Upcycling of spent LiCoO2: engineering the coordination-trapping behavior towards conversion-type anodes for advanced Li-storage†
Zihao
Zeng‡
,
Hai
Lei‡
,
Yunpeng
Wen
,
Chao
Zhu
,
Jiexiang
Li
,
Wei
Sun
 ,
Yue
Yang
,
Yue
Yang
 and
Peng
Ge
and
Peng
Ge
 *
*
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China. E-mail: gp-gepeng@csu.edu.cn
First published on 31st January 2025
Abstract
Based on its high economic/sustainability value, the upcycling of spent cathodes into anodes has been deemed to be an alternative strategy to traditional chemical synthesis. Supported by an effective acid leaching and coordination-trapping self-assembly reaction, a nano-scale CoS@NSC anode was successfully prepared from spent LiCoO2 and used as a promising anode for lithium ion batteries.
Given their high specific capacity and extraordinary conductivity, transition metal sulfides have been deemed to be promising anodes for energy storage systems, including lithium ion batteries (LIBs), sodium ion batteries (SIBs), and others.1–3 However, the large-scale production of metal sulfides suffers from the high cost of raw materials and cumbersome preparation process.4–6 To solve the problems above, a series of studies have focused on natural minerals, proposing short-process mineral regeneration processes such as gas-phase melting methods, and achieving the fabrication of metal sulfides with improved Li/Na ion storage capabilities.7–9 Compared to natural minerals, urban mineral resources, such as spent batteries, have captured greater attention due to their having higher metal enrichment and fewer impurities.10–12 At present, hydrometallurgy, pyrometallurgy and direct regeneration methods have been utilized to recover the cathode materials of spent LIBs, accompanied by considerable advances.13–15 Supported by the hydrometallurgy process, the metal ions could be recovered in the form of ions. Meanwhile, with the assistance of a flotation agent, the metal ions could be captured by the sulfhydryl group (–SH), leading to self-assembly into flotation agent–metal complexes.16 Similar to metal organic frameworks (MOFs), the as-obtained complexes consisted of two parts: the hydrocarbon chains and the sulfhydryl group–metal part. Thus, inspired by the flotation chemistry and structure traits of MOFs, it was expected that the prepared complexes could be transformed into anode materials to achieve upcycling-recovery of spent cathodes. Herein, sodium diethyldithiocarbamate (DDTC-Na) was utilized as a metal capture agent, and the leached Co ions could be complexed with the sulfhydryl functional group (–SH), resulting in the formation of DDTC-Co. In the molten salt, hexagonal-prism-like Co1-xS@N,S co-doped carbon composites could be derived from DDTC-Co. When used as the anode for Li ion storage, the as-fabricated composites displayed considerable electrochemical performance. In particular, tailoring of the carbonization temperature resulted in the DDTC-derived carbon coated showing different conductive traits, leading to diverse cycling stability. A detailed investigation of the physical–chemical and electrochemical properties was carried out.
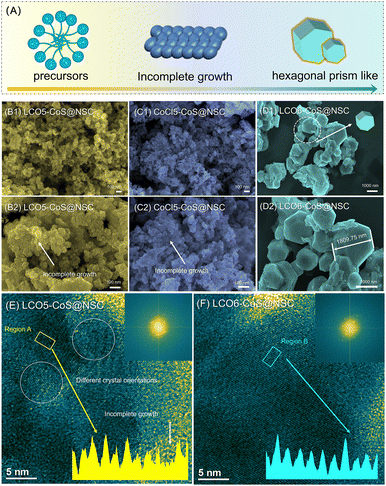
Fig. 1A illustrates the detailed steps of the spent LiCoO2 (LCO) upcycling process. Firstly, spent LCO was soaked in an HCl solution, and through the chemical reaction of LCO and H+ ions, all the Co ions could be collected in the solution, which is shown in Fig. 1B1. Later, as shown in Fig. 1B2, the as-prepared Co-rich solution was added to a DDTC solution, and Co–S bonds were constructed through the combination of –SH and Co2+ ions, resulting in the formation of DDTC-Co complexes. As shown in Fig. 1B3 and B4, through the sedimentation effect, the as-prepared DDTC-Co could be gathered in the bottle of the solution, and the powder-like DDTC-Co precursor was obtained after the drying process. Through the addition of molten salt, slight melting was triggered on the surface of the salt particles, facilitating the transformation of the precursors into carbon nanostructures. The as-obtained DDTC-Co precursors were mixed with Na2SO4 for further growth and carbonization process, and the samples prepared at 600 °C were named LCO6-CoS@NSC. Additionally, to explore the effect of the sintering temperature on the spent samples, a sample was processed at 500 °C and named LCO5-CoS@NSC; and samples utilizing CoCl2 as the Co source were obtained at 500 °C and 600 °C and named CoCl5-CoS@NSC and CoCl6-CoS@NSC, respectively.
Fig. 1C compares the XRD patterns of the as-fabricated samples, which illustrate their diverse crystallinity. Comparing the samples prepared at 500 °C (including CoCl5-CoS@NSC and LCO5-CoS@NSC), LCO5-CoS@NSC displayed a higher degree of crystallinity, indicating that its crystallization area was larger than that of the other samples, which was beneficial to construct the continuous ion diffusion channels. Additionally, the XRD patterns provided the phase composition. Based on the well-matching traits of the XRD patterns and the standard PDF card (PDF#42-0826), all the samples could be identified as the Co1−xS phase without any impurities. As denoted by the “1 − x” in the chemical formula provided for the standard PDF card, it could be concluded that diverse valence states of Co co-existed in the samples. Given this, XPS was utilized to analyze the element state of prepared composites. Fig. 1D illustrates the full spectra of the as-prepared samples. It should be noted that the types of elements were the same in the three samples. Fig. 1E illustrates the Co 2p high resolution XPS spectrum. The peaks located at 796.7 and 781.3 eV indicated the existence of Co3+, while the peaks located at 793.9 and 778.7 eV were related to the Co2+ in the CoS lattice.17,18 Thus, based on the analysis, two valence states of the element cobalt coexist synergistically, matching well with the XRD results. Fig. 1F illustrates the S 2p high-resolution spectra. The peaks located at 168.7, 164.5, 163 and 161.7 eV were related to the formation of C–SOx, C–S, Co–S and C–S–Co chemical bonds.19 The C–S bonds indicated that a fraction of the S atoms were doped into the carbon layer, which was beneficial for improving the conductivity of the as-prepared samples. Importantly, the C–S–Co chemical bonds could serve as a bridge between the carbon layer and CoS lattice, which would provide extra transportation channels for the electrons. In the C 1s high-resolution spectrum, peaks belonging to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–S, C–N and C–C bonds could be noted at 288.3, 286.3 285.6 and 284.4 eV.20 The formation of C–N bonds indicated that a fraction of the N atoms were also doped into the carbon of the composite, further improving the conductivity of the as-obtained samples.21Fig. 1H illustrates the Raman spectra of the as-prepared samples; the peaks belonging to the carbon could clearly be divided into the G peak and D peak. Compared to the LCO6-CoS@NSC, the intensity of the G and D peak of CoCl5-CoS@NSC and LCO5-CoS@NSC were lower. Thus, it could be concluded that a low sintering temperature would lead to a large amount of H atoms remaining at the carbon layer, resulting in the inferior conductivity.
O, C–S, C–N and C–C bonds could be noted at 288.3, 286.3 285.6 and 284.4 eV.20 The formation of C–N bonds indicated that a fraction of the N atoms were also doped into the carbon of the composite, further improving the conductivity of the as-obtained samples.21Fig. 1H illustrates the Raman spectra of the as-prepared samples; the peaks belonging to the carbon could clearly be divided into the G peak and D peak. Compared to the LCO6-CoS@NSC, the intensity of the G and D peak of CoCl5-CoS@NSC and LCO5-CoS@NSC were lower. Thus, it could be concluded that a low sintering temperature would lead to a large amount of H atoms remaining at the carbon layer, resulting in the inferior conductivity.
The different sintering temperatures would bring about diverse morphology traits in the resulting samples, leading to differences in their energy storage abilities. The SEM and TEM images in Fig. 2 illustrate interesting structure-evolution behaviors. As shown in Fig. 2B1–B2 and C1–C2, after the sintering process at 500 °C, a nano-dot-like structure could be noted in the SEM images, indicating that the lattice of CoS had not grown completely. As the temperature was improved to 600 °C, a series of hexagonal-like particles could be noted, as shown in Fig. 2D1, and the size of the hexagonal particles was detected to be 1809.75 nm, as shown in Fig. 2D2. Thus, as presented in Fig. 2A, the structural evolution during the sintering process could be summarized as follows. Firstly, the precursors connected with each other, and the CoS phase was generated during the 20–500 °C sintering process. However, at this stage, the growth behavior of the CoS lattice was incomplete, and the carbon layer was only partly carbonized, resulting in the inferior energy storage ability. As noted in Fig. 2E, regions with different crystal orientations can be observed in the TEM image, indicating a series of tiny unit cells that were not fully grown and fused co-existed in the 500 °C sintered samples. Additionally, the irregular crystal streaks shown in the illustration provide further evidence of incomplete grain growth at this stage. As shown in Table S1 (ESI†), from the ICP analysis, some Al and Na ions were present as impurities in the samples, which were derived from the Al foil and Na2SO4 molten salt.
In contrast, in the TEM images of LCO6-CoS@NSC, the crystal orientation was consistent across all regions, proving that 600 °C sintering process could provide enough energy for complete growth. Correspondingly, regular crystal streaks can be noted in Fig. 2F, further indicating the complete growth behavior of CoS. In summary, the sintering temperature was critical to the transition process from the precursor to the CoS@C composites, which mainly involves the completeness of the crystal growth process and the degree of carbonization of the hydrocarbon chain. Tailoring a suitable calcination temperature could ensure that the anode material that was converted from spent LCO could display better electrochemical performance.
Inspired by the attractive results of the physical–chemical properties investigation of the as-fabricated composites, exploration of their electrochemical properties was carried out, as shown in Fig. 3. Utilizing the three samples as the working electrodes and Li metal as the counter electrode, coin cells were assembled and further tested within the voltage range of 0.01–3.0 V. As illustrated in Fig. 3A, the cycling performance of the as-obtained samples was compared. At 1.0 A g−1, LCO6-CoS@NSC, LCO5-CoS@NSC and CoCl5-CoS@NSC displayed an initial capacity of 889.8, 754.2 and 705.4 mA h g−1, respectively, following the order LCO6-CoS@NSC > LCO5-CoS@NSC > CoCl5-CoS@NSC. It should be noted that obvious capacity-fading behaviors were observed for LCO5-CoS@NSC and CoCl5-CoS@NSC, which was related to their incomplete growth behaviors and the un-carbonized coated layer. After 200 cycles, LCO6-CoS@NSC retained a capacity of about 820.2 mA h g−1 with a capacity retention rate of 92.17%. As shown in Fig. S1 (ESI†), LCO6-CoS@NSC displayed considerable stability over 500 cycles. Fig. S2 (ESI†) illustrates the electrochemical performance of CoCl6-CoS@NSC. The electrochemical performance of CoCl6-CoS@NSC was similar to that of LCO6-CoS@NSC, indicating that the strategy of upcycling spent LCO into an anode was successful. Additionally, the rate capacity of the three samples is compared in Fig. 3B. At 0.2, 0.5, 1.0, 2.0 3.0 and 5.0 A g−1, the capacity of LCO6-CoS@NSC was 977.4, 891.9, 845.6, 810.2, 794.9 and 760.9 mA h g−1, which was higher than that of LCO5-CoS@NSC (888.9, 788.8, 720.3, 678.8, 630.1 and 568 mA h g−1 at 0.2, 0.5, 1.0, 2.0 3.0 and 5.0 A g−1) and CoCl5-CoS@NSC (862.5, 797.5, 748, 707.8, 652.8 and 616 mA h g−1 at 0.2, 0.5, 1.0, 2.0 3.0 and 5.0 A g−1). Furthermore, when applied as anode for SIBs, LCO6-CoS@NSC can achieve about 600 mA h g−1 at 1.0 C and displays considerable cycling stability (Fig. S3, ESI†). Thus, it could be concluded that composites derived from spent cathode materials could serve as anodes for LIBs and SIBs.
To probe the differences in the electrochemical properties of the three samples, the relationships between voltage and capacity were compared, as shown in Fig. 3C. It should be noted that three plateaus could be noted in the curves of LCO6-CoS@NSC. Based on previous work, the three plateaus indicated the three reactions during the charging and discharging plateaus.22 The plateaus located at 1.0–1.5 V were related to the formation of polymer film, which could contributed to the increase in capacity.23 However, only two plateaus could be noted for LCO5-CoS@NSC and CoCl5-CoS@NSC; the plateau corresponding to the polymer film vanished. Thus, it could be concluded that the incomplete growth behaviors with low sintering temperature would bring about the loss of the energy-storage plateaus, resulting in the decrease in capacity.
To further investigate the detailed mechanism of the sintering temperature and Li-ion storage ability, CV testing was carried out as shown in Fig. 3D1, E1 and F1. For comparison of the CV curves of the three as-obtained samples, the vertical current axis was set with a consistent range. It should be noted that the current intensity of LCO6-CoS@NSC was much larger than that of the other two samples, indicating its better electrochemical properties. Additionally, three peaks can be noted in the CV curves of LCO6-CoS@NSC, matching well with the analysis of the charging–discharging curves. In order to observe the differences in the CV maps of the three materials more intuitively, the CV maps were plotted in the form of contour maps, as shown in Fig. 3D2, E2 and F2. Compared to those of LCO6-CoS@NSC, the peaks of LCO5-CoS@NSC had half-vanished and those of CoCl5-CoS@NSC had fully vanished. Thus, a suitable sintering temperature was important for improving the capacity of the as-fabricated samples. Fig. 3H illustrates the relationship between the peak current and scan rate for the three as-prepared samples. By fitting the data to linear equations, the slope values were obtained, with the oxidation/reduction peak slopes following the order LCO6-CoS@NSC > LCO5-CoS@NSC > CoCl5-CoS@NSC. Using these slope values and eqn (1), the diffusion coefficient of the Li ions could be calculated. The peak current (Ip), number of electrons transferred (n), active electrode surface area (A), scan rate (v), and concentration of Li+ ions (CLi+) in the CoS lattice were defined in eqn (1). Thus, the diffusion rate of Li ions in LCO6-CoS@NSC was higher than that of other samples.24
| Ip = 2.69 × 105 × n2/3AD1/2ν1/2 | (1) |
Fig. 3I presents the Nyquist plots of the as-obtained samples before the first discharge process. The Nyquist plots clearly consist of two distinct components: a semicircle in the mid-to-high frequency region and a line in the low frequency region, consistent with previous reports.25,26 The semicircle observed in the mid-frequency region is attributed to the charge transfer resistance between the electrode material and the electrolyte. Thus, using Zview software, the Rct values of the as-prepared samples were calculated and are presented in Fig. 3J; they followed the order LCO6-CoS@NSC < LCO5-CoS@NSC < CoCl5-CoS@NSC.25,26 LCO6-CoS@NSC possessed a lower Rct value than other samples, indicating that the Li transportation rate of LCO6-CoS@NSC was higher than that of the other samples.
In this study, CoS@N,S co-doped carbon composites were successfully developed using a simple upcycling approach from spent LiCoO2, with a significant focus on optimizing the sintering temperature to enhance the lithium storage capability. By utilizing DDTC-Na as a capture agent, the Co ions were complexed and transformed into highly conductive CoS composites through controlled thermal treatment. The sample prepared at 600 °C (LCO6-CoS@NSC) exhibited superior crystallinity and complete carbonization compared to those prepared at lower temperatures. This well-developed crystal structure provided a continuous ion diffusion channel, resulting in a high initial capacity of 889.8 mA h g−1, excellent cycling stability, and superior rate performance. In contrast, the samples sintered at 500 °C, LCO5-CoS@NSC and CoCl5-CoS@NSC, demonstrated incomplete CoS lattice growth and uncarbonized layers, leading to reduced capacity and inferior electrochemical performance. In summary, the upcycling of spent LCO into high-performance CoS@N,S co-doped carbon composites has been demonstrated to be a promising strategy for the sustainable development of anode materials for lithium-ion batteries. This work provides an effective approach for improving electrochemical properties, offering valuable insights into the design of next-generation energy storage materials.
This work was financially supported by National Natural Science Foundation of China (52374288, 52204298), Young Elite Scientists Sponsorship Program by China Association for Science and Technology (2022QNRC001), National Key Research and Development Program of China (2022YFC3900805-4/7), Hunan Provincial Education Office Foundation of China (No. 21B0147), Collaborative Innovation Centre for Clean and Efficient Utilization of Strategic Metal Mineral Resources, Found of State Key Laboratory of Mineral Processing (BGRIMM-KJSKL-2017-13), Innovation Driven Project of Central South University (2023CXQD009).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.References
- W. Zhao, S. Lei, J. Li, F. Jiang, T. Wu, Y. Yang, W. Sun, X. Ji and P. Ge, Adv. Energy Mater., 2024, 14(19), 2304431 CrossRef CAS.
- Z. Zeng, W. Zhao, S. Yuan, Y. Dong, J. Zhu, F. Jiang, Y. Yang, S. Liu, L. Wang and P. Ge, ACS Appl. Energy Mater., 2022, 5, 9189–9213 CrossRef CAS.
- Y. Tang, Y. Wei, A. F. Hollenkamp, M. Musameh, A. Seeber, T. Jin, X. Pan, H. Zhang, Y. Hou, Z. Zhao, X. Hao, J. Qiu and C. Zhi, Nano-Micro Lett., 2021, 13, 178 CrossRef CAS PubMed.
- W. Zhao, S. Yuan, L. Zhang, F. Jiang, Y. Yang, G. Zou, H. Hou, P. Ge, W. Sun and X. Ji, Energy Storage Mater., 2022, 45, 1183–1200 CrossRef.
- M. Hu, Z. Ju, Z. Bai, K. Yu, Z. Fang, R. Lv and G. Yu, Small Methods, 2019, 4, 1900673 CrossRef.
- J. B. Zhao, Y. Y. Zhang, Y. H. Wang, H. Li and Y. Y. Peng, J. Energy Chem., 2018, 27, 1536–1554 CrossRef.
- Z. Zeng, Y. Dong, S. Yuan, W. Zhao, L. Wang, S. Liu, Y. Yang, P. Ge, W. Sun and X. Ji, Energy Storage Mater., 2022, 45, 442–464 CrossRef.
- S. Wang, Y. Cheng, H. Xue, W. Liu, Z. Yi, L. Chang and L. Wang, J. Mater. Chem. A, 2021, 9, 7838–7847 RSC.
- Y. X. Huang, Z. H. Wang, Y. Jiang, S. J. Li, M. Wang, Y. S. Ye, F. Wu, M. Xie, L. Li and R. J. Chen, Adv. Sci., 2018, 5, 1800613 CrossRef PubMed.
- S. Kang, S. Lee, H. Lee and Y.-M. Kang, Nat. Rev. Chem., 2024, 8, 587–604 CrossRef CAS PubMed.
- D. Lee, H. Kim and S. M. Jeong, ACS Energy Lett., 2024, 9(9), 4255–4264 CrossRef CAS.
- Z. Wang, L. Li, Z. Sun, P. Tang, G. Hu, J. Tan and F. Li, Prog. Mater. Sci., 2024, 143, 101247 CrossRef CAS.
- H. Yang, B. Deng, X. Jing, W. Li and D. Wang, Waste Manage., 2021, 129, 85–94 CrossRef CAS PubMed.
- Y. Gao, Y. Li, J. Li, H. Xie and Y. Chen, J. Alloys Compd., 2020, 845, 156234 CrossRef CAS.
- Z. Zeng, P. Xu, J. Li, C. Yi, W. Zhao, W. Sun, X. Ji, Y. Yang and P. Ge, Adv. Funct. Mater., 2023, 34, 2308671 CrossRef.
- Z. Zeng, S. Yuan, C. Yi, W. Zhao, Z. Yuan, Y. Dong, J. Zhu, Y. Yang and P. Ge, ACS Appl. Mater. Interfaces, 2022, 14, 52067–52078 CrossRef CAS PubMed.
- J. Zong, F. Wang, C. Nie, M. Zhao and S. Yang, J. Mater. Chem. A, 2022, 10, 10651–10661 RSC.
- T. Zhu, W. Shen, X. Wang, Y.-F. Song and W. Wang, Chem. Eng. J., 2019, 378, 122159 CrossRef CAS.
- W. Zhao, L. Zhang, F. Jiang, X. Chang, Y. Yang, P. Ge, W. Sun and X. Ji, J. Mater. Chem. A, 2020, 8, 5284–5297 RSC.
- G. Wang, C. Chen, Z. Zeng, Y. Chen, Z. Wang, W. Zhao, S. Yuan, Y. Dong, Z. Yuan and P. Ge, Mater. Chem. Phys., 2023, 310, 128234 Search PubMed.
- Q. Zhang, Y. Zeng, X. Wang, L. Wang, H. Wang, J. Xiao and X. Li, J. Alloys Compd., 2021, 881, 160594 CrossRef CAS.
- J. B. G. Youngsik Kim, J. Phys. Chem. C, 2008, 112, 15060 CrossRef.
- Q. Wang, L. Jiao, H. Du, W. Peng, Y. Han, D. Song, Y. Si, Y. Wang and H. Yuan, J. Mater. Chem., 2011, 21, 327–329 RSC.
- L. Zhang, W. Zhao, S. Yuan, F. Jiang, X. Chen, Y. Yang, P. Ge, W. Sun and X. Ji, J. Energy Chem., 2021, 60, 531–545 CrossRef CAS.
- C. Nithya and S. Gopukumar, J. Mater. Chem. A, 2014, 2, 10516–10525 RSC.
- M. Srout, M. Carboni, J.-A. Gonzalez and S. Trabesinger, Small, 2023, 19, 2206252 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: See DOI: https://doi.org/10.1039/d4cc05473c |
| ‡ Zihao Zeng and Hai Lei contributed equally to this work and should be regarded as the co-first authors. |
| This journal is © The Royal Society of Chemistry 2025 |