Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isolation and characterization of a new polyoxometalate ligand, H3SbW14O5010−, and its interactions with f-elements†
Ian
Colliard
 *ab and
Gauthier J.-P.
Deblonde
*ab and
Gauthier J.-P.
Deblonde
 *ac
*ac
aPhysical and Life Sciences Directorate, Glenn T. Seaborg Institute, Lawrence Livermore National Laboratory, Livermore, California 94550, USA. E-mail: Colliard1@LLNL.gov; Deblonde1@LLNL.gov
bMaterial Sciences Division, Lawrence Livermore National Laboratory, Livermore, California 94550, USA
cNuclear and Chemical Sciences Division, Lawrence Livermore National Laboratory, Livermore, California 94550, USA
First published on 10th December 2024
Abstract
We report the synthesis and characterization (Raman, FTIR, DLS, single crystal XRD) of Cs10H3SbW14O50·6H2O, which contains a new polyoxometalate building block: H3SbW14O5010− (SbW14). Solution-state fluorescence, DLS, and UV-vis absorbance results with Nd3+, Eu3+, Am3+, and Cm3+ confirm that SbW14 acts as an efficient complexant and fluorescence sensitizer for f-elements.
Polyoxometalates (POMs) represent a large family of molecules with numerous applications, and their range of compositions and structures covers most of the periodic table.1–3 While the POM core structures are typically built upon d-block metals4 (W, Mo, V, Cr, Nb, Ta), their compounds often incorporate other elements and complex metal ions, and are counter-balanced by cations. POM compounds that have been characterized thus far cover over 70 elements (from hydrogen to as heavy as curium5–8), representing one of the most diverse class of molecules.
POMs can be seen as the inorganic equivalents of organic chelators or as an ever-expanding family of molecules that uses d-block metal octahedra (e.g., WO62−) as unitary blocks, instead of carbon–carbon bonds. POM core structures include the Lindqvist9 (e.g., Mo6O192−, HxNb6O19x−8 and HxTa6O19x−8), Peacock–Weakley10 (e.g., W5O186−), Keggin11 (e.g., PMo12O403−, SiW11O398−), Well–Dawson12 (e.g., P2W18O626−), Anderson–Evans13,14 (e.g., Mo7O246−), and Preyssler15 (e.g., P5W30O11015−) ions. However, the Keggin, Wells–Dawson, and Lindqvist structures are the most widely studied.4,15–17 For instance, in the Keggin structure ([XW12O40]n−), over 25 candidates are available to be incorporated as heteroelement (X). This leads to an overwhelming number of Keggin derivatives,18 all with potentially distinct physiochemical properties.
In this context, the subset of antimono-polytungstates (Sb-POTs), has been relatively overlooked, despite having truly unique properties. In previously reported examples of Sb-POTs, the stereoactive lone pair19 of Sb(III) strongly influenced the structures, albeit still leading to Keggin derivatives.20 In addition, the low charge density of Sb3+ shifts the POMs’ stability window to slightly higher pHs, often leading to protonated Sb-POTs.21–23 This often results in larger POMs, yet still all derived from the Keggin structure. Several Sb-POTs have been reported in the last few decades—all based on successive condensations of [SbW9O33]9− (SbW9), which is a tri-lacunary Keggin ion. SbW9 is arguably the most used Sb-POT structure and has served as precursor for larger POMs ([NaSb9W21O86]18−, [Sb2W21O69]6−, [H2SbW22O76]14−, [Sb8W36O132]24−…).20
In recent years, POMs have also received increasing attention for use as chelators for f-elements. In fact, POMs offer an interesting alternative relative to the more traditional organic chelators as they can act as oxygen-donor ligands but contain elements that are not easily incorporable in organic ligands. POMs can also stabilize usually unstable oxidation states of actinides, such as Am(VI).8 The high-molecular weight of POMs also make them useful to perform microscale crystallization tests with radioisotopes.5 In our quest to expand the chemistry of f-elements with POM ligands, we attempted to transpose our synthetic protocols recently used5,7,24 for Keggin POMs with B3+, Ga3+, Si4+, Ge4+, and P5+ to antimony (Sb3+ – Fig. 1). Unexpectedly, the substitution of the heteroelements mentioned above for Sb3+ did not yield the expected Keggin POM. Not only does the behavior of antimony depart from that of the other heteroelements, but it also yielded a novel POM structure. This therefore unlocked an opportunity to explore f-element chemistry with a new type of ligand.
Herein we report the single crystal XRD structure of the POM compound Cs10H3SbW14O50·6H2O. This antimony(III) polytungstate is based on a new building block [H3SbW14O50]10− (SbW14). This POM was obtained by the typical synthetic route that has consistently led to Keggin-type structures with other p-block elements.15 Additionally, we probed the potential binding of SbW14 to trivalent lanthanides (Eu3+ and Nd3+) and actinides (Am3+ and Cm3+) in solution. Eu3+ and Cm3+ were used for their fluorescence properties while Nd3+ and Am3+ were used for their absorbance properties. All four f-elements also have similar ionic radii, facilitating intercomparisons. The behavior of SbW14 was also contrasted with the previously known POM SbW9. The results show that SbW14 can act as an effective aqueous ligand with f-elements. This represents the first study of interactions between antimony-containing ligands and heavy actinides.
SbW14 was obtained by conversion of the precursor [SbW9O33]9− (SbW9). This precursor was synthesized as reported by Bösing et al.20 without modification. The SbW9 to SbW14 conversion reaction was found remarkably straightforward – happening at room temperature, ambient pressure, and in aqueous solution. From the synthesis by Bösing et al., a 2 mM solution of SbW9 was prepared (30 mg in a 100 mM acetate buffer at pH 5.5). Then, an equal volume of 6 M CsCl was added (final concentration of 3 M). After 24–48 h crystals of SbW14 appeared. The pH was measured at various points during the crystallization period and was stable at 5.5. For a more detailed synthesis procedure see ESI.† Single crystals of SbW14 were collected for structure determination, plus Raman and FTIR analysis. Fig. S1 (ESI†) clearly shows distinct Raman and FTIR features between SbW9 and SbW14.
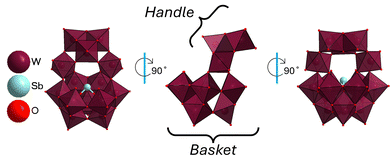
Interestingly, the new Sb-POT could only be crystallized through the addition of caesium counterions, as opposed to pH variation. For POMs, the most conventional manner to control their speciation is by controlling the pH, to tune their hydrolysis and condensation reactions. We assume that SbW14 had thus far remained undiscovered because there is generally very little information in the literature about Sb-POTs with caesium counterions. This new crystal structure is unique in its synthesis, obtained through counterion-mediated reactions as opposed to pH-controlled reactions. The new structure, fully formulated as Cs10H3SbW14O50·6H2O, crystallizes in the orthorhombic space group P212121 with a unit cell volume of 6609.5 (3) A3; for more information see Table S1 (ESI†). One manner to describe the new Sb-POTs is as a combination of the [SbW9O33]9− subunit, nicknamed the ‘basket’, with a [W5(OH)3O14]− ‘handle’ subunit (Fig. 2). The structure itself has only one symmetry element; a mirror plane along its long axis. Thus, the structure falls under the point group Cs. The ‘basket’ subunit, SbW9, is the original precursor and itself is often described as the tri-lacunary version of parent Keggin structure ([XW12O40]n−).25,26 In other words, removal of three adjacent tungstates from a parent but hypothetical structure [SbW12O40]5−, would results in the SbW9 subunit. We suspect that SbW9 does not convert to [SbW12O40]5− due to the size of Sb3+ and steric hindrance created by its lone electron pair. In SbW9, six tungstates surround the central [SbO3]3−, with the remaining three tungstates opposite to the lone pair on Sb.
Bond valence sum calculations in SbW14 confirms the oxidation state at +III for Sb (Table S2, ESI†). The six surrounding tungstates can be thought of as the ‘rim of the basket’ while the remaining three tungstates can be the ‘bottom’. The ‘handle’ itself, [W5(OH)3O14]−, can further be subdivided and described as a [W3(OH)3O10]5− with two edge-sharing cis-di-oxyl-tungstens, [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) W
W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]2+, binding to two of the three tungstates in the trimer. These two flanking tungstens then bind to the ‘basket’ completing their six-fold coordination. The ‘handle’ is attached to the basket via four out of the six tungstates surrounding Sb in the SbW9 subunit. As a result, all tungstates have a coordination number of six, as seen for most polyoxotungstates, with rare exceptions.27 Bond valence sum calculation on the O and W positions additionally reveal the potential location of three protons on the POMs, leading to the formula [H3SbW14O50]10−, Table S3 (ESI†).
O]2+, binding to two of the three tungstates in the trimer. These two flanking tungstens then bind to the ‘basket’ completing their six-fold coordination. The ‘handle’ is attached to the basket via four out of the six tungstates surrounding Sb in the SbW9 subunit. As a result, all tungstates have a coordination number of six, as seen for most polyoxotungstates, with rare exceptions.27 Bond valence sum calculation on the O and W positions additionally reveal the potential location of three protons on the POMs, leading to the formula [H3SbW14O50]10−, Table S3 (ESI†).
Due to its unique composition and structure, the complexation of SbW14 with f-elements became of particular interest. The solution behavior of this new POT was explored through fluorescent spectroscopy (Eu3+vs. Cm3+) and UV-Visible spectrophotometry (Nd3+vs. Am3+). This represents the first study on transplutonium elements with antimony-based ligands. To first probe the physiochemical properties and complexation behavior for SbW14, excitation and emission spectra were taken with Eu3+ at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (Eu3+
2 ratio (Eu3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SbW14), as we expected a 1
SbW14), as we expected a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex based on potential binding sites of the SbW14 structure (Fig. 2). Excess POM to f-element was also used to ensure full complexation. Parallel experiments were done with SbW9, for comparison purposes and to confirm that SbW14 does not convert back to its precursor upon binding to the f-elements. Both SbW9 and SbW14 were found to spontaneously bind to the trivalent lanthanides and actinides.
2 complex based on potential binding sites of the SbW14 structure (Fig. 2). Excess POM to f-element was also used to ensure full complexation. Parallel experiments were done with SbW9, for comparison purposes and to confirm that SbW14 does not convert back to its precursor upon binding to the f-elements. Both SbW9 and SbW14 were found to spontaneously bind to the trivalent lanthanides and actinides.
Fig. 3a and b show the normalized excitation and emission spectra, respectively for Eu3+ with SbW9 and SbW14. Both POMs bind to Eu3+ and sensitize its luminescence, but evidently via different pathways (Fig. 3a). The preferential excitation pathway for Eu3+ is via the POM band (λex = 290–310 nm) in the case of SbW14 but via direct excitation of Eu3+ (4f–4f sharp transitions at 360–410 nm, main peak λex = 396 nm) in the case of SbW9. The excitation peak via the POM has a maximum at 343 nm for SbW9, compared to 303 nm for SbW14. When comparing the emission spectra, SbW14 results in more defined Eu3+ emission peaks, with intensities about 16-fold higher (Fig. S3, ESI†).
 | ||
| Fig. 3 Fluorescence studies on Eu–SbW14 (purple curves) and Cm–SbW14 (blue curves) compared to Eu-SbW9 (green curves) and CmSbW99 (orange curves). Eu3+ fluorescence: (a) excitation and (b) emission. Cm3+ fluorescence: (c) excitation and (d) emission. Fig. 3a is normalized to the excitation peak at 396 nm as Eu3+ sensitization via the POM band of SbW9 is inefficient. See Fig. S1–S5 (ESI†) for raw intensity spectra. Solution conditions were as follows: [Eu3+] = 1 mM, and [Cm3+] = 100 μM, [SbW9] and [SbW14] at 2 mM and 200 μM, respectively. Sb-POTs were dissolved in 0.1 M acetate at pH of 5.5. *Indicates the POM excitation band. | ||
Lifetime dependent fluorescence further shows different complexation behaviors for SbW9versus SbW14 (Table S4, ESI†). While both SbW9 and SbW14 yield mono-exponential decay curves with Eu3+ (suggesting only one type of complex(es) present), the lifetimes are significantly different, at 336 μs and 621 μs, respectively. Based on the Kimura equations,28 which empirically correlate fluorescence lifetimes to hydration sphere, the number of coordinating water molecules decreases from three for Eu–SbW9 to one for Eu–SbW14. Although we were unable to crystallize a complex of SbW14 with an f-element, the solution-state fluorescence results suggest that SbW14 is more amenable to fulfil the f-element coordination sphere than SbW9, leaving it less exposed to solvent molecules. Additional fluorescence titration experiments (Fig. S6–S8, ESI†), revealed that the Eu–SbW14 likely undergoes a gradual complexation reaction when the ratio SbW14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu is varied. By analogy to other POMs,4 we tentatively ascribe the observed spectral changes to the sequential formation of 1
Eu is varied. By analogy to other POMs,4 we tentatively ascribe the observed spectral changes to the sequential formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes, however the precise solution-state speciation of the f-element/SbW14 systems will require further investigations.
2 complexes, however the precise solution-state speciation of the f-element/SbW14 systems will require further investigations.
When studies were extended from 4f to 5f-elements (Am3+ and Cm3+) some deviations could be discerned in their binding behavior and physiochemical properties. Fluorescence results (Fig. 3c, d and Fig. S4, S5, ESI†) confirmed that Cm3+ also binds to both SbW9 and SbW14. However, contrary to Eu3+, the excitation spectra revealed that, for both SbW9 and SbW14, sensitization of Cm3+via the POM is more efficient than direct excitation. The POM excitation band is at 276 nm for Cm–SbW9versus 303 nm for Cm–SbW14. Sensitization of Cm3+ is also more efficient via SbW14 than SbW9, by a factor of ∼4 (Fig. S4, ESI†). In other words, SbW14 leads to brighter complexes than SbW9 for both Eu3+ and Cm3+. The Cm3+ emission spectra are also distinct, with peak maximum at 606.0 nm for SbW9 and 609.0 nm for SbW14 and an increase in intensity of a factor of 1.5. Both Cm–SbW9 and Cm–SbW14 complexes exhibit a significant peak shift when compared to free Cm3+ under similar conditions (598.4 nm).5 While the Kimura equation has been shown to not be applicable for most reported curium POMs,5 apart from the simplest polytungstate [Cm(W5O18)2]9−,6 the time-dependent fluorescence herein is somewhat consistent with the Eu3+ analog (i.e., lifetime longer for Cm–SbW14vs. Cm–SbW9). The measured lifetimes for Cm3+ are 133 μs for SbW9 to 177 μs for SbW14 (Table S4, ESI†). This corresponds to four and three water molecules, respectively. The trend is consistent with the Eu3+ results and indicates a lower hydration number for the Cm–SbW14 complex, relative to Cm–SbW9, but the absolute number of water molecules may not be accurate. The difference in hydration between Eu3+ and Cm3+ complexes could arise from different binding sites on SbW14.
However, we posit that the Kimura equation fails to accurately describe the Cm3+ hydration for the Sb-POTs and starts to be less valid for Eu3+ as well, in this particular context. We attribute this to the alkali counterions, which are typically considered inert but have been shown to form strong ion pairs with certain POM complexes. Herein, SbW9 uses Na+ whereas SbW14 uses Cs+, which could impact fluorescence properties. Based on the emission spectra for SbW9, the observed species are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, [Eu(SbW9O33)(H2O)3]6− and [Cm(SbW9O33)(H2O)4]6−, but the speciation for Cm/Eu–SbW14, remains ambiguous as we do not know its coordination mode. We also performed dynamic light scattering (DLS) experiments on SbW14, and SbW9, with and without Eu3+ and Cm3+ (Fig. S9 and Table S5, ESI†). The measured hydrodynamic diameters could support the formation of 1
1 complexes, [Eu(SbW9O33)(H2O)3]6− and [Cm(SbW9O33)(H2O)4]6−, but the speciation for Cm/Eu–SbW14, remains ambiguous as we do not know its coordination mode. We also performed dynamic light scattering (DLS) experiments on SbW14, and SbW9, with and without Eu3+ and Cm3+ (Fig. S9 and Table S5, ESI†). The measured hydrodynamic diameters could support the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes for both POMs under the tested conditions, although the size difference between 1
1 complexes for both POMs under the tested conditions, although the size difference between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes may not be enough to discriminate with this technique. The role of counterions on the apparent hydrodynamic size measured by DLS also adds some uncertainty to the applicability of DLS to POM complexes.
2 complexes may not be enough to discriminate with this technique. The role of counterions on the apparent hydrodynamic size measured by DLS also adds some uncertainty to the applicability of DLS to POM complexes.
To gain more insight into the speciation, UV-Vis absorbance experiments with Nd3+ and Am3+ were performed (Fig. 4). Both metals previously demonstrated sensitive absorbance peak shifting for different Nd3+/Am3+-POT species.5,8 Am3+ typically exhibit significant peak shifting with the free ion absorbance at 504 nm, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Am3+
1 Am3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POT complexes at 506–512 nm, and 1
POT complexes at 506–512 nm, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes at 514–520 nm.5 For both Nd3+ and Am3+, there is evidence of complexation with both SbW9 and SbW14 (Fig. 4). The absorbance bands of Nd3+ and Am3+ are both affected in the presence of SbW9 and SbW14, relative to their free ion state. In the case of Am3+, SbW9 leads to asymmetric broadening of the peak, likely indicative of a lower symmetry relative to the aqua ion. For Am3+ with SbW14, the peak exhibits a more obvious change with a dominant peak appearing at 508.0 nm. Note that the Am3+–Sb-POT experiments were limited to 20 μM concentrations, as opposed to 1 mM for Nd3+ or 100 μM for Cm3+, which may have limited the complexation efficacy of the POMs. The results nonetheless confirm that both SbW9 and SbW14 bind to trivalent lanthanides and actinides in solution. The similar extinction coefficient also supports the formation of 1
2 complexes at 514–520 nm.5 For both Nd3+ and Am3+, there is evidence of complexation with both SbW9 and SbW14 (Fig. 4). The absorbance bands of Nd3+ and Am3+ are both affected in the presence of SbW9 and SbW14, relative to their free ion state. In the case of Am3+, SbW9 leads to asymmetric broadening of the peak, likely indicative of a lower symmetry relative to the aqua ion. For Am3+ with SbW14, the peak exhibits a more obvious change with a dominant peak appearing at 508.0 nm. Note that the Am3+–Sb-POT experiments were limited to 20 μM concentrations, as opposed to 1 mM for Nd3+ or 100 μM for Cm3+, which may have limited the complexation efficacy of the POMs. The results nonetheless confirm that both SbW9 and SbW14 bind to trivalent lanthanides and actinides in solution. The similar extinction coefficient also supports the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes under these conditions.
1 complexes under these conditions.
In conclusion, this study reports a new antimono-polytungstate structure, SbW14, and its solution behavior with lanthanide and actinide elements. The Cs10H3SbW14O50·6H2O structure was synthesized by an unconventional route of counterion-mediated conversion (versus traditional hydrolysis and condensation reactions). This compound was characterized via single crystal XRD, FTIR, Raman microscopy and compared to its precursor, SbW9. Solution-state fluorescence, UV-Vis spectroscopy, and DLS experiments with Eu3+vs. Cm3+, and Nd3+vs. Am3+, revealed consistent complexation of SbW9 and SbW14 to the lanthanides or actinides. Furthermore, we demonstrate that the new structure functions as a more efficient sensitizer and for both Eu3+ and Cm3+, relative to SbW9. Future work will focus on elucidating the solution-state speciation and expand the counterion conversion of other Sb-POTs and interactions with other f-elements.
This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Element Chemistry program at Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. Release number: LLNL-JRNL-870910.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- M. K. Kinnan, W. R. Creasy, L. B. Fullmer, H. L. Schreuder-Gibson and M. Nyman, Eur. J. Inorg. Chem., 2014, 2361–2367 CrossRef CAS.
- J.-J. Chen, M. D. Symes and L. Cronin, Nat. Chem., 2018, 10, 1042–1047 CrossRef CAS PubMed.
- A. Gaita-Ariño, F. Luis, S. Hill and E. Coronado, Nat. Chem., 2019, 11, 301–309 CrossRef PubMed.
- N. I. Gumerova and A. Rompel, Chem. Soc. Rev., 2020, 49, 7568–7601 RSC.
- I. Colliard, J. R. I. Lee, C. A. Colla, H. E. Mason, A. M. Sawvel, M. Zavarin, M. Nyman and G. J.-P. Deblonde, Nat. Chem., 2022, 14, 1357–1366 CrossRef CAS PubMed.
- I. Colliard and G. J.-P. Deblonde, Chem. Commun., 2024, 60, 5999–6002 RSC.
- I. Colliard and G. J.-P. Deblonde, JACS Au, 2024, 4, 2503–2513 CrossRef CAS PubMed.
- H. Zhang, A. Li, K. Li, Z. Wang, X. Xu, Y. Wang, M. V. Sheridan, H.-S. Hu, C. Xu, E. V. Alekseev, Z. Zhang, P. Yan, K. Cao, Z. Chai, T. E. Albrecht-Schönzart and S. Wang, Nature, 2023, 616, 482–487 CrossRef CAS PubMed.
- I. Lindqvist, Ark. Kemi, 1953, 5, 247–250 CAS.
- R. D. Peacock and T. J. R. Weakley, J. Chem. Soc. A, 1971, 1836–1839 RSC.
- J. F. Keggin and W. L. Bragg, Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, 1997, 144, 75–100.
- B. Dawson, Acta Crystallogr., 1953, 6, 113–126 CrossRef CAS.
- J. S. Anderson, Nature, 1937, 140, 850 CrossRef CAS.
- H. T. Evans, J. Am. Chem. Soc., 1948, 70, 1291–1292 CrossRef CAS.
- M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer, Berlin, Heidelberg, 1st edn, 1983 Search PubMed.
- N. I. Gumerova and A. Rompel, Sci. Adv., 2023, 9, eadi0814 CrossRef CAS PubMed.
- A. Misra, K. Kozma, C. Streb and M. Nyman, Angew. Chem., Int. Ed., 2020, 59, 596–612 CrossRef CAS PubMed.
- C. A. Ohlin, Phys. Chem. Chem. Phys., 2020, 22, 4043–4050 RSC.
- B. M. Benin, K. M. McCall, M. Wörle, D. Borgeaud, T. Vonderach, K. Sakhatskyi, S. Yakunin, D. Günther and M. V. Kovalenko, Chem. Mater., 2021, 33, 2408–2419 CrossRef CAS PubMed.
- M. Bösing, I. Loose, H. Pohlmann and B. Krebs, Chem. – Eur. J., 1997, 3, 1232–1237 CrossRef.
- H. Naruke and T. Yamase, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1992, 48, 597–599 CrossRef.
- Z. Tang, M. Wang, X. Jia, S. Xie, P. Chen, D. Wang, L. Chen and J. Zhao, Inorg. Chem., 2022, 61, 14648–14661 Search PubMed.
- X. Xin, Y. Ma, L. Hou, Y. Wang, X. Xue, J. Lin and Z. Han, Inorg. Chem., 2019, 58, 9567–9571 CrossRef CAS PubMed.
- I. Colliard and G. J.-P. Deblonde, Inorg. Chem., 2024, 63, 16293–16303 Search PubMed.
- J. F. Keggin, Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, 1934, 144, 75–100.
- C. Falaise, G. Mpacko Priso, N. Leclerc, M. Haouas and E. Cadot, Inorg. Chem., 2023, 62, 2494–2502 Search PubMed.
- G. Deblonde and I. Colliard, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2024, 80, 667–670 Search PubMed.
- T. Kimura, G. R. Choppin, Y. Kato and Z. Yoshida, Radiochim. Acta, 1996, 72, 61–64 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2326569. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc05558f |
| This journal is © The Royal Society of Chemistry 2025 |