Open Access Article
Open Access ArticleUnveiling the promise of peptide nucleic acids as functional linkers for an RNA imaging platform†
Aleksandra J.
Wierzba
 ab,
Erin M.
Richards
ab,
Erin M.
Richards
 ab,
Shelby R.
Lennon
ab,
Shelby R.
Lennon
 a,
Robert T.
Batey
a,
Robert T.
Batey
 *a and
Amy E.
Palmer
*a and
Amy E.
Palmer
 *ab
*ab
aDepartment of Biochemistry, University of Colorado, Boulder, CO 80309-0596, USA. E-mail: Robert.Batey@colorado.edu; Amy.Palmer@colorado.edu; Fax: +1 303 492 5894; Tel: +1 303 735 2159 Tel: +1 303 492 1945
bBioFrontiers Institute, University of Colorado, Boulder, CO 80303-0596, USA
First published on 19th December 2024
Abstract
Linkers in chemical biology provide more than just connectivity between molecules; their intrinsic properties can be harnessed to enhance the stability and functionality of chemical probes. In this study, we explored the incorporation of a peptide nucleic acid (PNA)-based linker into RNA-targeting probes to improve their affinity and specificity. By integrating a PNA linker into a small molecule probe of the Riboglow platform, we enabled dual binding events: cobalamin (Cbl)–RNA structure-based recognition and sequence-specific PNA–RNA interaction. We show that incorporating a six-nucleotide PNA sequence complementary to a region of wild type RNA aptamer (env8) results in a 30-fold improvement in binding affinity compared to the probe with a nonfunctional PEG linker. Even greater improvements are observed when the PNA probe was tested against truncated versions of the RNA aptamer, with affinity increasing by up to 280-fold. Additionally, the PNA linker is able to rescue the Cbl–RNA interaction even when the cobalamin binding pocket is compromised. We demonstrate that PNA probes effectively bind RNA both in vitro and in live cells, enhancing visualization of RNA in stress granules and U-bodies at low concentrations. The modular nature of the Riboglow platform allows for flexible modifications of the PNA linker, fluorophore, and RNA tag, while maintaining high specificity and affinity. This work establishes a new approach for enhancing RNA imaging platforms through the use of PNA linkers, highlighting the potential of combining short oligonucleotides with small molecules to improve the affinity and specificity of RNA-targeting probes. Furthermore, this dual-binding approach presents a promising strategy for driving advancements in RNA-targeted drug development.
Introduction
The ability to visualize macromolecules in live cells has driven many fundamental discoveries in biology over the last few decades. Tagging proteins with fluorophores has revolutionized our understanding of the behavior of individual proteins in the cellular context, in large part due to a diverse and easy-to-use set of tools that continues to expand to meet new experimental challenges.1 Conversely, while the roles of RNA in human biology are myriad and diverse, the ability to visualize RNA in live cells is much more limited. The most widely employed RNA imaging tool to date is the MS2-MCP system which uses fluorescently tagged proteins to track individual RNA molecules in live cells.2,3 However, this system has a set of well-established technical limitations that have inspired the development of additional live-cell RNA imaging tools.4–6 Over the past decade, much effort has focused on the development of tools that employ a small-molecule binding aptamer as a tag that is incorporated into the RNA of interest.7,8 While these next generation RNA imaging tools are beginning to make an impact in addressing important biological questions, their lack of widespread adoption indicates a need for improvements in their capabilities and performance.One class of RNA probes relies on binding of a conjugated fluorophore-quencher pair to an RNA aptamer, where the binding event separates the fluorophore from the quencher, giving rise to fluorescence turn-on. For example, a conjugate between tetramethylrhodamine (TMR) and a dinitroaniline quencher was created by connecting the pair with a small flexible polyethylene glycol (PEG) linker. When TMR binds to an in vitro selected RNA aptamer,9 the quencher is separated from the fluorophore, causing an increase in fluorescence.10 Fluorophore-binding aptamers suffer from the limitation that a new aptamer needs to be selected for every new fluorophore. To overcome this limitation, some RNA probes are designed so that the quencher binds the RNA tag.11–13 For example, Riboglow is an RNA imaging tool in which the RNA aptamer binds to the quencher, enabling the use of a wide array of fluorescent dyes.13 In this case, the quencher is cobalamin (Cbl) that binds a biological RNA aptamer derived from a bacterial mRNA riboswitch that represses translation of the message in response to cobalamin binding. In each case, the fluorophore and quencher are connected by a PEG linker, which was chosen for its flexibility and ease of installation into the probe.
Linkers are an indispensable tool in chemical biology, providing not only structural connectivity but also functional versatility in the design and synthesis of bioconjugates.14–16 PEG is widely used in chemical probes due to its biological inertness, hydrophilicity, and flexibility, all of which make it an attractive choice as a linker between two functional parts of a chemical probe, where it is presumed to be innocuous. We speculated, however, that using PEG linkers in RNA imaging probes might represent a missed opportunity to add chemical functionality to the linker. A functionalized linker could interact with the target or influence the probe's behavior. In our previous work on Riboglow, we tested a limited set of different linkers, focusing primarily on their length as a factor affecting probe properties, particularly the optimal fluorophore-quencher separation.13 We demonstrated that the optimal linker length resulted in effective quenching/dequenching platform properties. Moreover, a more rigid linker consisting of four glycine units (4xGly linker), compared to 5xPEG, improved the probe's turn-on efficiency. Building on this, we became particularly interested in incorporating a short, antisense oligonucleotide (ASO) that could base pair with a target RNA. This is a new approach to probe design that provides additional binding capabilities that could impact the functionality of RNA-targeted probes.
Peptide nucleic acids (PNAs) hold great promise as a nucleic acid mimic.17–19 These nucleic acid analogs have a backbone composed of repeating N-(2-aminoethyl)-glycine units linked by peptide bonds rather than sugar–phosphate groups. As a consequence, they exhibit enhanced stability against enzymatic degradation and form stronger duplexes with complementary DNA or RNA than either DNA or RNA homoduplexes due to the lack of charge repulsion between the strands.19 PNAs have been shown to function as antisense agents, gene-editing tools, and crosslinkers,17 as well as fluorogenic probes for nucleic acid sensing when tethered to a fluorescent dye (molecular beacons).20 Despite these advantages, there are notable challenges that deter scientists from using PNAs, such as poor cell penetration, limited solubility, and a tendency to form aggregates.21 However, these issues can be mitigated by coupling PNAs with appropriate molecules.22,23 Previous work has shown that PNAs can be coupled to cobalamin to facilitate their uptake through the bacterial cobalamin import system and act as an ASO to repress gene expression.24–28 Additionally, conjugation of PNA to hydrophilic cobalamin significantly improves the solubility of the synthetic oligonucleotide. Together, these observations suggest that PNAs may serve as ideal candidates as “smart” linkers in contact quenching RNA probes.
In this work, we explore the impact of functional linkers on an RNA imaging platform by substituting the neutral PEG linker in Riboglow with PNA linkers designed to interact with the target RNA via base pairing. We designed a six-nucleotide PNA sequence complementary to a single-stranded region within the aptamer that does not engage in cobalamin binding, which is crucial for maintaining cobalamin recognition. Using chemical probing, we demonstrate the direct engagement of the PNA linker with the target RNA sequence along with effective cobalamin binding. Base pairing of the PNA linker with the RNA aptamer combined with the cobalamin–RNA binding significantly increases the probe affinity. In vitro fluorescence turn-on assays revealed the KD decreases 30-fold to 0.11 ± 0.02 nM for the full-length wild type env8 cobalamin riboswitch aptamer when compared to a probe with a 5xPEG linker. We show that scrambling the PNA sequence in the probe abolishes its interaction with the RNA, but the sequence specific interaction can be rescued by making a compensatory change in the RNA. An even more beneficial impact of PNA was observed for truncated versions of the RNA aptamer, in which introduction of a PNA binding site on either the 3′ or 5′ end led to an increase in affinity by 60 to 80-fold (for two different sequences at the 3′ end) or 220 to 280-fold (for two different sequences at the 5′ end). Further, we show that the PNA linker can drive binding between the probe and the RNA, even when the binding pocket for cobalamin is significantly compromised. Cell studies show the superiority of the PNA probes compared to those with nonfunctional linkers. The PNA probes show stronger enrichment in RNA–protein granules (stress granules or U-bodies) in live cells expressing aptamer-tagged RNA-of-interest. In summary, we establish a new pathway for improvement of RNA imaging tools as well as the potential for using short oligonucleotides conjugated to small molecules to improve affinity and specificity of chemical probes targeting RNA.
Results
Synthesis of cobalamin–PNA–fluorophore conjugates
We set out to install chemical functionality in the linker of cobalamin–fluorophore probes to improve the affinity and specificity of the Riboglow RNA imaging platform. To accomplish this, we replaced the flexible polyethylene glycol linker in the original Riboglow probes13 with a PNA linker. Based on the crystal structure and secondary structural elements, we designed the PNA sequence to target the single-stranded J1/13 region in the wild type cobalamin env8 riboswitch (Fig. S1, ESI†).29 The targeted site within the riboswitch does not engage in cobalamin binding; therefore, it was envisioned as a suitable landing pad for the complementary linker. We chose a PNA linker rather than one of the other nucleic acid analogs because cobalamin–PNA conjugates have been previously synthesized and shown to function in bacterial cells as an antisense oligonucleotide.24–28 The mode of action of Cbl–PNA probe and the RNA tag, involving both cobalamin binding by the riboswitch and the engagement of sequence-specific interaction between the linker and the complementary RNA region, is shown in Fig. 1A.Cbl–PNA–dye probes were synthesized by preparing a specifically designed cobalamin derivative, followed by the subsequent attachment of a suitably tailored PNA oligomer to Cbl and the addition of a fluorescent dye (Fig. 1B and C). Two distinct bioorthogonal chemistries were employed to combine cobalamin with the PNA strand and the fluorescent dye. Maleimide–thiol conjugation was chosen to synthesize Cbl–PNA conjugates due to its chemoselectivity and the stability of the thiosuccinimide bond. Copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC), previously shown to be efficient for preparing Cbl conjugates, was used for the dye attachment.13,24 PNA oligomers were manually synthesized using solid-phase synthesis (4.2, ESI†). The strand was obtained by coupling six monomers together through Fmoc chemistry and tagging the N-terminus and C-terminus with two distinct functionalities: a sulfhydryl group and an azide group, respectively. To obtain the thiol-reactive cobalamin, its primary hydroxyl group was modified via carbamate chemistry, leading to the introduction of a maleimide moiety (Cbl–maleimide, Fig. 1C). This derivative was then successfully coupled with the thiol end of the PNA strand, yielding the thiosuccinimide product, the Cbl–PNA conjugate (Fig. S2, ESI†). The target Cbl–PNA–dye probe was obtained via CuAAC between the alkyne-modified dye and the azide end of the PNA, resulting in a covalently linked hybrid (Fig. 1B and C, 4.8, ESI†).
The Cbl–PNA–ATTO590 probe possesses a sequence targeting the complementary single-stranded region of the wild type env8 cobalamin riboswitch (env8-FL-3′antiPNA, Fig. S4, ESI†). To assess the specificity of PNA to base pair with the targeted RNA region, the Cbl–PNA–ATTO590 probe was tested against a “scrambled” variant, Cbl–PNAscr–ATTO590 (Fig. 1B and Fig. S3, ESI†), which is equipped with the same set of nucleobases, but arranged in a different order to abrogate base-pairing with the RNA aptamer. Additionally, we compared the PNA probe to the previously published probes with neutral chemical linkers, namely Cbl–5xPEG–ATTO590 and Cbl–4xGly–ATTO590 (Fig. 1B and Fig. S3, ESI†).13
The PNA probe productively base pairs with the riboswitch
To visualize RNA–PNA pairing, we used a chemical footprinting approach called “Selective 2′-hydroxyl acylation analyzed by primer extension” (SHAPE).30,31 This technique exploits small electrophilic reagents (here N-methylisatoic anhydride, NMIA) that react with 2′-hydroxyl groups in flexible regions of the RNA to interrogate its structure at single-nucleotide resolution. Thus, when a given nucleotide is involved in secondary structural elements (i.e., base pairing to form a helix), the 2′-hydroxyl has strongly reduced reactivity towards NMIA. We visualized chemical reactivity of each 2′-hydroxyl group in env8 RNA using radiolabeled primer extension and electrophoresis of the resulting cDNAs on a sequencing gel.SHAPE chemical probing revealed Cbl and PNA interactions between the Cbl-conjugates (Fig. S2, ESI†) and env8-FL-3′antiPNA. The regions relevant to Cbl-riboswitch binding are shown in Fig. S1 (ESI†). The Cbl-specific signature of protection within the L5, J6/3, and L13 RNA regions of the env8 riboswitch was the same for Cbl–PNA, Cbl–PNAscr, and Cbl alone (Fig. 2A, left gel, Fig. S5, ESI†). This reveals that conjugation of the PNA to Cbl does not impact the ability of Cbl to interact productively with the RNA. In contrast, the J1/13 region revealed ligand-dependent protection between Cbl–PNA and wild type env8-FL-3′antiPNA (Fig. 2A, left gel) but not with the Cbl–PNAscr. This suggests that the PNA interacts with the RNA in a sequence-specific fashion.
To demonstrate conclusively that the PNA–RNA interaction is sequence specific, the same chemical probing experiments were performed using an RNA whose J1/13 sequence was altered to be complementary to the PNAscr sequence.
These data revealed that the PNA-mediated base pairing interaction is rescued when the sequence of J1/13 was altered to match that of Cbl–PNAscr (env8-FL-3′antiPNAscr, Fig. 2A, right gel, Fig. S6, ESI†). Hence both Cbl–PNA and Cbl–PNAscr conjugates engaged in base pairing only when a complementary sequence was present within the RNA structure. Quantification of reactivity in the J1/13 regions of env8-FL-3′antiPNA and env8-FL-3′antiPNAscr indicated significant protections of this fragment for both complementary pairs (Fig. 2B, top and bottom, respectively) compared to the ligands lacking the sequence-specific fragment. Together, these data reveal specific RNA–PNA hybridization in the context of a small molecule–PNA conjugate. Further, we show the sequence of the RNA aptamer can be mutated to create a PNA binding site, indicating that PNA sequences are not limited to those that pair with wild type RNA but could be expanded to a large number of potential PNA sequences that pair with a complementary region of RNA.
Quantitative binding analysis reveals that the PNA linker enhances binding affinity of the probe
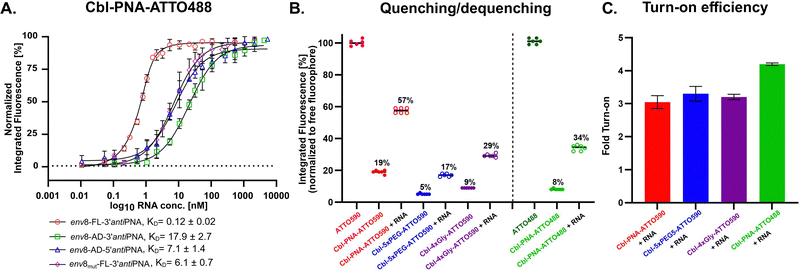
Since the PNA creates additional interactions with the RNA through base pairing, we predicted that the PNA linker would increase the affinity of Cbl–PNA probes compared to probes in which the linker does not interact with the RNA. To test this, we performed a series of titrations that leverage probe turn-on upon binding due to separation of the fluorophore from its quencher (Fig. 3). The affinity was determined by titrating an increasing amount of the RNA into a solution containing a constant amount of Cbl probe and the dissociation constant (KD) was determined by fitting the fluorescence data to a quadratic binding equation.32Binding analysis demonstrated that PNA significantly increased the affinity of Cbl probes for the RNA riboswitch. Cbl–PNA–ATTO590 exhibited tighter binding with wild type env8-FL-3′antiPNA, with a KD of 0.11 ± 0.02 nM, in comparison to Cbl–PNAscr–ATTO590 (KD = 1.4 ± 0.4 nM) and Cbl–5xPEG–ATTO590 (KD = 3.2 ± 0.2 nM) (Fig. 3A and G). The Cbl–PNAscr–ATTO590 probe bound slightly tighter compared to Cbl–5xPEG–ATTO590 which could be attributed to nonspecific interactions between the RNA and noncomplementary PNAscr sequences. Mutation of env8 J1/13 to make it complementary to the Cbl–PNAscr–ATTO590 probe (env8-FL-3′antiPNAscr) resulted in comparably tighter binding between the two with a KD of 0.12 ± 0.07 nM (Fig. 3B and G), further demonstrating that complementary sequence in the linker leads to significant enhancement of the probe affinity.
Truncated aptamer domain Cbl riboswitches display enhanced binding by PNA linker probes
The RNA imaging field is in pursuit of relatively small RNA tags to minimally perturb the behavior of the RNA of interest. We previously tested a truncated env8 containing only the aptamer domain (env8-AD)13 for binding to Cbl–5xPEG–ATTO590 probe and found it had significantly reduced affinity (KD = 1.3 ± 0.6 μM) compared to a full length aptamer (KD = 3.2 ± 0.2 nM). Here we tested whether addition of an antiPNA binding site could enhance the binding affinity of the truncated env8. In the case of the aptamer domain (AD), which lacks the P13 element, the PNA-pairing sequence could be installed on either the 5′- or 3′-side of the aptamer providing more opportunities to optimize the enhancement in affinity between the RNA and probe (Fig. 3C–F).Addition of the antiPNA sequence to the 3′ end of truncated env8 (env8-AD-3′antiPNA) led to an 80-fold decrease in the KD value for the PNA probe compared to the 5xPEG probe (Fig. 3C and G). The probe with the scrambled PNA sequence showed weaker binding to the noncomplementary aptamer, which was rescued when the complementary sequence was added to the 3′ end (env8-AD-3′antiPNAscr) (Fig. 3C and D). A similar trend was observed when the PNA sequence was added to the 5′ end of the truncated env8 (env8-AD-5′antiPNA), with an even greater decrease in the KD value, approximately 280-fold, compared to the 5xPEG probe (Fig. 3E and F). As in our previous work,13 the probe with the 5xPEG linker exhibited a KD in the micromolar range, whereas probes with complementary PNA sequences showed KD values in the low nanomolar range (Fig. 3C–F and G). Inserting the antiPNA complementary sequence at the 5′ end resulted in higher affinity (KD ∼ 5 nM) compared to the 3′ end (KD ∼ 17 nM) (Fig. 3G), indicating that binding can be further optimized by altering the placement of the PNA landing pad within the RNA aptamer. The PNA linker can drive the binding of cobalamin to an impaired riboswitch. Given that incorporation of an antiPNA landing pad into an RNA aptamer could decrease the KD up to 280-fold, we evaluated whether complementary PNA interactions could be the primary driver of binding between a probe and RNA rather than a structure-based recognition by the cobalamin. For this purpose we synthesized an env8 variant with four mutations in the Cbl binding pocket that debilitate binding to Cbl but retain the complementary sequence to the PNA (env8mut-FL-3′antiPNA, Fig. 4A). To evaluate Cbl–PNA conjugates binding to the mutated RNA, we used SHAPE chemical probing (Fig. 4B and Fig. S7, ESI†). Ligand-induced protections in the L5 and J6/3 regions of env8 are indicative of Cbl binding, as seen in Fig. 2. Quantification of the degree of protection in these regions revealed that as the ligand concentration was lowered from 30 μM to 0.3 μM, the Cbl–PNA conjugate showed significantly greater protection compared to Cbl or Cbl–PNAscr. This indicates that the PNA linker facilitates Cbl binding to the debilitated aptamer and demonstrates that the PNA can indeed drive binding (Fig. 4B and C). As observed for env8mut-FL-3′antiPNA, the J1/13 region showed significant protection upon interaction with Cbl–PNA, but not the other two non-complementary conjugates, confirming specific base pairing between PNA strand in the probe and its complementary sequence in the aptamer. Measurement of binding affinities for the mutant env8 via fluorescence turn-on studies revealed that the probe containing the complementary PNA linker was able to bind the mutated RNA within the nanomolar range (KD for Cbl–PNA–ATTO590 to env8mut-FL-3′antiPNA was 12.6 ± 1.2 nM and for the scrambled pair was 18.4 ± 1.6 nM) (Fig. 4D and E). This result shows a more than 300-fold increase in affinity over the Cbl–5xPEG–ATTO590 probe which bound to the mutant with KD = 3.8 ± 0.3 μM, demonstrating that PNA binding to a complementary sequence in the aptamer significantly enhances affinity between a weak RNA–Cbl binder.
The Cbl–PNA conjugate accommodates different fluorescent probes
The modular nature of our synthetic method allows us to install any type of alkyne-modified fluorophore at the end of the PNA strand. To demonstrate the application of an alternative fluorophore, we synthesized the PNA probe with the ATTO488 dye (Fig. S3, 4.8, ESI†) and measured its affinity towards a set of RNAs (Fig. 5A). Like the ATTO590 variant, Cbl–PNA–ATTO488 displayed high affinity binding to the complementary full length env8 aptamer (KD 0.12 ± 0.02 nM), the truncated variants, and the env8 mutant (Fig. 5A). The results reveal that the binding affinity is independent of the type of dye, and the probe can be tuned according to spectral needs.The specific fluorophore, however, may have different turn-on properties due to different quenching/dequenching mechanisms. We measured the level of probe quenching in the absence of the RNA and dequenching when the RNA was present and normalized the values to the free fluorophore (Fig. 5B). The Cbl–PNA–ATTO590 probe exhibited a higher level of residual fluorescence in its quenched state (19%) compared to the 5xPEG (5%) or 4xGly (9%) probe, likely due to less efficient quenching possibly caused by the increased length and/or increased rigidity of the PNA linker. Nevertheless, once dequenched, the probe turned on by ∼3-fold, comparable to the probes with neutral chemical linkers (Fig. 5C). In contrast, the probe with ATTO488 dye exhibited improved turn on (4.2-fold) compared to other linkers (Fig. 5C), demonstrating that the quenching/dequenching propensity can be fine-tuned by altering the fluorophore while maintaining a high binding affinity. Fluorescence emission spectra of the probes in the presence and absence of RNA are shown in Fig. S8 (ESI†). To verify whether the PNA linker contributes to the quenching process, we synthesized a PNA–ATTO590 conjugate (Fig. S3, 4.10, ESI†) and tested it against wild type env8 in a fluorescence turn-on assay. No significant turn-on was observed, indicating that cobalamin is responsible and essential for quenching the fluorophore (Fig. S9, ESI†).
Cbl–PNA probes show improved visualization of RNA in live mammalian cells
While the Cbl–PNA–ATTO590 probe performs better in vitro than probes with a neutral linker, we sought to test the probe in live human cells. To accomplish this, we visualized recruitment of mRNA to stress granules (SGs) in live cells; under sodium arsenite stress, cells sequester non-translating cytosolic RNA and proteins into SGs.33 This assay has been widely used to test RNA-tagging tools.13,34–40 U-2 OS cells stably expressing GFP-G3BP1, a SG marker protein,41 were transfected with a plasmid encoding ACTB tagged with four copies of env8-FL-3′antiPNA.42 The cells were subsequently beadloaded with 5 μM solution of Cbl-based probe, imaged on a laser scanning confocal microscope, and the enrichment ratio of the probe in SGs relative to the surrounding cytosol was measured (Fig. 6A and Fig. S10, ESI†). Riboglow probes with PNA, 5xPEG, and 4xGly linkers all showed significantly increased enrichment ratios compared to the negative control (untagged RNA), indicating effective binding and recruitment to SGs. However, probes with PNA and 4xGly linkers exhibited higher enrichment ratios and greater statistical significance compared to the 5xPEG probe (Fig. 6B and C). All images were collected with the same imaging settings and at the same probe concentration, but 4xGly probe was dimmer in cells than the PNA or 5xPEG probes (data not shown). Therefore, the PNA probe outperformed both the 5xPEG and 4xGly probes in the SG assay. The Cbl–PNA–ATTO590 probe is brighter when dequenched and has a higher binding affinity in vitro compared to probes with nonfunctional linkers (Fig. 3G and 5B). To transfer these advantages to an in vivo setting, we decreased the amount of probe 10-fold and performed the SG assay. The 4xGly probe was too dim to analyze the enrichment ratio, so instead we compared the percent colocalization of the probe with SGs. The PNA probe had a much higher percent of cytosolic colocalization with the SG marker compared to 4xGly probe (mean of 58% versus 15%, respectively, Fig. S11, ESI†). The average cytosol intensity of cells with PNA probe was higher in the presence of tagged RNA, showing fluorescence turn-on of the PNA probe upon binding (Fig. 6D). This result is consistent with findings from another study using different Riboglow probes.40 With the same imaging settings, the average cytosol intensity of cells with 4xGly probe did not show a difference in the presence of tagged RNA (Fig. 6D). The increased brightness and higher affinity of the PNA probe enable it to work at low probe concentrations while the probe with nonfunctional 4xGly linker fails.While recruitment of ACTB to SGs has been widely used to evaluate RNA imaging tools,13,35–40,42 a study that purified SGs and sequenced their RNA revealed that only a small percent of endogenous ACTB RNA localizes to SGs.42,43 To increase the dynamic range in the SG assay, we tested (1/2)NORAD, the first half of the NORAD ncRNA which was shown to be strongly localized to SGs during stress.40,44 As a negative control, we examined (1/8)NORAD, the first eighth of the NORAD ncRNA which has weaker SG colocalization. All RNAs used in this assay have the same 4xenv8-FL-3′antiPNA array appended to their 3′ end. The enrichment ratio of the Cbl–PNA–ATTO590 probe increased significantly in the presence of (1/2)NORAD, far above ACTB, but remained unchanged in the presence of (1/8)NORAD (Fig. 6E). In addition to demonstrating that the PNA probe functions with other RNA targets, we show that (1/2)NORAD is a more robust target than ACTB in the SG assay, leading to higher enrichment in SGs, as has been shown recently with FLIM and other Cbl probes.40
Because the RNA aptamer does not bind the fluorophore directly, the fluorophore can be easily exchanged. The Cbl–PNA–ATTO488 probe performs on par with the Cbl–PNA–ATTO590 in vitro, so we also tested Cbl–PNA–ATTO488 probe in live cells. Cbl–PNA–ATTO488 performs similarly to Cbl–PNA–ATTO590 and shows increased enrichment in SGs in the presence of (1/2)NORAD-4xenv8-FL-3′antiPNA (Fig. 6F). As was shown with the in vitro experiments, the probe fluorophore can be switched without altering the functionality of the system in vivo.
The Cbl–PNA–ATTO590 probe confers more than 200-fold increased affinity in vitro for the truncated aptamer by base-pairing with a complementary region (Fig. 3C–G). Therefore, we tested whether PNA could provide a distinct advantage for tracking RNAs with a truncated aptamer in live cells using a U-body assay. Under thapsigargin stress, cells accumulate small noncoding U-rich spliceosomal RNAs and associated proteins into cytoplasmic U-bodies.45,46 Previously, we used the truncated aptamer env8-AD-U1 and a Cbl–5xPEG–ATTO590 probe to track enrichment of the U1 snRNA into U-bodies upon stress.13 Importantly, this small snRNA cannot tolerate large fusions without perturbing its processing,47 making the truncated aptamer essential. In this U-body assay, we transfected U-2 OS cells with GFP-SMN1, a U-body marker protein,45 and tagged U1 with either env8-AD or env8-AD-5′antiPNA. The cells were then beadloaded with 50 μM Cbl-PNA-ATTO590 probe, imaged using a laser scanning confocal microscope, and analyzed for cytosolic colocalization of the PNA probe with the U-body marker (Fig. 7A and Fig. S12, ESI†). A significantly higher percentage of U-bodies could be visualized with the PNA probe in the presence of env8-AD-5′antiPNA-U1 compared to env8-AD-U1. Cells with env8-AD-5′antiPNA showed high cytoslic colocalization between PNA probe and U-bodies, although it should be noted that the Riboglow probe always shows some fluorescence signal in the nucleus (Fig. 7B and C). Binding of the Cbl portion alone (env8-AD-U1) is sufficient to observe colocalization, but the further increase in colocalization in cells transfected with RNA capable of engaging the PNA linker (env8-AD-5′antiPNA-U1) strongly suggests that the PNA probe's sequence-specific binding behavior observed in vitro is recapitulated in live cells.
Discussion
Linkers are vital in chemical biology, connecting functional groups, molecules, and biomolecules to form complex, multifunctional systems crucial for studying biological processes. However, their role extends well beyond mere connectivity, as their intrinsic chemical properties can be leveraged to enhance the stability, reactivity, and functionality of these systems.14 In our work, we explored the possibility of improving the affinity and specificity of chemical probes targeting RNA by incorporating a linker capable of interacting with the RNA target with high specificity. To achieve this, we integrated a PNA-based linker into the small-molecule probe which functions through contact-quenching. The PNA linker serves as an additional anchoring point for the probe, augmenting the RNA–ligand (Cbl) structure-based recognition. Therefore, by incorporating the chemical functionality within the linker, we enable two distinct binding events to operate in concert, resulting in increased affinity and specificity for the RNA imaging platform.Significantly tighter binding was observed for PNA probes compared to probes with neutral linkers, and the combined involvement of the two binding interactions (Cbl–RNA and PNA linker-complementary RNA) was confirmed through chemical probing. The difference in affinity was even more pronounced when the RNA aptamer was truncated. Additionally, the effect was preserved regardless of whether the PNA binding site is installed at the 5′ or 3′ end of the aptamer, indicating the adaptability of the approach. In vivo studies demonstrated the advantages of PNA probes, allowing for more robust visualization of RNA recruitment to stress granules and U-bodies. The increased affinity and brightness allowed the PNA probes to be used at much lower concentrations compared to those with non-functional linkers. Additionally, we have shown that the PNA–RNA sequence-specific interaction observed in vitro is also successfully occurring in live cells. We demonstrated that a linker as short as six nucleobases can effectively restore binding to a significantly impaired aptamer, increasing affinity by over 300-fold compared to a probe with a non-functional 5xPEG linker. This proves that specificity of the probe can be driven by the PNA linker. Moreover, this property can be utilized to achieve orthogonality by designing multiple aptamers with significantly different affinities and targeting them selectively with distinct PNA probes. This strategy paves the way for simultaneous multicolor tracking of various RNA types, a concept we intend to investigate further in the future.
The Riboglow platform can be readily adapted, and with the installation of the PNA linker, its versatility expands even further. We have demonstrated that both the PNA linker and the fluorophore can be modified while preserving high affinity and specificity. Additionally, the RNA tag itself can be altered to match the sequence present within the linker and/or modify the ligand interaction. However, there is still room for improvement and exploration. In this context, special emphasis can be placed on the modularity of PNAs, as their capabilities are not limited to the selective recognition of single-stranded RNAs. PNAs can be chemically modified48 and altered nucleobases can serve as a handle for bioactive molecule installation (e.g. fluorophore).49 Specifically designed PNAs can recognize double-stranded regions (triplex formation), form triplex invasion structures, duplex invasion, and double-duplex invasion structures.50–53 Furthermore, the synthetic nature of PNA allows for non-canonical pairings to optimize PNA–RNA interactions.54 Therefore, we believe that combining a small molecule with a PNA strand has the potential to enhance existing RNA imaging tools that rely on collisional quenching, as well as inspire the development of new ones.
In this work we establish a new approach for improving RNA imaging tools and demonstrate the potential for using short oligonucleotides conjugated to small molecules to improve affinity and specificity of chemical probes targeting RNA. Apart from new opportunities to improve RNA imaging tools, we speculate that the approach described here could also be exploited in RNA-targeted drug development. Though much effort has been devoted to targeting RNA, the field still remains in its infancy.55–57 Two main therapeutic strategies including antisense oligonucleotides (∼20–30 nucleotides) and small molecules have been employed to target disease-causing RNAs.58,59 Among small molecules, bifunctional structures such as dimeric miRNA binders enhance potency and selectivity by simultaneously targeting a functional site and an adjacent druggable motif.58 On the other hand, RNA cleavers and degraders, such as bleomycin A5-conjugates and RIBOTACs, offer novel therapeutic modalities to reduce levels of disease-causing RNAs.58 These examples support the importance of introducing more than one functionality within the RNA-targeting molecule to achieve the desired effects. Similarly, relying on just one binding event may not be sufficient and could result in binding promiscuity. Synthetic oligonucleotides open new possibilities in this area and can significantly improve specific RNA targeting. A recent example of PNA-based fluorogenic probes that sense the panhandle structure of the influenza A virus (IAV) RNA promoter region utilizes the PNA's ability to form triplexes with double-stranded RNA region.49 Consistent with our findings, conjugation of 8-mer PNA sequence equipped with a fluorogenic nucleobase to 7-dimethoxy-2-(1-piperazinyl)-4-quinazolinamine (DPQ) improved affinity toward RNA compared to the small molecule alone. Therefore, in the pursuit of more defined RNA-small molecule interactions, not only for imaging purposes, the combination of two different binding events operating in concert is essential. We foresee that the combination of a small-molecule targeting RNA via structure-based recognition, along with a sequence-specific synthetic oligonucleotide serving as an additional anchoring point, could advance the field of RNA drugging further.
Conclusions
Our work demonstrates the potential of using short peptide nucleic acid-based linkers to enhance RNA-targeting chemical probes. By integrating a PNA linker into the Riboglow platform, we achieved significant improvements in probe affinity and specificity through dual binding events. The PNA linker allows for stronger and more specific binding, enhancing probe performance both in vitro and in vivo. These improvements enable lower probe concentrations and more robust visualization of RNA dynamics in cellular environments.Our findings highlight the versatility of PNA linkers in RNA imaging platforms and suggest broader applications, including the design of orthogonal probes for multicolor tracking of different RNA species. Additionally, the adaptability of the Riboglow platform, combined with the tunable nature of PNAs, provides a pathway for further enhancing RNA imaging tools and developing new strategies for targeting RNA.
We also propose that this approach can be extended beyond imaging to RNA-targeted drug development. The ability to combine multiple binding interactions within a single probe opens new avenues for improving the selectivity and potency of RNA-targeting molecules. This dual-binding approach represents a promising strategy for advancing RNA drugging and holds potential for future innovations in both research and therapeutic applications.
Materials and methods
Probe synthesis
Synthesis and characterization of fluorescent probes are described in ESI:† 4 probe synthesis and characterization.RNA synthesis
For all in vitro experiments, RNAs were synthesized using template DNA amplified through PCR. DNA templates were amplified using a series of overlapping oligonucleotides ordered from IDT (Table S2, ESI†). Transcriptions used T7 RNA polymerase (for details see Table S3, ESI†).60 The RNAs were purified using denaturing PAGE (8% acrylamide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bisacrylamide 29
bisacrylamide 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8 M urea, 1× Tris–Borate–EDTA (TBE) buffer). For details see ESI:† Methods 3.1. In vitro transcription of wild type env8 RNA led to two major products. Analysis of Cbl binding to the upper band, the lower band, or a mixture of two bands revealed the lower band to be the correct species (Fig. S13, ESI†). All subsequent binding experiments were conducted with the purified lower band.
1, 8 M urea, 1× Tris–Borate–EDTA (TBE) buffer). For details see ESI:† Methods 3.1. In vitro transcription of wild type env8 RNA led to two major products. Analysis of Cbl binding to the upper band, the lower band, or a mixture of two bands revealed the lower band to be the correct species (Fig. S13, ESI†). All subsequent binding experiments were conducted with the purified lower band.
SHAPE experiments
Chemical structure probing was performed using N-methylisatoic anhydride (NMIA) as previously described31 with modifications. RNAs were synthesized with additional 5′ and 3′ sequences, called the SHAPE cassette, for purposes of reverse transcription and visualization.31 RNAs were refolded by heating at 90 °C for 3 minutes and placed on ice for a minimum of 10 minutes. RNA was added to folding buffer (333 mM Na-HEPES pH 8.0, 333 mM NaCl, 20 mM MgCl2) along with a ligand (dissolved in DMSO) and incubated at room temperature for 10 minutes before adding NMIA dissolved in DMSO or neat DMSO for control reactions. The final 10 μL probing reactions had concentrations of 100 nM RNA, 100 mM Na-HEPES pH 8.0, 100 mM NaCl, 6 mM MgCl2, 30 μM ligand unless stated otherwise, and 13 mM NMIA. Probing reactions were incubated at 37 °C for 41 minutes (5 half-lives). Following probing, reactions were moved straight into primer extension using reverse transcriptase as previously described61 without ethanol precipitation. A 32P 5′-end labeled reverse transcription primer complementary to the 3′ SHAPE cassette was used (Table S2, ESI†) along with the enzyme mix (250 mM KCl, 167 mM Tris–HCl at pH 8.3, 1.67 mM each dNTP, 17 mM DTT, 0.33 units Superscript III).61 Products were run on a 12% denaturing polyacrylamide gel at 55 W for several (4–6) hours until the areas of interest could be resolved. Gels were exposed to a storage phosphor screen at least overnight and for up to two weeks, and screens were imaged using a Typhoon FLA 9500 PhosphoroImager (GE) or an Amersham Typhoon 5 (Cytiva). Gel images were straightened and quantified by the SAFA software package.62 Data were also normalized by the software, using the built-in invariant residue determination. This normalizes all sequencing gel band intensities to those of nucleotides which do not show any change in response to ligand, to account for any loading differences. Finally, degree of protection (DOP) was calculated using the equation DOP = −((C − N)/N) where C is the sum of all bands of a region of interest in the Cbl lane and N is the sum of all bands of a region of interest in the NMIA lane.Fluorescence turn-on experiments
All in vitro experiments were conducted in RNA buffer (100 mM KCl, 10 mM NaCl, 1 mM MgCl2, 50 mM HEPES, pH 8.0) with the addition of 0.01% nonidet P40 and 10% DMSO (v/v). For the Cbl-fluorophore probes, the extinction coefficient of the fluorophores was used to determine the concentration (120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 for ATTO590 and 90
000 M−1 cm−1 for ATTO590 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 for ATTO488, source: ATTO-TEC). To determine the binding affinity of each probe to the RNA of interest, a series of titration experiments were performed. Relevant amounts of RNA were titrated into 120 μL reactions into Eppendorf tubes, ensuring a final concentration of 1 nM cobalamin probe. For details see ESI:† Methods 3.2 and Table S4 (ESI†). The fold turn-on of 1 nM probes was calculated by dividing the fluorescence value obtained from titration point at cRNA = 50 nM (for Cbl–PNA–ATTO590 and Cbl–PNA–ATTO488) and cRNA = 500 nM (for Cbl–5xPEG–ATTO590 and Cbl–4xGly–ATTO590) by the fluorescence value obtained for the free probe. Same fluorescence values were used to determine quenching/dequenching levels for each probe. Quenching levels were derived from titration point at cRNA = 50 nM (for Cbl–PNA–ATTO590 and Cbl–PNA–ATTO488) and cRNA = 500 nM (for Cbl–5xPEG–ATTO590 and Cbl–4xGly–ATTO590) while dequenching levels from fluorescence values obtained for free probe at 1 nM. Each value was divided by the fluorescence level obtained for the free fluorophore (cfree
000 M−1 cm−1 for ATTO488, source: ATTO-TEC). To determine the binding affinity of each probe to the RNA of interest, a series of titration experiments were performed. Relevant amounts of RNA were titrated into 120 μL reactions into Eppendorf tubes, ensuring a final concentration of 1 nM cobalamin probe. For details see ESI:† Methods 3.2 and Table S4 (ESI†). The fold turn-on of 1 nM probes was calculated by dividing the fluorescence value obtained from titration point at cRNA = 50 nM (for Cbl–PNA–ATTO590 and Cbl–PNA–ATTO488) and cRNA = 500 nM (for Cbl–5xPEG–ATTO590 and Cbl–4xGly–ATTO590) by the fluorescence value obtained for the free probe. Same fluorescence values were used to determine quenching/dequenching levels for each probe. Quenching levels were derived from titration point at cRNA = 50 nM (for Cbl–PNA–ATTO590 and Cbl–PNA–ATTO488) and cRNA = 500 nM (for Cbl–5xPEG–ATTO590 and Cbl–4xGly–ATTO590) while dequenching levels from fluorescence values obtained for free probe at 1 nM. Each value was divided by the fluorescence level obtained for the free fluorophore (cfree![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dye = 1 nM) to obtain quenching/dequenching levels. Data were normalized to values between 0 and 100, where 0 was the value of the lower baseline and 100 was the value of the upper baseline.
dye = 1 nM) to obtain quenching/dequenching levels. Data were normalized to values between 0 and 100, where 0 was the value of the lower baseline and 100 was the value of the upper baseline.
Cloning plasmids for mammalian expression
To create PB-HaloTag-ACTB-0x, pUC57-HaloTag-ACTB-18xenv8-FL was ordered as a custom plasmid from GeneWiz. Lyophilized plasmid was dissolved and transformed into C3040 recombinase-deficient E. coli chemically competent cells (NEB). Colonies were selected on LB + Amp agar plates and plasmid was isolated for downstream use (Qiagen Midiprep Kit). pUC57-HaloTag-ACTB-18xenv8-FL and PB510B-1 (System Biosciences) were digested with the unique restriction sites SwaI and XbaI (NEB). Fragments were gel purified (Macherey Nagel Gel and PCR Cleanup Kit) and ligated with T4 DNA ligase (NEB). The ligation reaction was then transformed into C3040 cells and selected on LB + Amp agar plates. Colonies were screened for the insertion and sent for sequencing. PB-HaloTag-ACTB-18xenv8 was then digested with the unique restriction sites BlpI and PmeI (NEB) to remove the 18xenv8 fragment, blunted with DNA polymerase I Klenow fragment (Thermo Fisher Scientific), and ligated with T4 DNA ligase. The ligation reaction was then transformed into C3040 cells and selected on LB + Amp agar plates. Colonies were screened for deletion of the array and sent for whole plasmid nanopore sequencing.To create env8-AD-5′antiPNA-U1, env8-AD-U1 (Addgene plasmid #112059) was first transformed into C2925 dam-/dcm-E. coli chemically competent cells (NEB) and selected on LB + Kan agar plates. Plasmid was isolated for downstream use. A custom gBlock of env8-AD-5′antiPNA and primers to amplify it were ordered from IDT (Table S2, ESI†). env8-AD-5′antiPNA was PCR amplified, ethanol precipitated, digested with the unique restriction sites BglII and BclI (NEB), and purified. env8-AD-U1 was digested with the unique restriction sites BglII and BclI, CIP (NEB) treated, and gel purified. Fragments were ligated with T7 DNA ligase (NEB), transformed into Stellar E. coli chemically competent cells (Takara Bio), and selected on LB + Kan agar plates. Colonies were screened and sent for whole plasmid nanopore sequencing.
Cell lines and cell culture
U-2 OS cells were obtained from ATCC (ATCC number HTB-96) and cultured in McCoy's 5A medium supplemented with 10% FBS and 1% P/S. Cells were routinely passaged into 10-cm cell culture-treated plates before they reached confluency and were mycoplasma free for the course of these experiments. All cells used in experiments were used on or before their 25th passage. The GFP-G3BP1 stable U-2 OS cells and the HaloTag-G3BP1 CRISPR stable U-2 OS cells were generated previously.13Stress granule (SG) assay and U-body assay
For details on assay setup, imaging and analysis see ESI:† Methods 3.3–3.6 and Table S5 (ESI†).Author contributions
AJW: investigation and methodology (synthesis and characterization of the probes, RNA synthesis and purification, fluorescence turn-on assay, data analysis). EMR: investigation and methodology (preparation of in vivo constructs, in vivo imaging and quantification). SRL: investigation and methodology (RNA synthesis and purification, SHAPE experiments and quantification). AJW, RTB, AEP: conceptualization of the project. AJW, EMR, SRL: writing – original draft. AJW, RTB, AEP: writing – review and editing. RTB, AEP: supervision, funding acquisition, project administration.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare the following competing financial interest(s): R. T. B. serves on the Scientific Advisory Boards of Expansion Therapeutics, SomaLogic, and MeiraGTx.Acknowledgements
We would like to acknowledge the following financial support: R01 GM133184 (AEP and RTB), R35 GM139644 (AEP), R35 GM152029 (RTB), T32 GM065103 (EMR, SRL), F31 5F31ES033919 (EMR), Polish National Agency for Academic Exchange PPN/BEK/2020/1/00219/U/0000 (AJW). We thank the Shared Instruments Pool (RRID: SCR_018986) of the Department of Biochemistry at the University of Colorado Boulder for the use of the Typhoon 5. The Typhoon 5 is funded by NIH Shared Instrumentation Grant S10OD034218-01. The imaging work was performed at the BioFrontiers Institute's Advanced Light Microscopy Core (RRID: SCR_018302). Laser scanning confocal microscopy was performed on an Nikon A1R microscope supported by NIST-CU Cooperative Agreement award number 70NANB15H226. We would like to thank Dr Joe Dragavon of the BioFrontiers Institute Advanced Light Microscopy Core for assistance with microscopy. We would like to acknowledge the University of Colorado Biochemistry Cell Culture Core Facility, especially Dr Theresa Nahreini, for providing resources and support for all our cell work. We would like to thank Dr Esther Braselmann for helpful comments regarding the stress granule assay.References
- K. M. Dean and A. E. Palmer, Nat. Chem. Biol., 2014, 10, 512–523 CrossRef PubMed.
- E. Tutucci, N. M. Livingston, R. H. Singer and B. Wu, Annu. Rev. Biophys., 2018, 47, 85–106 CrossRef PubMed.
- E. Tutucci, M. Vera, J. Biswas, J. Garcia, R. Parker and R. H. Singer, Nat. Methods, 2018, 15, 81–89 CrossRef PubMed.
- B. Wu, V. Miskolci, H. Sato, E. Tutucci, C. A. Kenworthy, S. K. Donnelly, Y. J. Yoon, D. Cox, R. H. Singer and L. Hodgson, Genes Dev., 2015, 29, 876–886 CrossRef PubMed.
- J. F. Garcia and R. Parker, RNA, 2016, 22, 657–659 CrossRef PubMed.
- J. F. Garcia and R. Parker, RNA, 2015, 21, 1393–1395 CrossRef PubMed.
- E. Braselmann, C. Rathbun, E. M. Richards and A. E. Palmer, Cell Chem. Biol., 2020, 27, 891–903 CrossRef PubMed.
- P. Le, N. Ahmed and G. W. Yeo, Nat. Cell Biol., 2022, 24, 815–824 CrossRef PubMed.
- M. Sunbul, J. Lackner, A. Martin, D. Englert, B. Hacene, F. Grün, K. Nienhaus, G. U. Nienhaus and A. Jäschke, Nat. Biotechnol., 2021, 39, 686–690 CrossRef PubMed.
- M. Sunbul and A. Jäschke, Angew. Chem., Int. Ed., 2013, 52, 13401–13404 CrossRef PubMed.
- A. Murata, S. Ichi Sato, Y. Kawazoe and M. Uesugi, Chem. Commun., 2011, 47, 4712–4714 RSC.
- A. Arora, M. Sunbul and A. Jäschke, Nucleic Acids Res., 2015, 43(21), e144 Search PubMed.
- E. Braselmann, A. J. Wierzba, J. T. Polaski, M. Chromiński, Z. E. Holmes, S. T. Hung, D. Batan, J. R. Wheeler, R. Parker, R. Jimenez, D. Gryko, R. T. Batey and A. E. Palmer, Nat. Chem. Biol., 2018, 14, 964–971 CrossRef CAS PubMed.
- X. Yang, Z. Pan, M. R. Choudhury, Z. Yuan, A. Anifowose, B. Yu, W. Wang and B. Wang, Med. Res. Rev., 2020, 40, 2682–2713 CrossRef PubMed.
- C. Borsari, D. J. Trader, A. Tait and M. P. Costi, J. Med. Chem., 2020, 63, 1908–1928 CrossRef CAS PubMed.
- G. Leriche, L. Chisholm and A. Wagner, Bioorg. Med. Chem., 2012, 20, 571–582 CrossRef CAS PubMed.
- J. Saarbach, P. M. Sabale and N. Winssinger, Curr. Opin. Chem. Biol., 2019, 52, 112–124 CrossRef CAS.
- R. Brazil, ACS Cent. Sci., 2023, 9, 3–6 CrossRef CAS PubMed.
- P. E. Nielsen, M. Egholm, R. H. Berg and O. Buchardt, Science, 1991, 254, 1497–1500 CrossRef CAS PubMed.
- T. Vilaivan, Beilstein J. Org. Chem., 2018, 14, 253–281 CrossRef CAS PubMed.
- U. Koppelhus and P. E. Nielsen, Adv. Drug Delivery Rev., 2003, 55, 267–280 CrossRef CAS.
- U. Tsylents, I. Siekierska and J. Trylska, Eur. Biophys. J., 2023, 52, 533–544 CrossRef CAS.
- M. Hawner and C. Ducho, Cellular Targeting of Oligonucleotides by Conjugation with Small Molecules, 2020, vol. 25 Search PubMed.
- M. Równicki, M. Wojciechowska, A. J. Wierzba, J. Czarnecki, D. Bartosik, D. Gryko and J. Trylska, Sci. Rep., 2017, 7, 7644 CrossRef PubMed.
- M. Równicki, Z. Dąbrowska, M. Wojciechowska, A. J. Wierzba, K. Maximova, D. Gryko and J. Trylska, ACS Omega, 2019, 4, 819–824 CrossRef.
- M. Giedyk, A. Jackowska, M. Równicki, M. Kolanowska, J. Trylska and D. Gryko, Chem. Commun., 2019, 55, 763–766 RSC.
- T. Pieńko, J. Czarnecki, M. Równicki, M. Wojciechowska, A. J. Wierzba, D. Gryko, D. Bartosik and J. Trylska, Biophys. J., 2021, 120(4), 725–737 CrossRef.
- A. J. Wierzba, M. Wojciechowska, J. Trylska and D. Gryko, in Vitamin B12 - Peptide Nucleic Acid Conjugates, ed. W. M. Hussein, R. J. Stephenson and I. Toth, Springer US, New York, NY, 2021, pp. 65–82 Search PubMed.
- J. E. Johnson Jr, F. E. Reyes, J. T. Polaski and R. T. Batey, Nature, 2012, 492, 133–137 CrossRef PubMed.
- E. J. Merino, K. A. Wilkinson, J. L. Coughlan and K. M. Weeks, J. Am. Chem. Soc., 2005, 127, 4223–4231 CrossRef.
- K. A. Wilkinson, E. J. Merino and K. M. Weeks, Nat. Protoc., 2006, 1, 1610–1616 CrossRef PubMed.
- I. Jarmoskaite, I. Alsadhan, P. P. Vaidyanathan and D. Herschlag, Elife, 2020, 9, 1–34 CrossRef.
- H. Unworth, S. Raguz, H. J. Edwards, C. F. Higgins and E. Yagüe, FASEB J., 2010, 24, 3370–3380 CrossRef PubMed.
- S. Mollet, N. Cougot, A. Wilczynska, F. Dautry, M. Kress, E. Bertrand and D. Weil, Mol. Biol. Cell, 2008, 19, 4469–4479 CrossRef.
- D. A. Nelles, M. Y. Fang, M. R. O’Connell, J. L. Xu, S. J. Markmiller, J. A. Doudna and G. W. Yeo, Cell, 2016, 165, 488–496 CrossRef.
- C. Zurla, A. W. Lifland and P. J. Santangelo, PLoS One, 2011, 6(5), e19727 CrossRef.
- J. Wu, S. Zaccara, D. Khuperkar, H. Kim, M. E. Tanenbaum and S. R. Jaffrey, Nat. Methods, 2019, 16, 862–865 CrossRef PubMed.
- X. Chen, D. Zhang, N. Su, B. Bao, X. Xie, F. Zuo, L. Yang, H. Wang, L. Jiang, Q. Lin, M. Fang, N. Li, X. Hua, Z. Chen, C. Bao, J. Xu, W. Du, L. Zhang, Y. Zhao, L. Zhu, J. Loscalzo and Y. Yang, Nat. Biotechnol., 2019, 37, 1287–1293 CrossRef.
- X. Li, H. Kim, J. L. Litke, J. Wu and S. R. Jaffrey, Angew. Chem., Int. Ed., 2020, 59, 4511–4518 CrossRef.
- N. Sarfraz, E. Moscoso, T. Oertel, H. J. Lee, S. Ranjit and E. Braselmann, Nat. Commun., 2023, 14, 1–9 Search PubMed.
- H. Tourrière, K. Chebli, L. Zekri, B. Courselaud, J. M. Blanchard, E. Bertrand and J. Tazi, J. Cell Biol., 2023, 222, e200212128072023new CrossRef PubMed.
- Q. Wang, F. Xiao, H. Su, H. Liu, J. Xu, H. Tang, S. Qin, Z. Fang, Z. Lu, J. Wu, X. Weng and X. Zhou, Nucleic Acids Res., 2022, 50(14), e84 CrossRef CAS PubMed.
- A. Khong, T. Matheny, S. Jain, S. F. Mitchell, J. R. Wheeler and R. Parker, Mol. Cell, 2017, 68, 808–820.e5 CrossRef.
- T. Matheny, B. van Treeck, T. N. Huynh and R. Parker, RNA, 2021, 27, 174–189 CrossRef.
- J. L. Liu and J. G. Gall, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 11655–11659 CrossRef.
- J. Tsalikis, I. Tattoli, A. Ling, M. T. Sorbara, D. O. Croitoru, D. J. Philpott and S. E. Girardin, J. Biol. Chem., 2015, 290, 20904–20918 CrossRef PubMed.
- A. McCloskey, I. Taniguchi, K. Shinmyozu and M. Ohno, Science, 2012, 335, 1643–1646 CrossRef.
- G. Singh and V. Monga, Bioorg. Chem., 2023, 141, 106860 CrossRef PubMed.
- Y. Sato, H. Miura, T. Tanabe, C. U. Okeke, A. Kikuchi and S. Nishizawa, Anal. Chem., 2022, 94, 7814–7822 CrossRef.
- X. Zhan, L. Deng and G. Chen, Biopolymers, 2021, 113(2), e23476 CrossRef PubMed.
- J. Lohse, O. Dahl and P. E. Nielsen, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 11804–11808 CrossRef PubMed.
- T. Bentin, H. J. Larsen and P. E. Nielsen, Biochemistry, 2003, 42, 13987–13995 CrossRef.
- Y. Liang, S. Willey, Y.-C. Chung, Y.-M. Lo, S. Miao, S. Rundell, L.-C. Tu and D. Bong, Nat. Commun., 2023, 14, 2987 CrossRef PubMed.
- A. Kiliszek, K. Banaszak, Z. Dauter and W. Rypniewski, Nucleic Acids Res., 2015, 44, 1937–1943 CrossRef PubMed.
- J. P. Falese, A. Donlic and A. E. Hargrove, Chem. Soc. Rev., 2021, 50, 2224–2243 RSC.
- J. L. Childs-Disney, X. Yang, Q. M. R. Gibaut, Y. Tong, R. T. Batey and M. D. Disney, Nat. Rev. Drug Discovery, 2022, 21, 736–762 CrossRef PubMed.
- Y. Shao and Q. C. Zhang, Essays Biochem., 2020, 64, 955–966 CrossRef.
- S. M. Meyer, C. C. Williams, Y. Akahori, T. Tanaka, H. Aikawa, Y. Tong, J. L. Childs-Disney and M. D. Disney, Chem. Soc. Rev., 2020, 49, 7167–7199 RSC.
- S. P. Pradeep, S. Malik, F. J. Slack and R. Bahal, RNA, 2023, 29, 434–445 CrossRef.
- A. L. Edwards, A. D. Garst and R. T. Batey, in Determining Structures of RNA Aptamers and Riboswitches by X-Ray Crystallography, ed. G. Mayer, Humana Press, Totowa, NJ, 2009, pp. 135–163 Search PubMed.
- C. D. Stoddard, S. D. Gilbert and R. T. Batey, RNA, 2008, 14, 675–684 CrossRef.
- R. Das, A. Laederach, S. M. Pearlman, D. Herschlag and R. B. Altman, RNA, 2005, 11, 344–354 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cb00274a |
| This journal is © The Royal Society of Chemistry 2025 |