Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deuterated oxazines are bright near-infrared fluorophores for mitochondrial imaging and single molecule spectroscopy†
Blaise
Gatin-Fraudet
 *a,
Julius
Seifert
a,
Kris
Sarach
a,
Christina Holmboe
Olesen
*a,
Julius
Seifert
a,
Kris
Sarach
a,
Christina Holmboe
Olesen
 a,
Ramona
Birke
a,
Sigrid
Milles
a,
Ramona
Birke
a,
Sigrid
Milles
 a,
Melissa
Birol
b,
Martin
Lehmann
a and
Johannes
Broichhagen
a,
Melissa
Birol
b,
Martin
Lehmann
a and
Johannes
Broichhagen
 *a
*a
aLeibniz-Forschungsinstitut für Molekulare Pharmakologie, Robert-Rössle-Str. 10, 13125 Berlin, Germany. E-mail: gatinfraudet@fmp-berlin.de; broichhagen@fmp-berlin.de
bBerlin Institute of Medical Systems Biology (BIMSB), Max Delbrück Center for Molecular Medicine, Hannoversche Str. 28, 10115 Berlin, Germany
First published on 6th February 2025
Abstract
Bright near-infrared fluorophores are in demand for microscopy. We showcase a deuterated oxazine being 23% brighter vs. ATTO700. With a longer lifetime of 1.85 nanoseconds, we find the best-in-class SulfoOxazine700-d10 to stain mitochondria for confocal microscopy, and demonstrate unaffected diffusion properties in single molecule fluorescence correlation spectroscopy.
Fluorescence is a fascinating phenomenon that has changed the life science to observe cellular structures and to measure biomolecule behaviour on the single molecule level. The observed fluorophore comes with important parameters, such as its excitation and emission wavelengths, its absorptivity, fluorescence quantum yield and lifetime. Dyes in the near-infrared region are particularly interesting,1 since less autofluorescence is present in this regime, and light of longer wavelengths penetrates tissues deeper. As such, they have gathered increasing interest in photodynamic therapy,2 biological imaging,3,4 protein labelling,5 and material science.6 However, they still suffer from poorer brightness and shorter lifetimes. Given our recent exploration of ATTO700-one of the best performing molecules in the NIR range in Förster resonance energy transfer (FRET) and stimulated emission by depletion (STED) microscopy,7 we aimed to improve this oxazine dye. One strategy that has proven highly successful is the replacement of carbon-hydrogen (CH) by carbon deuterium (CD). This approach has entered chemical biology to augment rhodamine8,9 and cyanine fluorophores,10 as well as azobenzene photoswitches11 for photopharmacology and has furthermore found applications for increasing stability in organic light emitting diodes.12 A big advantage of this method is its minimal impact on the structure, and that one may use existing synthetic pathways, instead of de novo design and synthesis. Another benefit is that excitation and emission wavelengths do not change by this approach, making predictions about fluorescence behaviour solid.
In this study, we set out to improve ATTO700 based on a report by Pauff et al.,13 to first synthesize oxazine scaffolds in order to obtain insight as to whether this strategy works on these molecular structures and by adding a sulfonate, installing a negative charge for water solubility (Fig. 1A). Commencing with m-anisidine (1) in an Ytterbium(III) catalyzed reaction with acetone, we obtained dihydroquinoline 2 in 65% yield, which was alkylated using ethyl iodide or ethyl bromide-d5 to access 3a and 3b, respectively (62% and 46% yield) (Fig. 1B). Sulfonation on the allylic methyl position was achieved using oleum in aqueous sulfuric acid, giving access to 4a and 4b in 67% and 66% yield, respectively. We designed the synthesis for a mix and match strategy to build oxazines with varying deuteration and sulfonation patterns. To do so, 3 and 4 were converted to four para-nitro azobenzenes 5 using the stable and storable 4-nitrobenzenediazonium tetrafluoroborate salt in a mixture of methanol and aqueous H2SO4 in moderate to excellent yields (5a: 82%; 5b: 78%; 5c: 93%; 5d: 65%) by precipitation, in sufficient purity, neglecting the need for further purification. In parallel, 3 was demethylated to the dihydroquinoline-7-ol using BBr3 in 73% and 80% yield for the non-deuterated and deuterated version 6a and 6b, respectively. We then were able to build oxazine700 derivatives with varying degrees of ethyl group deuteration: Oxazine700-d0 (5a + 6a; 32% yield), Oxazine700-d5 (5b + 6a; 24% yield), and Oxazine700-d10 (5b + 6b; 6% yield) (Fig. 1B). We noticed that this reaction was challenging, and equally successful using purified or crude 6, so the notable drop in isolated yield may stem either from the kinetic isotope effect, or from less pure 6b compared to 6a. With these compounds in hand, we were able to determine key photophysical properties, i.e. maxima of excitation and emission wavelength, molar extinction coefficient, and quantum yield. While the excitation and emission profiles in PBS were quite similar (λExc/Em in nm: Oxazine700-d0: 701/716; Oxazine700-d5: 701/716; Oxazine700-d10: 700/718) (Fig. 1B), we observed a remarkable increase in extinction and quantum yield for Oxazine700-d10 compared to the non- or half-deuterated species in PBS (ε in M−1 cm−1 and Φ: Oxazine700-d0: 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 0.17; Oxazine700-d5: 124
000 and 0.17; Oxazine700-d5: 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 0.17; Oxazine700-d10: 146
000 and 0.17; Oxazine700-d10: 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 0.20) (Fig. 1C). The observation that half-deuteration does not show any improvements leads us to the suggestion that one non-deuterated ethyl group is sufficient for vibrational, non-radiative decays, supported by the timescale such processes occur (τvibr ∼ 10−12 s; τfluo ∼ 10−9 s). Our efforts to obtain lifetimes on a confocal microscope equipped with a FLIM module were unsuccessful since the dyes seemed to form aggregates on the solvent boundaries in the small volumes used. We then compared the small oxazine library to commercially available ATTO700, for which ε and Φ were reported to be 125
000 and 0.20) (Fig. 1C). The observation that half-deuteration does not show any improvements leads us to the suggestion that one non-deuterated ethyl group is sufficient for vibrational, non-radiative decays, supported by the timescale such processes occur (τvibr ∼ 10−12 s; τfluo ∼ 10−9 s). Our efforts to obtain lifetimes on a confocal microscope equipped with a FLIM module were unsuccessful since the dyes seemed to form aggregates on the solvent boundaries in the small volumes used. We then compared the small oxazine library to commercially available ATTO700, for which ε and Φ were reported to be 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and 0.25 (ref. 14), respectively. We assessed these parameters in-house, and found ε and Φ to be 165
000 M−1 cm−1 and 0.25 (ref. 14), respectively. We assessed these parameters in-house, and found ε and Φ to be 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and 0.20, respectively. As we measured higher extinction yet lower quantum yield, the absolute brightness is comparable with a value of 33.0, that we report here (Fig. 1C). As such, ATTO700 performed better than the oxazines. However, and importantly, the improving trend of deuteration from d0 to d5 to d10 stayed promising. In addition, sulfonation on dyes is reported to increase brightness15 and such a moiety is present on ATTO700. Accordingly, we next set out to synthesize SulfoOxazine700-d0 and SulfoOxazine700-d10 (consciously neglecting the half-deuterated compound since no or less improvement was observed with oxazines) by using 5c, d in a reaction with 6a, b. Interestingly, the isolated yields were much higher compared to the non-sulfonated versions (50% and 22%). With these in hand, we pursued with characterization, and while λExc = 700 nm remained constant, a small hypsochromic shift by 6 nm was observed for SulfoOxazine700-d10 (Fig. 1C and D). Gratifyingly, while SulfoOxazine700-d0 was close to ATTO700, we recorded an increase in extinction and quantum yield for SulfoOxazine700-d10 with 194
000 M−1 cm−1 and 0.20, respectively. As we measured higher extinction yet lower quantum yield, the absolute brightness is comparable with a value of 33.0, that we report here (Fig. 1C). As such, ATTO700 performed better than the oxazines. However, and importantly, the improving trend of deuteration from d0 to d5 to d10 stayed promising. In addition, sulfonation on dyes is reported to increase brightness15 and such a moiety is present on ATTO700. Accordingly, we next set out to synthesize SulfoOxazine700-d0 and SulfoOxazine700-d10 (consciously neglecting the half-deuterated compound since no or less improvement was observed with oxazines) by using 5c, d in a reaction with 6a, b. Interestingly, the isolated yields were much higher compared to the non-sulfonated versions (50% and 22%). With these in hand, we pursued with characterization, and while λExc = 700 nm remained constant, a small hypsochromic shift by 6 nm was observed for SulfoOxazine700-d10 (Fig. 1C and D). Gratifyingly, while SulfoOxazine700-d0 was close to ATTO700, we recorded an increase in extinction and quantum yield for SulfoOxazine700-d10 with 194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and 0.21, respectively, resulting in a total increase in brightness of 23% (Fig. 1B and E). Fluorescence lifetime of SulfoOxazine700-d10 was also significantly increased by 15% at 100 nM in PBS, with τ = 1.85 ns (τATTO700 = 1.61 ns and τSulfoOxazine700-d0 = 1.64 ns) (Fig. 1B and F). Lastly, we assessed photobleaching on a confocal microscope using a 640 nm laser with I = 43 kW cm−2, and observed that both SulfoOxazines700 were indeed more resistant (Fig. 1B and G), with the non-deuterated version showing a bit more stability, however, non-significant at any given time point.
000 M−1 cm−1 and 0.21, respectively, resulting in a total increase in brightness of 23% (Fig. 1B and E). Fluorescence lifetime of SulfoOxazine700-d10 was also significantly increased by 15% at 100 nM in PBS, with τ = 1.85 ns (τATTO700 = 1.61 ns and τSulfoOxazine700-d0 = 1.64 ns) (Fig. 1B and F). Lastly, we assessed photobleaching on a confocal microscope using a 640 nm laser with I = 43 kW cm−2, and observed that both SulfoOxazines700 were indeed more resistant (Fig. 1B and G), with the non-deuterated version showing a bit more stability, however, non-significant at any given time point.
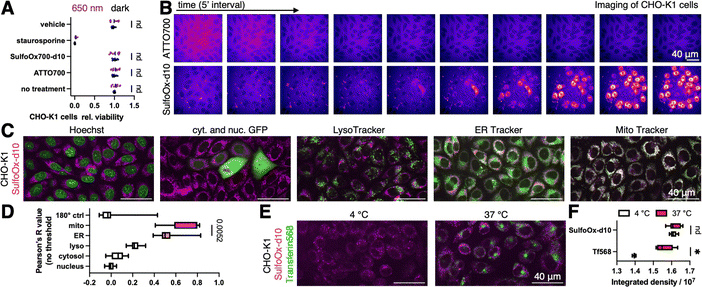
Next, we wanted to investigate if SulfoOxazine700-d10 is compatible in biological settings. We performed a WST-1 assay for cell viability, for which we incubated CHO-K1 cells for 24 hours with 1 μM and 10 μM of either ATTO700 or SulfoOxazine700-d10 in the dark, or with a 10 minute illumination period using 650 nm of light, before assessing metabolic activity (Fig. 2A and Fig. S1A (ESI†); no treatment, vehicle or 10 μM staurosporine served as controls). We were pleased that under no conditions, except positive control, cell viability was affected. Given the charged nature of the dyes, we aimed to use confocal microscopy to perform shadow imaging of CHO-K1 cells, a technique that is amenable to long-term morphological observations, due to the extracellular replenishment of the dye.16 We incubated CHO-K1 cells with 1 μM of ATTO700 or SulfoOxazine700-d10 and acquired images in 5 minute intervals (Fig. 2B). While we observed some bleaching for ATTO700, cellular outlines were identifiable over 45 minutes. Applying SulfoOxazine700-d10, however, lead to intracellular signals over the same time period. In order to test for organellar accumulation, we co-incubated with Hoechst (nucleus), transfected with nuclear and cytosolic GFP, LysoTracker, endoplasmic reticulum (ER) tracker or mitochondrial (Mito) Tracker (Fig. 2C). Running Pearson's R value co-localization tests, we confirmed the by eye observation that SulfoOxazine predominantly stains mitochondria (Fig. 2D). To obtain mechanistic information, we performed SulfoOxazine700-d10 uptake in the presence of transferrin568 (by endocytosis) at 4 °C (for blocking endocytosis) and at 37 °C (Fig. 2E), and found, when integrating total channel densities, that our dye enters cells presumably due to passive diffusion and/or aggregation, the mechanisms of which are of ongoing debate.17 Importantly, SulfoOxazine700-d10 might serve as a potential non-toxic carrier and/or contrast agent in biological systems.18 Nevertheless, imaging mitochondria remains a challenge, as these highly dynamic organelles are closely associated with cellular metabolism and function. MitoTracker derivatives are useful, but they sometimes affect mitochondrial function and miss certain mitochondrial populations.19SulfoOxazine700-d10 could represent an alternative for experiments on living cells in the near-infrared window.
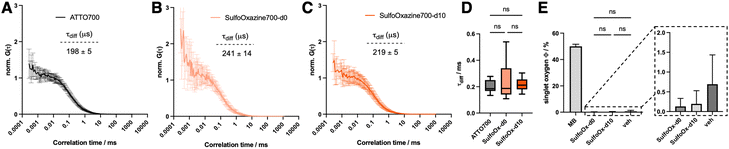
Lastly, we were interested in the photophysics of SulfoOxazines in more detail. We did not observe significant pH dependence (Fig. S1B, ESI†) and performed fluorescence correlation spectroscopy on 100 pM concentrated samples in buffer (20 mM Tris, 50 mM NaCl, pH = 7.5). ATTO700, SulfoOxazine700-d0 and SulfoOxazine700-d10 gave clear autocorrelation curves (Fig. 3A–C), which were fit to a single component diffusion model to extract τ-values for diffusion, which remained for all three tested molecules around τdiffusion ∼ 0.2 ms (Fig. 3D). Given the small differences in size and molecular weight of the measured species, and the fact that we did not observe large changes in Φ, this was to be expected, and demonstrates that SulfoOxazine700-d10 might be used as a brighter replacement for ATTO700, which has recently been used in different settings, such as DNA-PAINT,20 as local water sensing molecules,21 and to probe ligand-receptor interactions.22 This is also reflected by low singlet oxygen quantum yields (0.2% and 0.1%) for SulfoOxazine700-d0/d10 (Fig. 3E) versus methylene blue. In addition to this, derivatizing ATTO700 to SulfoOxazine700-d10 has a minimal impact on its molecular structure and size. With a molecular weight of 517 Da, it is in a similar regime as JF690 (458 Da),1 SiR700 (423 Da)23 and 2XR-1 (468 Da),5 which are dimmer (ε × Φ ∼ 12, 36 and 12, respectively), and have been used for in vivo and cellular imaging, respectively. Other available NIR dyes, for which structures are not always available, show a markedly increased size, for instance Alexa Fluor 700 with a Mw ∼ 1300 Da (ref. 24) or CF700 with a Mw ∼ 2315 Da (ref. 25). Although the former is reportedly brighter (ε × Φ ∼ 51), their size may impact their use in dynamic single molecule recordings. Together, our results may play a role in single molecule (sm) imaging approaches, where every photon counts, e.g. in smFRET, where a larger extinction coefficient of the acceptor will lead to a larger overlap integral. As such, an increased Förster distance allows measuring longer ranges with a SulfoOx-700 d10 acceptor, compared to ATTO700.
In summary, the deuteration strategy of chromophores was applicable to oxazines to boost brightness, photostability and fluorescence lifetime. Given the interest in near-infrared dyes for better imaging quality in the life sciences due to better tissue penetration of light and less phototoxicity, we highlight SulfoOxazine700-d10 as a negatively charged dye that is non-cytotoxic and enters cells actively, and might become a superior alternative in single molecule microscopy.
BGF, JS, and KS performed chemical synthesis and characterization, ML and MB photophysical characterization. BGF, MB and JB recorded single molecule spectroscopy. CHO and RB performed cell experiments. BGF, SM and JB conceived and supervised the study. BGF and JB wrote the manuscript with input from all authors. This project has received funding from the European Research Council (ERC) under the European Union's Horizon Europe Framework Programme (deuterON, Grant agreement no. 101042046 to JB) and under the European Union's Horizon 2020 research and innovation program (MultiMotif, grant agreement no. 802209 to SM). Funded by Boehringer Ingelheim Foundation (Exploration Grant to JB). We thank Michael Krauss and Volker Haucke for support (both FMP).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- J. B. Grimm, A. N. Tkachuk, L. Xie, H. Choi, B. Mohar, N. Falco, K. Schaefer, R. Patel, Q. Zheng, Z. Liu, J. Lippincott-Schwartz, T. A. Brown and L. D. Lavis, Nat. Methods, 2020, 17, 815–821 CrossRef CAS PubMed.
- N. I. Rubtsova, M. C. Hart, A. D. Arroyo, S. A. Osharovich, B. K. Liebov, J. Miller, M. Yuan, J. M. Cochran, S. Chong, A. G. Yodh, T. M. Busch, E. J. Delikatny, N. Anikeeva and A. V. Popov, Bioconjug. Chem., 2021, 32, 1852–1863 CrossRef CAS PubMed.
- J. Ast, A. N. Novak, T. Podewin, N. H. F. Fine, B. Jones, A. Tomas, R. Birke, K. Roßmann, B. Mathes, J. Eichhorst, M. Lehmann, A. K. Linnemann, D. J. Hodson and J. Broichhagen, JACS Au, 2022, 2, 1007–1017 CrossRef CAS PubMed.
- X.-X. Zhang, F. Yang, X. Zhao, Q. Wu, L. He, Z. Li, Z. Zhou, T.-B. Ren, X.-B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2024, 63, e202410666 CAS.
- W. Wu, K. Yan, Z. He, L. Zhang, Y. Dong, B. Wu, H. Liu, S. Wang and F. Zhang, J. Am. Chem. Soc., 2024, 146, 11570–11576 CAS.
- C. T. Jackson, S. Jeong, G. F. Dorlhiac and M. P. Landry, iScience, 2021, 24, 102156 CrossRef CAS PubMed.
- M. Trumpp, A. Oliveras, H. Gonschior, J. Ast, D. J. Hodson, P. Knaus, M. Lehmann, M. Birol and J. Broichhagen, Chem. Commun., 2022, 58, 13724–13727 RSC.
- J. B. Grimm, L. Xie, J. C. Casler, R. Patel, A. N. Tkachuk, N. Falco, H. Choi, J. Lippincott-Schwartz, T. A. Brown, B. S. Glick, Z. Liu and L. D. Lavis, JACS Au, 2021, 1, 690–696 CrossRef CAS PubMed.
- K. Roßmann, K. C. Akkaya, P. Poc, C. Charbonnier, J. Eichhorst, H. Gonschior, A. Valavalkar, N. Wendler, T. Cordes, B. Dietzek-Ivanšić, B. Jones, M. Lehmann and J. Broichhagen, Chem. Sci., 2022, 13, 8605–8617 RSC.
- H. Janeková, H. C. Friedman, M. Russo, M. Zyberaj, T. Ahmed, A. S. Hua, A. V. Sica, J. R. Caram and P. Štacko, Chem. Commun., 2024, 60, 1000–1003 RSC.
- K. Roßmann, A. J. Gonzalez-Hernandez, R. Bhuyan, C. Schattenberg, H. Sun, K. Börjesson, J. Levitz and J. Broichhagen, Angew. Chem., 2024, 63, e202408300 Search PubMed.
- S. Jung, W.-L. Cheung, S. Li, M. Wang, W. Li, C. Wang, X. Song, G. Wei, Q. Song, S. S. Chen, W. Cai, M. Ng, W. K. Tang and M.-C. Tang, Nat. Commun., 2023, 14, 6481 CrossRef CAS PubMed.
- S. M. Pauff and S. C. Miller, Org. Lett., 2011, 13, 6196–6199 CrossRef CAS PubMed.
- https://www.atto-tec.com/ATTO-700.html .
- N. Panchuk-Voloshina, R. P. Haugland, J. Bishop-Stewart, M. K. Bhalgat, P. J. Millard, F. Mao, W.-Y. Leung and R. P. Haugland, J. Histochem. Cytochem., 1999, 47, 1179–1188 CrossRef CAS PubMed.
- V. V. G. K. Inavalli, V. Puente Muñoz, J. E. Draffin and J. Tønnesen, Front. Cell. Neurosci., 2024, 18, 1330100 CrossRef CAS PubMed.
- S. Behzadi, V. Serpooshan, W. Tao, M. A. Hamaly, M. Y. Alkawareek, E. C. Dreaden, D. Brown, A. M. Alkilany, O. C. Farokhzad and M. Mahmoudi, Chem. Soc. Rev., 2017, 46, 4218–4244 RSC.
- A. Albanese and W. C. W. Chan, ACS Nano, 2011, 5, 5478–5489 CrossRef CAS PubMed.
- K. Neikirk, A. G. Marshall, B. Kula, N. Smith, S. LeBlanc and A. Hinton, Eur. J. Cell Biol., 2023, 102, 151371 CrossRef CAS PubMed.
- N. Gimber, S. Strauss, R. Jungmann and J. Schmoranzer, Nano Lett., 2022, 22, 2682–2690 CrossRef CAS PubMed.
- J. Maillard, C. A. Rumble and A. Fürstenberg, J. Phys. Chem. B, 2021, 125, 9727–9737 CrossRef CAS PubMed.
- K.-T. Lam, E. L. Taylor, A. J. Thompson, M.-D. Ruepp, M. Lochner, M. J. Martinez and J. A. Brozik, J. Phys. Chem. B, 2020, 124, 7791–7802 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, W. Piao, M. Kusakabe, N. Saito, T. Terai, T. Okabe and T. Nagano, J. Am. Chem. Soc., 2012, 134, 5029–5031 CrossRef CAS PubMed.
- https://www.thermofisher.com/order/catalog/product/A20010 .
- https://biotium.com/technology/cf-dyes/nir-cf-dyes/ .
Footnote |
| † Electronic supplementary information (ESI) available: Chemical synthesis and characterization; details of photophysical characterization and cell culture; Fig. S1. See DOI: https://doi.org/10.1039/d4cc03807j |
| This journal is © The Royal Society of Chemistry 2025 |