Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Halogenation of nucleic acid structures: from chemical biology to supramolecular chemistry
Catalina
Nicolau
a,
Julia
Requena-Ramírez
 b,
Jorge
González-García
b,
Jorge
González-García
 *b and
Antonio
Bauzá
*b and
Antonio
Bauzá
 *a
*a
aCtra. de Valldemossa, km. 7.5, Universitat de les Illes Balears, 07122, Palma de Mallorca, Islas Baleares, Spain. E-mail: antonio.bauza@uib.es
bDepartment of Inorganic Chemistry, Institute of Molecular Science (ICMol), University of Valencia, Catedrático José Beltrán 2, 46980 Paterna, Spain. E-mail: jorge.gonzalez@uv.es
First published on 22nd May 2025
Abstract
Halogenation of organic molecules is a widespread resource used in the fields of chemical biology and rational drug design to improve the binding affinity or solubility of a given compound. Interestingly, the incorporation of Br and I in DNA/RNA bases has been routinely carried out during decades to facilitate the structural determination of nucleic acids, without accounting for its impact in the DNA/RNA structure and molecular recognition events involving other biological entities (e.g. proteins and peptides). This is of critical importance, since halogens undergo non-covalent binding (specially Br and I) through the formation of halogen bonding interactions, thus structurally influencing peptide/protein–DNA/RNA binding poses or altering the supramolecular architecture of isolated nucleic acid structures. In this review, the physical nature of halogen bonds involving nucleobases as well as their implications in (i) the formation of protein–DNA/RNA complexes and (ii) the stabilization of non-canonical DNA/RNA structures will be discussed, focusing on the role of this non-covalent interaction as a promising tool in nucleic acid chemistry.
Introduction
Chemical modification of nucleic acids
Chemically modifying DNA and RNA bases is a crucial process in the regulation of gene expression,1,2 being essential for understanding the fundamental mechanisms of epigenetics.1 These modifications affect both the secondary nucleic acid structure and the protein–DNA/RNA binding mechanisms, thereby regulating processes related to cellular survival.3 During the last few stages of the past century, there was a significant increase in research related to the covalent modification of DNA/RNA, primarily due to its promising applications in clinical medicine, such as the use of biomarkers for disease prevention and diagnosis, which has led to a better understanding of the role of epigenetics in various diseases.4–7The chemical modification of DNA/RNA bases has been detected across all domains of life, including eukaryotic and prokaryotic organisms as well as viruses.8 More than 100 types of chemical modifications have been identified in RNA. Their functions are not yet clear, and they are mostly involved in tuning the translational process, controlling their stability and proper folding and determining the identity of the corresponding RNA.9,10 The plethora of non-canonical RNA nucleobases includes simple modifications such as methylation or acetylation in addition to hypermodified ribonucleosides containing different heterocycles, such as queuosine and wyosine.11,12 In contrast, there is a small fraction of DNA modifications in comparison with RNA. One notable example is 5-methylcytosine (m5C), discovered over 50 years ago, which remains one of the most extensively studied epigenetic marks in mammals to date.13 In addition to m5C, other canonical DNA modifications include 5-hydroxymethylcytosine (hm5C), 5-formylcytosine (f5C), 5-carboxylcytosine (ca5C), N4-methylcytosine (m4C), N6-methyladenine (m6A), and N6-hydroxymethyladenine (hm6A), among others (see Fig. 1).
These DNA modifications affect not only its conformation and stability but also its protein-binding mechanism. In fact, the malfunction of the epigenetic regulatory mechanisms has deep implications for human health, particularly in diseases related to (1) cancer,14–17 (2) obesity18 and diabetes,19,20 and (3) neurodegenerative processes.21–24
On the other hand, the non-canonical modification of nucleic acids encompasses various transformations, such as the incorporation of methyl (–Me), halogen (–X), or aryl (–Ar) groups likely at the C8 position of purine rings25–27 and the C5 position of pyrimidine rings (Fig. 2),28–30 although N4 and N6 positions are also used. Interestingly, these modifications play a key role in the formation of alternative DNA and RNA structures.31,32 For instance, modifications at the C8 position of the purine ring are often used as inducting agents of Z-conformation and guanine quadruplex formation in both DNA and RNA. These modifications have also been employed to study alternative structures, including H-DNA and DNA triple helices,33 such as methylation in tRNA,34 which led to the formation of Levitt base pairs instead of the canonical Watson–Crick base pairs.
The conformation of ribose rings (in both DNA and RNA) is also crucial in determining the adoption of a particular nucleic acid structure (see Fig. 3). An example of this can be observed in purines, where conformational equilibrium exists between the anti and syn conformations of the ribose rings. Under physiological conditions, the typical duplex DNA conformation is dominated by the B-form, where A⋯T and G⋯C base pairs35 preferentially adopt the anti conformation. However, several studies have demonstrated that the introduction of bulky substituents at the C8 position of the purine ring shifts is enough to shift the equilibrium towards the syn conformation,36,37 thereby promoting the transition to alternative DNA structures such as Z-DNA38–40 or guanine quadruplexes.41–43 Additionally, the incorporation of halogens at the C2′ position of the ribose ring has been studied for its role in stabilizing i-motif DNA structures. This phenomenon is correlated with genes that predispose individuals to certain diseases and plays a vital role in DNA's biological functions, as Z-DNA sequences are often located near transcription factor binding sites.44,45
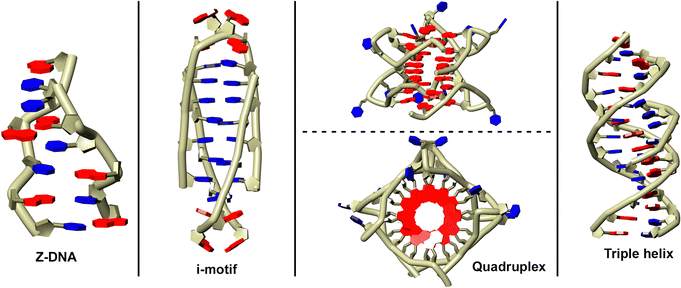 | ||
| Fig. 3 Schematic representation of several non-canonical DNA structures: Z-DNA (a), i-motif (b), G-quadruplex (c), and triple helix (d). | ||
Despite the growing number of studies in this field, the role and conformational properties of non-canonical DNA structures are not yet fully understood. Advancing our knowledge of the effects of such nucleotide and oligonucleotide modifications is therefore crucial for gaining a deeper understanding of their structural implications for nucleic acid stability and protein–DNA binding mechanisms. To achieve this, both experimental techniques46,47 (such as mass spectrometry48,49 and DNA sequencing studies)50 and computational tools (primarily molecular dynamics simulations and docking studies)51 are employed.
In this context, ongoing efforts to develop novel theoretical tools for studying and characterizing DNA and other biomolecules have established molecular dynamics simulations as a highly valuable tool for nucleic acid analysis. Furthermore, the combination of molecular dynamics and quantum mechanics calculations enables precise insights into the structure and dynamics of large biological systems (such as protein–DNA complexes), which is crucial for deciphering, at an atomistic level, the molecular mechanisms governing nucleic acid modification and recognition.
Nucleic acid halogenation and DNA damage
The presence of mutations in DNA is associated with genomic instability and, consequently, an increased predisposition to cancer.52–55 Concretely, the accumulation of DNA damage over time through these mutations acts as a trigger for uncontrolled cell proliferation and dysfunctional cellular migration mechanisms, both of which are hallmarks of cancer cells.56,57There is a well-established correlation between mutations and DNA damage, with inflammatory processes being a major cause of mutagenic damage to DNA. A key feature of inflammatory responses is the production of chemical species designed to neutralize pathogenic agents. While these reactive species are essential for protecting the body against infection, they can also cause DNA damage. As a result, DNA repair mechanisms play a critical role in preventing mutations in the genetic code. However, these mechanisms may fail or become overwhelmed by excessive damage, allowing mutations to persist. Since cancer develops through the accumulation of mutations, it is concluded that unrepaired DNA damage due to inflammation contributes to cancer progression by increasing mutagenesis.
Inflammation and genomic instability are closely interconnected (Fig. 4, top), as inflammation promotes mutagenesis through the production of reactive oxygen and nitrogen species (RONS), which can directly damage DNA. In a parallel way, DNA damage can further exacerbate inflammation, creating a self-perpetuating feedback loop being regulated by a plethora of DNA repair mechanisms, transcription factors, and cellular signaling pathways. Due to the complexity of the molecular mechanisms involved in inflammation, DNA damage, and repair, these processes can become dysregulated relatively easily, ultimately leading to cancer.
 | ||
| Fig. 4 Schematic representation of the relationship between inflammation and DNA damage, as well as its contribution to cancer (top). The biological chemistry of cancer, adapted from Mangerich and collaborators (bottom).69 | ||
In this context, various molecules have been classified as RONS, including nitric oxide (NO), which serves as a crucial cellular signaling molecule.58–62 Additionally, neutrophils and macrophages produce superoxide anions (O2−), while other enzymatic pathways contribute to a cascade of chemical reactions leading to the generation of a diverse array of RONS (Fig. 4, bottom). These include radicals (hydroxyl radical OH˙ and nitrogen dioxide radical NO2˙), anions (peroxynitrite ONOO− and nitrosoperoxocarbonate ONOOCO2−), anhydrides (nitrous anhydride N2O3), hypohalogenous acids (hypochlorous acid HOCl and hypobromous acid HOBr), and hydrogen peroxide (H2O2).63–65 Finally, studies have demonstrated that, in addition to RONS produced by immune cells, pro-inflammatory cytokines can also stimulate intracellular RONS production.66–68
The mutagenic character of halogenated DNA is associated with the generation of hypohalous acids by inflammatory cells. Specifically, neutrophils secrete the enzyme myeloperoxidase, which in turn produces hypochlorous acid (HOCl).70–72 On the other hand, eosinophils secrete eosinophil peroxidase, generating hypobromous acid (HOBr).73,74 Both molecules react with DNA during inflammatory processes, leading to the formation of halogenated products such as 5-halocytosine (5-haloC), 8-haloguanine (8-haloG), and 8-haloadenine (8-haloA) (Fig. 5).75–78
Among these halogenated products, the most abundant halogenated base is 5-chlorocytosine (5ClC),79,80 which has been detected at significantly higher levels than other DNA lesions resulting from oxidation, deamination, or peroxidation, as demonstrated in in vivo studies.81,82 Due to its substantial and persistent accumulation in tissues under inflammatory stress—possibly due to inefficient repair mechanisms—as well as in cancers associated with inflammatory pathways,83 5ClC has been designated as a biomarker of chronic inflammation.84
Incorporation of halogenated bases into DNA and RNA
Several studies have analyzed the effect of halogenation on the stability of base pairs in DNA and RNA, both theoretically and experimentally,85–100 partly due to its application as radiosensitizing agents in cancer therapies.101,102 In the following sections of this review, several selected examples are discussed in more detail.Evaluating the stability of halogenated base pairs
The first selected example involves the study by Koseki and collaborators,85 where they performed quantum mechanics calculations and molecular dynamics simulations of nucleic acids modified with 5-trifluorothymine (FTD) and 5-fluorouracil (5FU). These modified nucleotides, particularly FTD, had previously been used as antitumor agents;103–105 however, their structural effects on a DNA strand had not been thoroughly investigated. To address this, theoretical calculations (MP2/6-31++G**) were performed to analyze the stability of A⋯FTD and A⋯5FU base pairs (Fig. 6a).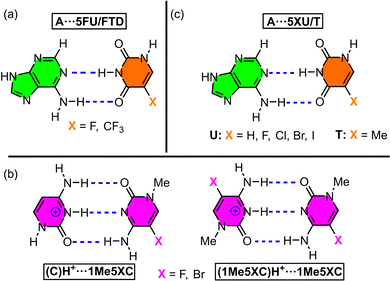 | ||
| Fig. 6 Schematic representation of the base pairs used in the studies by Koseki85 (a), Yang86 (b), and Heshmati87 (c). | ||
The results were compared with the canonical, non-halogenated base pairs A⋯T and A⋯U, revealing a slight increase in stability due to the electron-accepting effects of the –F and –CF3 substituents. Subsequently, molecular dynamics simulations were carried out on a DNA fragment to assess the stability and structural impact of incorporating halogenated nitrogenous bases. The authors concluded that the antitumor activity exhibited by 5-trifluoromethyl-thymine incorporation was due to the destabilizing effect of the –CF3 group, which was involved in the formation of intra-strand hydrogen and halogen bonds. Finally, experimental analyses were conducted using UV/visible spectroscopy, along with in vivo assays, to complement and corroborate the data obtained from the simulations.
Another relevant study was conducted by Yang and coworkers,86 where they evaluated the stability of cytosine (C)H+⋯C base pairs incorporating fluorine (F) and bromine (Br) atoms, and these modifications were involved in the transition from B-DNA to the i-motif structure. The authors employed the threshold collision induced dissociation technique using tandem ion beam mass spectrometry to study the formation of cytosine homodimers and heterodimers (Fig. 6b).
The authors concluded that halogenation at the C5 position reduced the strength of the interactions between the studied base pairs.106,107 This effect was significantly more pronounced in the stability of base pairs that incorporated a methylated cytosine at the C1 position. The authors suggested that the i-motif formation could be destabilized through C5 halogenation; however, these modified base pairs still exhibited greater stability than their neutral nitrogenous base counterparts, thus suggesting that halogenation is not sufficient to prevent i-motif formation but can alter the number of nucleotides required to induce its transition from a canonical Watson–Crick helix.
Another example is the work of Heshmati and collaborators,87 who conducted computational analyses (B3LYP/6-311G* level of theory) to assess the stability of the A⋯5FT base pair (Fig. 6c). Their aim was to explore the potential use of halogenated thymine derivatives as DNA radiosensitizers in anticancer therapy108–110 and observed that halogen atom incorporation led to substantial alterations in both the geometry and charge distribution of the thymine molecule, consequently affecting the stability of the A⋯5FT base pair and eventually provoking base pair opening.
Other interesting studies encompass those conducted by Guerra91 and Parker92 and collaborators, where the stability of A⋯T and G⋯C base pairs incorporating halogenated nucleotides was analyzed (Fig. 7a and b). Concretely, Guerra and collaborators91 carried out theoretical evaluations (BP86/TZVP level of theory) to understand the stability of the base pairs 8XA⋯6XT and 8XG⋯6XC (X = H, F, Cl, and Br), in which the hydrogen atom at positions 8 (A, G) and 6 (T, C) was replaced by halogens (Fig. 7a). The authors concluded that the effects varied significantly depending on both the halogenation position and the base pair under study. They observed contrasting effects, such as an enhancement in hydrogen bond donor capacity (N–H) alongside a reduction in hydrogen bond acceptor capacity (O, N). In general, fluorine incorporation into the pyrimidine base reinforced base pair stability, whereas its incorporation into the purine base had the opposite effect. Additionally, the authors highlighted the significant role of orbital interactions as an additional stabilization source beyond electrostatic contributions in these halogenated base pairs.
 | ||
| Fig. 7 Schematic representation of the base pairs used in the studies by Guerra91 (a), Parker92 (b), and Zhao88 (c). | ||
A final example worth mentioning is the study by Zhao and coworkers,88 who investigated the stability of halogenated uracil tetrads (Fig. 7c) using DFT calculations (B3LYP/6-31+G** level of theory). Their results showed that stabilization energy of this ternary complex followed the order H > F > Cl > Br. Furthermore, electrostatic potential maps (MEP) revealed that the typical cation-binding site (Na+/K+) located at the center of the tetrad disappeared upon incorporation of the 5XU bases. Instead, an electron-deficient region emerged, favoring interactions with electron-rich species.
Beyond their role in modulating base pair stability through their electron-acceptor properties, halogen atoms have also been explored as substituents for conventional hydrogen bonding between bases. Specifically, Parker and collaborators92 performed computational studies (B3LYP/6-31G* level of theory) to assess the potential of halogen atoms as direct stabilizers of base pairs through halogen bond formation. They examined the stability of Watson–Crick base pairs G⋯C and A⋯T, in which Cl, Br, and I replaced hydrogen atoms (Fig. 7b), and compared their stability with non-halogenated base pairs. The base pairs stabilized through halogen bonding exhibited greater stability, following the trend Cl < Br < I. Among these, bromine emerged as the ideal halogen bond donor, offering a higher polarizability than chlorine and a smaller atomic volume than iodine.
Halogen bonds in DNA and RNA supramolecular assemblies
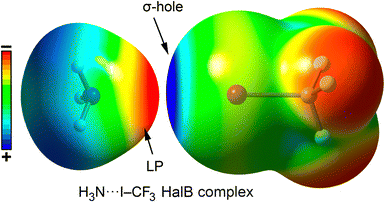
Currently, halogen bonding (a non-covalent attractive interaction between a halogen and a Lewis base)111,112 has been established as a valuable molecular tool in various areas of modern chemistry, including solid-state chemistry, materials science, and supramolecular chemistry.113 In the field of chemical biology, halogen bonding has been mostly investigated in protein–ligand complex formation, becoming an essential tool in the design of novel therapeutic agents within pharmaceutical chemistry.114–118The stability of halogen bond (HalB) interactions arises from multiple contributions, including charge transfer,119 dispersion forces,120 polarization,121 and electrostatics.122,123 Typically, the electrostatic component is based on the attraction between an electron-rich molecule (or region) and a localized region of positive electrostatic potential along the extension of the Y–X bond (X = F, Cl, Br, I; Y = C), known as the σ-hole.124 This phenomenon is due to the polarization of halogen atoms when covalently bonded to another atom (e.g., C), leading to an anisotropic electrostatic potential distribution around the halogen. Consequently, a σ-hole typically appears along the C–X bond direction, surrounded by a negatively charged σ-lump, associated with the lone electron pairs of the halogen (Fig. 8 and 9).
 | ||
| Fig. 9 Molecular electrostatic potential (MEP) surfaces of CH3F, CH3Cl, CH3Br and CH3I. Electropositive regions are shown in blue color and electronegative in red color. | ||
The σ-hole present on the halogen atom acts as an electrophilic region, facilitating favorable interactions with electron-rich species such as oxygen (O), nitrogen (N), sulfur (S), and π-systems. The polarizability of the halogen atom (which increases in the order F < Cl < Br < I) as well as the electron-withdrawing capacity of the group to which the halogen is bonded are key factors in determining the strength and directionality of the interaction. Additionally, the orbital term also contributes to the stabilization of HalBs, being usually based on the electronic donation from a lone pair or a π-system (such as a double/triple bond or an aromatic ring) belonging to the Lewis base to the σ-antibonding orbital* of the C–X bond, further strengthening the interaction.
Ho and collaborators125–129 have extensively studied HalB interactions in biological systems, establishing a series of criteria to consider a halogen bond as biologically relevant. These criteria are as follows:
1. It must affect the functionality of the biological system.
2. It must influence the structures and stability of biomolecules that define the system's function.
3. It must possess sufficient stability to impact biological structures and their functions.
4. It must exhibit strength comparable to that of a hydrogen bond.
Recently, our group proposed an additional criterion130 based on Bader's “atoms in molecules” (QTAIM) theory,131 consisting of characterizing the HalB interaction through a bond critical point, as outlined in the definition of this interaction proposed by the International Union of Pure and Applied Chemistry (IUPAC).108
Halogenated nucleotides are also incorporated into DNA or RNA to facilitate the resolution of their crystal structures using techniques such as single-wavelength anomalous dispersion (SAD) or multi-wavelength anomalous dispersion (MAD).132,133 These modifications can lead to minimal or localized effects as well as drastic and global structural changes,134 which have been studied both experimentally and theoretically.135–140 The following sections describe various structures in which halogen bonding plays a fundamental role in stabilizing DNA and RNA architectures.
The first example is the X-ray structure of the sequence d(ACBrUCGGABrUGA), which was used to resolve its non-brominated analog.141 Both sequences crystallized in a similar architecture (Fig. 10), exhibiting a duplex pattern stabilized by “head-to-head” interactions and intercalated base pairs of C⋯C, A⋯A, and G⋯G. As shown in the right side of the image, the bromine atom present in bromouridine (BrU) forms an inner-strand HalB with an oxygen atom from the phosphate group of an adjacent nucleotide, contributing to local duplex stabilization. Additionally, an inter-strand HalB is observed between two BrU nucleotides, where the bromouridine acts as both the HalB donor and the acceptor, which further stabilizes the supramolecular architecture (the left side of the image).
 | ||
| Fig. 10 X-ray structure of a duplex corresponding to the sequence d(ACBrUCGGABrUGA). HalB interactions are highlighted in purple and cyan and magnified within the rectangular parts of the figure.141 | ||
HalB interactions have also been employed to regulate the formation of a supramolecular biological complex known as the Holliday junction,142 which is an intermediate structure formed by four DNA strands during cellular recombination mechanisms.143 To achieve this, Voth and collaborators142 replaced a thymine in the sequence d(CCAGTACTGG) with iodouridine (5IU), thereby facilitating the obtention of an X-ray structure (Fig. 11). The results showed that the halogenated structure corresponded to a Holliday junction, whereas the native sequence ultimately crystallized as a standard DNA duplex.144
 | ||
| Fig. 11 Stabilizing effect of the HalB in the formation of a Holliday junction structure.144 The interaction is magnified in the rectangular part of the figure. | ||
Crystallographic analysis of both structures revealed HalB between the 5IU base and an oxygen atom from a neighboring phosphate group, effectively replacing the hydrogen bond typically observed in such structures.145,146 Subsequent competitive studies involving other halouridines (5XU, X = F, Cl, Br) demonstrated that O⋯X HalB exhibited a more stable enthalpy of formation than the hydrogen bond. This finding was further corroborated through differential scanning calorimetry (DSC) and melting studies in solution.
HalBs have also been observed in RNA fragments, including the MS2–RNA complex,147 which is considered a clear example of halogen bonding in nucleic acid structures,148 as well as in X-ray structures of U2AF65–RNA complexes (Fig. 12).149,150 In the latter, 5BrU was used to accurately determine the position of the pyrimidine base (cytosine or uracil) through X-ray diffraction. More in detail, in the 3VAF structure, the incorporation of 5BrU led to the formation of an O⋯Br halogen bond with an oxygen atom from a neighboring phosphate group, which played a crucial role in stabilizing the conformation required for protein–RNA recognition.
 | ||
| Fig. 12 X-ray structures of 3VAF (a) and 5EV3 (b). The HalB interaction is magnified in the circular parts of the figure. The O⋯Br distances and C–Br⋯O angles are also indicated. Visualization of the halogen bond interaction using the NCIplot analysis has been included in ref. 151. Reprinted (adapted) with permission from ref. 152. Copyright 2020 American Chemical Society. | ||
Halogen bonds also play a role in protein–DNA/RNA recognition mechanisms. One example involves the Tdt protein, which belongs to the family of DNA polymerases responsible for nucleotide addition at the V(D)J union in T-cell receptors. In the study by Gouge and collaborators,153 a series of DNA oligomers with different lengths and compositions were described as analogs of the enzyme's pre-catalytic and post-catalytic states. To solve the 4I29 structure (Fig. 13a), an adenine was replaced by its brominated analog at the C8 position (8BrA), forming a halogen bond interaction with an oxygen atom from the carboxylate group of the ASP379 amino acid.
 | ||
| Fig. 13 X-ray structures of 4I29 (a),153 5A3D (b),154 and 3LWP (c).155 The HalB interaction is magnified in the circular parts of the figure. The O⋯Br/I distances and C–Br/I⋯O angles are also indicated. Visualization of the halogen bond interaction using the NCIplot analysis has been included in ref. 151. Reprinted (adapted) with permission from ref. 156. Copyright 2020 American Chemical Society. | ||
The second example involves the nucleotide excision repair (NER) enzyme, which belongs to the family of proteins responsible for DNA repair and serves as a primary defense against DNA damage. In this context, the study by Koch and collaborators154 analyzed the mechanism by which the NER protein binds to damaged DNA. In the 5A3D structure, some bases were replaced with 5-iodouracil (5IU) to facilitate crystallographic structure resolution. Two of these bases were located in the protein–DNA binding region and involved in O⋯I halogen bonds with an oxygen atom from the carboxylate group of the ASP240 amino acid (Fig. 13b), representing an additional non-covalent force contributing to protein–DNA recognition.
The final selected example involves the H/ACA RNP pseudouridine synthase enzyme, which catalyzes the isomerization of uridine to pseudouridine in RNA, a modification that plays a regulatory role in post-transcriptional RNA expression. The study by Zhou and coworkers155 focuses on the catalytic mechanism of pseudouridylation through the conformational analysis of the glycosidic bond of uridine in a pre-reactive state. To achieve this, the authors utilized both native RNA and RNA containing 5BrU, both labeled with 32P. Their findings revealed that 5BrU interacted with the protein in a way that stabilized the anti conformation of the nucleoside. This interaction was identified as a C⋯Br halogen bond between the bromine atom of uridine and the π-system of the aromatic amino acid TYR113 (Fig. 13c). By obtaining this non-reactive enzyme variant, the study advanced the understanding of its mechanism of action, providing an example of a biologically relevant halogen bond.
Future perspectives
The examples shown above highlighted the impact of nucleic acid halogenation in supramolecular biology. As a future perspective, we propose the use of this non-covalent force to guide the molecular recognition of specific nucleic acid sequences, since the selective recognition of target sites in single-stranded nucleic acids (ssDNA and ssRNA) as well as in more complex structures (e.g. G-quadruplexes) is of crucial importance. A routine tool used in the biological area for DNA/RNA detection and sensing is peptide nucleic acids (PNAs), a family of DNA analogs first reported by Nielsen's group in 1991.157 In the PNA structure, the sugar-phosphate backbone of DNA is replaced by poly[N-(2-aminoethyl)glycine], while the nucleobases (A, G, C, and T) are attached to the central N atom in the monomeric unit through a –COCH2– linking group (Fig. 14). PNAs exhibit remarkable ability to form highly stable duplexes with complementary ssDNA and ssRNA. We believe that the incorporation of brominated/iodinated nucleobases into PNAs might trigger a specific base to base recognition motifs and form striking supramolecular DNA assemblies based on HalBs, thus paving the way for new avenues in the field of supramolecular biology. One recent example is the study by Patil and collaborators158 involving halogenated-U PNAs for dsRNA recognition; however, they rely on base-to-base hydrogen bonding interactions to achieve nucleic acid binding. | ||
| Fig. 14 Schematic representation of a PNA–nucleic acid complex through complementary A⋯T and G⋯C pairs. | ||
Conclusions
In this review, a literature survey related to chemical modifications of nucleic acids, focusing on halogenation, has been carried out. More in detail, the structural and biological consequences of nucleobase halogenation have been discussed alongside several examples that investigate the influence of this chemical modification on the stability of nucleic acid base pairs. Furthermore, we have extended our analysis to the use of halogen bonding interactions involving brominated and iodinated nucleobases as a stabilization source of (i) novel biological supramolecular assemblies, such as “Holliday junctions”, (ii) protein conformations and (iii) protein–nucleic acid complexes. Lastly, we propose the inclusion of halogenated nucleobases into PNAs capable of forming a base to base halogen bonds as new supramolecular tools for the recognition of specific nucleic acid sequences.Author contributions
C. N. and J. R.-R. performed the data search and J. G.-G and A. B. designed the study and wrote the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. N. and A. B. thank the MICIU/AEI for financial support (PID2020-115637GB-I00 FEDER funds). J. M. R.-R. and J. G. G. thank the Spanish Ministry for Science and Innovation, The National Research Agency and FEDER funds from the EU (grant MFA/2022/014). J. G.-G. acknowledges the financial support from project CNS2024-154644 funded by MICIU/AEI/10.13039/501100011033. This study forms part of the Advanced Materials program and was supported by MCIN with funding from the European Union Next Generation EU (PRTR-C17.I1) and by Generalitat Valenciana.Notes and references
- C. H. Waddington, The epigenotype, Endeavour, 1942, 1, 18 Search PubMed.
- C. H. Waddington, Nature, 1942, 150, 563–565 CrossRef.
- S. Huang, M. Litt and C. Ann Blakey, Epigenetic Gene Expression and Regulation, Elsevier, New York, 2015 Search PubMed.
- Y. Chen, T. Hong, S. Wang, J. Mo, T. Tian and X. Zhou, Chem. Soc. Rev., 2017, 46, 2844–2872 RSC.
- S. M. Freier and K. H. Altmann, Nucleic Acids Res., 1997, 25, 4429–4443 CrossRef CAS PubMed.
- V. B. Pinheiro, A. I. Taylor, C. Cozens, M. Abramov, M. Renders, S. Zhang, J. C. Chaput, J. Wengel, S. Y. Peak-Chew, S. H. McLaughlin, P. Herdewijn and P. Holliger, Science, 2012, 336, 341–344 CrossRef CAS PubMed.
- S. Ni, H. Yao, L. Wang, J. Lu, F. Jiang, A. Lu and G. Zhang, Int. J. Mol. Sci., 2017, 18, 1683 CrossRef PubMed.
- Y. Motorin and M. Helm, Wiley Interdiscip. Rev.: RNA, 2011, 2, 611–631 CrossRef CAS PubMed.
- T. Carell, C. Brandmayr, A. Hienzsch, M. Müller, D. Pearson, V. Reiter, I. Thoma, P. Thumbs and M. Wagner, Angew. Chem., Int. Ed., 2012, 51, 7110–7131 CrossRef CAS PubMed.
- I. A. Roundtree, M. E. Evans, T. Pan and C. He, Cell, 2017, 169, 1187–1200 CrossRef CAS PubMed.
- R. J. Ontiveros, J. Stoute and K. F. Liu, Biochem. J., 2019, 476, 1227–1245 CrossRef CAS PubMed.
- W. A. Cantara, P. F. Crain, J. Rozenski, J. A. McCloskey, K. A. Harris, X. Zhang, F. A. P. Vendeix, D. Fabris and P. F. Agris, Nucleic Acids Res., 2011, 39, D195–D201 CrossRef CAS PubMed.
- C. Luo, P. Hajkova and J. R. Ecker, Science, 2018, 361, 1336–1340 CrossRef CAS PubMed.
- K. Chen, J. Zhang, Z. Guo, Q. Ma, Z. Xu, Y. Zhou, Z. Xu, Z. Li, Y. Liu, X. Ye, X. Li, B. Yuan, Y. Ke, C. He, L. Zhou, J. Liu and W. Ci, Cell Res., 2015, 26, 103–118 CrossRef PubMed.
- Y. Tang, S. J. Zheng, C. B. Qi, Y. Q. Feng and B. F. Yuan, Anal. Chem., 2015, 87, 3445–3452 CrossRef CAS PubMed.
- A. Seidel, P. Seidel, O. Manuwald and O. Herbarth, Environ. Toxicol., 2015, 30, 956–967 CrossRef CAS PubMed.
- K. L. Mahon, W. Qu, J. Devaney, C. Paul, L. Castillo, R. J. Wykes, M. D. Chatfield, M. J. Boyer, M. R. Stockler, G. Marx, H. Gurney, G. Mallesara, P. L. Molloy, L. G. Horvath and S. J. Clark, Br. J. Cancer, 2014, 111, 1802–1809 CrossRef CAS PubMed.
- S. Pfeiffer, J. Krüger, A. Maierhofer, Y. Böttcher, N. Klöting, N. el Hajj, D. Schleinitz, M. R. Schön, A. Dietrich, M. Fasshauer, T. Lohmann, M. Dreßler, M. Stumvoll, T. Haaf, M. Blüher and P. Kovacs, Sci. Rep., 2016, 6, 27969 CrossRef CAS PubMed.
- H. Kirchner, I. Sinha, H. Gao, M. A. Ruby, M. Schönke, J. M. Lindvall, R. Barrès, A. Krook, E. Näslund, K. Dahlman-Wright and J. R. Zierath, Mol. Metab., 2016, 5, 171–183 CrossRef CAS PubMed.
- C. Ling and L. Groop, Diabetes, 2009, 58, 2718–2725 CrossRef CAS PubMed.
- R. B. Maccioni, J. P. Munoz and L. Barbeito, Arch. Med. Res., 2001, 32, 367–381 CrossRef CAS PubMed.
- A. J. Phipps, J. C. Vickers, P. C. Taberlay and A. Woodhouse, Neurobiol. Aging, 2016, 45, 30–42 CrossRef CAS PubMed.
- L. de Boni, L. Riedel, I. Schmitt, T. F. Kraus, O. Kaut, D. Piston, S. Akbarian and U. Wullner, Neurobiol. Aging, 2015, 36, 3334–3337 CrossRef PubMed.
- X. Su, Y. Chu, J. H. Kordower, B. Li, H. Cao, L. Huang, M. Nishida, L. Song, D. Wang and H. J. Federoff, PLoS One, 2015, 10, e134087 Search PubMed.
- J. Goodchild, Bioconjugate Chem., 1990, 1, 165–187 CrossRef CAS PubMed.
- O. Doluca, J. M. Withers and V. V. Filichev, Chem. Rev., 2013, 113, 3044–3083 CrossRef CAS PubMed.
- Y. Saito and R. H. E. Hudson, J. Photochem. Photobiol., C, 2018, 36, 48–73 CrossRef CAS.
- L. K. McKenzie, R. El-Khoury, J. D. Thorpe, M. J. Damha and M. Hollenstein, Chem. Soc. Rev., 2021, 50, 5126–5164 RSC.
- R. E. Cline, R. M. Fink and K. Fink, J. Am. Chem. Soc., 1959, 81, 2521–2527 CrossRef CAS.
- D. M. Frisch and D. W. Visser, J. Am. Chem. Soc., 1959, 81, 1756–1758 CrossRef CAS.
- R. A. Manderville and S. D. Wetmore, Chem. Sci., 2016, 7, 3482–3493 RSC.
- R. J. Ontiveros, J. Stoute and K. F. Liu, Biochem. J., 2019, 476, 1227–1245 CrossRef CAS PubMed.
- L. E. Xodo, G. Manzini, F. Quadrifoglio, G. A. van der Marel and J. H. van Boom, Nucleic Acids Res., 1991, 19, 5625–5631 CrossRef CAS PubMed.
- V. Y. P. Väre, E. R. Eruysal, A. Narendran, K. L. Sarachan and P. F. Agris, Biomolecules, 2017, 7, 29 CrossRef PubMed.
- J. D. Watson and F. H. C. Crick, Nature, 1953, 171, 737–738 CrossRef CAS PubMed.
- S. Uesugi and M. J. Ikehara, Am. Chem. Soc., 1977, 99, 3250–3253 CrossRef CAS PubMed.
- V. Vongsutilers, D. J. Phillips, B. C. Train, G. R. McKelvey, N. M. Thomsen, K. H. Shaughnessy, J. P. Lewis and P. M. Gannett, Biophys. Chem., 2011, 154, 41–48 CrossRef CAS PubMed.
- A. Rich and S. Zhang, Nat. Rev. Genet., 2003, 4, 566–572 CrossRef CAS PubMed.
- A. H. Wang, G. J. Quigley, F. J. Kolpak, J. L. Crawford, J. H. van Boom, G. van der Marel and A. Rich, Nature, 1979, 282, 680–686 CrossRef CAS PubMed.
- S. C. Ha, K. Lowenhaupt, A. Rich, Y. G. Kim and K. K. Kim, Nature, 2005, 437, 1183–1186 CrossRef CAS PubMed.
- J. Gros, F. Rosu, S. Amrane, A. De Cian, V. Gabelica, L. Lacroix and J. L. Mergny, Nucleic Acids Res., 2007, 35, 3064–3075 CrossRef CAS PubMed.
- J. T. Davis, Angew. Chem., Int. Ed., 2004, 43, 668–698 CrossRef CAS PubMed.
- Z. Y. Sun, X. N. Wang, S. Q. Cheng, X. X. Su and T. M. Ou, Molecules, 2019, 24, 396 CrossRef CAS PubMed; H. Abou Assi, R. W. Harkness, N. Martin-Pintado, C. J. Wilds, R. Campos-Olivas, A. K. Mittermaier, C. González and M. J. Damha, Nucleic Acid Res., 2016, 44, 4998–5009 CrossRef PubMed.
- B. Wittig, S. Wölfl, T. Dorbic, W. Vahrson and A. Rich, EMBO J., 1992, 11, 4653–4663 CrossRef CAS PubMed.
- A. Maruyama, J. Mimura, N. Harada and K. Itoh, Nucleic Acids Res., 2013, 41, 5223–5234 CrossRef CAS PubMed.
- W. Y. Lai, J. Z. Mo, J. F. Yin, C. Lyu and H. L. Wang, Trends Anal. Chem., 2019, 110, 173–182 CrossRef CAS.
- M. Berney and J. F. McGouran, Nat. Rev. Chem., 2018, 2, 332–348 CrossRef CAS.
- J. Xiong, B. F. Yuan and Y. Q. Feng, Chem. Res. Toxicol., 2019, 32, 808–819 Search PubMed.
- M. D. Lan, B. F. Yuan and Y. Q. Feng, Chin. Chem. Lett., 2019, 30, 1–6 CrossRef CAS.
- M. Berney and J. F. McGouran, Nat. Rev. Chem., 2018, 2, 332–348 CrossRef CAS.
- M. Karplus and J. A. McCammon, Nat. Struct. Biol., 2002, 9, 646–652 CrossRef CAS PubMed.
- F. Colotta, P. Allavena, A. Sica, C. Garlanda and A. Mantovani, Carcinogenesis, 2009, 30, 1073–1081 CrossRef CAS PubMed.
- L. Pikor, K. Thu, E. Vucic and W. Lam, Cancer Metastasis Rev., 2013, 32, 341–352 CrossRef CAS PubMed.
- S. Negrini, V. G. Gorgoulis and T. D. Halazonetis, Nat. Rev. Mol. Cell Biol., 2010, 11, 220–228 CrossRef CAS PubMed.
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS PubMed.
- M. R. Stratton, P. J. Campbell and P. A. Futreal, Nature, 2009, 458, 719–724 CrossRef CAS PubMed.
- B. Vogelstein, N. Papadopoulos, V. E. Velculescu, S. Zhou, L. A. Diaz Jr. and K. W. Kinzler, Science, 2013, 339, 1546–1558 CrossRef CAS PubMed.
- D. S. Bredt and S. H. Snyder, Annu. Rev. Biochem., 1994, 63, 175–195 CrossRef CAS PubMed.
- S. Moncada, R. M. Palmer and E. A. Higgs, Pharmacol. Rev., 1991, 43, 109–142 CrossRef CAS PubMed.
- D. D. Thomas, L. A. Ridnour, J. S. Isenberg, W. Flores-Santana, C. H. Switzer, S. Donzelli, P. Hussain, C. Vecoli, N. Paolocci, S. Ambs, C. A. Colton, C. C. Harris, D. D. Roberts and D. A. Wink, Free Radical Bio. Med., 2008, 45, 18–31 CrossRef CAS PubMed.
- R. S. Lewis, S. Tamir, S. R. Tannenbaum and W. M. Deen, J. Biolo. Chem., 1995, 270, 29350–29355 CrossRef CAS PubMed.
- D. J. Stuehr and M. A. Marletta, Cancer Res., 1987, 47, 5590–5594 CAS.
- R. Takahashi, K. Edashige, E. F. Sato, M. Inoue, T. Matsuno and K. Utsumi, Arch. Biochem. Biophys., 1991, 285, 325–330 CrossRef CAS PubMed.
- J. A. Badwey and M. L. Karnovsky, Annu. Rev. Biochem., 1980, 49, 695–726 CrossRef CAS PubMed.
- E. Van den Worm, C. J. Beukelman, A. J. Van den Berg, B. H. Kroes, R. P. Labadie and H. Van Dijk, Eur. J. Pharmacol., 2001, 433, 225–230 CrossRef CAS PubMed.
- I. M. Colin, S. Poncin, P. Leveque, B. Gallez and A. C. Gerard, Thyroid, 2014, 24, 441–452 CrossRef CAS PubMed.
- M. Sundaresan, F. V. J. Yu ZX, D. J. Sulciner, J. S. Gutkind, K. Irani, P. J. Goldschmidt-Clermont and T. Finkel, Biochem. J., 1996, 318, 379–382 CrossRef CAS PubMed.
- D. Yang, S. G. Elner, Z. M. Bian, G. O. Till, H. R. Petty and V. M. Elner, Exp. Eye Res., 2007, 85, 462–472 CrossRef CAS PubMed.
- A. Mangerich, P. C. Dedon, J. G. Fox, S. R. Tannenbaum and G. N. Wogan, Free Radic. Res., 2013, 47, 958–986 CrossRef CAS PubMed.
- B. S. van der Veen, M. P. de Winther and P. Heeringa, Antioxid. Redox Signaling, 2009, 11, 2899–2937 CrossRef CAS PubMed.
- C. C. Winterbourn, M. B. Hampton, J. H. Livesey and A. J. Kettle, J. Bio. Chem., 2006, 281, 39860–39869 CAS.
- J. P. Henderson, J. Byun and J. W. Heinecke, J. Bio. Chem., 1999, 274, 33440–33448 CAS.
- J. P. Henderson, J. Byun, M. V. Williams, M. L. McCormick, W. C. Parks, L. A. Ridnour and J. W. Heinecke, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 1631–1636 CrossRef CAS PubMed.
- H. Ohshima, M. Tatemichi and T. Sawa, Arch. Biochem. Biophys., 2003, 417, 3–11 CrossRef CAS PubMed.
- T. Asahi, H. Kondo, M. Masuda, H. Nishino, Y. Aratani, Y. Naito, T. Yoshikawa, S. Hisaka, Y. Kato and T. Osawa, J. Biol. Chem., 2010, 285, 9282–9291 CrossRef CAS PubMed.
- C. Badouard, M. Masuda, H. Nishino, J. Cadet, A. Favier and J. L. Ravanat, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 827, 26–31 CrossRef CAS PubMed.
- M. Masuda, T. Suzuki, M. D. Friesen, J. L. Ravanat, J. Cadet, B. Pignatelli, H. Nishino and H. Ohshima, J. Biol. Chem., 2001, 276, 40486–40496 CrossRef CAS PubMed.
- Z. Shen, W. Wu and S. L. Hazen, Biochemistry, 2000, 39, 5474–5482 CrossRef CAS PubMed.
- Y. Kawai, H. Morinaga, H. Kondo, N. Miyoshi, Y. Nakamura, K. Uchida and T. Osawa, J. Bio. Chem., 2004, 279, 51241–51249 CAS.
- J. I. Kang Jr. and L. C. Sowers, Chem. Res. Toxicol., 2008, 21, 1211–1218 Search PubMed.
- A. Mangerich, C. G. Knutson, N. M. Parry, S. Muthupalani, W. Ye, E. Prestwich, L. Cui, J. L. McFaline, M. Mobley, Z. Ge, K. Taghizadeh, J. S. Wishnok, G. N. Wogan, J. G. Fox, S. R. Tannenbaum and P. C. Dedon, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E1820–1829 CrossRef PubMed.
- B. I. Fedeles, B. D. Freudenthal, E. Yau, V. Singh, S. C. Chang, D. Li, J. C. Delaney, S. H. Wilson and J. M. Essigmann, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, E4571–4580 CrossRef CAS PubMed.
- Y. Sato, S. Takahashi, Y. Kinouchi, M. Shiraki, K. Endo, Y. Matsumura, Y. Kakuta, M. Tosa, A. Motida, H. Abe, G. Imai, H. Yokoyama, E. Nomura, K. Negoro, S. Takagi, H. Aihara, K. I. Masumura, T. Nohmi and T. Shimosegawa, Carcinogenesis, 2006, 27, 1068–1073 CrossRef CAS PubMed.
- C. G. Knutson, A. Mangerich, Y. Zeng, A. R. Raczynski, R. G. Liberman, P. Kang, W. Ye, E. G. Prestwich, K. Lu, J. S. Wishnok, J. R. Korzenik, G. N. Wogan, J. G. Fox, P. C. Dedon and S. R. Tannenbaum, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, E2332–2341 CAS.
- J. Koseki, M. Konno, A. Asai, N. Horie, K. Tsunekuni, K. Kawamoto, S. Obika, Y. Doki, M. Mori and H. Ishii, Sci. Rep., 2020, 10, 1138 CrossRef CAS PubMed.
- B. Yang, R. R. Wu and M. T. Rodgers, J. Am. Soc. Mass Spectrom., 2015, 26, 1469–1482 CrossRef CAS PubMed.
- E. Heshmati, P. Abdolmaleki, H. Mozdarani and A. S. Sarvestani, Bioorg. Med. Chem. Lett., 2009, 19, 5256–5260 CrossRef CAS PubMed.
- X. Zhao and F. Meng, Theochem, 2006, 770, 157–162 CrossRef CAS.
- Z. Yang and M. T. Rodgers, J. Am. Chem. Soc., 2004, 126, 16217–16226 CrossRef CAS PubMed.
- M. Hanus, M. Kabelac, D. Nachtigallova and P. Hobza, Biochemistry, 2005, 44, 1701–1707 CrossRef CAS PubMed.
- C. F. Guerra, T. van der Wijst and F. M. Bickelhaupt, Struct. Chem., 2005, 16, 211–221 CrossRef CAS.
- A. J. Parker, J. Stewart, K. J. Donald and C. A. Parish, J. Am. Chem. Soc., 2012, 134, 5165–5172 CrossRef CAS PubMed.
- R. Xu, Z. Hu, Z. Wang, Y. Liu, Z. Zhou, J. Xu, Z. Sun, H. Sun and J. Chen, J. Phys. Chem. B, 2020, 124, 2560–2567 CrossRef CAS PubMed.
- V. Valinluck, W. Wu, P. Liu, J. W. Neidigh and L. C. Sowers, Chem. Res. Toxicol., 2006, 19, 556–562 Search PubMed.
- Z. Yang and M. T. Rodgers, J. Am. Chem. Soc., 2004, 126, 16217–16226 CrossRef CAS PubMed.
- F. Seela, X. Peng and H. Li, J. Am. Chem. Soc., 2005, 127, 7739–7751 CrossRef CAS PubMed.
- M. Hanus, M. Kabelác, D. Nachtigallová and P. Hobza, Biochemistry, 2005, 44, 1701–1707 CrossRef CAS PubMed.
- E. Cubero, E. C. Sherer, F. J. Luque, M. Orozco and C. A. Laughton, J. Am. Chem. Soc., 1999, 121, 8653 CrossRef CAS.
- E. Cubero, C. A. Laughton, F. J. Luque and M. Orozco, J. Am. Chem. Soc., 2000, 122, 6891 CrossRef CAS.
- M. Orozco, B. Hernández and F. J. Luque, J. Phys. Chem. B, 1998, 102, 5228 CrossRef CAS.
- C. J. McGinn, D. S. Shewach and T. S. Lawrence, J. Natl. Cancer Inst., 1996, 88, 1193 CrossRef CAS PubMed.
- A. K. Vallerga, D. A. Zarling and T. J. Kinsella, Clin. Adv. Hematol. Oncol., 2004, 2, 793 Search PubMed.
- C. Heidelberger, D. G. Parsons and D. C. Remy, J. Med. Chem., 1964, 7, 1–5 CrossRef CAS PubMed.
- E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed.
- H. Wójtowicz-Rajchel, J. Fluorine Chem., 2012, 143, 11–48 CrossRef.
- B. Yang, R. R. Wu and M. T. Rodgers, Anal. Chem., 2013, 85, 11000–11006 CrossRef CAS PubMed.
- B. Yang and M. T. Rodgers, J. Am. Chem. Soc., 2014, 136, 282–290 CrossRef CAS PubMed.
- F. J. Sullivan, L. L. Herscher, J. A. Cook, J. Smith, S. M. Steinberg, A. H. Epstein, E. H. Oldfield, T. E. Goffman, T. J. Kinsella, J. B. Mitchell and E. Glatstein, Int. J. Radiat. Oncol., Biol., Phys., 1994, 30, 583–590 CrossRef CAS PubMed.
- R. C. Urtasun, T. J. Kinsella, N. Farnan, J. D. Delrowe, S. G. Lester and D. S. Fulton, Int. J. Radiat. Oncol., Biol., Phys., 1996, 36, 1163–1167 CrossRef CAS PubMed.
- J. A. Williams, X. Yuan, L. E. Dillehay, V. R. Shastri, H. Brem and J. R. Williams, Int. J. Radiat. Oncol., Biol., Phys., 1998, 42, 631–639 CrossRef CAS PubMed.
- G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711–1713 CrossRef CAS.
- P. Metrangolo, T. Pilati and G. Resnati, CrystEngComm, 2006, 8, 946–947 RSC.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed.
- P. Y. S. Lam, C. G. Clark, A. M. Smallwood and R. S. Alexander, Presented in part at The 238th ACS National Meeting, Washington, DC, 2009 Search PubMed.
- Y. Lu, T. Shi, Y. Wang, H. Yang, X. Yan, X. Luo, H. Jiang and W. Zhu, J. Med. Chem., 2009, 52, 2854–2862 CrossRef CAS PubMed.
- Z. Xu, Z. Liu, T. Chen, T. Chen, Z. Wang, G. Tian, J. Shi, X. Wang, Y. Lu, X. Yan, G. Wang, H. Jiang, K. Chen, S. Wang, Y. Xu, J. Shen and W. Zhu, J. Med. Chem., 2011, 54, 5607–5611 CrossRef CAS PubMed.
- P. S. Ho, Future Med. Chem., 2017, 9, 637–640 CrossRef CAS PubMed.
- R. Wilcken, M. O. Zimmermann, A. Lange, A. C. Joerger and F. M. Boeckler, J. Med. Chem., 2013, 56, 1363–1388 CrossRef CAS PubMed.
- O. Hassel, Presented in part at Nobel Lectures, Chemistry 1963–1970, Amsterdam, 1972 Search PubMed.
- K. E. Riley, M. Vazquez, C. Umemura, C. Miller and K. A. Tran, Chem. – Eur. J., 2016, 22, 17690–17695 CrossRef CAS PubMed.
- T. Clark, J. S. Murray and P. Politzer, Aust. J. Chem., 2013, 67, 451–456 CrossRef.
- K. Eskandari and H. Zariny, Chem. Phys. Lett., 2010, 492, 9–13 CrossRef CAS.
- H. J. Jahromi and K. Eskandari, Struct. Chem., 2013, 24, 1281–1287 CrossRef CAS.
- T. Clark, M. Hennemann, J. S. Murray and P. Politzer, J. Mol. Model., 2007, 13, 291–296 CrossRef CAS PubMed.
- P. Auffinger, F. A. Hays, E. Westhof and P. S. Ho, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16789–16794 CrossRef CAS PubMed.
- P. S. Ho, in Halogen Bonding I: Impact on Materials Chemistry and Life Sciences, ed P. Metrangolo and G. Resnati, Springer, 2015, vol. 8, pp. 241–276 Search PubMed.
- P. S. Ho, Top. Curr. Chem., 2015, 358, 241–276 CrossRef CAS PubMed.
- M. C. Ford and P. S. Ho, J. Med. Chem., 2016, 59, 1655–1670 CrossRef CAS PubMed.
- R. S. Czarny, A. N. Ho and P. S. Ho, Chem. Rec., 2021, 21, 1240–1251 CrossRef CAS PubMed.
- A. Frontera and A. Bauzá, Org. Biomol. Chem., 2021, 19, 6858–6864 RSC.
- R. F. W. Bader, Chem. Rev., 1991, 91, 893–928 CrossRef CAS.
- C. M. Ogata, W. A. Hendrickson, X. Gao and D. Patel, J. Abstr. Cryst. Assoc. Mtg. Ser., 1989, 217, 53 Search PubMed.
- Y. Xiong and M. Sundaralingam, Nucleic Acids Res., 2000, 28, 2171–2176 CrossRef CAS PubMed.
- E. Ennifar, S. Bernacchi, P. Wolff and P. Dumas, RNA, 2007, 13, 1445–1452 CrossRef CAS PubMed.
- N. J. Thornton and T. van Mourik, Int. J. Mol. Sci., 2020, 21, 6571 CrossRef CAS PubMed.
- M. N. Piña, A. Frontera and A. Bauzá, ACS Chem. Biol., 2020, 15, 1942–1948 CrossRef PubMed.
- D. Mitra, N. Bankoti, D. Michael, K. Sekar and T. N. Guru Row, J. Chem. Sci., 2020, 132, 1–11 CrossRef.
- A. Frontera and A. Bauzá, J. Chem. Theory Comput., 2020, 16, 4744–4752 CrossRef CAS PubMed.
- M. Marín-Luna, I. Alkorta and J. Elguero, Comput. Theor. Chem., 2016, 1076, 101–108 CrossRef.
- L. Xu, P. Sang, J. W. Zou, M. B. Xu, X. M. Li and Q. S. Yu, Chem. Phys. Lett., 2011, 509, 175–180 CrossRef CAS.
- S. Tripathi, D. Zhang and P. J. Paukstelis, Nucleic Acids Res., 2015, 43, 1937–1944 CrossRef CAS PubMed.
- A. R. Voth, F. A. Hays and P. S. Ho, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6188–6193 CrossRef CAS PubMed.
- F. A. Hays, A. Teegarden, Z. J. Jones, M. Harms, D. Raup, J. Watson, E. Cavaliere and P. S. Ho, Proc. Natl. Acad. Sci. U. S. A., 2015, 102, 7157–7162 CrossRef PubMed.
- F. A. Hays, J. M. Vargason and P. S. Ho, Biochemistry, 2003, 42, 9586–9597 CrossRef CAS PubMed.
- B. F. Eichman, J. M. Vargason, B. H. M. Mooers and P. S. Ho, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3971–3976 CrossRef CAS PubMed.
- B. F. Eichman, M. Ortiz-Lombardia, J. Aymami, M. Coll and P. S. Ho, J. Mol. Biol., 2002, 320, 1037–1051 CrossRef CAS PubMed.
- E. Grahn, T. Moss, C. Helgstrand, K. Fridborg, M. Sundaram, K. Tars, H. Lago, N. J. Stonehouse, D. R. Davis, P. G. Stockley and L. Liljas, RNA, 2001, 7, 1616–1627 CAS.
- M. H. Kolář and O. Tabarrini, J. Med. Chem., 2017, 60, 8681–8690 CrossRef PubMed.
- J. L. Jenkins, A. A. Agrawal, A. Gupta, M. R. Green and C. L. Kielkopf, Nucleic Acids Res., 2013, 41, 3859–3873 CrossRef CAS PubMed.
- A. A. Agrawal, E. Salsi, R. Chatrikhi, S. Henderson, J. L. Jenkins, M. R. Green, D. N. Ermolenko and C. L. Kielkopf, Nat. Commun., 2016, 7, 10950 CrossRef CAS PubMed.
- J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J. P. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625–632 CrossRef PubMed.
- M. N. Piña, A. Frontera and A. Bauzá, ACS Chem. Biol., 2020, 15, 1942–1948 CrossRef PubMed.
- J. Gouge, S. Rosario, F. Romain, P. Beguin and M. Delarue, J. Mol. Biol., 2013, 425, 4334–4352 CrossRef CAS PubMed.
- S. C. Koch, J. Kuper, K. L. Gasteiger, N. Simon, R. Strasser, D. Eiser, S. Geiger, S. Schneider, C. Kisker and T. Carell, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8272–8277 CrossRef CAS PubMed.
- J. Zhou, C. Lv, B. Liang, M. Chen, W. Yang and H. Li, J. Mol. Biol., 2010, 401, 690–695 CrossRef CAS PubMed.
- A. Frontera and A. Bauzá, J. Chem. Theory Comput., 2020, 16, 4744–4752 CrossRef CAS PubMed.
- P. E. Nielsen, M. Egholm, R. H. Berg and O. Buchardt, Science, 1991, 254, 1497–1500 CrossRef CAS PubMed.
- K. M. Patil, D. F. K. Toh, Z. Yuan, Z. Meng, Z. Shu, H. Zhang, A. A. Lerk Ong, M. S. Krishna, L. Lu, Y. Lu and G. Chen, Nucleic Acids Res., 2018, 46, 7506–7521 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |