P5NH-DCA change from negative to positive due to the hydrolysis of acid-labile amide groups. The hydrolysis products with multiple positive charges can bind to the cell membrane and efficiently disrupt the cancer cell membrane. However, in the neutral microenvironment of healthy cells, the negatively charged P5NH-DCA remains stable, significantly reducing cytotoxicity. The strategy of killing cancer cells by membrane disruption may be a new approach for cancer chemotherapy (Fig. 18).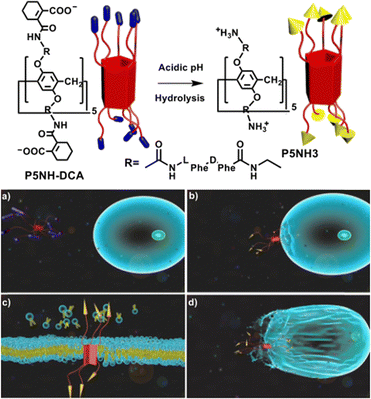 | ||
| Fig. 18 (a) Charge reversal process of a charge-reversal amphiphilic pillar[5]arene (5NH-DCA); (b) binding to cancer cell membrane; (c) disrupting cancer cell membrane; (d) killing cancer cells. Reproduced with permission from ref. 96. Copyright 2019 American Chemical Society. | ||
Interestingly, Prof. Yang and colleagues prepared an amphiphilic pillar[5]arene pseudo[1]rotaxane (PPR) modified with polyethylene glycol. PPR can self-assemble to form vesicles, and these vesicles exhibit a high drug loading capacity, successfully encapsulating DOX and releasing the encapsulated DOX sustainably in an environment with high GSH concentration like that of tumor cells, showing significant GSH responsiveness.97 The cytotoxicity assays revealed that the vesicles loaded with DOX exhibited a remarkable inhibitory effect on cancer cell proliferation. This is the first use of pseudo[1]rotaxane-based materials to protect a vulnerable responsive bond in drug delivery systems, specifically realizing zero premature release of drugs, leaving healthy cells unharmed, and offering a new possibility for the application of supramolecular amphiphiles and molecular machinery in precise cancer therapy (Fig. 19).
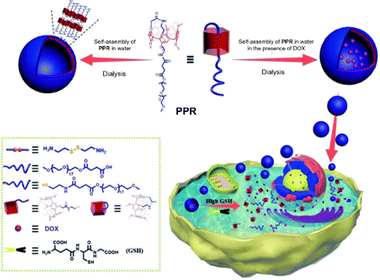 | ||
| Fig. 19 Illustration of the construction of a redox-responsive smart vesicular nanocarrier based on amphiphilic pillar[5]arene pseudo[1]rotaxane (PPR), and its loading and controlled release of a hydrophobic drug in response to GSH. Reproduced with permission from ref. 97. Copyright 2019 Royal Society of Chemistry. | ||
Besides, our group prepared an A1/B1 type amphiphilic pillar[5]arene by modifying glycol chains on one repeating unit of pillar[5]arene (WP5-8C-2PEG).98 On the other hand, we also successfully synthesized a novel A–D–A small molecule photosensitizer, named DPTTIC. Due to its strong D–A effect, DPTTIC has broad wavelength range light absorption capabilities and a narrow bandgap. The amphiphilic WP5-8C-2PEG can self-assemble into vesicles in water, which can induce hydrophobic DPTTIC into the vesicles. The prepared DPTTIC nanoparticles have good water solubility and uniform spherical morphology, facilitating cellular uptake. In terms of photothermal therapy and photodynamic therapy activities, DPTTIC NPs exhibit excellent mild photothermal conversion and the ability to generate 1O2 and ˙OH under laser irradiation. Therefore, these DPTTIC NPs with PTT and PDT capabilities show promising results in anti-cancer treatment both in vitro and in vivo (Fig. 20).
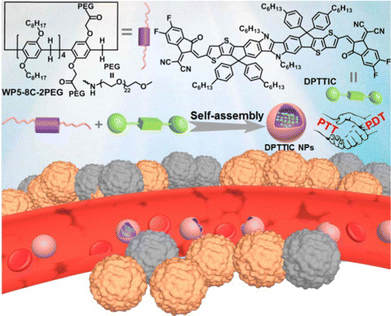 | ||
| Fig. 20 Chemical structures of A1/B1 type amphiphilic pillar[5]arene (WP5-8C-2PEG) and DPTTIC, and a schematic diagram of the application of DPTTIC NPs in combined PTT and PDT therapy. Reproduced with permission from ref. 98. Copyright 2022 Royal Society of Chemistry. | ||
Amphiphilic pillar[5]arene self-assembled materials possess good amphiphilicity, can self-assemble to form various morphological structures, have excellent biocompatibility and are conducive to drug encapsulation and transportation. Disadvantages: the self-assembly process is easily affected by environmental factors and the stability fluctuates. Applicable cancer models include gastric cancer and esophageal cancer models, etc., which can help with targeted drug release and improve the efficacy of cancer treatment.
7. Conclusions
The review explores the utilization of pillar[5]arene-based nanomaterials in tumor therapy. Through their combination with various types of nanomaterials, such as inorganic metal nanoparticles, organic porous materials, organic porous/inorganic metal hybrid materials, nanosystem self-assembly, and amphiphilic pillar[5]arene self-assembly, these nanomaterials have the potential to enhance therapeutic efficacy and reduce side effects in chemotherapy, photodynamic therapy, photothermal therapy, and multimodal synergistic therapy. Supramolecular strategies based on pillar[5]arenes offer innovative approaches for tumor therapy by enabling the development of smart drug delivery systems, controllable photosensitizers, and anticancer agents that target cancer cell membranes.99,100 However, pillar[5]arene based nanomaterials started relatively late in clinical trials and are still in the exploratory stage. Meanwhile, there are also some potential regulatory hurdles, such as challenges in safety assessment, difficulties in quality control and standardization, the definition of criteria for evaluating efficacy, and so on.Despite the promising applications of pillar[5]arenes and nanomaterials in cancer therapy, there are still some shortcomings and challenges that need to be addressed:
1. Biocompatibility and toxicity: while pillar[5]arenes generally exhibit good biocompatibility on their own, further studies are required to assess their toxic properties when combined with other nanomaterials.
2. Targeting: effective strategies need to be developed for targeted delivery of nanomaterials to tumor sites while minimizing non-specific accumulation in healthy tissue.
3. Stability and controlled release: optimization is needed for interactions between pillar[5]arenes and nanomaterials to ensure stability within the body and controlled release of drugs.
4. Mass production: developing scalable and cost-effective synthesis methods is essential for mass production of pillar[5]arene-based nanomaterials with consistent properties.
5. In vivo behavior: thorough studies are necessary to evaluate the safety, efficacy, and long-term effects of nanomaterials’ biological distribution, metabolism, and clearance within the body.
Pillar[5]arenes and other synthetic macrocycles offer unique opportunities for the construction and functionality of therapeutic nanomaterials. Their well-defined cavities can host guest molecules, enabling precise drug loading and controlled release. The modular nature of these macrocycles allows for facile chemical modification, tailoring their properties to specific therapeutic needs. For example, they can be conjugated with targeting ligands to enhance the specificity of nanomaterials towards cancer cells. Additionally, their self-assembly capabilities can lead to the formation of nanostructures with enhanced stability and bioavailability. However, challenges remain in optimizing the synthesis and assembly processes to ensure reproducibility and scalability for clinical applications.
Data availability
Data for this article are available at main article of this paper.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21801139), and College Students’ Innovation and Entrepreneurship Project (202410304102Y). We also thank Nantong University Analysis & Testing Center for characterization.Notes and references
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263 CrossRef PubMed.
- W. H. Almalki, Curr. Drug Delivery, 2024, 21, 509–524 CrossRef CAS PubMed.
- P. Puente, M. J. Luderer, C. Federico, A. Jin, R. C. Gilson, C. Egbulefu, K. Alhallak, S. Shah, B. Muz, J. Sun, J. King, D. Kohnen, N. N. Salama, S. Achilefu, R. Vij and A. K. Azab, J. Controlled Release, 2018, 270, 158–176 CrossRef PubMed.
- D. Jin, J. Zhang, Y. Huang, X. Qin, J. Zhuang, W. Yin, S. Chen, Y. Wang, P. Hua and Y. Yao, Dalton Trans., 2021, 50, 1189–1196 RSC.
- T. Kumeria, ACS Biomater. Sci. Eng., 2022, 8, 4025–4027 CrossRef CAS PubMed.
- L. Xu, Y. Xue, J. Xia, X. Qu, B. Lei, T. Yang, X. Zhang, N. Li, H. Zhao, M. Wang, M. Luo, C. Zhang, Y. Du and C. Yan, Biomaterials, 2020, 230, 119670 CrossRef CAS PubMed.
- J.-R. Wu and Y.-W. Yang, Chem. Commun., 2019, 55, 1533–1543 RSC.
- Q. Wu, Q. Lei, H.-C. Zhong, T.-B. Ren, Y. Sun, X.-B. Zhang and L. Yuan, Chem. Commun., 2019, 55, 1533–1543 RSC.
- Y.-H. Song, Q. Bian, F. Wang, J. Liu, Y.-H. Yang, Y.-M. Zhang and Y. Liu, Coord. Chem. Rev., 2025, 524, 216299 CrossRef CAS.
- R. Kashapov, Y. Razuvayeva, E. Fedorova and L. Zakharova, Soft Matter, 2024, 20, 8549–8560 RSC.
- C. Wang, L. Xu, Z. Jia and T.-P. Loh, Chin. Chem. Lett., 2024, 35, 109075 CrossRef CAS.
- N. Qian, X.-F. Hou, Y. Tang, S. Zhang, X.-M. Chen and Q. Li, ChemPhotoChem, 2023, 7, e202300018 CrossRef CAS.
- S.-Q. Cheng, Q. Lin, S.-L. Li, Y.-X. Guo, X.-L. Han, Y. Sun and Y. Liu, Green Chem., 2023, 25, 7026–7040 RSC.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668–5671 CrossRef CAS PubMed.
- H. Zhu, J. Liu, Y. Wu, L. Wang, H. Zhang, Q. Li, H. Wang, H. Xing, J. L. Sessler and F. Huang, J. Am. Chem. Soc., 2023, 145, 11130–11139 CrossRef CAS PubMed.
- K. Yang, S. Chao, F. Zhang, Y. Pei and Z. Pei, Chem. Commun., 2019, 55, 13198–13210 RSC.
- K. Kato, S. Fa, S. Ohtani, T.-H. Shi, A. M. Brouwer and T. Ogoshi, Chem. Soc. Rev., 2022, 51, 3648–3687 RSC.
- T. Ogoshi, K. Kitajima, T. Yamagishi and Y. Nakamoto, Org. Lett., 2010, 12, 636–638 CrossRef CAS PubMed.
- J.-F. Chen, G. Tian, K. Liu, N. Zhang, N. Wang, X. Yin and P. Chen, Org. Lett., 2022, 24, 1935–1940 CrossRef CAS PubMed.
- X. Yang, W. Cai, S. Dong, K. Zhang, J. Zhang, F. Huang, F. Huang and Y. Cao, ACS Macro Lett., 2017, 6, 647–651 CrossRef CAS PubMed.
- M. Bojtár, A. Simon, P. Bombicz and I. Bitter, Org. Lett., 2017, 19, 4528–4531 CrossRef PubMed.
- Y. Cai, X. Yan, S. Wang, Z. Zhu, M. Cen, C. Ou, Q. Zhao, Q. Yan, J. Wang and Y. Yao, Inorg. Chem., 2021, 60, 2883–2887 CrossRef CAS PubMed.
- T. Ogoshi, N. Ueshima and T. Yamagishi, Org. Lett., 2013, 15, 3742–3745 CrossRef CAS PubMed.
- H. Butkiewicz, S. Kosiorek, V. Sashuk, M. Zimnicka and O. Danylyuk, Cryst. Growth Des., 2022, 22, 2854–2862 CrossRef CAS.
- L. Ma, R. Tang, Y. Zhou, J. Bei, Y. Wang, T. Chen, C. Ou, Y. Han, C.-G. Yan and Y. Yao, Chem. Commun., 2022, 58, 8978–8981 RSC.
- B. Lu, X. Yan, J. Wang, D. Jing, J. Bei, Y. Cai and Y. Yao, Chem. Commun., 2022, 58, 2480–2483 RSC.
- X. Li, M. Shen, J. Yang, L. Liu and Y.-W. Yang, Adv. Mater., 2024, 18, 2313317 CrossRef PubMed.
- X.-Y. Lou and Y.-W. Yang, Adv. Mater., 2020, 32, 2003263 CrossRef CAS PubMed.
- Z. Li, N. Song and Y.-W. Yang, Matter, 2019, 1, 345–368 CrossRef.
- X.-Y. Lou, Y.-P. Li and Y.-W. Yang, Biotechnol. J., 2019, 14, 1800354 CrossRef PubMed.
- J. Yang, D. Dai, L. Ma and Y.-W. Yang, Chin. Chem. Lett., 2021, 32, 729–734 CrossRef CAS.
- J. Gao, H.-M. Yu, M. Wu, Q. Chen, Y. Yang, Y. Qu, M. Sun, J.-C. Qin, L. Ma and Y.-W. Yang, Mater. Today Chem., 2022, 23, 100716 CrossRef CAS.
- S. Liang, M.-H. Li, M.-L. Qi, H. Hui, H.-P. Zhang, L. Wang and Y.-W. Yang, Nano Lett., 2024, 24, 13708–13717 CrossRef CAS PubMed.
- G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS PubMed.
- Y. Wen, X. Di, Z. Chen, X. Zhang, Z. Pei and Y. Pei, Chem. Commun., 2024, 60, 12694–12697 RSC.
- L. Ma, Y. Dai, Y. Meng, W. Yu, Y. Bai, Y. Cai, Y. Han, J. Wang, L. Yao and Y. Yao, Chem. Commun., 2024, 60, 8387–8390 RSC.
- J. Zhou, J. Gu, X. Sun, Q. Ye, X. Wu, J. Xi, J. Han and Y. Liu, Adv. Sci., 2024, 11, 2308493 CrossRef CAS PubMed.
- H. Wang, Y. Wang, W. Xu, H. Zhang, J. Lv, X. Wang, Z. Zheng, Y. Zhao, L. Yu, Q. Yuan, L. Yu, B. Zheng and L. Gao, ACS Appl. Mater. Interfaces, 2023, 15, 54266–54279 CrossRef CAS PubMed.
- R. Tang, L. Zhou, Y. Dai, Y. Wang, Y. Cai, T. Chen and Y. Yao, Chem. Commun., 2024, 60, 1160–1163 RSC.
- P. Huang, D. Lian, H. Ma, N. Gao, L. Zhao, P. Luan and X. Zeng, Chin. Chem. Lett., 2021, 32, 3696–3704 CrossRef CAS.
- E. C. S. Santos, T. C. Santos, T. S. Fernandes, F. L. Jorge, V. Nascimento, V. G. C. Madriaga, P. S. Cordeiro, N. R. Checca, N. M. Da Costa, L. F. R. Pinto and C. M. Ronconi, J. Mater. Chem. B, 2020, 8, 703–714 RSC.
- J. Yang, D. Dai, X. Lou, L. Ma, B. Wang and Y.-W. Yang, Theranostics, 2020, 10, 615–629 CrossRef CAS PubMed.
- X. Wang, J. Sheng and M. Yang, Chin. Chem. Lett., 2024, 30, 533–540 CrossRef.
- J. Qi, Y. Fang, R. T. Kwok, X. Zhang, X. Hu, J. W. Y. Lam, D. Ding and B. Z. Tang, ACS Nano, 2017, 11, 7177–7188 CrossRef CAS PubMed.
- B. Huang, P. Wang, Y. Ouyang, R. Pang, S. Liu, C. Hong, S. Ma, Y. Gao, J. Tian and W. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 41038–41046 CrossRef CAS PubMed.
- B. Huang, J. Tian, Z. Cui, S. Weng, W. Wang, X. Jiang and W. Zhang, Chem. Eng. J., 2022, 444, 136164 CrossRef CAS.
- G. Lu, X. Gao, H. Zhang, Y. Zhang, Y. Yu, Z. Sun, W. Li, W. Wu, Y. Lu and H. Zou, Chin. Chem. Lett., 2022, 33, 1923–1926 CrossRef CAS.
- M. Cen, Y. Ding, J. Wang, X. Yuan, B. Lu, Y. Wang and Y. Yao, ACS Macro Lett., 2020, 9, 1558–1562 CrossRef CAS PubMed.
- X. Wu, Y. Zhang, Y. Lu, S. Pang, K. Yang, Z. Tian, Y. Pei, Y. Qu, F. Wang and Z. Pei, J. Mater. Chem. B, 2017, 5, 3483–3487 RSC.
- D. N. Shurpik, D. A. Sevastyanov, P. V. Zelenikhin, P. L. Padnya, V. G. Evtugyn, Y. N. Osin and I. I. Stoikov, Beilstein J. Nanotechnol., 2020, 11, 421–431 CrossRef CAS PubMed.
- N. Song, Z. Zhang, P. Liu, D. Dai, C. Chen, Y. Li, L. Wang, T. Han, Y.-W. Yang, D. Wang and B. Z. Tang, Adv. Funct. Mater., 2021, 31, 2009924 CrossRef CAS.
- T. Skorjanc, D. Shetty and A. Trabols, Chem., 2021, 7, 882–918 CAS.
- M. K. Nguyen, J. S. Gwag, L. Nguyen-Dinh, H. B. Truong, H. H. Do, Y.-C. Lee, N. T. Tran and L. G. Trung, Nano Today, 2024, 55, 102211 CrossRef CAS.
- Y. Wang, D. Wang, J. Wang, C. Wang, J. Wang, Y. Ding and Y. Yao, Chem. Commun., 2022, 58, 1689–1692 RSC.
- J. García-Ben, L. N. McHugh, T. D. Bennett and J. M. Bermúdez-García, Coord. Chem. Rev., 2022, 455, 214337 CrossRef.
- J. Ahn, H. Jin, J. Park, B. Lee, M. Ok, J. Lee, J. Bae and J. H. Jung, Part. Part. Syst. Charact., 2020, 37, 2000136 CrossRef CAS.
- Y. Wang, Y. Wen, Y. Qu, Z. Pei and Y. Pei, J. Colloid Interface Sci., 2022, 615, 386–394 CrossRef CAS PubMed.
- Y. Cai, Z. Zhang, Y. Ding, L. Hu, J. Wang, T. Chen and Y. Yao, Chin. Chem. Lett., 2021, 32, 1267–1279 CrossRef CAS.
- S. Chao, X. Lv, N. Ma, Z. Shen, F. Zhang, Y. Pei and Z. Pei, Chem. Commun., 2020, 56, 8861–8864 RSC.
- H. Peng, B. Xie, X. Yang, J. Dai, G. Wei and Y. He, Chem. Commun., 2020, 56, 8115–8118 RSC.
- J.-J. Li, H.-Y. Zhang, X.-Y. Dai, Z.-X. Liu and Y. Liu, Chem. Commun., 2020, 56, 5949–5952 RSC.
- Y. Wang, H. Zhong, J. Yang, Y. Yao and L. Li, Chin. Chem. Lett., 2023, 34, 108452 CrossRef CAS.
- X. Lv, D. Xia, Y. Zuo, X. Wu, X. Wei and P. Wang, Langmuir, 2019, 35, 8383–8388 CAS.
- Y. Cao, Y. Chen, Z. Zhang, J. Wang, X. Yuan, Q. Zhao, Y. Ding and Y. Yao, Chin. Chem. Lett., 2021, 32, 349–352 CrossRef CAS.
- X. Yan, Y. Huang, M. Cen, J. Wang, J. Shi, B. Lu, Y. Wang and Y. Yao, Nanoscale Adv., 2021, 3, 1906–1909 RSC.
- W. Chen, Y. Zhang, J. Li, X. Lou, Y. Yu, X. Jia and C. Li, Chem. Commun., 2013, 49, 7956–7958 RSC.
- X. Chi, X. Ji, D. Xia and F. Huang, J. Am. Chem. Soc., 2015, 137, 1440–1443 CrossRef CAS PubMed.
- T. Chen, J. Wang, R. Tang, Y. Huang, Q. Zhao and Y. Yao, Chin. Chem. Lett., 2023, 34, 108088 CrossRef CAS.
- J. Wu, J. Tian, L. Rui and W. Zhang, Chem. Commun., 2018, 54, 7629–7632 RSC.
- Y. Sun, L. Liu, L. Jiang, Y. Chen, H. Zhang, X. Xu and Y. Liu, J. Am. Chem. Soc., 2023, 145, 16711–16717 CrossRef CAS PubMed.
- H. Yan, X. Yin, D. Wang, T. Han and B. Z. Tang, Adv. Sci., 2023, 10, 2305149 CrossRef CAS PubMed.
- A. J. Taylor, J. T. Wilmore and P. D. Beer, Chem. Commun., 2024, 60, 11916–11919 RSC.
- T. Ogoshi, K. Demachi, K. Kitajima and T. Yamagishi, Chem. Commun., 2011, 47, 7164–7166 RSC.
- Y. Zhou, H. Tang, Z.-H. Li, L. Xu, L. Wang and D. Cao, Chem. Commun., 2021, 57, 13114–13117 RSC.
- M. Schmidt and B. Esser, Chem. Commun., 2021, 57, 9582–9585 RSC.
- Y. Wang, R. Tang, Y. Zhang, Y. Dai, Q. Zhou, Y. Zhou, C.-G. Yan, B. Lu, J. Wang and Y. Yao, Inorg. Chem., 2023, 62, 7605–7610 CrossRef CAS PubMed.
- Y. Fan, S. Fan, L. Liu, S. Guo, J. He, X. Li, Z. Lian, W. Guo, X. Chen, Y. Wang and H. Jiang, Chem. Sci., 2023, 14, 11121–11130 RSC.
- T. Ogoshi, M. Hashizume, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 3708–3710 RSC.
- X. Min, F. Yi, X. Han, M. Li, Q. Gao, X. Liang, Z. Chen, Y. Sun and Y. Liu, Chem. Eng. J., 2022, 432, 134327 CrossRef CAS.
- G. Sun, Z. He, M. Hao, M. Zuo, Z. Xu, X.-Y. Hu, J.-J. Zhu and L. Wang, J. Mater. Chem. B, 2019, 7, 3944–3949 RSC.
- X. Liu, K. Jia, Y. Wang, W. Shao, C. Yao, L. Peng, D. Zhang, X.-Y. Hu and L. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 4843–4850 CrossRef CAS PubMed.
- Q. Wang, P. Zhang, J. Xu, B. Xia, L. Tian, J. Chen, J. Li, F. Lu, Q. Shen, X. Lu, W. Huang and Q. Fan, ACS Appl. Bio Mater., 2018, 1, 70–78 CrossRef CAS.
- H. Liu, J. Yang, X. Yan, C. Li, M. Elsabahy, L. Chen, Y.-W. Yang and H. Gao, J. Mater. Chem. B, 2021, 9, 9594–9605 RSC.
- J. Wang, M. Cen, J. Wang, D. Wang, Y. Ding, G. Zhu, B. Lu, X. Yuan, Y. Wang and Y. Yao, Chin. Chem. Lett., 2022, 33, 1475–1478 CrossRef CAS.
- Z. Shen, N. Ma, F. Wang, J. Ren, C. Hou, S. Chao, Y. Pei and Z. Pei, Chin. Chem. Lett., 2022, 33, 4563–4566 CrossRef CAS.
- M. Yang, K. Yang, B. Gao, P. Wang, T. Li, Y. Zheng, Y. Pei, Z. Pei and Y. Lv, Chem. Commun., 2022, 58, 11147–11150 RSC.
- S. Chao, P. Huang, Z. Shen, Y. Pei, Y. Lv, Y. Lu and Z. Pei, Org. Chem. Front., 2023, 10, 3491–3497 RSC.
- K. Yang, K. Ma, M. Yang, Y. Lv, Y. Pei and Z. Pei, Chem. Commun., 2023, 59, 3779–3782 RSC.
- Y. Zhou, L. Yang, L. Ma, Y. Han, C.-G. Yan and Y. Yao, Molecules, 2022, 27, 6428 CrossRef CAS PubMed.
- Y. Ding, C. Wang, Y. Ma, L. Zhu, B. Lu, Y. Wang, J. Wang, T. Chen, C. Dong and Y. Yao, Acta Biomater., 2022, 143, 381–391 CrossRef CAS PubMed.
- K. Wan, S.-C. Gao, X. Fang, M.-Y. Xu, Y. Yang and M. Xue, Chem. Commun., 2020, 56, 10155–10158 RSC.
- X. Tian, M. Zuo, P. Niu, K. Velmurugan, K. Wang, Y. Zhao, L. Wang and X. Hu, ACS Appl. Mater. Interfaces, 2021, 13, 37466–37474 CrossRef CAS PubMed.
- X. Tian, S. Li, K. Velmurugan, Z. Bai, Q. Liu, K. Wang, M. Zuo and X. Hu, Mater. Chem. Front., 2023, 7, 2484–2492 RSC.
- Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712–15715 CrossRef CAS PubMed.
- Y. Chang, J. Chen, J. Yang, T. Lin, L. Zeng, J. Xu, J. Hou and X. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 38497–38502 CrossRef CAS PubMed.
- Y.-H. Cui, R. Deng, Z. Li, X.-S. Du, Q. Jia, X.-H. Wang, C.-Y. Wang, K. Meguellati and Y. Yang, Mater. Chem. Front., 2019, 3, 1427–1432 RSC.
- B. Lu, Z. Zhang, Y. Huang, Y. Zhang, J. Wang, Y. Ding, Y. Wang and Y. Yao, Chem. Commun., 2022, 58, 10353–10356 RSC.
- F. A. Mohammed, T. Xiao, L. Wang and R. B. P. Elmes, Chem. Commun., 2024, 60, 11812–11836 RSC.
- S. Ren, G.-Y. Qiao and J.-R. Wu, Chem. Soc. Rev., 2024, 53, 10312–10334 RSC.
Footnote |
| † Y. Dai and W. Yu make equal contributions to this work. |
| This journal is © The Royal Society of Chemistry 2025 |

