 Open Access Article
Open Access ArticleIsolation of mixed valence charge-neutral Ag12, and dicationic Ag10 nano-clusters stabilized by carbene-phosphaalkenides†
Maria
Francis‡
 a,
Asutosh
Patra‡
a,
Asutosh
Patra‡
 a,
Farsana Abdul
Salam
a,
Farsana Abdul
Salam
 a,
Siriyara Jagannatha
Prathapa
b and
Sudipta
Roy
a,
Siriyara Jagannatha
Prathapa
b and
Sudipta
Roy
 *a
*a
aDepartment of Chemistry, Indian Institute of Science Education and Research (IISER) Tirupati, Tirupati 517619, India. E-mail: roy.sudipta@iisertirupati.ac.in
bBruker India Scientific Pvt. Ltd, India
First published on 12th December 2024
Abstract
Cyclic alkyl(amino) carbene (cAAC)-supported phosphaalkenides (cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)− have been employed as ligands for the isolation of two atomically precise mixed valence paramagnetic AgI/012Cl3, and AgI/010, nano-clusters [(Me2-cAAC
P)− have been employed as ligands for the isolation of two atomically precise mixed valence paramagnetic AgI/012Cl3, and AgI/010, nano-clusters [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6Ag12Cl3] (2), and [(Me2-cAAC
P)6Ag12Cl3] (2), and [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6Ag10](NTf2)2 (4). 2 and 4 have been structurally characterized by single-crystal X-ray diffraction revealing the presence of three Ag0 atoms, nine AgI ions (2); and two Ag0 atoms, eight AgI ions (4), respectively. The clustering inorganic unit Ag12Cl3 in 2 has been found to be surrounded by six mono-anionic μ3-cAAC
P)6Ag10](NTf2)2 (4). 2 and 4 have been structurally characterized by single-crystal X-ray diffraction revealing the presence of three Ag0 atoms, nine AgI ions (2); and two Ag0 atoms, eight AgI ions (4), respectively. The clustering inorganic unit Ag12Cl3 in 2 has been found to be surrounded by six mono-anionic μ3-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P moieties having 3-bar symmetry. 2 and 4 have been studied by cyclic voltammetry, UV-vis, ESI-MS, XPS, EPR spectroscopy, and DFT calculations.
P moieties having 3-bar symmetry. 2 and 4 have been studied by cyclic voltammetry, UV-vis, ESI-MS, XPS, EPR spectroscopy, and DFT calculations.
Silver nanoclusters (NCs) are known to exhibit unique electronic, and optical properties, such as strong photoluminescence, and various catalytic activities.1 They are utilised as attractive luminescent probes for sensing and bioimaging.2 Structurally well-defined NCs possessing Ag0 atoms are highly reactive, and easier to oxidize compared to their heavier Au analogues, which makes their synthesis challenging. Metallic silver is non-magnetic in its bulk form. However, Pereiro et al. theoretically predicted that the small nuclearity silver NCs, Agn, could be magnetic with variation of the magnetic moment depending on the value of n [n = 2–22].3 Liu et al. reported the isolation of the chiral open-shell Ag23 NC with five Ag0 atoms, which was structurally characterized by single-crystal X-ray diffraction.4 The open-shell behaviour of this Ag23 NC was confirmed by a weak electron paramagnetic resonance (EPR) signal, characteristic of s
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, with g
1/2, with g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
= ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.959 and 1.955.4 The first atomically precise mixed valence Ag0/I22 NC displaying thermally activated delayed fluorescence (TADF) was reported in 2022 by Sun and co-workers.5 In the same year, two other mixed valence NCs Ag0/I8 and Ag0/I29 exhibiting strong EPR signals were reported.6 The heavily Ag0-doped KCl:AgCl crystals in different chemical defect positions [cationic and anionic holes] were studied by EPR spectroscopy.7a The generation of Ag43+, and Ag6+ clusters was also reported under different chemical conditions.7b,c However, the mixed valence silver NCs are rarely reported.8 The first closed-shell mixed valence AgI/III2 [Ag⋯Ag 7.4 Å] containing N8-donor macrocyclic ligand was isolated by Qin-Hui et al. in 1994.9a The 1D zig-zag chain of AgI/II2 (ref. 9b) stabilized by the cyclic alkyl(amino) carbene (cAAC)-supported mono-anion of the inversely polarized phosphaalkene, viz., the phosphaalkenide10 was reported by Roy and co-workers. N-heterocyclic carbene (NHC), and P-SiMe3 ligated diamagnetic AgI12, and AgI26 clusters were reported by Corrigan et al.11 A plethora of other AgI clusters have been synthesized, and characterized employing alkyne, thiolate, sulfide, selenide, phosphine, perchlorate, etc. as ligands.12 Moreover, clusters with Ag–H moieties have also been isolated, and further studied by mass spectrometry.13 However, isolation of stable silver NCs introducing carbene-phosphaalkenides as ligands is still scarce. Herein, we report on the solid-state isolation, and structural characterization of two novel structurally well-defined mixed valence paramagnetic silver NCs with AgI/012Cl3, and AgI/010 metallic cores.
1.959 and 1.955.4 The first atomically precise mixed valence Ag0/I22 NC displaying thermally activated delayed fluorescence (TADF) was reported in 2022 by Sun and co-workers.5 In the same year, two other mixed valence NCs Ag0/I8 and Ag0/I29 exhibiting strong EPR signals were reported.6 The heavily Ag0-doped KCl:AgCl crystals in different chemical defect positions [cationic and anionic holes] were studied by EPR spectroscopy.7a The generation of Ag43+, and Ag6+ clusters was also reported under different chemical conditions.7b,c However, the mixed valence silver NCs are rarely reported.8 The first closed-shell mixed valence AgI/III2 [Ag⋯Ag 7.4 Å] containing N8-donor macrocyclic ligand was isolated by Qin-Hui et al. in 1994.9a The 1D zig-zag chain of AgI/II2 (ref. 9b) stabilized by the cyclic alkyl(amino) carbene (cAAC)-supported mono-anion of the inversely polarized phosphaalkene, viz., the phosphaalkenide10 was reported by Roy and co-workers. N-heterocyclic carbene (NHC), and P-SiMe3 ligated diamagnetic AgI12, and AgI26 clusters were reported by Corrigan et al.11 A plethora of other AgI clusters have been synthesized, and characterized employing alkyne, thiolate, sulfide, selenide, phosphine, perchlorate, etc. as ligands.12 Moreover, clusters with Ag–H moieties have also been isolated, and further studied by mass spectrometry.13 However, isolation of stable silver NCs introducing carbene-phosphaalkenides as ligands is still scarce. Herein, we report on the solid-state isolation, and structural characterization of two novel structurally well-defined mixed valence paramagnetic silver NCs with AgI/012Cl3, and AgI/010 metallic cores.
The dark red crystals of Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–K (1)14 (Me2-cAAC
P–K (1)14 (Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N-2,6-iPr2C6H3)(CMe2)2(CH2)) were reacted with AgNTf2 in a 3
C(N-2,6-iPr2C6H3)(CMe2)2(CH2)) were reacted with AgNTf2 in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in toluene at 0 °C to room temperature (rt) for 12 h to obtain a dark brown-red reaction mixture. Upon drying, the resultant crystalline solid obtained was dissolved in freshly distilled DCM, and stored for crystallization upon concentration to ∼1 mL in a freezer at −40 °C. After one-week, dark red blocks of [(Me2-cAAC
2 molar ratio in toluene at 0 °C to room temperature (rt) for 12 h to obtain a dark brown-red reaction mixture. Upon drying, the resultant crystalline solid obtained was dissolved in freshly distilled DCM, and stored for crystallization upon concentration to ∼1 mL in a freezer at −40 °C. After one-week, dark red blocks of [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6(Ag)12(Cl)3] (2) were isolated in 40% yield (Scheme 1). The presence of the three chloride ions in 2 can be attributed to the decomposition of DCM, the usage of which is found to be crucial for the isolation of 2. A different mixed valence dicationic cluster [(Me2-cAAC
P)6(Ag)12(Cl)3] (2) were isolated in 40% yield (Scheme 1). The presence of the three chloride ions in 2 can be attributed to the decomposition of DCM, the usage of which is found to be crucial for the isolation of 2. A different mixed valence dicationic cluster [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6(Ag)10](NTf2)2 (4) was isolated in 60% yield as yellow blocks when [Me2-cAAC
P)6(Ag)10](NTf2)2 (4) was isolated in 60% yield as yellow blocks when [Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–B(N(iPr)2)2] (3)9b was reacted with AgNTf2 in 2
P–B(N(iPr)2)2] (3)9b was reacted with AgNTf2 in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in toluene at rt for 12 h, followed by crystallization of the resultant precipitate from concentrated DCM solution, stored at 0 °C in a refrigerator (Scheme 2). The (cAAC
1 molar ratio in toluene at rt for 12 h, followed by crystallization of the resultant precipitate from concentrated DCM solution, stored at 0 °C in a refrigerator (Scheme 2). The (cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)− ligands are found to be redox non-innocent, and undergo oxidation to produce the corresponding radical followed by dimerization, resulting in the formation of (Me2-cAAC)2P2 with a subsequent reduction of AgI to Ag0 in solution affording the mixed valence Ag-NCs 2 and 4.6 The presence of Ag0 and AgI centres in 2 and 4 is supported by XPS spectroscopy (see ESI†). The formation of (Me2-cAAC)2P2 in the reaction mixture is confirmed by 31P NMR spectroscopy (δ = 55.1 ppm).
P)− ligands are found to be redox non-innocent, and undergo oxidation to produce the corresponding radical followed by dimerization, resulting in the formation of (Me2-cAAC)2P2 with a subsequent reduction of AgI to Ag0 in solution affording the mixed valence Ag-NCs 2 and 4.6 The presence of Ag0 and AgI centres in 2 and 4 is supported by XPS spectroscopy (see ESI†). The formation of (Me2-cAAC)2P2 in the reaction mixture is confirmed by 31P NMR spectroscopy (δ = 55.1 ppm).
The red/yellow blocks of 2/4 are found to be stable under an argon atmosphere for six months inside a glove box at rt. The powders of 2 and 4 decompose above 205 and 165 °C, respectively. 2 and 4 are found to be NMR silent, and EPR active.
2 and 4 have been structurally characterized by single-crystal X-ray diffraction. The dodeca-nuclear Ag-NC [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6(Ag)12(Cl)3] (2) crystallizes in the trigonal R
P)6(Ag)12(Cl)3] (2) crystallizes in the trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (Fig. 1). 2 comprises six (Me2-cAAC
space group (Fig. 1). 2 comprises six (Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)− ligands, three chloride ions, and twelve Ag-atoms/ions. Fig. 2 shows that the asymmetric unit of 2 starting at position-1, generates six different symmetry equiv. points (1–6). Positions 1, 3 and 5 are non-inverted, while positions 2, 4 and 6 are three inverted-symmetry equiv. positions. There are three Ag0 atoms in 2, which is suggested from charge balance consideration, since there are a total of nine mono-anionic ligands present.
P)− ligands, three chloride ions, and twelve Ag-atoms/ions. Fig. 2 shows that the asymmetric unit of 2 starting at position-1, generates six different symmetry equiv. points (1–6). Positions 1, 3 and 5 are non-inverted, while positions 2, 4 and 6 are three inverted-symmetry equiv. positions. There are three Ag0 atoms in 2, which is suggested from charge balance consideration, since there are a total of nine mono-anionic ligands present.
 | ||
Fig. 1 Molecular structure of NC [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6(Ag)12(Cl3)] (2). Hydrogen atoms are omitted for clarity. P)6(Ag)12(Cl3)] (2). Hydrogen atoms are omitted for clarity. | ||
The space filling model of 2 has been shown in Fig. 3 (left), which represents how the central Ag12 metallic core is well protected by the surrounding cAAC-supported phosphaalkenide ligands. The natural bond orbital (NBO) analyses of NCs 2 and 4 at neutral doublet, and dicationic triplet states, respectively, were performed at the UBP86-D3(BJ)/Def2-SVP level of theory (see ESI† for details). The NBO analysis of 2 revealed that the α-LUMO+1, α-LUMO, and α-SOMO are very close in energy (ΔE = 0.01–0.05 eV) suggesting that the unpaired electron in 2 can span all these three orbitals involving the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–P unit, Cl and Ag atoms (see ESI†). This is also reflected in the Mulliken α-spin densities of 2 showing delocalization of spin densities due to the unpaired electron (Fig. 3 (right), see ESI†).
N–P unit, Cl and Ag atoms (see ESI†). This is also reflected in the Mulliken α-spin densities of 2 showing delocalization of spin densities due to the unpaired electron (Fig. 3 (right), see ESI†).
 | ||
Fig. 3 Space filling model (left), and Mulliken α-spin densities (right) of [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6Ag12Cl3] (2). P)6Ag12Cl3] (2). | ||
The Ag–Ag distances of charge-neutral NC 2 are found to be 2.883(1), and 3.024(2) Å, which are significantly shorter than those of the previously isolated tri-cationic closed-shell Ag NC 23+(OTf−)3 (2.9593(14), 2.9174 (12), 3.2026(15), 3.1541(13) Å).6 The Ag–P distances of 2 are 2.379(4), 2.401(3), and 2.455(2) Å, which are close to those of 23+(OTf−)3 (Ag–P 2.393(4)–2.431(4) Å).6 The C–P bond in 2 is 1.75(1) Å, which is slightly shorter than those of 23+(OTf−)3 (1.780(11)–1.801(11) Å).6 The Ag–Cl distances in 2 are found to be 2.4530(14), and 2.572(7) Å, which are either very similar in value or significantly smaller than those of 23+ (2.475(9), 2.705(5), 2.638 (4), 2.7143(11), 2.732(4), 2.8254(10) Å).6 The diameter of the outer/peripheral Ag6 ring of 2 having a three-fold symmetry is 8.37 Å, which is very close to those of 23+ with two-fold symmetry (8.26, 8.36, 8.48 Å).6 The Cl–Cl distance between the two extreme Cl-ions in 2 is 5.8 Å, which is shorter than that of 23+ (6.5 Å).6 The P–P distance in 2 is 9.02 Å, which is slightly greater than the average value of 23+ (8.46 Å; 8.26, 8.36, 4.48, 8.88, 9.22 Å).62 represents the first example of an AgI/0 based mixed valence NC, which has been structurally characterized in two different charged (0 (2) vs. +3 (23+)], and spin states [doublet (2) vs. singlet (23+)).6
The dicationic deca-nuclear Ag NC [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6Ag10](NTf2)2 (4) crystallizes in triclinic space group P
P)6Ag10](NTf2)2 (4) crystallizes in triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Fig. 4). The entire molecule of 4 appears in the crystallographic asymmetric unit, which possesses six mono-anionic ligands (Me2-cAAC
(Fig. 4). The entire molecule of 4 appears in the crystallographic asymmetric unit, which possesses six mono-anionic ligands (Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)−, and ten Ag-atoms/ions. There are two non-coordinating/free mono-anionic NTf2− anions present for the electrical charge balance. The charge balance consideration suggests that there are two Ag0 atoms in 4. The four arms of the central Ag4 unit of 4 have been bridged by four P-atoms of mono-anionic Me2-cAAC
P)−, and ten Ag-atoms/ions. There are two non-coordinating/free mono-anionic NTf2− anions present for the electrical charge balance. The charge balance consideration suggests that there are two Ag0 atoms in 4. The four arms of the central Ag4 unit of 4 have been bridged by four P-atoms of mono-anionic Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P− ligands producing a (Me2-cAAC
P− ligands producing a (Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)4Ag4 unit. Two Ag2 units have been placed in anti-fashion above and below the irregular square-like Ag4 unit (Fig. 4 and 5).
P)4Ag4 unit. Two Ag2 units have been placed in anti-fashion above and below the irregular square-like Ag4 unit (Fig. 4 and 5).
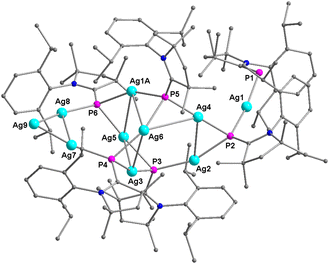 | ||
Fig. 4 Molecular structure of NC [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6Ag10](NTf2)2 (4). Hydrogen atoms are omitted for clarity. Two triflimide anions [N(SO2CF3)2−] are omitted for clarity. P)6Ag10](NTf2)2 (4). Hydrogen atoms are omitted for clarity. Two triflimide anions [N(SO2CF3)2−] are omitted for clarity. | ||
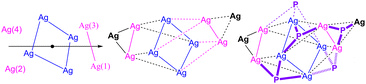 | ||
Fig. 5 Core topology of [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6Ag10](NTf2)2 (4). Two Ag6 square prisms sharing the blue colored pseudo-Ag4-square with a 4-bar symmetry operation. P)6Ag10](NTf2)2 (4). Two Ag6 square prisms sharing the blue colored pseudo-Ag4-square with a 4-bar symmetry operation. | ||
An additional Ag-atom is present above the Ag2 unit (Fig. 4 and 5), while another Ag atom is bridged by a μ3-P atom (right, Fig. 5). The Ag–Ag distances of the central four-membered Ag4 ring in 4 are 2.90, 3.0, and 3.20 Å, which are longer than those (∼2.96 Å) of the Ag0/I8 complex.6
The Ag2 unit, which is situated on the top of the central Ag4 unit, possesses the Ag–Ag distance of 2.91 Å, which is significantly shorter than that of the Ag3 triangle of 4 (3.04 Å). The corresponding Ag–Ag distance in previously reported Ag0/I8 complexes is 3.07 Å.6 Notably, the distal Ag atom (Ag9) (Ag7–Ag9, Ag8–Ag9; 2.472(3), 2.458(3) Å) is significantly closer to the Ag2 arm (Ag8–Ag9), which is situated above the central Ag4 unit of 4. The Ag–P bond lengths of NC Ag0/I10 (4) range from 2.23 Å to 2.48 Å, and are significantly different than those of the neutral Ag8 cluster (2.404(5)–2.417(4) Å).6 The space filling diagram of a ten Ag atoms/ions unit of 4 has been shown in Fig. 6 (left). The Ag atom placed above the Ag2 unit is situated in between the two aromatic rings of the Dipp ligands. The NBO, and Mulliken spin density analyses of 4 (Fig. 6 (right)) showed the major spin densities on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–P unit with very small values on the Ag-atoms except for one Ag-atom (5.6%) and thus typical EPR features of Ag–P systems were not observed6 in the experimental EPR spectrum of 4.
N–P unit with very small values on the Ag-atoms except for one Ag-atom (5.6%) and thus typical EPR features of Ag–P systems were not observed6 in the experimental EPR spectrum of 4.
 | ||
Fig. 6 Space filling model of total molecule (left), and Mulliken α-spin densities (right) of [(Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)6Ag10](NTf2)2 (4). P)6Ag10](NTf2)2 (4). | ||
The EPR spectra of 2 and 4 (in DCM) are shown in Fig. 7 and 8. The EPR simulation/fitting of 2 considering coupling of the unpaired electron with the nuclei of 107,109Ag, 31P and 35,37Cl atoms is quite satisfactory (Fig. 7). Three board lines near g = 2.0033 were observed for the previously reported Ag0/I8 cluster containing four Ag0 atoms.6,9b However, the EPR spectrum of 2 shows three sets of multiple hyperfine lines, which have been simulated with EasySpin.
The coupling constants of one 107,109Ag0, two 31P and one 35,37Cl nuclei are 9.43, 111.79/111.75, and 9.81 MHz, respectively, with a slight rhombic nature of g [gx = 2.00766, gy = 2.00885, gz = 1.99959]. The EPR spectrum of 4 appears as unsymmetrical showing two resonances around g ≈ 2 [gx = 2.00165, gy = 2.00006, gz = 1.99703] (Fig. 8). The coupling constant of Ag0 is 2.51 MHz, which is significantly smaller than that of 2 (9.43 MHz). The ESI-MS studies show that 2 (see ESI†) and 4 can fly as dications. The ESI-MS spectrum of 42+ corroborates the loss of two isopropyl groups with the intake of a sodium ion as [(Me2cAACP)6Ag10 + Na–2C3H7–H]2+. The cyclic voltammetry studies of 2 and 4 suggest the possible oxidation (see ESI†). The UV-vis spectra (in THF at 298 K) of 2 and 4 exhibited the absorption maxima (λmax) at 365 and 367 nm, respectively (see ESI†). 2 and 4 were observed to be non-emissive.
In conclusion, two novel mixed valence Ag-NCs (Ag10, 2; Ag12, 4) were isolated as red/yellow blocks. 2 and 4 have been structurally characterized by X-ray single-crystal diffraction. The Ag10, and Ag12 NCs possess three and two Ag0 atoms, respectively. The NMR silent NCs 2 and 4 were found to be EPR active. The unpaired electrons couple with the 35,37Cl, 31P and 107,109Ag nuclei. The strongest coupling constant was obtained for 31P-nuclei (111.75 MHz) in 2. The coupling constant of 107,109Ag of 2 (9.43 MHz) is four times stronger than that of 4 (2.51 MHz). The distributions of electron densities in 2 and 4 were estimated by computation of the Mulliken spin densities, and further correlated with ERP simulation.
SR gratefully acknowledges STARS-IISC, MoE (MoE-STARS/STARS-2/2023-0666), IISERT, CSIR for the generous financial support. We thank Dr S. S. Sen (NCL-Pune), and KB for the XPS measurements.
Data availability
The data supporting this article (experimental details, UV-vis, HRMS, EPR, XPS, single-crystal X-ray data, and computational details) have been included as part of the ESI.† Crystallographic data for 2 and 4 have been deposited at the CCDC (2242239, 2194453†), and can be obtained from https://www.ccdc.cam.ac.uk/.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. M. Aikens, J. Phys. Chem. Lett., 2011, 2, 99 CrossRef CAS PubMed; (b) Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526 CrossRef CAS PubMed.
- (a) T. Udayabhaskararao and T. Pradeep, J. Phys. Chem. Lett., 2013, 4, 1553 CrossRef CAS PubMed; (b) K. Zheng, X. Yuan, N. Goswami, Q. Zhang and J. Xie, RSC Adv., 2014, 4, 60581 RSC.
- M. Pereiro, D. Baldomir and J. E. Arias, Phys. Rev. A, 2007, 75, 063204 CrossRef.
- C. Liu, T. Li, H. Abroshan, Z. Li, C. Zhang, H. J. Kim, G. Li and R. Jin, Nat. Commun., 2018, 9, 744 CrossRef PubMed.
- Z.-R. Yuan, Z. Wang, B.-L. Han, C.-K. Zhang, S.-S. Zhang, Z.-Y. Zhu, J.-H. Yu, T.-D. Li, Y.-Z. Li, C.-H. Tung and D. Sun, Angew. Chem., Int. Ed., 2022, 61, e202211628 CrossRef CAS PubMed.
- E. Nag, S. Battuluri, K. C. Mondal and S. Roy, Chem. – Eur. J., 2022, 28, e202202324 CrossRef CAS PubMed.
- (a) P. G. Baranov, N. G. Romanov, V. A. Khramtsov and V. S. Vikhnin, J. Phys.: Condens. Matter, 2001, 13, 2651 CrossRef CAS; (b) J. Sadło, J. Michalik and L. Kevan, EPR and ESEEM study of silver clusters in ZK-4 molecular sieves, Nukleonika, 2006, 51, 49 Search PubMed; (c) A. Baldansuren, R. Eichel and E. Roduner, Phys. Chem. Chem. Phys., 2009, 11, 6664 RSC.
- H. Hirai, S. Ito, S. Takano, K. Koyasu and T. Tsukuda, Chem. Sci., 2020, 11, 12233 RSC.
- (a) Y. Shu-Yan, L. Qin-Hui and S. Meng-Chang, Inorg. Chem., 1994, 33, 1251 CrossRef CAS; (b) E. Nag, S. Battuluri, B. B. Sinu and S. Roy, Inorg. Chem., 2022, 61, 3007 CrossRef PubMed.
- L. Weber, U. Lassahn, H.-G. Stammler and B. Neumann, Eur. J. Inorg. Chem., 2005, 4590 CrossRef CAS.
- B. K. Najafabadi and J. F. Corrigan, Chem. Commun., 2014, 51, 665 RSC.
- (a) D. Fenske and F. Simon, Angew. Chem., Int. Ed. Engl., 1997, 36, 230 CrossRef CAS; (b) X. He, H.-X. Liu and L. Zhao, Chem. Commun., 2016, 52, 5682 RSC; (c) X.-J. Wang, T. Langetepe, C. Persau, B.-S. Kang, G. M. Sheldrick and D. Fenske, Angew. Chem., Int. Ed., 2002, 41, 3818 CrossRef CAS; (d) D. Fenske, C. Persau, S. Dehnen and C. E. Anson, Angew. Chem., Int. Ed., 2004, 43, 305 CrossRef CAS PubMed; (e) M. Ueda, Z. L. Goo, K. Minami, N. Yoshinari and T. Konno, Angew. Chem., Int. Ed., 2019, 58, 14673 CrossRef CAS PubMed; (f) Z.-A. Nan, Y. Wang, Z.-X. Chen, S.-F. Yuan, Z.-Q. Tian and Q.-M. Wang, Chem. Commun., 2018, 1, 1 CrossRef; (g) J.-W. Liu, H.-F. Su, Z. Wang, Y.-A. Li, Q.-Q. Zhao, X.-P. Wang, C.-H. Tung, D. Sun and L.-S. Zheng, Chem. Commun., 2018, 54, 4461 RSC; (h) L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. Del Gobbo, R. G. AbdulHalim, M. Eddaoudi, D.-E. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970 CrossRef CAS PubMed; (i) S.-F. Yuan, Z.-J. Guan, W.-D. Liu and Q.-M. Wang, Nat. Commun., 2019, 10, 1 CrossRef PubMed; (j) C. Sun, B. K. Teo, C. Deng, J. Lin, G.-G. Luo, C.-H. Tung and D. Sun, Coord. Chem. Rev., 2021, 427, 213576 Search PubMed; (k) M. S. Bootharaju, R. Dey, L. E. Gevers, M. N. Hedhili, J.-M. Basset and O. M. Bakr, J. Am. Chem. Soc., 2016, 138, 13770 CrossRef CAS PubMed.
- M. Jash, A. C. Reber, A. Ghosh, D. Sarkar, M. Bodiuzzaman, P. Basuri, A. Baksi, S. N. Khanna and T. Pradeep, Nanoscale, 2018, 10, 15714 RSC.
- A. Kulkarni, S. Arumugam, M. Francis, P. G. Reddy, E. Nag, S. M. N. V. T. Gorantla, K. C. Mondal and S. Roy, Chem. – Eur. J., 2021, 27, 200 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2242239 (2), 2194453 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc05628k |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |





